Hydrothermal Carbonization of Municipal Woody and Herbaceous Prunings: Hydrochar Valorisation as Soil Amendment and Growth Medium for Horticulture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrochar Post-Treatments and Characterization
2.3. Lettuce Germination Tests
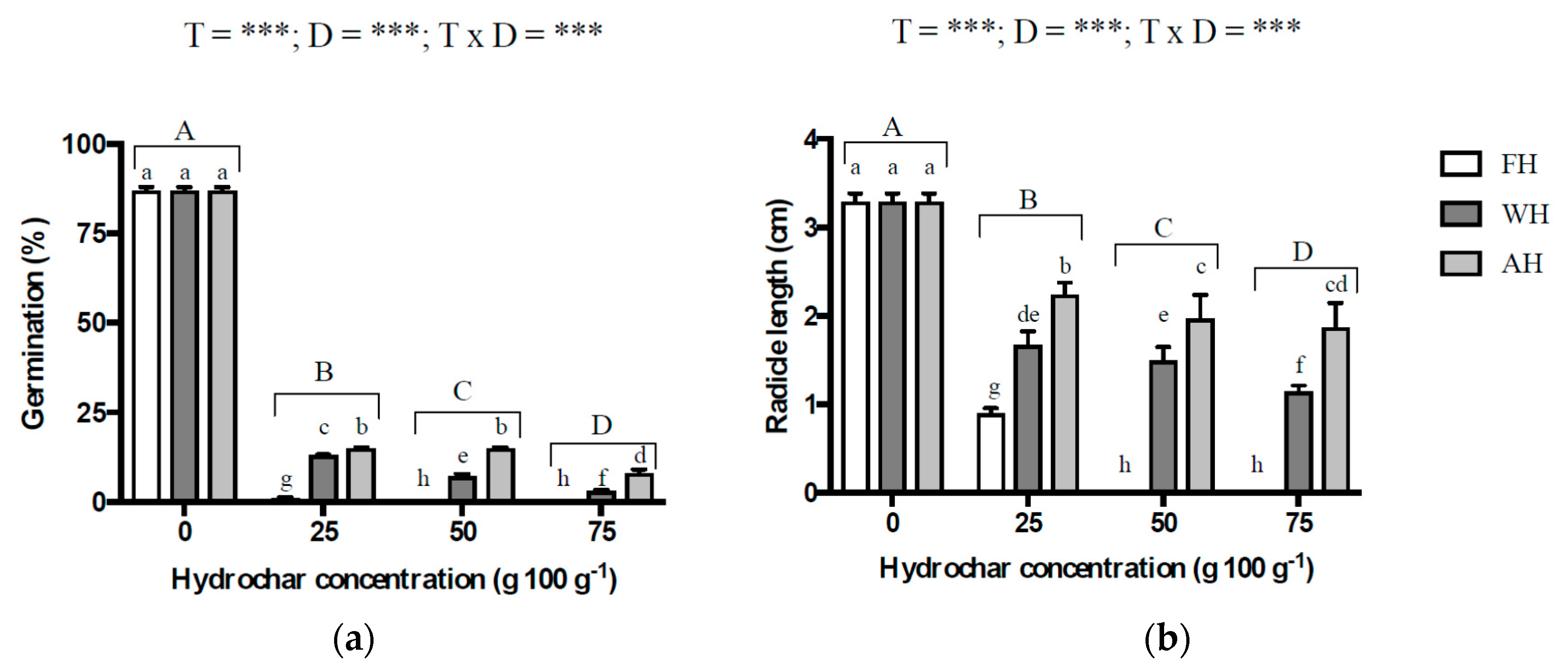
3. Results
4. Discussion
4.1. Fresh Hydrochar (FH) Chemical Properties
4.2. Effects of Post-Treatments on Hydrochar Properties
4.3. Effect of Hydrochar Application on Lettuce Germination
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Libra, J.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Pavlovič, I.; Knez, Ž.; Škerget, M. Hydrothermal reactions of agricultural and food processing wastes in sub- and supercritical water: A review of fundamentals, mechanisms, and state of research. J. Agric. Food Chem. 2013, 61, 8003–8025. [Google Scholar] [CrossRef] [PubMed]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Balasubramanian, R. Preparation and characterization of fuel pellets from woody biomass, agro-residues and their corresponding hydrochars. Appl. Energy 2014, 113, 1315–1322. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Lu, L.; Namioka, T.; Yoshikawa, K. Effects of hydrothermal treatment on characteristics and combustion behaviors of municipal solid wastes. Appl. Energy 2011, 88, 3659–3664. [Google Scholar] [CrossRef]
- Reza, M.T.; Uddin, M.H.; Lynam, J.G.; Coronella, C.J. Engineered pellets from dry torrefied and HTC biochar blends. Biomass Bioenergy 2014, 63, 229–238. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Falco, C.; Sevilla, M. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796–6822. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Ok, Y.S.; Niazi, N.K.; Rizwan, M.; Al-Wabel, M.I.; Usman, A.R.A.; Moon, D.H.; Lee, S.S. Effect of corn residue biochar on the hydraulic properties of sandy loam soil. Sustainability 2017, 9, 266. [Google Scholar] [CrossRef]
- Sethupathi, S.; Zhang, M.; Rajapaksha, A.U.; Lee, S.R.; Nor, N.M.; Mohamed, A.R.; Al-Wabel, M.; Lee, S.S.; Ok, Y.S. Biochars as potential adsorbers of CH4, CO2 and H2S. Sustainability 2017, 9, 121. [Google Scholar] [CrossRef]
- Puccini, M.; Stefanelli, E.; Hiltz, M.; Seggiani, M. Activated Carbon from Hydrochar Produced by Hydrothermal Carbonization of Wastes. Chem. Eng. Trans. 2017, 57, 169–174. [Google Scholar] [CrossRef]
- Nguyen, M.V.; Lee, B.K. Removal of dimethyl sulfide from aqueous solution using cost-effective modified chicken manure biochar produced from slow pyrolysis. Sustainability 2015, 7, 15057–15072. [Google Scholar] [CrossRef]
- Hitzl, M.; Corma, A.; Pomares, F.; Renz, M. The hydrothermal carbonization (HTC) plant as a decentral biorefinery for wet biomass. Catal. Today 2015, 257, 154–159. [Google Scholar] [CrossRef]
- Bargmann, I.; Rillig, M.C.; Buss, W.; Kruse, A.; Kuecke, M. Hydrochar and Biochar Effects on Germination of Spring Barley. J. Agron. Crop Sci. 2013, 199, 360–373. [Google Scholar] [CrossRef]
