1. Introduction
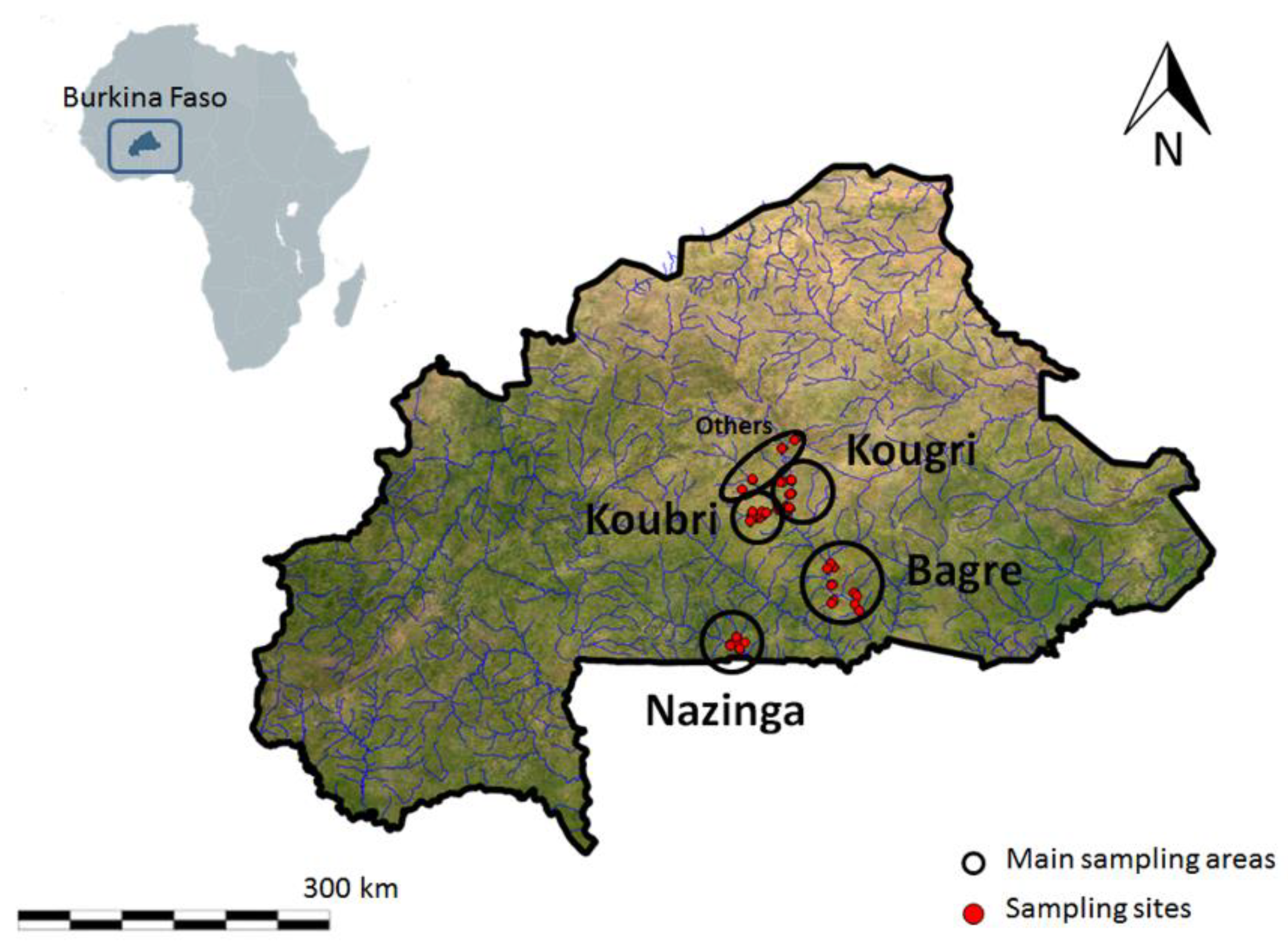
The Upper Volta basin is located in Burkina Faso, a sub-Saharan landlocked country in the central part of West Africa. It is one of the poorest and the least developed countries in the world, and rising population density is driving an increasing demand for fish protein and is jeopardizing it as an important source for food security. The country has a tropical climate with two seasons. In the rainy season, from May/June to September, the country receives between 600 and 1000 mm of rainfall per year, decreasing with latitude. In the dry season, high temperatures (up to 45 °C) result in massive losses of water due to high evapotranspiration rates (2000 mm/year) [
1,
2,
3]. This leads to temporal changes in freshwater habitat availability in terms of quantity and quality [
4].
The natural habitat conditions are altered by human activities. Frequent pressures on African water bodies include (1) water shortage and water abstraction, (2) pollution (suspended solids, solid wastes, organic and agricultural chemicals), (3) damming, (4) deforestation, and (5) overfishing [
5,
6,
7]. Similar pressures are also reported in Burkina’s water bodies, e.g., over abstraction, reservoir degradation, and pollution with agricultural chemicals [
8]. The Volta Basin has lost almost 97% of its original forest cover [
7,
9], which is a good indicator for watershed degeneration [
10]. As a reaction to severe droughts in Burkina Faso, more than 1400 reservoirs of 1 to 25,000 ha surface area were built since 1950. These are mainly used for agriculture, livestock farming, and fisheries [
3]. About 85% of all built reservoirs are smaller than one million cubic meters (Mm
3), whereas the two largest ones contribute to more than 60% of the national capacity [
11]. The construction of reservoirs increased fishery landings by 15 times since 1950, employing more than 30,000 fishermen and several thousand women for processing and selling the fish [
3]. According to Boelee [
12] and Mahe et al. [
13], more than 50% of the annual discharge of the Nakanbé basin is held in these reservoirs by dams. Many reservoirs are not passable for fish due to lacking or poorly built fish passages [
3]. Moreover, large reservoirs have severe downstream effects such as altered flow and sediment regime, a change of water parameters (temperature, oxygen, suspended solids, etc.), erosion, missing flood pulses, decreased fish recruitment, and disturbed river biota [
14,
15,
16].
