Efficacy of EDTA and Olive Mill Wastewater to Enhance As, Pb, and Zn Phytoextraction by Pteris vittata L. from a Soil Heavily Polluted by Mining Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Soil Properties
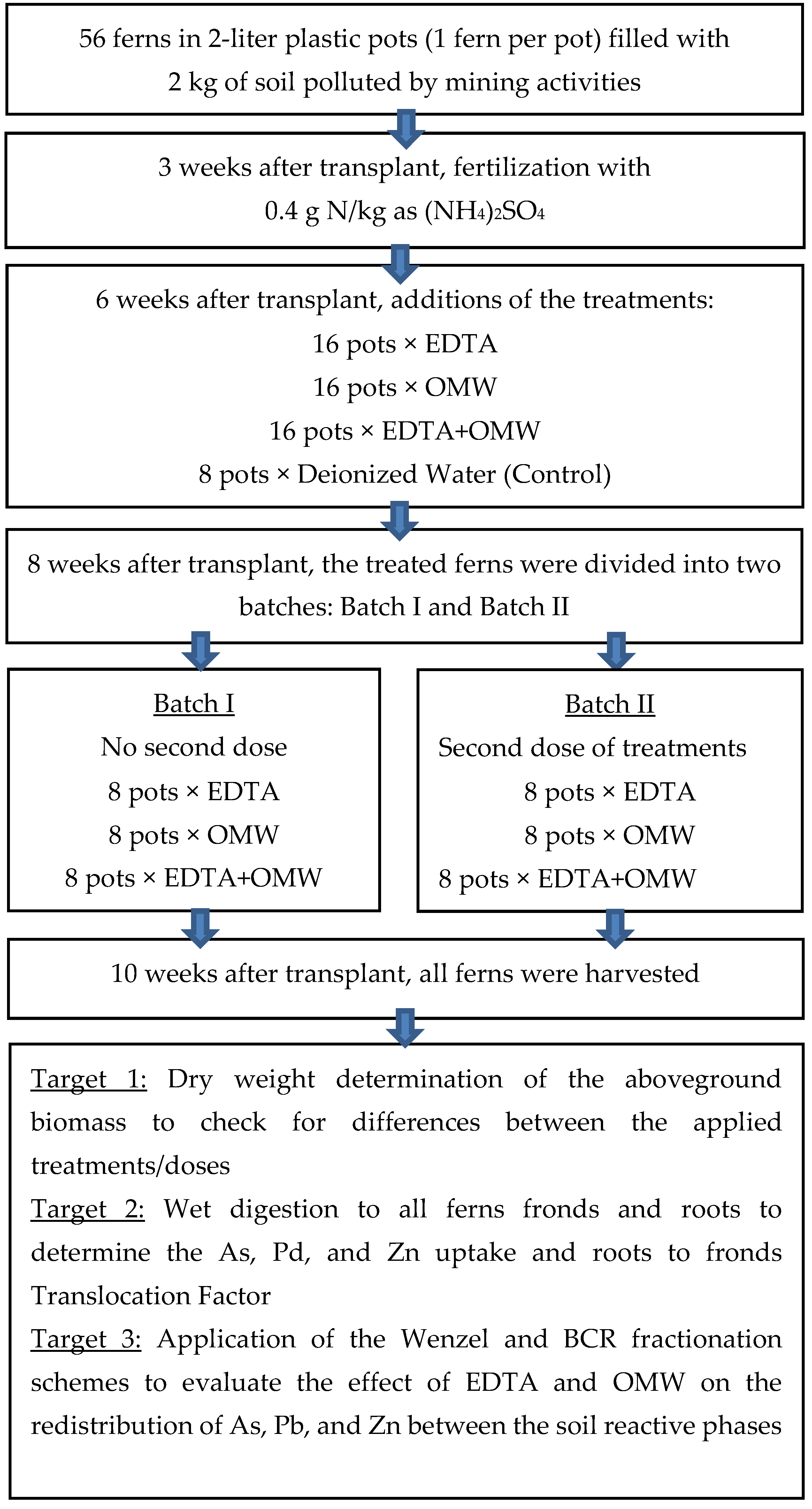
2.2. Greenhouse Experiment
2.3. Plant Analysis
2.4. Sequential Extraction Procedures
2.5. Analytical Determinations
2.6. Statistical Analysis
3. Results
3.1. Plant Growth
3.2. Elements Concentrations and Translocation in P. vittata
3.3. Distribution of Arsenic and Metals in Soil Fractions
4. Discussion
4.1. Effect of Added Amendments on As and Metal Accumulation in P. vittata Fronds
4.2. Arsenic, Lead, and Zinc Extraction per Pot
4.3. Element Partitioning
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Kalyvas, G.; Gasparatos, D.; Papassiopi, N.; Massas, I. Topsoil pollution as ecological footprint of historical mining activities in Greece. Land Degrad. Dev. 2018, 1–11. [Google Scholar] [CrossRef]
- Gasparatos, D. Fe-Mn concretions and nodules to sequester heavy metals in soils. In Environmental Chemistry for a Sustainable World, Vol 2: Remediation of Air and Water Pollution; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer Science + Business Media B.V: Dordrecht, The Netherlands, 2012; pp. 443–474. [Google Scholar]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Willscher, S.; Jablonski, L.; Fona, Z.; Rahmi, R.; Wittig, J. Phytoremediation experiments with Helianthus tuberosus under different pH and heavy metal soil concentrations. Hydrometallurgy 2017, 168, 153–158. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.; Barceló, J.; Bernal, M.P.; Navari-Izzo, F.; Poschenrieder, C.; Shilev, S.; Clemente, R.; Monterroso, C. Trace element behavior at the root–soil interface: Implications in phytoremediation. Environ. Exp. Bot. 2009, 67, 243–259. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.; Poonia, P. Role of hyperaccumulators in phytoextraction of metals from contaminated mining sites: A review. Crit. Rev. Environ. Sci. Technol. 2011, 41, 168–214. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, J.; Lombi, E. Phytoremediation of metals, metalloids, and radionuclides. Adv. Agron. 2002, 75, 1–56. [Google Scholar] [CrossRef]