- Busch, D.; Kammann, C.; Grünhage, L.; Müller, C. Simple Biotoxicity Tests for Evaluation of Carbonaceous Soil Additives: Establishment and Reproducibility of Four Test Procedures. J. Environ. Qual. 2012, 41, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Fornes, F.; Belda, R.M.; Lidón, A. Analysis of two biochars and one hydrochar from different feedstock: Focus set on environmental, nutritional and horticultural considerations. J. Clean. Prod. 2015, 86, 40–48. [Google Scholar] [CrossRef]
- Jandl, G.; Eckhardt, K.-U.; Bargmann, I.; Kücke, M.; Greef, J.-M.; Knicker, H.; Leinweber, P. Hydrothermal Carbonization of Biomass Residues: Mass Spectrometric Characterization for Ecological Effects in the Soil–Plant System. J. Environ. Qual. 2013, 42, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Burguete, P.; Corma, A.; Hitzl, M.; Modrego, R.; Ponce, E.; Renz, M. Fuel and chemicals from wet lignocellulosic biomass waste streams by hydrothermal carbonization. Green Chem. 2016, 18, 1051–1060. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable Cations. In Agronomy Monograph, Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar] [CrossRef]
- Mehlich, A. Determination of Cation- and Anion-Exchange Properties of Soils. Soil Sci. 1948, 66, 429–446. [Google Scholar] [CrossRef]
- Isermeyer, H. Estimation of soil Respiration in Closed Jars. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1952; ISBN 9780125138406. [Google Scholar]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995; ISBN 9780125138406. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Schanderl, S.H. Methods in Food Analysis. In Physical, Chemical and Instrumental Methods of Analysis; Academic Press Inc.: New York, NY, USA, 1970. [Google Scholar]
- Peacock, W.; Christensen, L. Interpretation of soil and water analysis. In Raisin Production Manual, Publication 3393 ed; University of California Division of Agricultural and Natural Resources: Oakland, CA, USA, 2000. [Google Scholar]
- Gomez, A.A.; Gomez, K.A. Statistical procedures for agricultural research. In Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984; Volume 6, p. 680. [Google Scholar]
- Jess, A.; Wasserscheid, P. Chemical Technology: An Integral Textbook; Wiley-VCH: Weinheim, Germany, 2013; ISBN 3527304460. [Google Scholar]
- Giandon, P. L’Interpretazione delle Analisi del Terreno; ARPAV: Vicenza, Italy, 2007; ISBN 8875041156. [Google Scholar]
- Horneck, D.A.; Sullivan, D.M.; Owen, J.S.; Hart, J.M. Soil Test Interpretation Guide; Oregon State University Extension Service: Corvallis, OR, USA, 2011; pp. 1–12. [Google Scholar] [CrossRef]
- Bonner, J.; Varner, J.E. Mineral Metabolism, Plant Biochemistry; Academic Press: London, UK, 1976; ISBN 0121148602. [Google Scholar]