The specific composition of communities is mainly influenced by the interaction between animals and their biotic and abiotic environments [
17]. Fish habitat encompasses a variety of physical, biological, and chemical features of the environment that affect assemblages, populations, and individuals [
18]. The understanding of these interrelations also is crucial with regard to future changes caused by climate change [
19,
20,
21].
The loss of habitat and human pressures led to a decline in fish populations in terms of total population, biodiversity, and average fish size [
3,
22]. Native fish species are well adapted to their environment to an extent that some species use the entire river continuum from the headwaters to the estuaries within their life cycle [
15]. Because fish cannot easily migrate between aquatic systems, they have to adapt to changing conditions or die. Therefore, they are potential indicators of environmental changes and trends in general aquatic biodiversity, since they interact with other aquatic organisms via predation, nutrient input, and mechanical effects [
4]. The knowledge of essential fish habitat for spawning, breeding, feeding, or growth to maturity is an important management tool for a healthy ecosystem and sustainable fisheries [
23,
24]. Thus far, for Burkinabe and Sub-Sahelian fish species, little knowledge exists about fish assemblages regarding their composition, their habitat preferences, or their sensitivity to or tolerance of human pressures [
3,
25]. Hugueny et al. [
26] noted, already 20 years ago, that fish must become a well-established indicator to support better management that secures an important source of food (protein), especially for Burkina Faso and other less developed countries.
This study highlights important findings and generalizations regarding the roles of abiotic factors and human pressures that influence fish community composition and abundances for sub-Sahelian countries. The specific objectives are: (1) to describe fish assemblages, map available habitat parameters, and link them to habitat use by four key species, and (2) to identify typical human impacts on aquatic habitats and associated fish assemblages by proving the response of fish to human induced pressure impacts, eventually leading to modern fisheries management.
4. Discussion
In Burkina Faso and globally, fish are a very important protein source, and their sustainable management continues to be a long-term goal. Based on measurements in Burkina Faso, we introduce, for the first time, species lists and indices as management tools that link characteristics and presence of fish species and assemblages with associated characteristics of habitat and human pressures.