- Manouchehri, N.; Bermond, A. EDTA in Soil Science: A Review of its Application in Soil Trace Metal Studies. Terr. Aquat. Environ. Toxicol. 2009, 3, 1–15. [Google Scholar] [CrossRef]
- Pardo, T.; Bernal, P.; Clemente, R. The use of olive mill waste to promote phytoremediation. In Olive Mill Waste: Recent Advances for Sustainable Management; Academic Press: Cambridge, MA, USA, 2017; pp. 183–204. [Google Scholar]
- Morillo, J.A.; Antizar-Ladislao, B.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A.; Russell, N.J. Bioremediation and biovalorisation of olive-mill wastes. Appl. Microbiol. Biotechnol. 2009, 82, 25–39. [Google Scholar] [CrossRef] [PubMed]
- McBride, M. Adsorption and oxidation of phenolic compounds by iron and manganese oxides. Soil Sci. Soc. Am. J. 1987, 51, 1466–1472. [Google Scholar] [CrossRef]
- Lestan, D.; Luo, C.L.; Li, X.D. The use of chelating agents in the remediation of metal-contaminated soils: A review. Environ. Pollut. 2008, 153, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Mekki, A.; Dhouib, A.; Sayadi, S. Effects of olive mill wastewater application on soil properties and plants growth. Int. J. Recycl. Org. Waste Agric. 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, X.; Lei, M. Application of arsenic hyperaccumulator Pteris vittata L. to contaminated soil in Northern China. J. Geochem. Explor. 2017, 182, 132–137. [Google Scholar] [CrossRef]
- Lessl, J.T.; Luo, J.; Ma, L.Q. Pteris vittata continuously removed arsenic from non-labile fraction in three contaminated-soils during 3.5-year of phytoextraction. J. Hazard. Mater. 2014, 279, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, T.; An, Z.; Lei, M.; Huang, Z.; Liao, X.; Liu, Y. Potential of Pteris vittata L. for phytoremediation of sites co-contaminated with cadmium and arsenic: The tolerance and accumulation. J. Environ. Sci. 2008, 20, 62–67. [Google Scholar] [CrossRef]
