Adsorption of Hexavalent Chromium Using Banana Pseudostem Biochar and Its Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Banana Pseudostem Biochars
2.3. Characterization of Banana Pseudostem Biochars
2.4. Adsorption Experiments
3. Results and Discussion
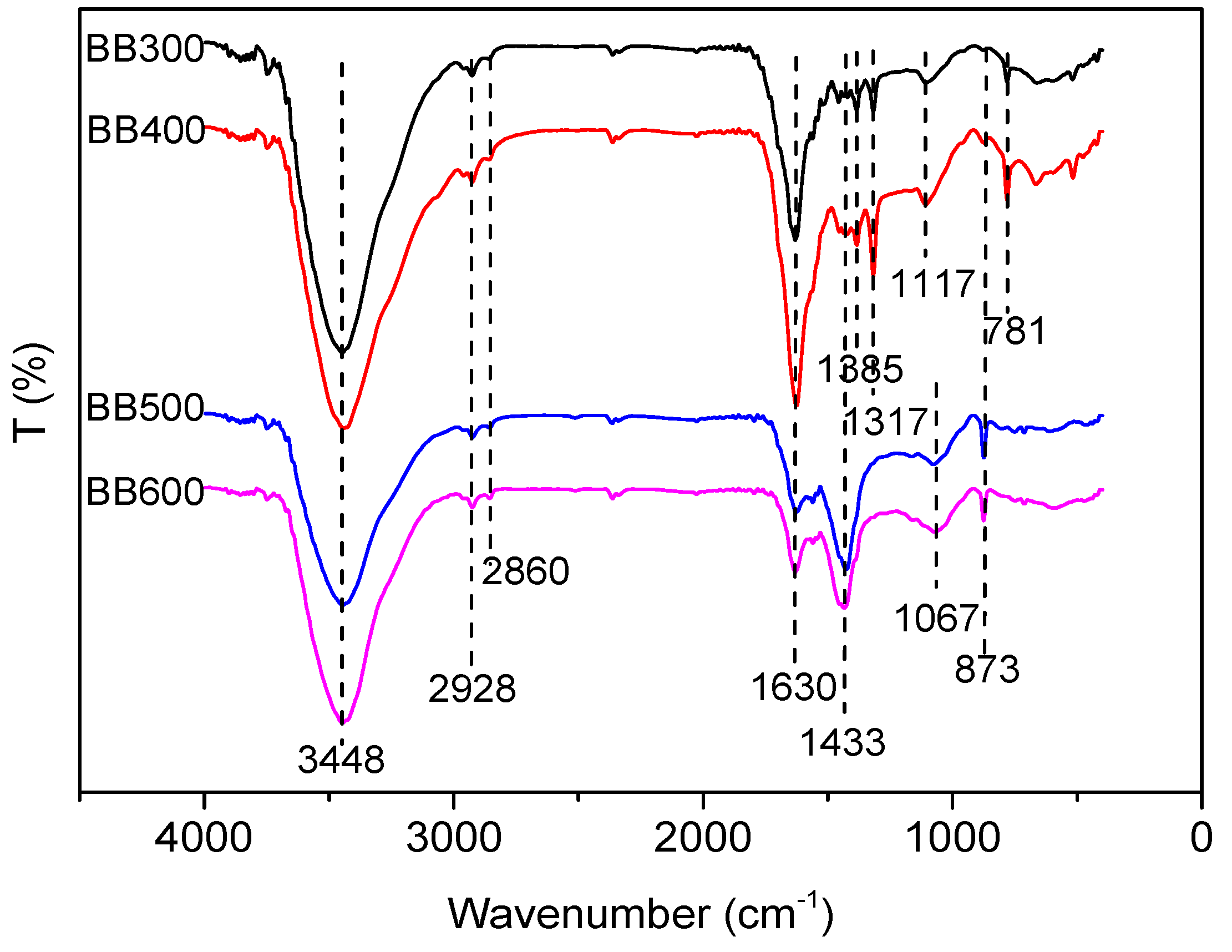
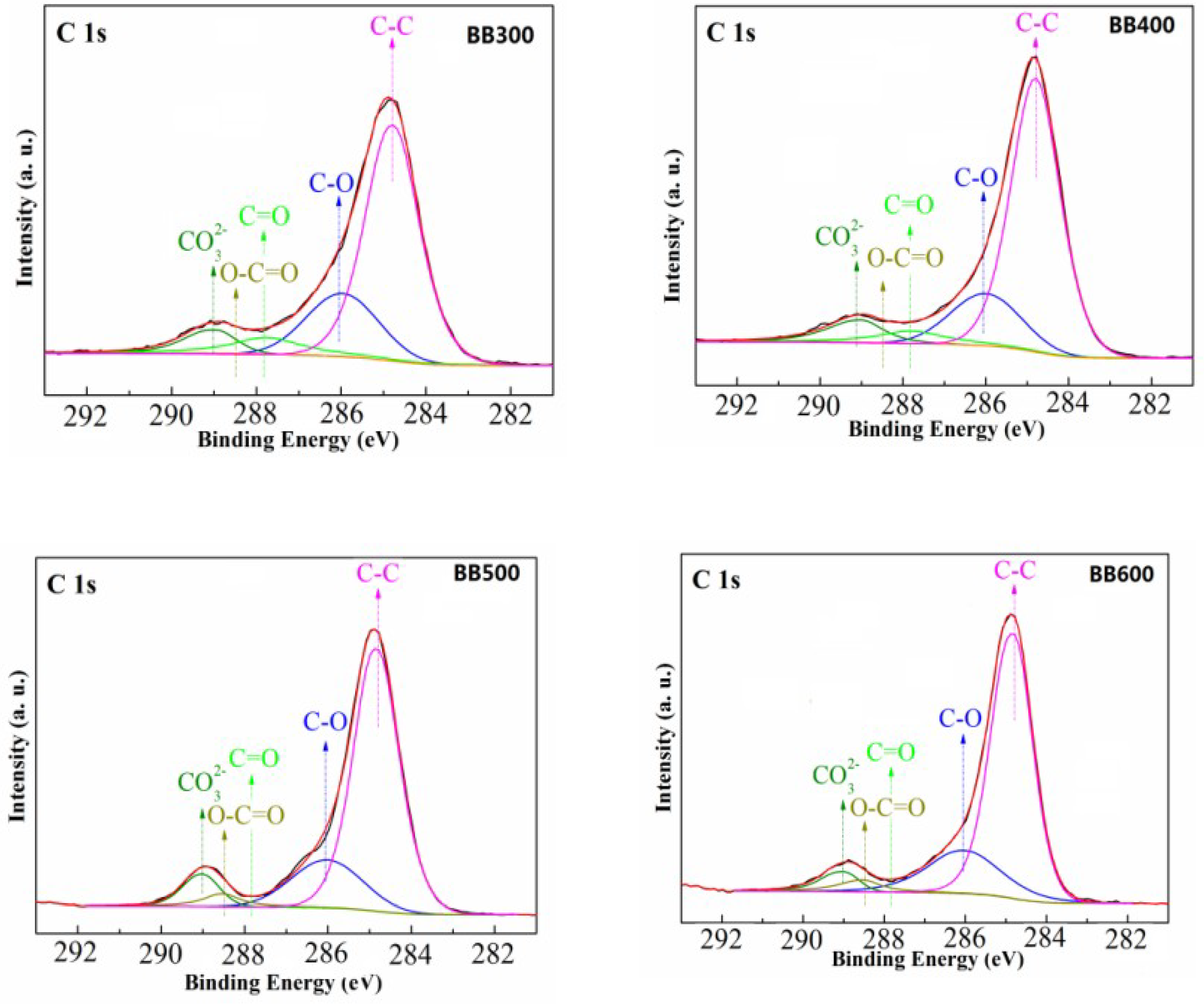
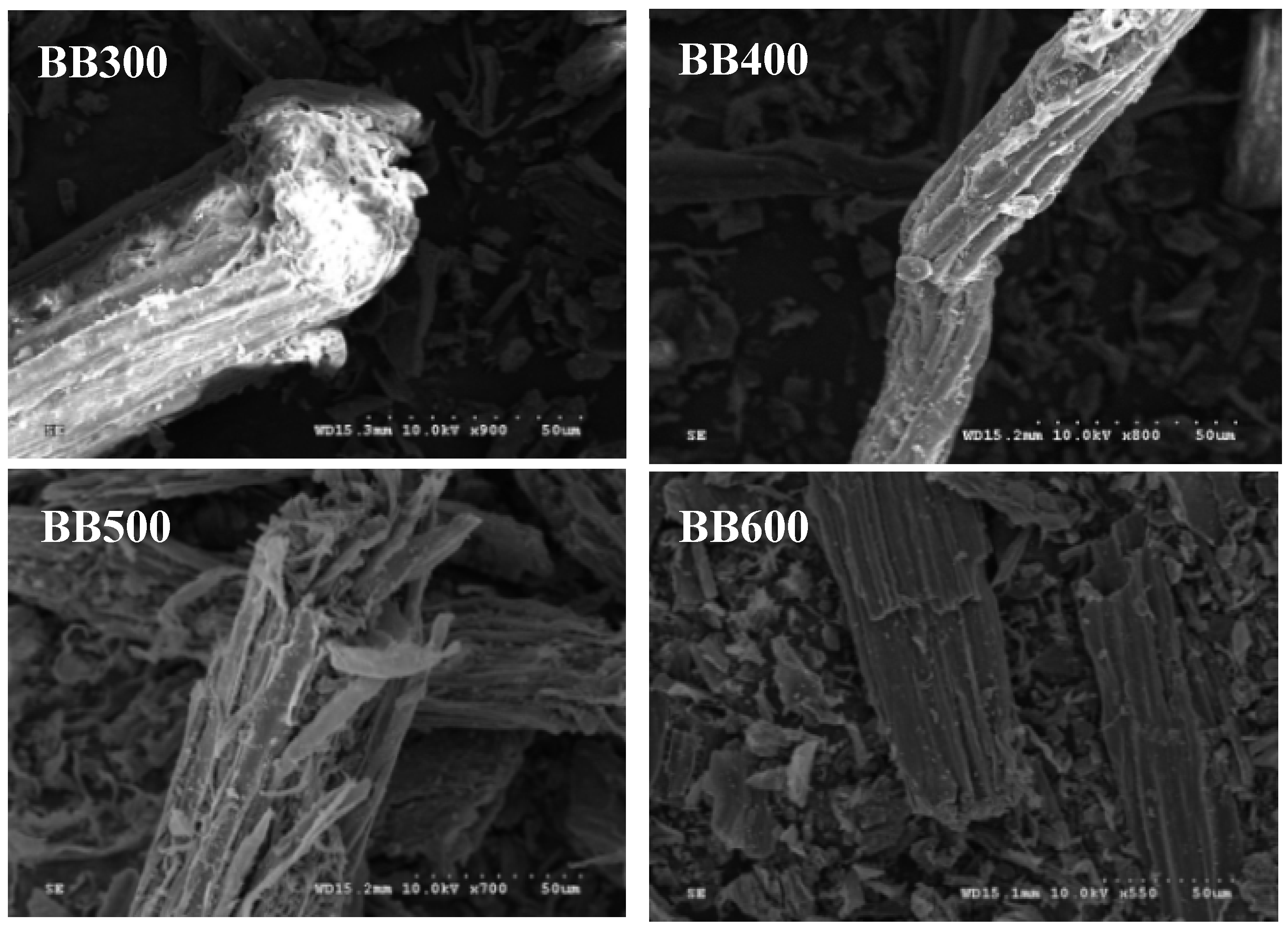
3.1. Characterization of Banana Pseudostem Biochars
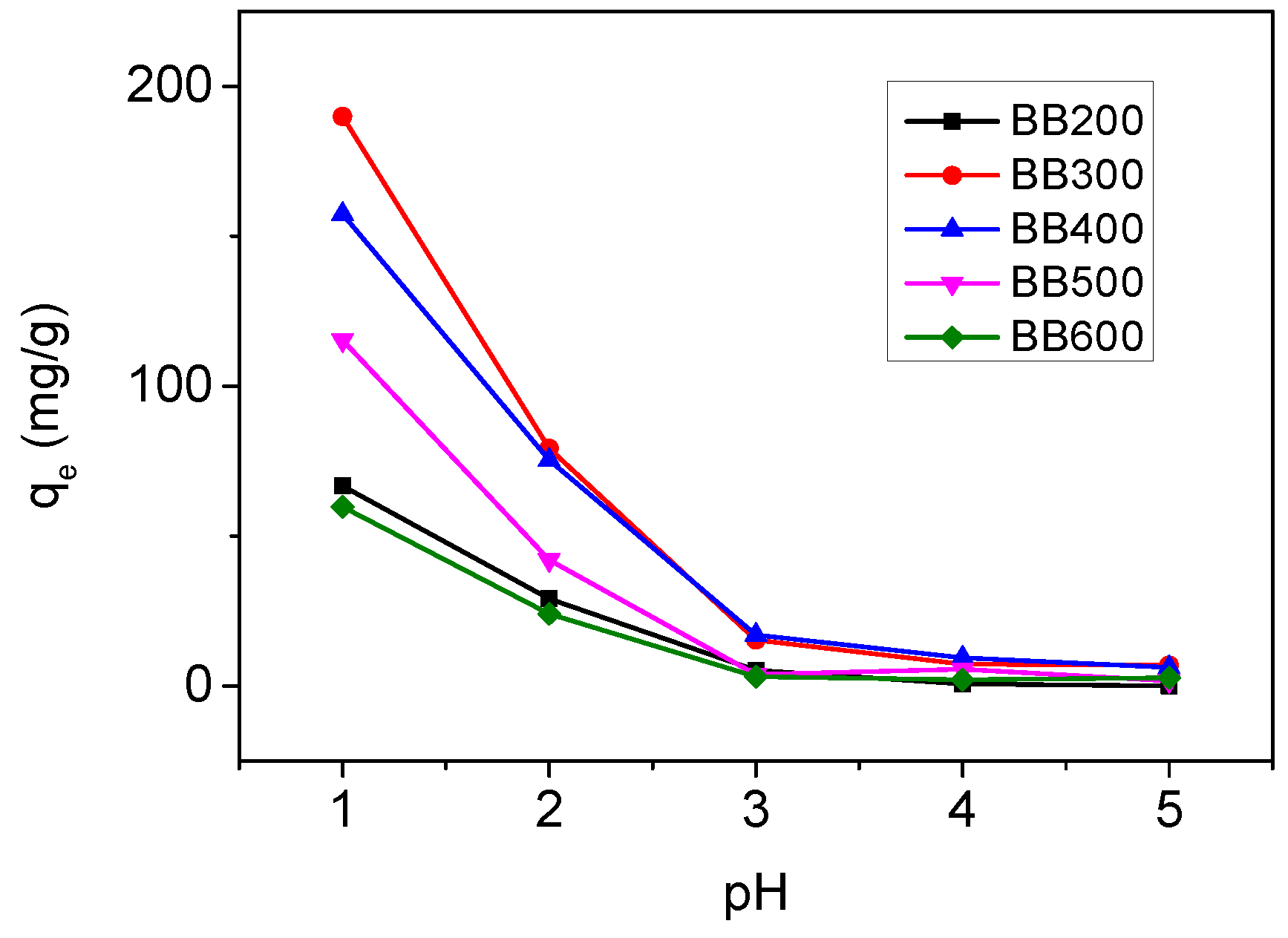
3.2. Effects of pH and Pyrolysis Temperature
3.3. Adsorption Kinetics
3.4. Adsorption Isotherms
3.5. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bayazit, Ş.S.; Kerkez, Ö. Hexavalent chromium adsorption on superparamagnetic multi-wall carbon nanotubes and activated carbon composites. Chem. Eng. Res. Des. 2014, 92, 2725–2733. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K.S.M. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Costa, M.; Klein, C.B. Toxicity and carcinogenicity of chromium compounds in humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, H.; Alattar, M.; Jiang, S.; Han, J.; Ma, Y.; Jiang, C. The preferential accumulation of heavy metals in different tissues following frequent respiratory exposure to PM2.5 in rats. Sci. Rep. 2015, 5, 16936–16944. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Jiao, W.; Yu, H.; Niu, Y.; Pang, Y.; Xu, X.; Guo, X. Spatial evaluation of heavy metals concentrations in the surface sediment of Taihu Lake. Int. J. Environ. Res. Public Health 2015, 12, 15028–15039. [Google Scholar] [CrossRef] [PubMed]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 2002, 180, 5–22. [Google Scholar] [PubMed]