4.1. Fish Assemblages and Associated Habitats
By sampling more than 69 fish species and 34 genera, we defined the composition of distinct fish assemblages in 157 habitats around Burkina Faso undergoing different kinds and levels of human pressure. Through quantification, we linked freshwater habitat conditions with their effects on fish community composition and abundances at the beginning of the dry season. Most of the Sahelian fish species spawn in the wet season [
4]. This is also reflected in the average fish size of the caught species indicating that most of them were juveniles. Indices based on such links allow managers to detect challenges from habitat (aquatic and terrestrial) and human pressures as well as fish assemblages and develop policies for managing them.
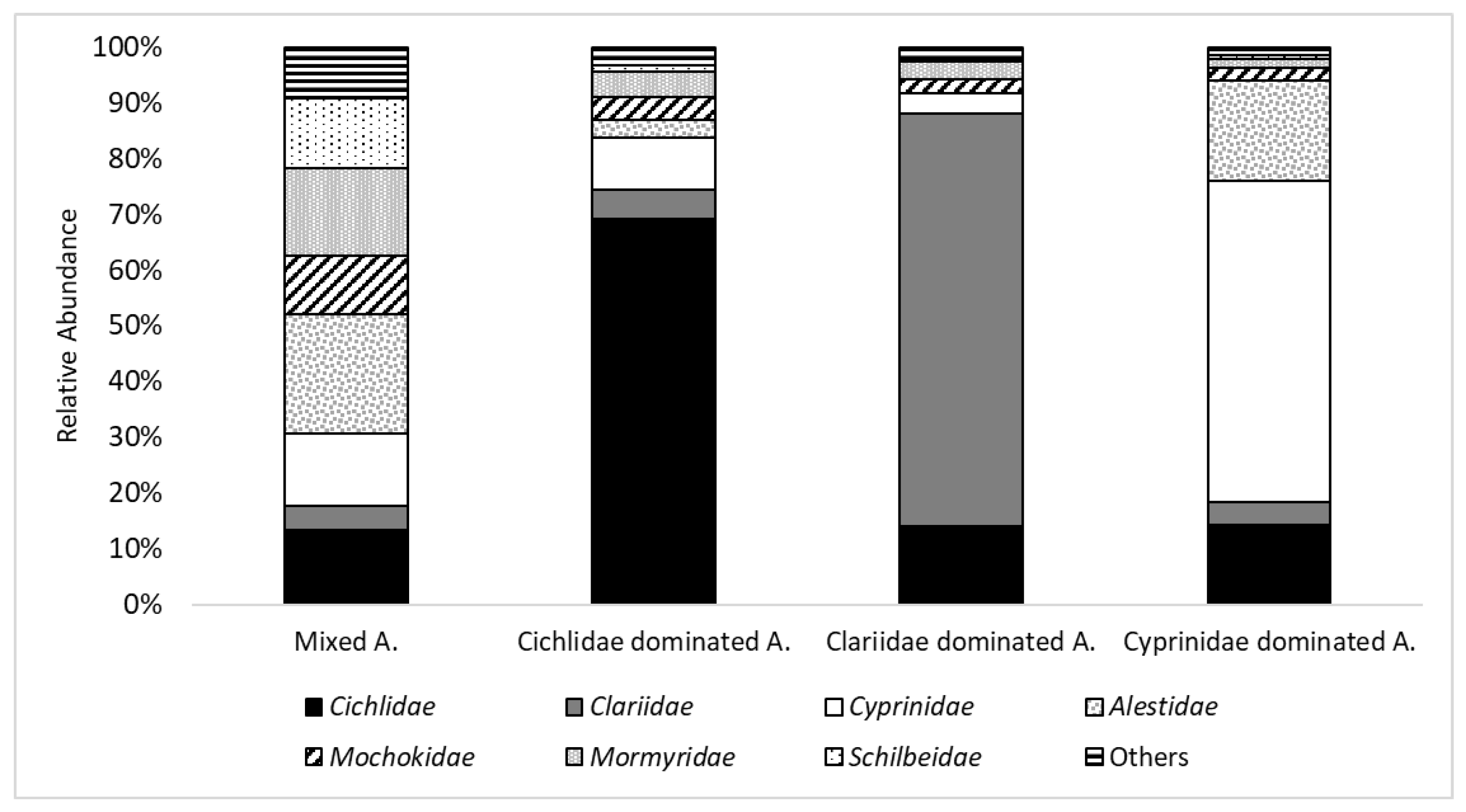
The four discrete fish community assemblages describe the typically observed co-existence of Burkinabe fish species in distinct clusters. One is dominated by Alestidae, one by Cichlidae, one by Clariidae, and one by Cyprinidae. Further studies that analyze the parameters and the relationships determining the community’s structure should consider such factors as: habitat parameters, feeding types, predator–prey relations, and human impacts. Species found in the same assemblage indicate similar requirements/tolerances for their biotic and/or abiotic environment. Further study in Burkina Faso is also mandated by the fact that fully one third of species with red list status have not yet been classified at the world level, much less at the national level.
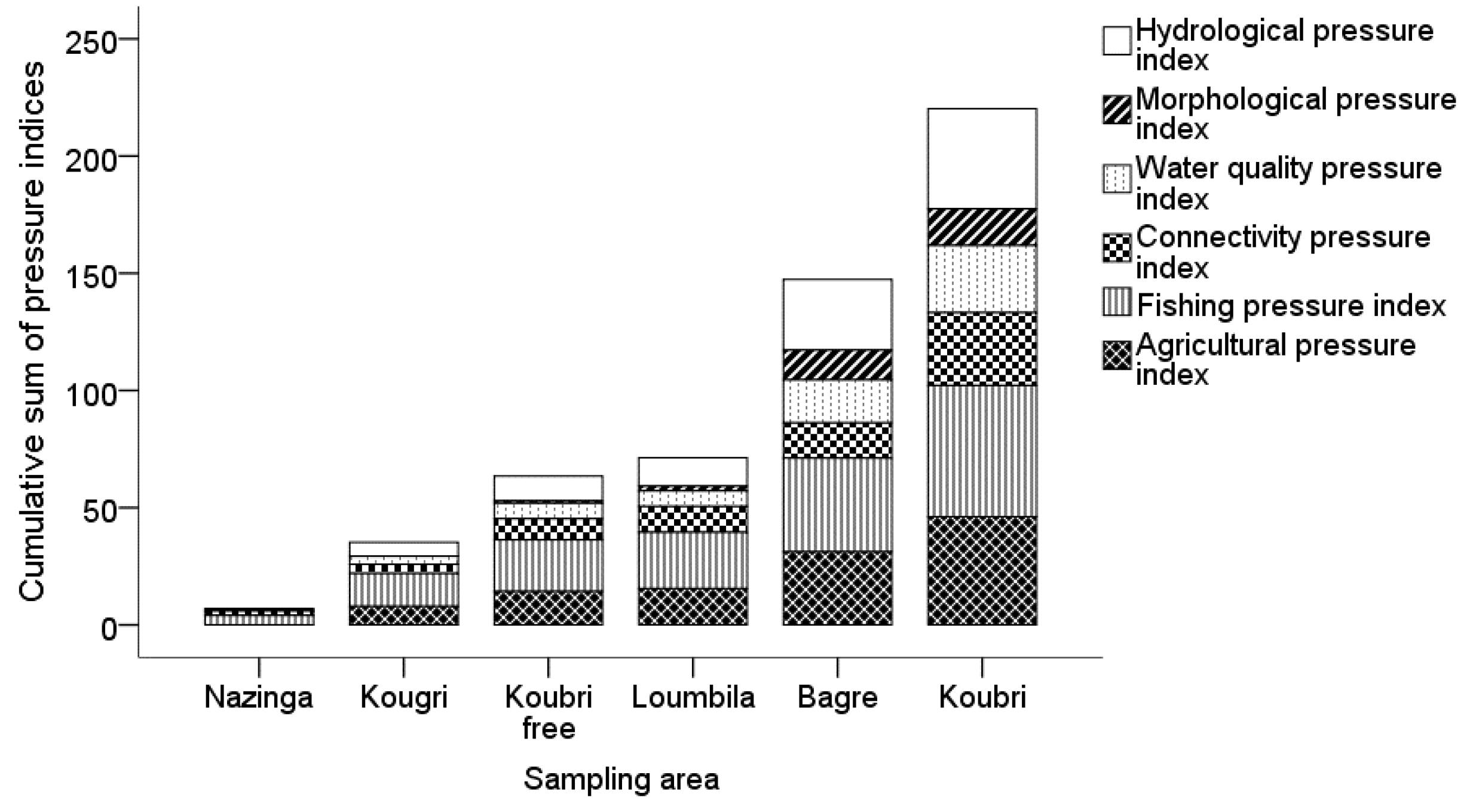
The data collected on available habitat conditions represent a picture at a certain time, which is strongly influenced by seasonal hydrology just before the dry season [
22]. The benthic substrate composition of every site was dominated by a fine fraction. In addition, the heavily impacted and obstructed area of Koubri showed a notable proportion of concrete caused by erosion of reservoir and irrigation channel infrastructure. These man-made aquatic zones were also used as fish habitat, mainly by
Enteromius macrops,
Clarias spp.,
Oreochromis niloticus, and
Coptodon zillii. This indicates the tolerance of these species for obstructions. The protected area of Nazinga exhibited a higher percentage of coarser substrate conditions, likely the result of no agriculture, fewer reservoirs, and more free-flowing sections [
3,
22]. The high proportion of fine fractions in Bagré can be explained by the selection of the sampling points, which are mostly located at tributaries to the reservoir with agriculture along them, which deposit high sediment loads. This can also explain high conductivity values for these sites.