- Chen, T.B.; Wei, C.Y.; Huang, Z.C.; Huang, Q.F.; Lu, Q.G.; Fan, Z.L. Arsenic hyperaccumulator Pteris Vittata L. and its arsenic accumulation. Chin. Sci. Bull. 2002, 47, 902–905. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Chartzoulakis, K.; Psarras, G.; Moutsopoulou, M.; Stefanoudaki, E. Application of olive mill wastewater to a Cretan olive orchard: Effects on soil properties, plant performance and the environment. Agric. Ecosyst. Environ. 2010, 138, 293–298. [Google Scholar] [CrossRef]
- Neugschwandtner, R.W.; Tlustoš, P.; Komárek, M.; Száková, J. Phytoextraction of Pb and Cd from a contaminated agricultural soil using different EDTA application regimes: Laboratory versus field scale measures of efficiency. Geoderma 2008, 144, 446–454. [Google Scholar] [CrossRef]
- Vaxevanidou, K.; Papassiopi, N.; Paspaliaris, I. Removal of heavy metals and arsenic from contaminated soils using bioremediation and chelant extraction techniques. Chemosphere 2008, 70, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Bouyoucos, G.H. A recalibration of the hydrometer method for making mechanical analysis of soils. Agron. J. 1951, 43, 434–438. [Google Scholar] [CrossRef]
- NF ISO 10693. Détermination de la Teneur en Carbonate—Méthode Volumétrique; Qualité des sols AFNOR: Paris, France, 1995; pp. 177–186. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA SSSA: Madison, WI, USA, 1982. [Google Scholar]
- Rhoades, J.D. Cation Exchange Capacity. In Methods of soil Analysis, Part 2, Chemical and Microbiological Properties, 2nd ed.; Agron. Monogr. 9; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA and SSSA: Madison, WI, USA, 1982; pp. 149–157. [Google Scholar]
- Gasparatos, D.; Haidouti, C. A comparison of wet oxidation methods for determination of total phosphorus in soils. J. Plant Nutr. Soil Sci. 2001, 164, 435–439. [Google Scholar] [CrossRef]