- Tu, W.; Li, K.; Shu, X.; Yu, W.W. Reduction of hexavalent chromium with colloidal and supported palladium nanocatalysts. J. Nanopart. Res. 2013, 15, 1593–1602. [Google Scholar]
- Kongsricharoem, N.; Polprasert, C. Chromium removal by a bipolar electro-chemical precipitation process. Water Sci. Technol. 1996, 34, 109–116. [Google Scholar]
- Song, Z.; Williams, C.J.; Edyvean, R.G.J. Sedimentation of tannery wastewater. Water Res. 2000, 34, 2171–2176. [Google Scholar] [CrossRef]
- Wang, G.; Chang, Q.; Han, X.; Zhang, M. Removal of Cr(VI) from aqueous solution by flocculant with the capacity of reduction and chelation. J. Hazard. Mater. 2013, 248–249, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, T.; Rajgopal, S.; Miranda, L.R. Chromium(VI) adsorption from aqueous solution by Hevea brasilinesis sawdust activated carbon. J. Hazard. Mater. 2005, 124, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, G.; Bhattacharya, P.K. Hexavalent chromium ion removal through micellar enhanced ultrafiltration. Chem. Eng. J. 2006, 119, 45–53. [Google Scholar] [CrossRef]
- Rengaraj, S.; Yeon, K.-H.; Moon, S.-H. Removal of chromium from water and wastewater by ion exchange resins. J. Hazard. Mater. 2001, B87, 273–287. [Google Scholar] [CrossRef]
- Achouri, O.; Panico, A.; Bencheikh-Lehocine, M.; Derbal, K.; Pirozzi, F. Effect of chemical coagulation pretreatment on anaerobic digestion of tannery wastewater. J. Environ. Eng. 2017, 143, 04017039. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P.; Singh, V.K. Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. J. Hazard. Mater. 