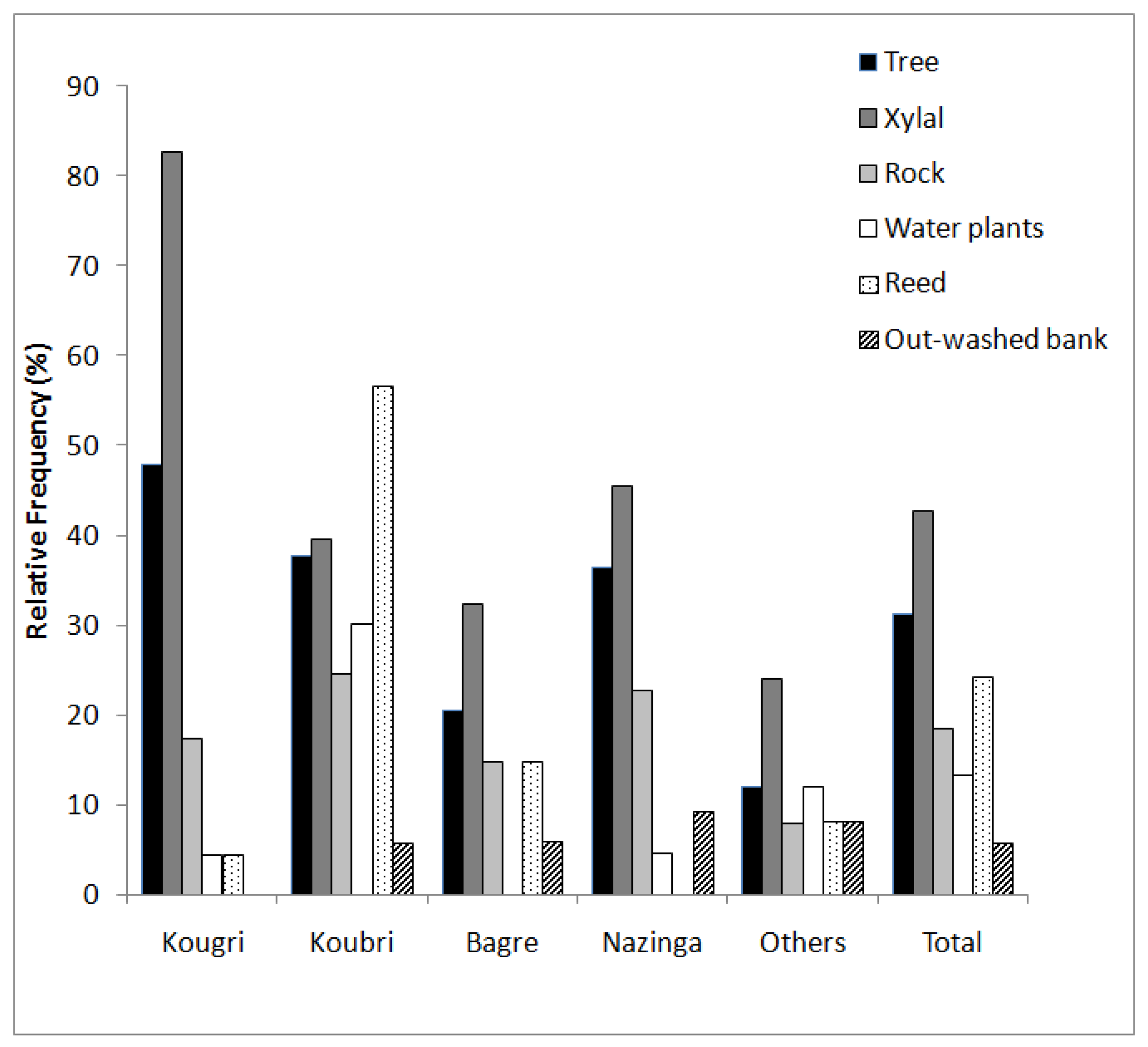
The availability of structural elements is a key determinant of the diversity of in-stream fish communities [
31,
43]. These elements can be necessary as an essential fish habitat for spawning, feeding, breeding, or hiding for certain species and life stages [
24,
43]. The relatively high frequencies of reed (56%) and water plants (30%) in Koubri were caused by high nutrient input from intensive agriculture. Our results show that Burkinabe waters do not exhibit a lack of in-stream structures as in Europe [
44].