- Karpouzas, D.; Ntougias, S.; Iskidou, E.; Rousidou, C.; Papadopoulou, K.; Zervakis, G.; Ehaliotis, C. Olive mill wastewater affects the structure of soil bacterial communities. Appl. Soil Ecol. 2010, 45, 101–111. [Google Scholar] [CrossRef]
- Jones, J.B., Jr.; Case, V.W. Sampling, handling and analyzing plant tissue samples. In Soil Testing and Plant Analysis; Westerman, R.L., Ed.; SSSA, Inc.: Madison, WI, USA, 1990; pp. 389–427. [Google Scholar]
- Ma, J.; Lei, E.; Lei, M.; Liu, Y.; Chen, T. Remediation of Arsenic contaminated soil using malposed intercropping of Pteris vittata L. and maize. Chemosphere 2018, 194, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Kalyvas, G.; Gasparatos, D.; Massas, I. A critical assessment of arsenic partitioning in mine-affected soils by using two sequential extraction protocols. Arch. Agron. Soil Sci. 2018. [Google Scholar] [CrossRef]
- Fiorentino, A.; Gentili, A.; Isidori, M.; Monaco, P.; Nardelli, A.; Parrella, A.; Temussi, F. Environmental effects caused by olive mill wastewaters: Toxicity comparison of low-molecular-weight phenol components. J. Agric. Food Chem. 2003, 51, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Karpouzas, D.G.; Rousidou, C.; Papadopoulou, K.K.; Bekris, F.; Zervakis, G.; Singh, B.K.; Ehaliotis, C. Effect of continuous olive mill wastewater applications, in the presence and absence of N fertilization, on the structure of rhizopshere soil fungal communities. FEMS Microbiol. Ecol. 2009, 70, 56–69. [Google Scholar] [CrossRef] [PubMed]
- An, Z.Z.; Huang, Z.C.; Lei, M.; Liao, X.Y.; Zheng, Y.M.; Chen, T.B. Zinc tolerance and accumulation in Pteris vittata L. and its potential for phytoremediation of Zn- and As-contaminated soil. Chemosphere 2006, 62, 796–802. [Google Scholar] [CrossRef] [PubMed]