2006, 135, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Kong, L.; Qu, Z.; Li, L.; Shen, G. Magnetic biochar decorated with ZnS nanocrytals for Pb (II) removal. ACS Sustain. Chem. Eng. 2014, 3, 125–132. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.H.; Zeng, R.J.; Jiang, H. Recent developments of post-modification of biochar for electrochemical energy storage. Bioresour. Technol. 2017, 246, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U., Jr. Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [PubMed]
- Zhou, L.; Liu, Y.; Liu, S.; Yin, Y.; Zeng, G.; Tan, X.; Hu, X.; Hu, X.; Jiang, L.; Ding, Y.; et al. Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Ma, W.; Pan, Y.; Meng, F.; Yu, S.; Wu, L. Synthesis of magnetic biochar from iron sludge for the enhancement of Cr (VI) removal from solution. J. Taiwan Inst. Chem. Eng. 2017, 80, 835–841. [Google Scholar] [CrossRef]
- Dong, X.; Ma, L.Q.; Li, Y. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 2011, 190, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.S.; Wang, S.L.; Tzou, Y.M.; Yan, Y.Y.; Kuan, W.H. Removal of hexavalent Cr by coconut coir and derived chars—The effect of surface functionality. Bioresour. Technol. 2012, 104, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Chen, Z. Biosorption of Cr(VI) from aqueous solution by biochar derived from the leaf of Leersia hexandra Swartz. Environ. Earth Sci. 2017, 76, 67–74. [Google Scholar] [CrossRef]
- Shah, M.P.; Reddy, G.V.; Banerjee, R.; Ravindra Babu, P.; Kothari, I.L. Microbial degradation of banana waste under solid state bioprocessing using two lignocellulolytic fungi (Phylosticta spp. MPS-001 and Aspergillus spp. MPS-002). Process. Biochem. 2005, 40, 445–451. [Google Scholar] [CrossRef]