- Sonon, L.S.; Kissel, D.E.; Saha, U.K. Cation Exchange Capacity and Base Saturation, Cicular 1040 ed; University of Georgia Extension: Athens, GA, USA, 2014. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils. In USDA Ag. Handbook 60; United States Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Natural Resources Conservation Service Soil. Available online: www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/health/assessment/?cid=nrcs142p2_053870 (accessed on 11 January 2017).
- Hammes, K.; Smernik, R.J.; Skjemstad, J.O.; Herzog, A.; Vogt, U.F.; Schmidt, M.W.I. Synthesis and characterisation of laboratory-charred grass straw (Oryza sativa) and chestnut wood (Castanea sativa) as reference materials for black carbon quantification. Org. Geochem. 2006, 37, 1629–1633. [Google Scholar] [CrossRef]
- Kuhlbusch, T.A.J.; Crutzen, P.J. Toward a global estimate of black carbon in residues of vegetation fires representing a sink of atmospheric CO2 and a source of O2. Glob. Biogeochem. Cycles 1995, 9, 491–501. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Glaser, B. One Step Forward toward Characterization: Some Important Material Properties to Distinguish Biochars. J. Environ. Qual. 2012, 41, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Wiedner, K.; Naisse, C.; Rumpel, C.; Pozzi, A.; Wieczorek, P.; Glaser, B. Chemical modification of biomass residues during hydrothermal carbonization—What makes the difference, temperature or feedstock? Org. Geochem. 2013, 54, 91–100. [Google Scholar] [CrossRef]
- Carrion, C.; Garcia-de-la-Fuente, R.; Fornes, F.; Puchades, R.; Abad, M. Acidifying Composts from Vegetable Crop Wastes to Prepare Growing Media for Containerized Crops. Compost Sci. Util. 2008, 16, 20–29. [Google Scholar] [CrossRef]
- Busch, D.; Stark, A.; Kammann, C.I.; Glaser, B. Genotoxic and phytotoxic risk assessment of fresh and treated hydrochar from hydrothermal carbonization compared to biochar from pyrolysis. Ecotoxicol. Environ. Saf. 2013, 97, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.P.; Dalal, R.C.; Mayer, D.G.; McDonald, M.; Routley, R.; Schwenke, G.D.; Buck, S.R.; Daniells, I.G.; Singh, D.K.; Manning, W.; et al. High subsoil chloride concentrations reduce soil water extraction and crop yield on Vertosols in north-eastern Australia. Aust. J. Agric. Res. 2008, 59, 321–330. [Google Scholar] [CrossRef]
- Saharinen, M.H.; Vuorinen, A.H.; Hostikka, M. Effective cation exchange capacity of manure compost of varying maturity stages determined by the saturation-displacement method. Commun. Soil Sci. Plant Anal. 1996, 27, 2917–2923. [Google Scholar] [CrossRef]
- Miller, J.; Beasley, B.; Drury, C.; Larney, F.; Hao, X. Influence of long-term application of composted or stockpiled feedlot manure with straw or wood chips on soil cation exchange capacity. Compost Sci. Util. 2016, 24, 54–60. [Google Scholar] [CrossRef]
- Costantini, E.A.C. Metodi di Valutazione dei Suoli e Delle Terre; Cantagalli: Firenze, Italy, 2006; Volume 7. [Google Scholar]