- January, M.C.; Cutright, T.J.; Van Keulen, H.; Wei, R. Hydroponic phytoremediation of Cd, Cr, Ni, As, and Fe: Can Helianthus annuus hyperaccumulate multiple heavy metals? Chemosphere 2008, 70, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, M.I.S.; Santos, J.A.G.; Ma, L.Q. Phytoextraction by arsenic hyperaccumulator Pteris vittata L. from six arsenic-contaminated soils: Repeated harvests and arsenic redistribution. Environ. Pollut. 2008, 154, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Meers, E.; Ruttens, A.; Hopgood, M.J.; Samson, D.; Tack, F.M.G. Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere 2005, 58, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.H.H.; Abdelhafez Ahmed, A.A. Role of EDTA in arsenic mobilization and its uptake by maize grown on an As-polluted soil. Chemosphere 2013, 90, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Mühlbachová, G. Soil microbial activities and heavy metal mobility in long-term contaminated soils after addition of EDTA and EDDS. Ecol. Eng. 2011, 37, 1064–1071. [Google Scholar] [CrossRef]
- Wan, X.M.; Lei, M.; Chen, T.B.; Zhou, G.D.; Yang, J.; Zhou, X.Y.; Zhang, X.; Xu, R.X. Phytoremediation potential of Pteris vittata L. Under the combined contamination of As and Pb: Beneficial interaction between As and Pb. Environ. Sci. Pollut. Res. 2014, 21, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Vamerali, T.; Bandiera, M.; Mosca, G. Field crops for phytoremediation of metal-contaminated land. Environ. Chem. Lett. 2010, 8, 1–17. [Google Scholar] [CrossRef]
- Madrid, L.; Diaz-Barrientos, E. Retention of heavy metals by soils in the presence of a residue from olive-oil industry. Eur. J. Soil Sci. 1994, 45, 71–77. [Google Scholar] [CrossRef]
- De la Fuente, C.; Clemente, R.; Martínez-Alcalá, I.; Tortosa, G.; Bernal, M.P. Impact of fresh and composted solid olive husk and their water-soluble fractions on soil heavy metal fractionation, microbial biomass and plant uptake. J. Hazard. Mater. 2011, 186, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Violante, A.; Pigna, M. Competitive sorption of arsenate and phosphate on different clay minerals and soils. Soil Sci. Soc. Am. J. 2002, 66, 1788–1796. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Saha, U.K. Arsenic hyperaccumulating fern: Implications for remediation of arsenic contaminated soils. Geoderma 2016, 284, 132–143. [Google Scholar] [CrossRef]