- Jayaprabha, J.S.; Brahmakumar, M.; Manilal, V.B. Banana pseudostem characterization and its fiber property evaluation on physical and bioextraction. J. Nat. Fibers 2011, 8, 149–160. [Google Scholar] [CrossRef]
- Li, D.-C.; Ding, J.-W.; Qian, T.-T.; Zhang, S.; Jiang, H. Preparation of high adsorption performance and stable biochar granules by FeCl3-catalyzed fast pyrolysis. RSC Adv. 2016, 6, 12226–12234. [Google Scholar] [CrossRef]
- Özçimen, D.; Ersoy-Meriçboyu, A. Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials. Renew. Energy 2010, 35, 1319–1324. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 2009, 76, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Xing, B.; Zhu, L. Comparative study on composition, structure, and adsorption behavior of activated carbons derived from different synthetic waste polymers. J. Colloid Interface Sci. 2011, 360, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Lian, F.; Yu, Z.; Zhu, L.; Xing, B.; Qiu, W. Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem. Eng. J. 2014, 242, 36–42. [Google Scholar]
- Li, G.; Zhu, W.; Zhang, C.; Zhang, S.; Liu, L.; Zhu, L.; Zhao, W. Effect of a magnetic field on the adsorptive removal of methylene blue onto wheat straw biochar. Bioresour. Technol. 2016, 206, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, W.; Yan, J.; Han, L.; Chen, Y.; Ouyang, D.; Chen, M. Nanoscale zero-valent iron supported by biochars produced at different temperatures: Synthesis mechanism and effect on Cr(VI) removal. Environ. Pollut. 2017, 223, 153–160. [Google Scholar] [PubMed]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [PubMed]