Physicochemical parameters are also important for fish assemblage composition. pH values ranged from 6.4 to 10.1 with a mean value of 7.8. The lowest and the highest values were measured in Koubri (
Table 4). These extreme values can be explained by the high agricultural and livestock activities in this area [
3,
22,
45,
46].
There were noticeable temperature differences between Bagré/Nazinga and Kougri/Koubri (
Table 6). One reason is that Kougri and Koubri are located in a warmer area than Nazinga and Bagré [
47]. Secondly, the selection of the sampling points and the influence of reservoirs can explain these circumstances. In Kougri and Koubri, most of the sampling points are located downstream of reservoirs, while in Bagré, we mainly sampled tributaries. Nazinga generally shows a lower density of reservoirs than Koubri [
22]. Nearly all reservoirs have a spillover, which releases the warm water from the surface and therefore heats up the waters downstream [
48].
4.2. Habitat Use
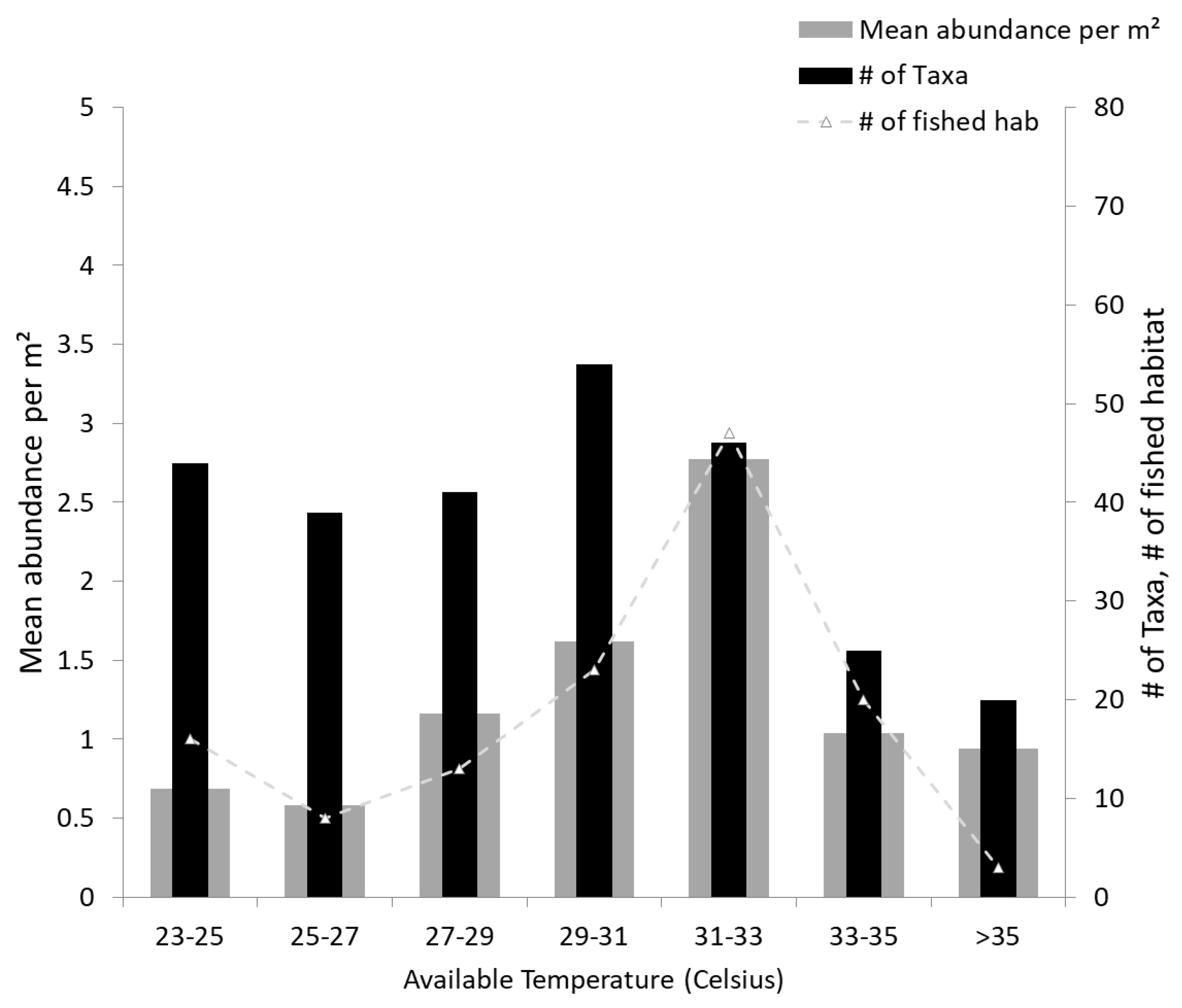
With increasing temperature, productivity of a water body increases as well, resulting in higher abundances and more diverse species richness until a maximum threshold is reached (
Figure 5). The described maximum between 29–31 °C and the sudden drop of abundance and species richness indicates the maximum temperature tolerance of some of local fish species [
49]. For West Africa and Burkina Faso, one indication of the potential negative effects of climate change, e.g., increasing desertification and decreasing annual precipitation with more frequent extreme events, is that increasing variability, rising trends, episodic extremes of temperature, or precipitation might have dramatic effects on the fish fauna and the fisheries. Desertification is directly linked with loss of habitat for aquatic species [
19,
20,
21].