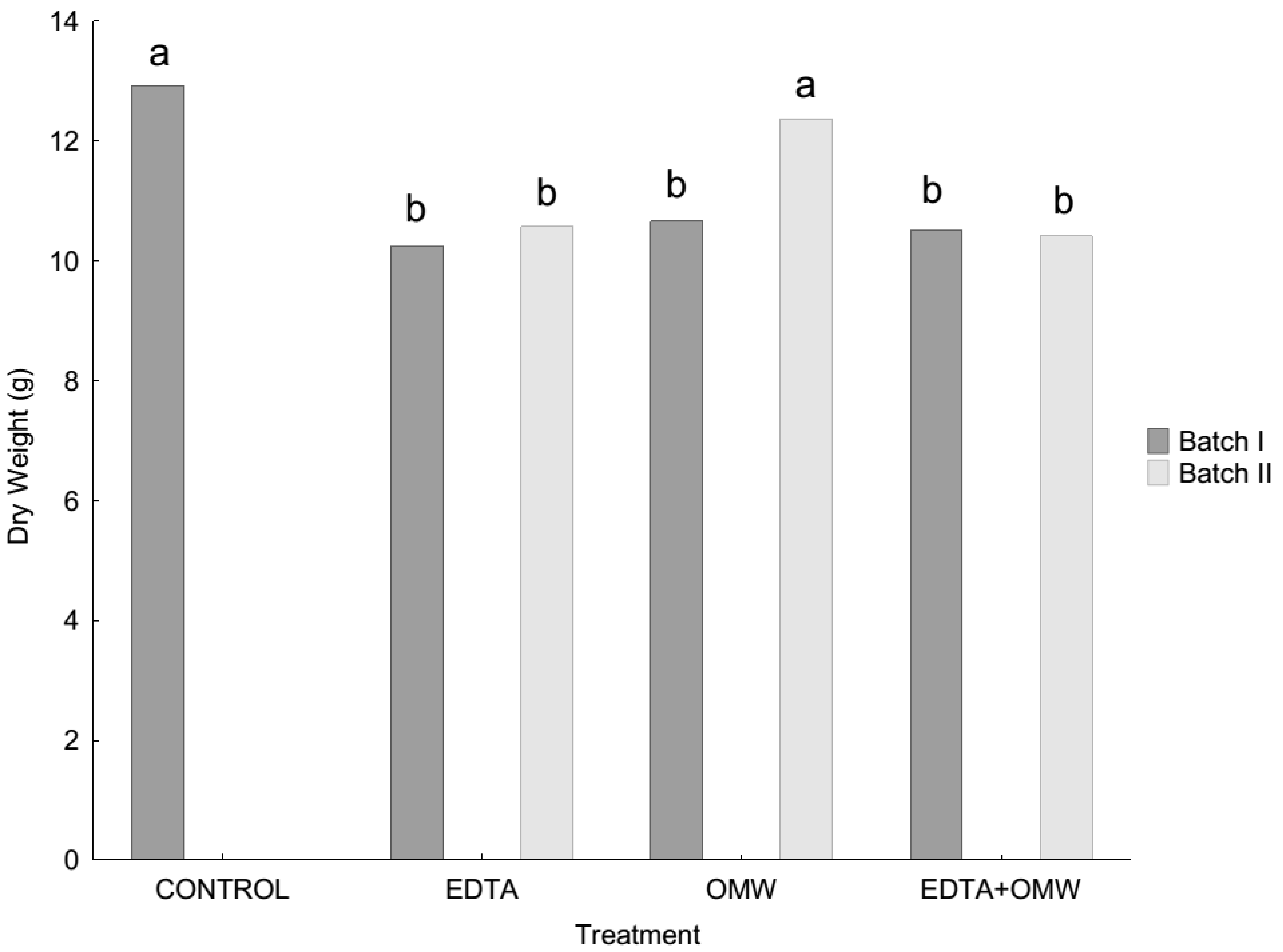
| ANOVA RESULTS | |||||
|---|---|---|---|---|---|
| Effect | SS | DF | MS | F | p |
| Treatment | 66.35 | 3 | 22.12 | 11.96 | 0.000 * |
| Batch | 3.72 | 1 | 3.72 | 2.01 | 0.162 |
| Treatment × Batch | 8.31 | 3 | 2.77 | 1.5 | 0.225 |
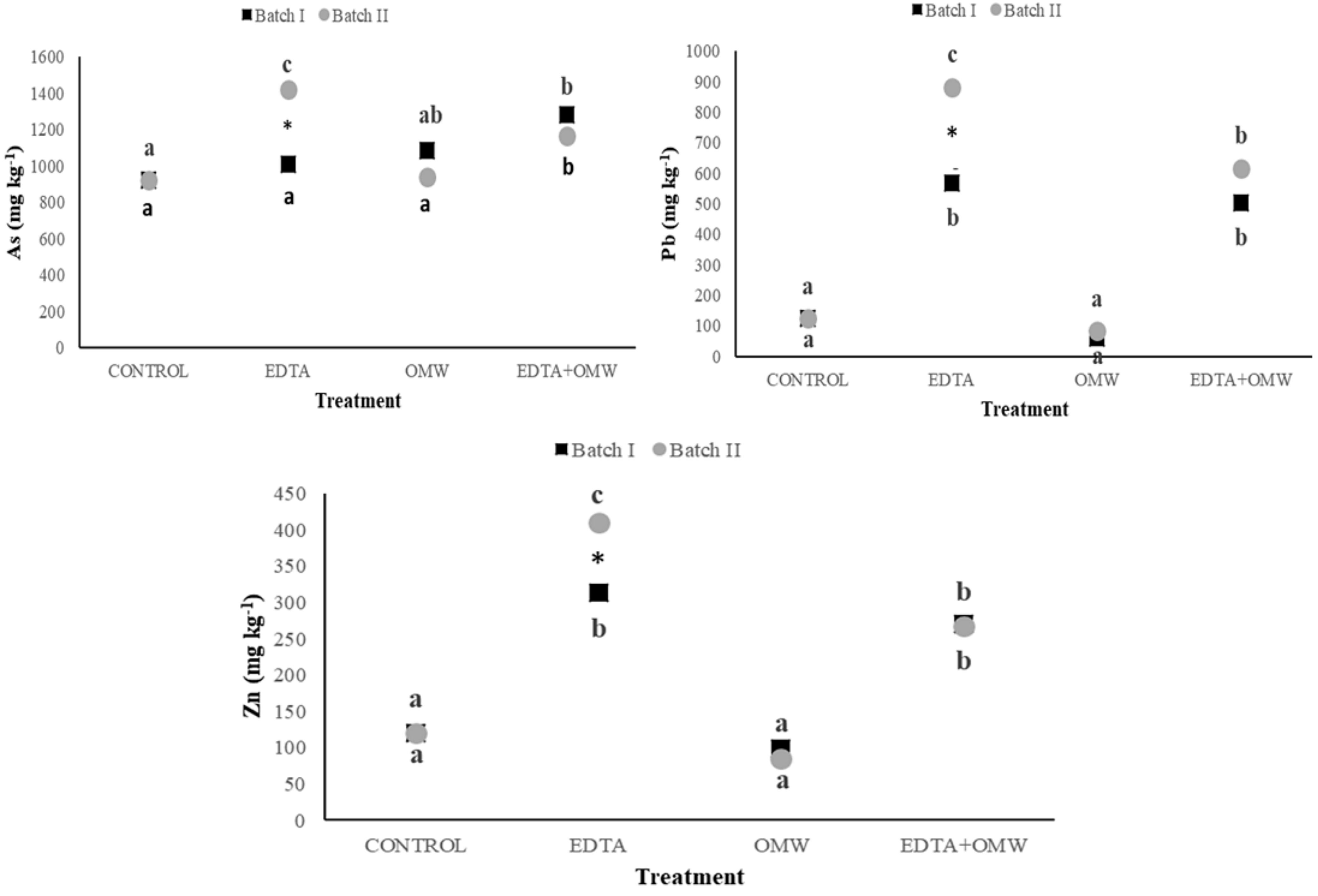
| ANOVA RESULTS | |||||
|---|---|---|---|---|---|
| Effect | SS | DF | MS | F | p |
| As | |||||
| Treatment | 108 × 104 | 3 | 359 × 103 | 10.71 | 0.000 * |
| Batch | 231 × 102 | 1 | 231 × 102 | 0.69 | 0.410 |
| Treatment × Batch | 788 × 103 | 3 | 263 × 103 | 7.84 | 0.000 * |
| Pb | |||||
| Treatment | 499 × 104 | 3 | 166 × 104 | 63.80 | 0.000 * |
| Batch | 202 × 103 | 1 | 202 × 103 | 7.73 | 0.007 * |
| Treatment × Batch | 245 × 103 | 3 | 817 × 102 | 3.13 | 0.033 * |
| Zn | |||||
| Treatment | 779 × 103 | 3 | 260 × 103 | 135.7 | 0.000 * |
| Batch | 6381 | 1 | 6381 | 3.3 | 0.073 |
| Treatment × Batch | 317 × 102 | 3 | 106 × 102 | 5.5 | 0.002 * |
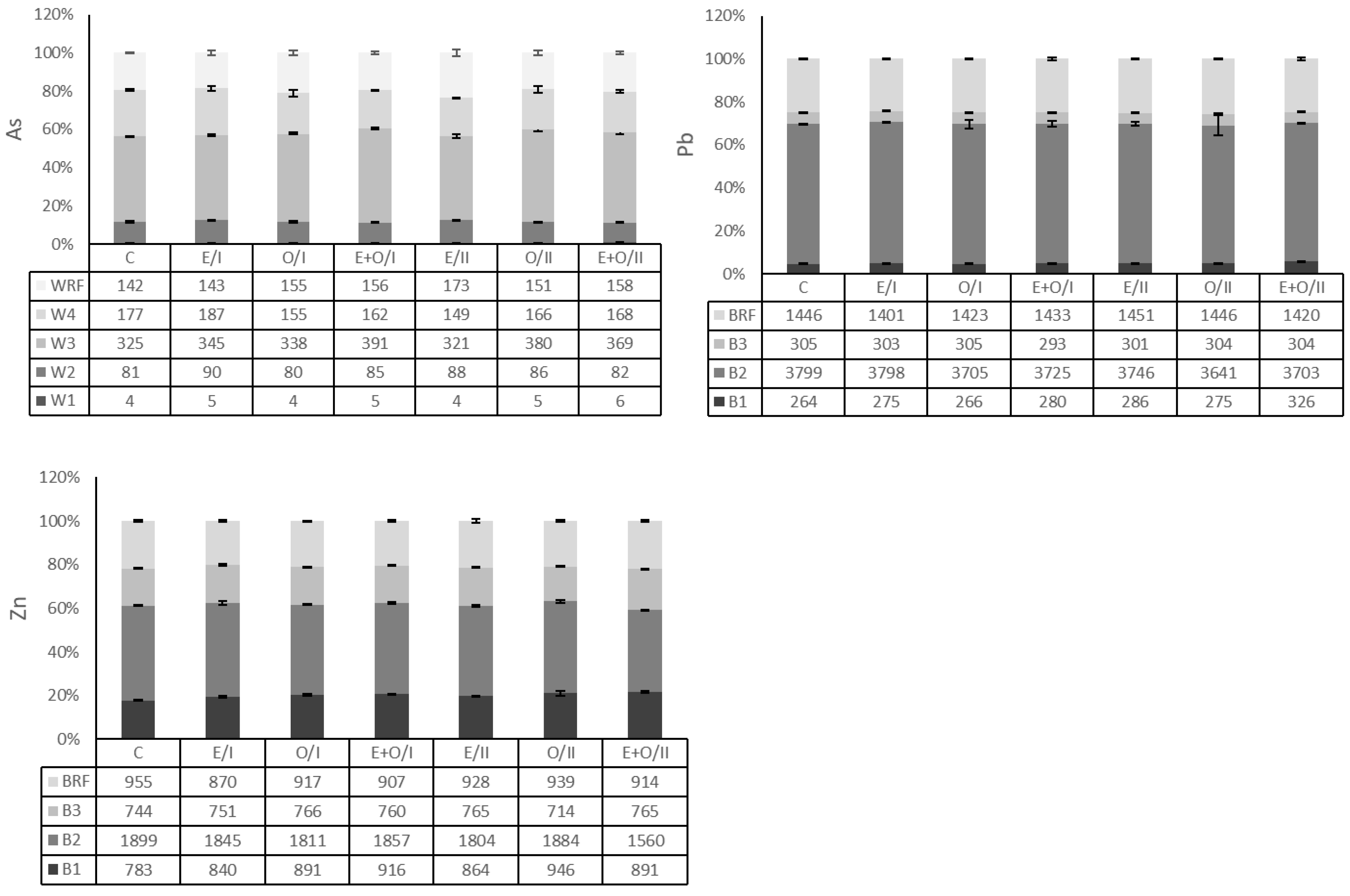
| Clay g kg−1 | Silt g kg−1 | Sand g kg−1 | pH (1:1) | Total CaCO3 g kg−1 | OM 1 g kg−1 | CEC 2 cmolckg−1 | Total As mg kg−1 | Total Pb mg kg−1 | Total Zn mg kg−1 |
|---|---|---|---|---|---|---|---|---|---|
| 198 | 301 | 501 | 7.72 | 69 | 45 | 18 | 822 | 5677 | 4428 |
| Step | Fraction | Extractant | Extraction Conditions | Extractant Volume (mL) for 1 g of Soil |
|---|---|---|---|---|
| BCR a | ||||
| B1 | Exchangeable/acid soluble | Acetic acid 0.11 mol L−1 | 16 h shaking at 22 ± 5 °C | 40 |
| B2 | Bound to Fe/Mn oxides (reducible) | Hydroxylammonium chloride 0.5 mol L−1 pH = 1.5 | 16 h shaking at 22 ± 5 °C | 40 |
| B3 | Bound to organic matter (oxidizable) | Hydrogen peroxide 8.8 mol L−1 Ammonium acetate 1.0 mol L−1, Ph = 2 ± 0.1 | 1 h digestion at 85 ± 2 °C 16 h shaking at 22 ± 5 °C | 10 + 10 50 |