- Zeng, Z.-W.; Tian, S.-R.; Liu, Y.-G.; Tan, X.-F.; Zeng, G.-M.; Jiang, L.-H.; Yin, Z.-H.; Liu, N.; Liu, S.-B.; Li, J. Comparative study of rice husk biochars for aqueous antibiotics removal. J. Chem. Technol. Biotechnol. 2017, 93, 1075–1084. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition on nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Sulaiman, F.; Miskam, M.A.; Taib, R.M. Characterization of banana (Musa spp.) pseudo-stem and fruit-bunch-Stem as a potential renewable energy resource. Int. J. Biol. Veter. Agric. Food Eng. 2014, 8, 712–716. [Google Scholar]
- Chen, D.; Yu, X.; Song, C.; Pang, X.; Huang, J.; Li, Y. Effect of pyrolysis temperature on the chemical oxidation stability of bamboo biochar. Bioresour. Technol. 2016, 218, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, W.; Li, J.; Zhang, Y. Characterization of cellulose from banana pseudo-stem by polyhydric alcohols liquefaction. Renew. Energy Resour. 2016, 34, 285–291. [Google Scholar]
- Chen, T.; Zhou, Z.; Xu, S.; Wang, H.; Lu, W. Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Bioresour. Technol. 2015, 190, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Tytlak, A.; Oleszczuk, P.; Dobrowolski, R. Sorption and desorption of Cr(VI) ions from water by biochars in different environmental conditions. Environ. Sci. Pollut. Res. Int. 2015, 22, 5985–5994. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, F.; Erto, A.; Lancia, A.; Musmarra, D. Equilibrium and dynamic study on hexavalent chromium adsorption onto activated carbon. J. Hazard. Mater. 