Regarding the mean shading of the water surface, Nazinga exhibited the highest mean value (Nazinga 19%, Bagré 14%, Koubri 12%, Kougri 6%). Additionally, shading can lower the impact of solar gain on water body temperature [
50]. Consequently, increasing riparian vegetation for sun protection lowers the average water body temperature as well as the scale of temperature fluctuations [
51,
52].
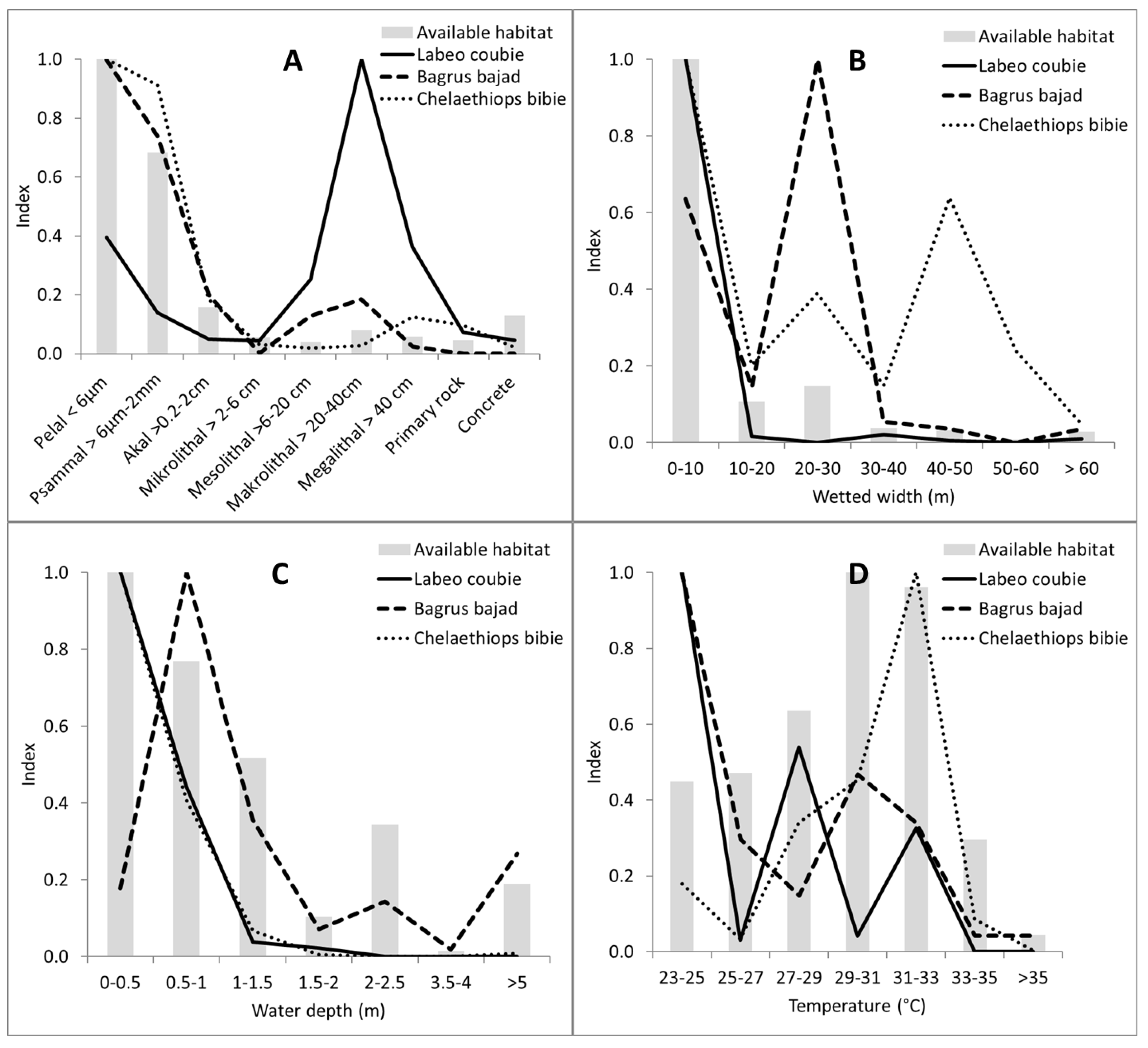
In ephemeral water bodies such as in sub-Saharan Burkina Faso, it is difficult to distinguish between habitat preferences and minimum requirements or tolerances. As the sampling took place at the beginning of the dry season, some species might have been trapped in unfavorable habitats. However, the results highlight that the Burkinabe fish species do have different habitat preferences. Regarding the habitat usage curves (
Figure 6), it is obvious that some habitat categories are used mainly according to their availability, and therefore the selection for this habitat feature is low, e.g., habitat use of substrate size for
Chelaethiops bibie or
Bagrus bajad. In contrast, the habitat selection of Makrolithal for
Labeo coubie is high (
Figure 6).
Summarizing the habitat use curves and the physicochemical parameters, one can see differences in the habitat use of four species (
Figure 6,
Table 4 and
Table 5).