| BRF b | Residual | Aqua regia (HCl/HNO3) | 16 h digestion | 25 |
| WENZEL c | ||||
| W1 | Non-specifically sorbed | (NH4)2 SO4 0.05 mol L−1 | 4 h shaking at 20 °C | 25 |
| W2 | Specifically sorbed | NH4H2PO4 0.05 mol L−1 | 16 h shaking at 20 °C | 25 |
| W3 | Amorphous and poorly crystalline hydrous oxides of Fe and Al | NH4+-oxalate buffer 0.2 mol L−1, pH = 3.25 | 4 h shaking in the dark at 20 °C | 25 |
| W4 | Well-crystallized hydrous oxides of Fe and Al | NH4+-oxalate buffer 0.2 mol L−1 + ascorbic acid 0.1 mol L−1, pH = 3.25 | 30 min shaking in the light at 96 °C | 25 |
| WRF | Residual | Aqua regia (HCl/HNO3) d | 16 h digestion d | 25 |
| ANOVA RESULTS | |||||
|---|---|---|---|---|---|
| Effect | SS | DF | MS | F | p |
| As | |||||
| Treatment | 61.1 | 3 | 20.4 | 0.662 | 0.583 |
| Batch | 138.7 | 1 | 138.7 | 4.511 | 0.044 * |
| Treatment × Batch | 70.9 | 3 | 23.6 | 0.769 | 0.523 |
| Pb | |||||
| Treatment | 0.110 | 3 | 0.037 | 6.705 | 0.002 * |
| Batch | 0.014 | 1 | 0.014 | 2.633 | 0.118 |
| Treatment × Batch | 0.013 | 3 | 0.004 | 0.794 | 0.509 |
| Zn | |||||
| Treatment | 0.296 | 3 | 0.099 | 4.234 | 0.015 * |
| Batch | 0.130 | 1 | 0.130 | 5.584 | 0.027 * |
| Treatment × Batch | 0.157 | 3 | 0.052 | 2.251 | 0.108 |
| TRANSLOCATION FACTOR (TF) | ||||||||
|---|---|---|---|---|---|---|---|---|
| BATCH I | BATCH II | |||||||
| CONTROL | EDTA | OMW | EDTA + OMW | CONTROL | EDTA | OMW | EDTA + OMW | |
| As | 6.91 a | 6.30 a | 7.99 a | 5.11 a | 6.91 a | 14.45 a | 11.18 a | 10.44 a |
| Pb | 0.08 abc | 0.14 abc | 0.04 b | 0.14 abc | 0.08 abc | 0.25 c | 0.06 b | 0.18 abc |
| Zn | 0.16 a | 0.20 a | 0.12 a | 0.19 a | 0.16 a | 0.57 b | 0.16 a | 0.29 ab |
| mg pot−1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| BATCH I | BATCH II | |||||||
| CONTROL | EDTA | OMW | EDTA + OMW | CONTROL | EDTA | OMW | EDTA + OMW | |
| As | 73.29 a/A | 102.45 ab | 106.08 ab/A | 123.11 b/A | 73.29 a/A | 135.24 b/A | 76.87 a/B | 114.17 b/A |