2015, 281, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Nityanandi, D.; Subbhuraam, C.V. Kinetics and thermodynamic of adsorption of chromium(VI) from aqueous solution using puresorbe. J. Hazard. Mater. 2009, 170, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Mishra, B.K. Oxidation of alcohol by lipopathic Cr(VI): A mechanistic study. J. Org. Chem. 2006, 71, 6759–6766. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ma, T.; Zhou, L.; Hu, Z.; Shi, L. Chromium isotopic fractionation during Cr(VI) reduction by Bacillus sp. under aerobic conditions. Chemosphere 2015, 130, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Mo, J.; Glocheux, Y.; Allen, S.; Walker, G.; Mangwandi, C. Preliminary investigation of mixed adsorbents for the removal of copper and methylene blue from aqueous solutions. Chem. Eng. J. 2014, 255, 525–534. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Özcan, A.S.; Erdem, B.; Özcan, A. Adsorption of acid blue 193 from aqueous solutions onto BTMA-bentonite. Colloids Surf. Physicochem. Eng. Asp. 2005, 266, 73–81. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Schaferlb, H. Chromic acid oxidation of isopropyl alcohol. preoxidation equilibria. J. Am. Chem. Soc. 1969, 91, 927–932. [Google Scholar] [CrossRef]
- Hiran, B.L.; Chaplot, S.L.; Joshi, V.; Chaturvedi, G. Kinetics of the effect of some bidentate amino acid ligands in the oxidation of lactic acid by chromium(VI). J. Am. Chem. Soc. 2002, 43, 657–661. [Google Scholar]
- Rorek, J.; Radkowsky, A.E. Mechanism of the chromic acid oxidation of cyclobutanol. J. Am. Chem. Soc. 1973, 95, 7123–7132. [Google Scholar]