Chelaethiops bibie, which feeds mainly on terrestrial insects [
53] and seeds found at the water surface [
54], uses shallow, large, warm water bodies with fine sediments and high proportions of Xylal. The frequent occurrence of Xylal (90%) is evidence that this structural element is an essential one for its lifecycle.
Bagrus bajad uses deeper, cool, mid-size water bodies also dominated by fine substrates. It lives and feeds on or near the bottom [
55] on insects, crustaceans, mollusks, and vegetable matter [
54], and adults are exclusively piscivorous [
56]. Worthington and Ricardo [
57] observed them at depths up to 60 m.
Lates niloticus shows similar habitat characteristics. It inhabits streams, lakes, and irrigation channels. Adults inhabit deep water, while juveniles are found in shallow water. It feeds on fish, especially
Clupeids and
Alestes [
58]. Smaller specimens also feed on larger crustaceans and insects. Juveniles are planktivorous [
54]. Maximum reported length for this species is 2000 mm [
59]. By contrast,
Labeo coubie inhabits water bodies that are characterized by coarse substrate and higher flow velocity. It occurs in narrow and shallow streams. For the described habitat of
Labeo coubie, one should note that we only caught young fish (maximum size of 150 mm).
4.3. Human Pressures, Overfishing, and Loss of Habitat
Efficient monitoring tools for the assessment of stream ecosystem response to human pressures are urgently needed but still lacking in West Africa. Apart from one method for macroinvertebrate [
60], there are no established biological assessment methods for Burkina Faso. All sampled Burkinabe sites were exposed to fishing activities (
Figure 9). In protected areas, e.g., Nazinga, fishermen need to buy a license in order to fish, and one season is closed for fishing. Despite such protections, camps of professional fishermen sell huge amounts of fish to nearby cities and Ouagadougou without incurring negative impacts on the fish assemblages. Therefore, Nazinga and a few remote sites were judged as medium fishing pressure sites, and all others were judged as high fishing pressure sites. Almost all sites (89%) were exposed to hydrological modifications. This was mainly due to the high reservoir density in the sampling areas. Agricultural activities were present in 87% of all sites, but rice occurred less than the other pressures. Additionally, 74% of the sites were affected by water quality pressures, and eutrophication was the most frequent pressure. Both larger and smaller dams created connectivity pressures on more than half of the sites. Morphological pressures or alterations were already present in nearly half (47%) of the sites. Cross-section and in-stream habitat modification were the most frequent kinds of habitat loss created by morphological pressure impacts.
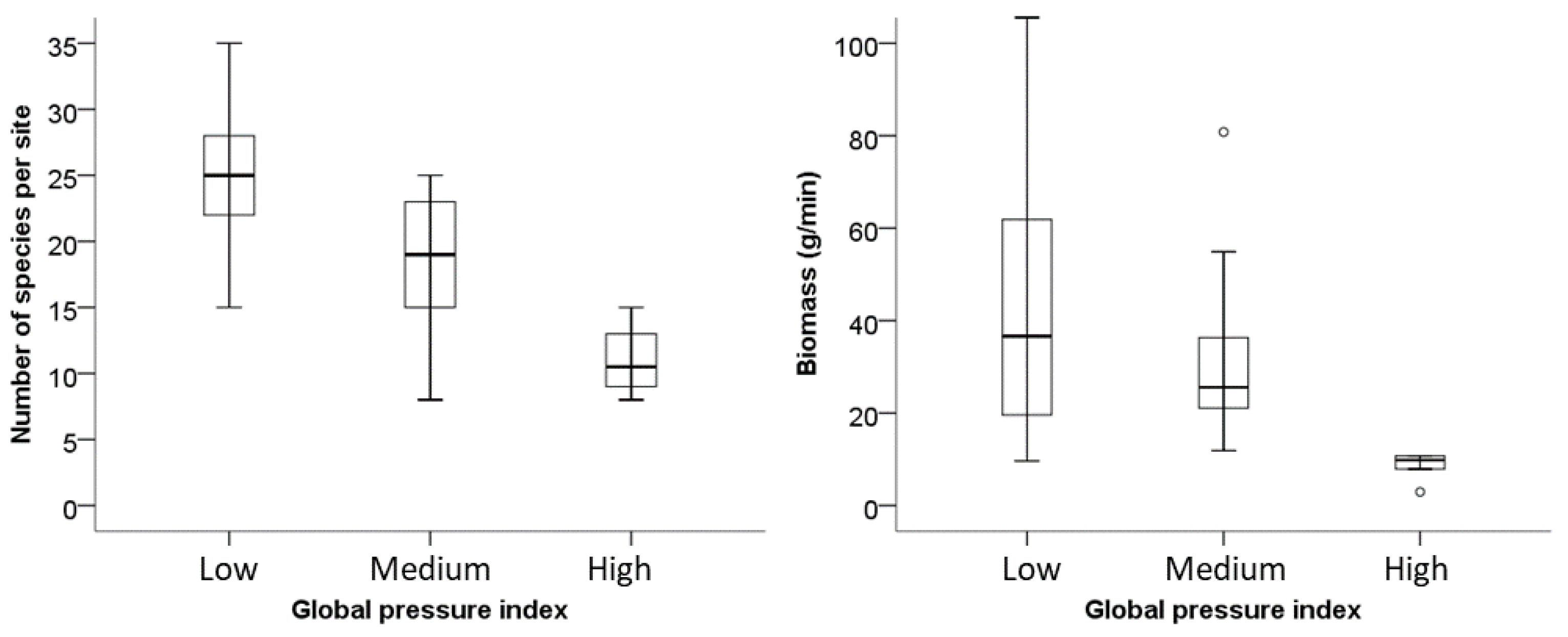
In response to different pressure classifications, the sharp and the distinct reactions of different fish assemblages in terms of taxa richness, total density (g/min, individuals/min), and share of intolerant species suggest that such measures could serve as core metrics. As pressures increase from medium to high, the trophic level drops from 3 to 2.5.