- Cochran, V.L.; Elliott, L.F.; Papendick, R.I. The Production of Phytotoxins from Surface Crop Residues1. Soil Sci. Soc. Am. J. 1977, 41, 903–908. [Google Scholar] [CrossRef]
- Patrick, Z.A. Phytotoxic substances associated with the decomposition in soil of plant residues. Soil Sci. 1971, 111, 13–18. [Google Scholar] [CrossRef]
- Bremner, J.M.; Krogmeier, M.J. Effects of urease inhibitors on germination of seeds in soil. Commun. Soil Sci. Plant Anal. 1990, 21, 311–321. [Google Scholar] [CrossRef]
- Lynch, J.M. Effects of organic acids on the germination of seeds and growth of seedlings. Plant Cell Environ. 1980, 3, 255–259. [Google Scholar] [CrossRef]
- Wallace, J.M.; Elliott, L.F. Phytotoxins from anaerobically decomposing wheat straw. Soil Biol. Biochem. 1979, 11, 325–330. [Google Scholar] [CrossRef]
- Wanniarachchi, S.D.; Voroney, R.P. Phytotoxicity of canola residues: Release of water-soluble phytotoxins. Can. J. Soil Sci. 1997, 77, 535–541. [Google Scholar] [CrossRef]
- Zak, D.; Roth, C.; Gelbrecht, J.; Fenner, N.; Reuter, H. Polyphenols as enzyme inhibitors in different degraded peat soils: Implication for microbial metabolism in rewetted peatlands. In Proceedings of the EGU General Assembly Conference, Vienna, Austria, 12–17 April 2015. [Google Scholar]
- Sharma, H.S.S.; McCall, D.; Lyons, G. Chemical changes in Peat as a Result of Neutralizing with Lime during the Preparation of Mushroom Casing. In Mushroom Biology and Mushroom Products, Proceedings of the 2nd International Conference, University Park, PA, USA, 9–12 June 1996; Pennsylvania State University: University Park, PA, USA, 1996; pp. 363–372. [Google Scholar]
- European Commision, Working Group On Polycyclic Aromatic Hydrocarbons. Ambient Air Pollution by Polycyclic Aromatic Hydrocarbons (PAH); Office for Official Publications of the European Communities: Luxembourg, 2001; ISBN 928942057X. [Google Scholar]
- Voroney, R.P.; Farquharson, B.J.; Janovicek, K.J.; Bauchamp, E.G.; Vyn, T.J.; Fortin, M. Technology Evaluation and Development Sub-Program: Yield Reduction Effects of Crop Residues in Conservation Tillage, Agriculture Canada Research Station; Agriculture Canada, Research Station: Harrow, ON, Canada, 1992.
- Siqueira, J.O.; Nair, M.G.; Hammerschmidt, R.; Safir, G.R.; Putnam, A.R. Significance of phenolic compounds in plant-soil-microbial systems. CRC Crit. Rev. Plant Sci. 1991, 10, 63–121. [Google Scholar] [CrossRef]
- Eibisch, N.; Helfrich, M.; Don, A.; Mikutta, R.; Kruse, A.; Ellerbrock, R.; Flessa, H. Properties and Degradability of Hydrothermal Carbonization Products. J. Environ. Qual. 2013, 42, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Buss, W.; Mašek, O. Mobile organic compounds in biochar—A potential source of contamination—Phytotoxic effects on cress seed (Lepidium sativum) germination. J. Environ. Manag. 