| Pb | 9.87 a | 56.95 bc | 5.90 a | 48.73 b | 9.87 a | 83.43 c | 6.74 a | 62.17 bc |
| Zn | 9.48 a | 31.62 bc | 9.60 a | 26.11 b | 9.48 a | 39.02 c | 6.91 a | 26.65 b |
| ANOVA RESULTS | |||||
|---|---|---|---|---|---|
| Effect | SS | DF | MS | F | p |
| As | |||||
| Treatment | 238 × 102 | 3 | 7932 | 11.89 | 0.000 * |
| Batch | 29 | 1 | 29 | 0.04 | 0.836 |
| Treatment × Batch | 8004 | 3 | 2668 | 4.00 | 0.012 * |
| Pb | |||||
| Treatment | 498 × 102 | 3 | 166 × 102 | 53.52 | 0.000 * |
| Batch | 1662 | 1 | 1662 | 5.36 | 0.024 * |
| Treatment × Batch | 1869 | 3 | 623 | 2.01 | 0.123 |
| Zn | |||||
| Treatment | 8382 | 3 | 2794 | 80.92 | 0.000 * |
| Batch | 27 | 1 | 27 | 0.80 | 0.376 |
| Treatment × Batch | 222 | 3 | 74 | 2.14 | 0.105 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalyvas, G.; Tsitselis, G.; Gasparatos, D.; Massas, I. Efficacy of EDTA and Olive Mill Wastewater to Enhance As, Pb, and Zn Phytoextraction by Pteris vittata L. from a Soil Heavily Polluted by Mining Activities. Sustainability 2018, 10, 1962. https://doi.org/10.3390/su10061962
Kalyvas G, Tsitselis G, Gasparatos D, Massas I. Efficacy of EDTA and Olive Mill Wastewater to Enhance As, Pb, and Zn Phytoextraction by Pteris vittata L. from a Soil Heavily Polluted by Mining Activities. Sustainability. 2018; 10(6):1962. https://doi.org/10.3390/su10061962
Chicago/Turabian StyleKalyvas, Georgios, Gerasimos Tsitselis, Dionisios Gasparatos, and Ioannis Massas. 2018. "Efficacy of EDTA and Olive Mill Wastewater to Enhance As, Pb, and Zn Phytoextraction by Pteris vittata L. from a Soil Heavily Polluted by Mining Activities" Sustainability 10, no. 6: 1962. https://doi.org/10.3390/su10061962
APA StyleKalyvas, G., Tsitselis, G., Gasparatos, D., & Massas, I. (2018). Efficacy of EDTA and Olive Mill Wastewater to Enhance As, Pb, and Zn Phytoextraction by Pteris vittata L. from a Soil Heavily Polluted by Mining Activities. Sustainability, 10(6), 1962. https://doi.org/10.3390/su10061962