Except for
Hemichromis and
Coptodon, all genera had a negative correlation between the pressure indices and the relative abundance of genera, indicating that these two are generalists. The share of
Cichlids and
Enteromius increased with pressure intensity. These taxa are noted as quite tolerant of pressures and are frequently found in reservoir communities [
26,
61]. Some genera such as
Auchenoglanis or
Hydrocynus correlated with many pressure groups, showing that they are very sensitive and indicating the correlation amongst the pressure groups.
Adams [
62] reported the loss of
Lates niloticus,
Hydrocynus sp.,
Gymnarchus niloticus,
Auchenoglanis sp., and
Bagrus sp. downstream of reservoirs in Nigeria. We also caught
Lates niloticus more frequently in free-flowing stretches.
Hydrocynus was found only in Nazinga, indicating that it is generally sensitive. We could find
Auchenoglanis in Nazinga and Bagré (in tributaries to the reservoir) but also downstream of the reservoir of Loumbila.
Bagrus was caught in all areas except for Nazinga and Loumbila.
The study shows a variation in spatial distribution of fish species richness. Median species richness decreased to 40% of values found at the low-pressure sites (GPI). However, if connectivity is intact and species can still migrate to the appropriate habitats for their life stages, this can compensate for some other pressures. About 50% of the caught species are reported as potamodromous [
63]. This way, connectivity could serve as a core metric in a multi-metric index. Since river morphology is still widely intact in Burkina Faso, restoring and preserving connectivity could be an important contribution to protect the Burkinabe fish communities.
While the presence of cichlids increased with pressure,
Mormyridae and
Mochokidae decreased. Hugueny, Camara, Samoura, and Magassouba [
26] came to similar results, where
Mormyridae were almost extinct at impacted sites and were proposed as intolerant species for a future Index of Biotic Integrity in Africa. Abundance of
Alestidae was similar in low and medium pressure sites but had a drop at high pressure sites.
Clariidae were frequent in medium pressure sites but less frequent in low and high pressure sites. Anne, Lelek, and Tobias [
61] experienced a considerable reduction in the abundance of the genus
Citharinus in reservoir environments and a substitution by cichlids and pelagic species such as cyprinids. We could only find
Citharinus in very low numbers in the protected area of Nazinga.
5. Conclusions
Rising risk of climate change, population growth, and associated human pressures give urgency to efforts to link science and policy for sustainable fisheries management in Burkina Faso, West Africa. Inland fisheries production has plateaued at the same time that the quantity of riverine fish has dramatically declined. There is a clear need for habitat protection; thus, the tools provided here open the door to habitat management based on quantification. This addresses the need for modernizing the governance of fisheries based on scientific measurements.
This study revealed that most waters of Burkina Faso were exposed to multiple human pressures, with overfishing, hydrological changes, agricultural pressures, and water quality being the most frequent. The results from Nazinga—higher abundances, larger mean length, and highest diversity—highlight the importance of protected areas as a refuge habitat for sensitive and rare species and as a genetic reserve for future restoration measures. The differences of total fish length at managed vs. non-managed fishing sites showed the importance of protections provided by sustainable fisheries management. These findings provide the scientific basis for policy recommendations, e.g., a licensing system for fishermen and closed seasons with better controls that can assure a higher fish yield long-term and improve the food security. Water quality is still a big problem in Burkina Faso. Urban areas urgently need better waste water treatment. Water quality in agricultural areas could be improved with re-vegetation of riparian buffer forests along the water bodies. This could also slow down soil erosion. This research shows that West African fish prefer specific temperature niches, and the shading effect of trees can buffer rising temperatures and increase fish species and fish community resilience to rising temperatures, especially in light of the extremes anticipated under various climate change scenarios [
64].
Some taxa reacted distinctly to specific levels of pressure intensity. As shown in this study and proposed by several authors, such sensitive taxa as
Mormyridae,
Polypterus,
Brycinus,
Hydrocynus,
Heterobranchus,
Synodontis,
Labeo,
Citharinus,
Lates niloticus, and
Heterotis niloticus could serve as indicator taxa for managing waters in Burkina Faso [
3,
65]. Additionally, in this study,
Auchenoglanis,
Ctenopoma, and
Schilbe showed distinct reactions to specific pressures. Other species such as
Coptodon zillii are tolerant of human pressures and increase significantly as pressures intensify. Additional sampling campaigns at reference sites in Burkina Faso as proposed by Kaboré, Moog, Alp, Guenda, Koblinger, Mano, Ouéda, Ouédraogo, Trauner, and Melcher [
60] are recommended to gain more valuable information on these aspects. Many problems have emerged with the creation of dams, e.g., siltation and reservoir volume loss [
3], downstream effects on biota, migration obstacles, the changes in hydrological and sediment regime, and the impacts on rain fed agriculture (e.g., [
16]). To address these problems, we propose to focus on alternative solutions, e.g., Martin and Van De Giesen [
66] showed that only 5% of the average annual groundwater recharge in large parts of the Burkinabe Nakanbé basin is exploited at present. They conclude that, from a geo-scientific view, further development of groundwater abstraction would be sustainable and desirable.