2014, 137, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Wagner, M.; Salem, M.; Antunes, P.M.; George, C.; Ramke, H.-G.; Titirici, M.-M.; Antonietti, M. Material derived from hydrothermal carbonization: Effects on plant growth and arbuscular mycorrhiza. Appl. Soil Ecol. 2010, 45, 238–242. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Müller, C.; Grünhage, L.; Koch, C.; Kammann, C. Biochar, hydrochar and uncarbonized feedstock application to permanent grassland—Effects on greenhouse gas emissions and plant growth. Agric. Ecosyst. Environ. 2014, 191, 39–52. [Google Scholar] [CrossRef]
- Makoi, J.; Ndakidemi, P. Biological, ecological and agronomic significance of plant phenolic compounds in rhizosphere of the symbiotic legumes. Afr. J. Biotechnol. 2007, 6, 1358–1368. [Google Scholar]

| Sample | C (wt %) * | H (wt %) * | N (wt %) * | O (wt %) * | Moisture (wt %) | Ash (wt %) ** | Volatiles (wt %) * | Fixed C (wt %) * |
|---|---|---|---|---|---|---|---|---|
| FH | 62.0 | 6.50 | 1.40 | 30.1 | 8.2 | 12.3 | 73.3 | 73.3 |
| WH | 60.9 | 6.35 | 1.65 | 31.1 | 2.7 | 10.7 | 73.1 | 73.1 |
| AH | 60.8 | 6.10 | 1.40 | 31.7 | 6.9 | 18.2 | 72.2 | 72.2 |
| BF | 54.7 | 6.56 | 1.74 | 37.0 | 46.5 | 25.9 | 87.2 | 87.2 |
| Peat | 49–60 | 5–8 | 1–4 | 28–45 | 70–80 | 70–80 | ||
| Lignite | 65–73 | 5–8 | 0.5–1.5 | 16–30 | 47–60 | 47–60 |
| Parameter | Unit | Limitation a | Limitation b | |
|---|---|---|---|---|
| pH | 5.8 | 6–8.5 | 6–8.5 | |
| Moisture | wt % | 8.2 | <50 | <50 |
| Organic C | wt % d.b. | 52.2 | >40 | >20 |
| Organic N | % of T.N. † | >99.8% | >80% | >80% |
| C/N | 44 | - | <50 | |
| Humic/Fulvic C | wt % d.b. | 17.3 | - | >2.5 |
| Pb | mg kg−1 d.b. | <60 | <140 | <140 |
| Cd | mg kg−1 d.b. | 0.22 | <1.5 | <1.5 |
| Ni | mg kg−1 d.b. | <15 | <100 | <100 |
| Zn | mg kg−1 d.b. | 84 | <500 | <500 |
| Cu | mg kg−1 d.b. | <60 | <230 | <230 |
| Hg | mg kg−1 d.b. | 0.06 | <1.5 | <1.5 |
| Cr(VI) | mg kg−1 d.b. | <0.3 | <0.5 | <0.5 |
| Parameter | Unit | |
|---|---|---|
| Total N | 0.12 | wt % d.b. |
| Exchangeable K | 46.0 | mg kg–1 d.b. |
| Calcium | 26,640 | mg kg–1 d.b. |
| Magnesium | 1859 | mg kg–1 d.b. |
| SO42− | 1312 | mg kg–1 d.b. |
| Cl− | 675 | mg kg–1 d.b. |
| Iron | 1414 | mg kg–1 d.b. |
| Boron | <25 | mg kg–1 d.b. |
| Manganese | <55 | mg kg–1 d.b. |
| Zn | 84 | mg kg–1 d.b. |
| Cu | <60 | mg kg–1 d.b. |
| C.E.C. (cation exchange capacity) | 3.8 | meq 100 g−1 |
| EC1:5 | 2.07 | mS cm−1 |
| Sodium | 873 | mg kg–1 d.b. |
| Total polyphenols (as tannic acid) | 27,204 | mg kg–1 d.b. |
| Total tannins (as tannic acid) | 22,822 | mg kg–1 d.b. |
| VFAs | 390 | mg kg–1 d.b. |
| Soil respiration | 4247 | mg C CO2 kg−1 |
| Parameter | Unit | FH | Rings Number |
|---|---|---|---|
| Benzo(a) anthracene | mg kg−1 d.b. | <0.1 | 4 |
| Benzo(a) pyrene | mg kg−1 d.b. | <0.1 | 5 |
| Benzo(b)fluoranthene | mg kg−1 d.b. | <0.1 | 5 |
| Benzo(k)fluoranthene | mg kg−1 d.b. | <0.1 | 5 |