- Fahim, N.F.; Barsoum, B.N.; Eid, A.E.; Khalil, M.S. Removal of chromium (III) from tannery wastewater using activated carbon from sugar industrial waste. J. Hazard. Mater. 2006, 136, 303–309. [Google Scholar] [CrossRef] [PubMed]
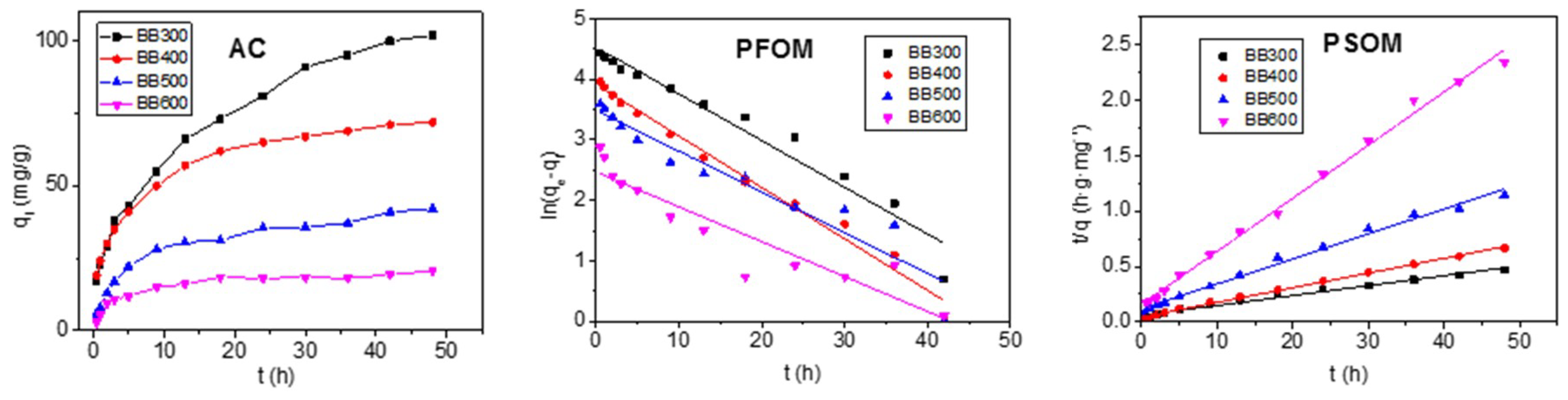
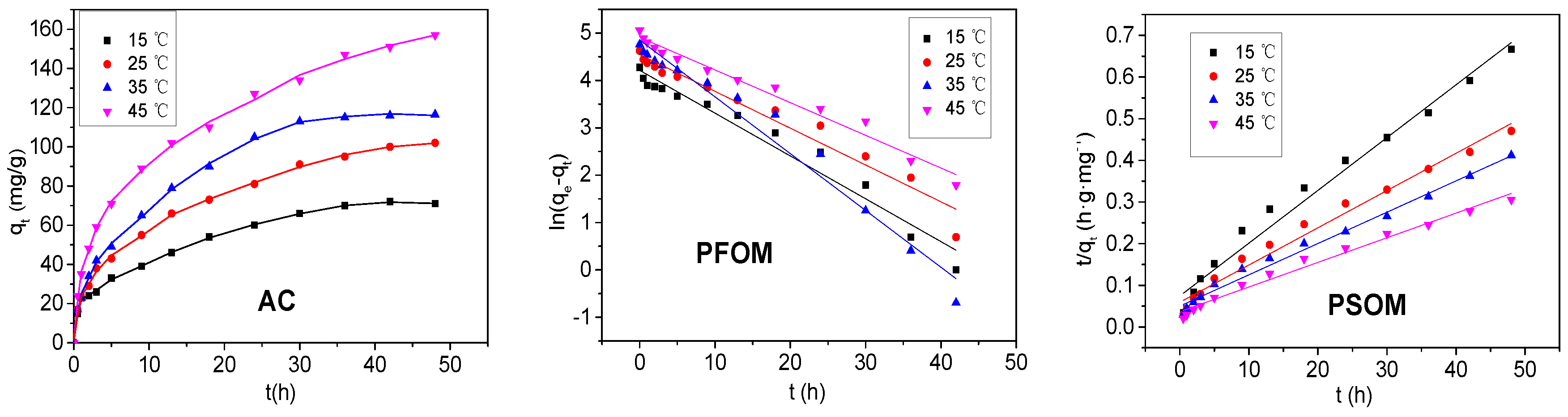
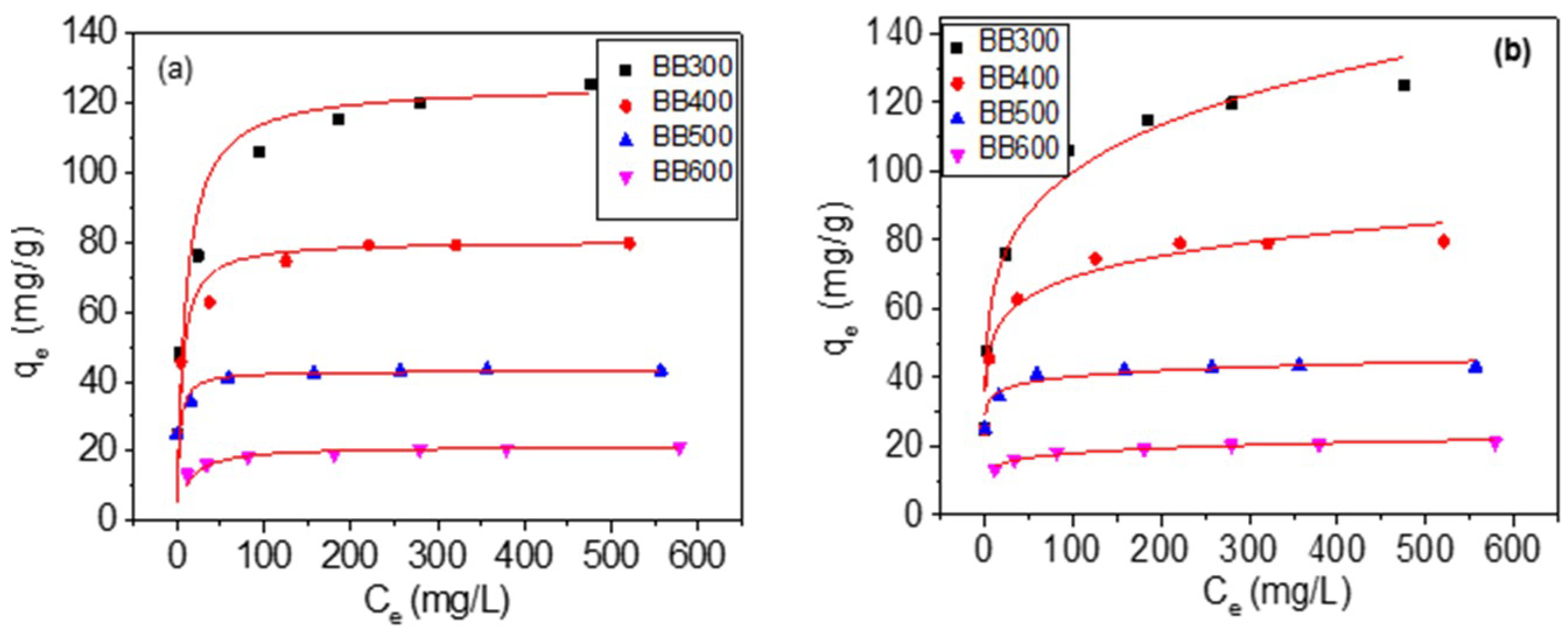

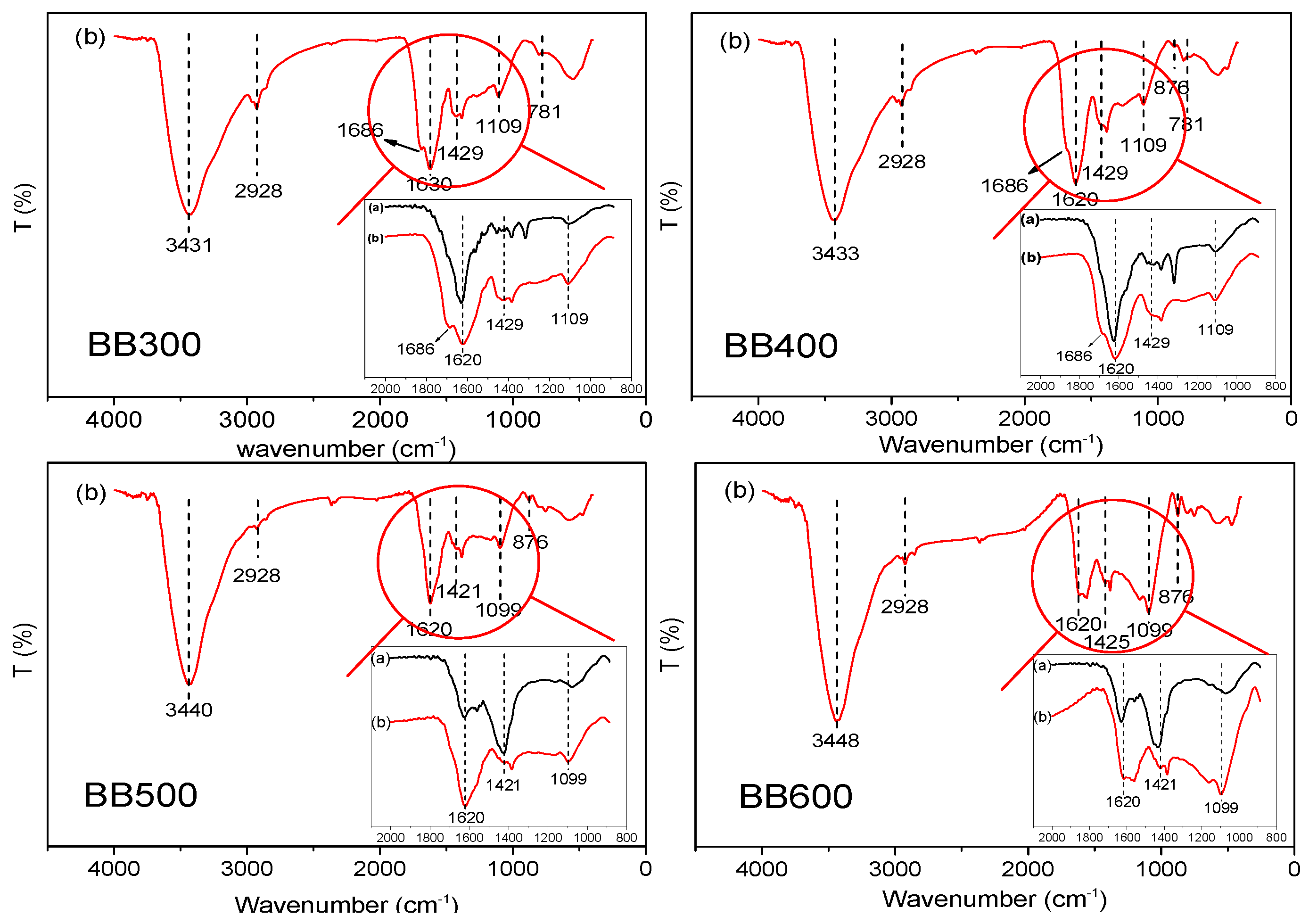
| BC | BB300 | BB400 | BB500 | BB600 |
|---|---|---|---|---|
| SA (m2/g) | 4.98 | 6.62 | 11.27 | 8.53 |
| PV (cm3/g) | 0.00959 | 0.01458 | 0.03932 | 0.04389 |
| PD (nm) | 10.39 | 11.85 | 15.60 | 19.45 |
| B.E. (eV) | Ass. | BB300 | BB400 | BB500 | BB600 | BB300+Cr |
|---|---|---|---|---|---|---|
| C1 (286.0) | C–O (%) | 34.14 | 26.25 | 26.94 | 29.56 | 43.52 |
| C2 (287.8) | C=O (%) | 12.62 | 8.50 | 0.11 | 0.13 | 0.52 |
| C3 (288.5) | COO (%) | 0.74 | 0.85 | 5.20 | 5.73 | 8.81 |
| C4 (289.0) | CO32− (%) | 10.47 | 10.39 | 9.85 | 7.19 | 5.35 |
| TCOG | 57.97 | 45.99 | 42.10 | 42.61 | 58.20 |
| Pseudo-First-Order Model | ||||
|---|---|---|---|---|
| BB300 | BB400 | BB500 | BB600 | |
| qe,exp | 102.45 | 72.21 | 42.01 | 20.58 |
| qe,cal | 93.03 | 50.25 | 32.70 | 11.35 |
| k1 | 7.72 × 10−2 | 8.51 × 10−2 | 6.74 × 10−2 | 5.63 × 10−2 |
| R2 | 0.96073 | 0.98212 | 0.90456 | 0.86206 |
| Pseudo-Second-Order Model | ||||
| BB300 | BB400 | BB500 | BB600 | |
| qe,exp | 102.45 | 72.21 | 42.01 | 20.58 |
| qe,cal | 111.35 | 75.20 | 44.40 | 20.88 |
| k2 | 1.35 × 10−3 | 3.92 × 10−3 | 4.29 × 10−3 | 1.45 × 10−2 |
| R2 | 0.98054 | 0.99660 | 0.99096 | 0.99493 |
| Pseudo-First-Order Model | ||||
|---|---|---|---|---|