| Chrysene | mg kg−1 d.b. | <0.1 | 4 |
| Dibenzo(a,h) anthracene | mg kg−1 d.b. | <0.1 | 5 |
| Benzo(e) pyrene | mg kg−1 d.b. | <0.1 | 5 |
| Benzo(j) fluoranthene | mg kg−1 d.b. | <0.1 | 5 |
| Naphthalene | mg kg−1 d.b. | 0.6 | 2 |
| Acenaphthene | mg kg−1 d.b. | <0.1 | 3 |
| Acenaphthylene | mg kg−1 d.b. | <0.1 | 3 |
| Anthracene | mg kg−1 d.b. | <0.1 | 3 |
| Benzo(g,h,i) perylene | mg kg−1 d.b. | <0.1 | 6 |
| Phenanthrene | mg kg−1 d.b. | <0.1 | 3 |
| Fluorene | mg kg−1 d.b. | <0.1 | 3 |
| Indeno(1,2,3-c,d) pyrene | mg kg−1 d.b. | <0.1 | 6 |
| Pyrene | mg kg−1 d.b. | <0.1 | 4 |
| ∑ PAHs | mg kg−1 d.b. | <2.2 |
| Parameter | Unit | FH † | WH † | AH † |
|---|---|---|---|---|
| pH | 5.82 c | 6.31 b | 6.90 a | |
| EC1:5 | mS cm−1 | 2.07 a | 0.66 c | 1.10 b |
| SO42− | mg kg−1 d.b. | 1312 a | 538 b | 565 b |
| Cl− | mg kg−1 d.b. | 675 a | 68 c | 436 b |
| Na | mg kg−1 d.b. | 873 a | 128 c | 245 b |
| Corg | wt % d.b. | 52.2 a | 50.8 a | 48.7 b |
| Norg | % of T.N. | >99.8 | >99.6 | >99.6 |
| Humic/Fulvic C | wt % d.b. | 17.3 a | 12.9 b | 13.8 b |
| VFAs | mg kg−1 d.b. | 390 a | 288 b | 8 c |
| Total polyphenols (as tannic acid) | mg kg−1 d.b. | 27,204 a | 23,140 b | 16,128 c |
| Total tannins (as tannic acid) | mg kg−1 d.b. | 22,822 a | 19,404 b | 14,320 c |
| Biomass respiration | mg C CO2 kg−1 | 4247 a | 4124 a | 3731 b |
| Parameter | Unit | |
|---|---|---|
| VFAs | mg kg−1 d.b. | n.d. |
| Total polyphenols (as tannic acid) | mg kg−1 d.b. | 1450 |
| Total tannins (as tannic acid) | mg kg−1 d.b. | 209 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puccini, M.; Ceccarini, L.; Antichi, D.; Seggiani, M.; Tavarini, S.; Hernandez Latorre, M.; Vitolo, S. Hydrothermal Carbonization of Municipal Woody and Herbaceous Prunings: Hydrochar Valorisation as Soil Amendment and Growth Medium for Horticulture. Sustainability 2018, 10, 846. https://doi.org/10.3390/su10030846
Puccini M, Ceccarini L, Antichi D, Seggiani M, Tavarini S, Hernandez Latorre M, Vitolo S. Hydrothermal Carbonization of Municipal Woody and Herbaceous Prunings: Hydrochar Valorisation as Soil Amendment and Growth Medium for Horticulture. Sustainability. 2018; 10(3):846. https://doi.org/10.3390/su10030846
Chicago/Turabian StylePuccini, Monica, Lucia Ceccarini, Daniele Antichi, Maurizia Seggiani, Silvia Tavarini, Marisa Hernandez Latorre, and Sandra Vitolo. 2018. "Hydrothermal Carbonization of Municipal Woody and Herbaceous Prunings: Hydrochar Valorisation as Soil Amendment and Growth Medium for Horticulture" Sustainability 10, no. 3: 846. https://doi.org/10.3390/su10030846
APA StylePuccini, M., Ceccarini, L., Antichi, D., Seggiani, M., Tavarini, S., Hernandez Latorre, M., & Vitolo, S. (2018). Hydrothermal Carbonization of Municipal Woody and Herbaceous Prunings: Hydrochar Valorisation as Soil Amendment and Growth Medium for Horticulture. Sustainability, 10(3), 846. https://doi.org/10.3390/su10030846