| 15 °C | 25 °C | 35 °C | 45 °C | |
| qe,exp | 72.04 | 102.45 | 116.50 | 156.56 |
| qe,cal | 67.63 | 94.36 | 128.17 | 135.94 |
| k1 | 9.04 × 10−2 | 7.78 × 10−2 | 1.20 × 10−1 | 6.90 × 10−2 |
| R2 | 0.96007 | 0.95519 | 0.96748 | 0.97669 |
| Pseudo-Second-Order Model | ||||
| 15 °C | 25 °C | 35 °C | 45 °C | |
| qe,exp | 72.04 | 102.45 | 116.50 | 156.56 |
| qe,cal | 61.46 | 111.86 | 132.98 | 169.20 |
| k2 | 3.56 × 10−3 | 1.35 × 10−3 | 1.13 × 10−3 | 9.50 × 10−4 |
| R2 | 0.97909 | 0.98054 | 0.98770 | 0.98285 |
| LM | ||||
|---|---|---|---|---|
| BB300 | BB400 | BB500 | BB600 | |
| qmax (mg/g) | 125.44 | 80.33 | 43.47 | 21.53 |
| KL (L/mg) | 0.1024 | 0.1910 | 0.3929 | 0.0796 |
| R2 | 0.9977 | 0.9996 | 0.9999 | 0.9997 |
| FM | ||||
| BB300 | BB400 | BB500 | BB600 | |
| KF (mg/g)·(mg/L)−n | 42.64 | 39.07 | 30.25 | 10.67 |
| n | 0.185 | 0.124 | 0.062 | 0.115 |
| R2 | 0.9813 | 0.9609 | 0.8030 | 0.9554 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Yu, W.; Liu, S.; Xu, C.; Li, J.; Zhang, Y. Adsorption of Hexavalent Chromium Using Banana Pseudostem Biochar and Its Mechanism. Sustainability 2018, 10, 4250. https://doi.org/10.3390/su10114250
Xu S, Yu W, Liu S, Xu C, Li J, Zhang Y. Adsorption of Hexavalent Chromium Using Banana Pseudostem Biochar and Its Mechanism. Sustainability. 2018; 10(11):4250. https://doi.org/10.3390/su10114250
Chicago/Turabian StyleXu, Shuang, Weiguang Yu, Sen Liu, Congying Xu, Jihui Li, and Yucang Zhang. 2018. "Adsorption of Hexavalent Chromium Using Banana Pseudostem Biochar and Its Mechanism" Sustainability 10, no. 11: 4250. https://doi.org/10.3390/su10114250
APA StyleXu, S., Yu, W., Liu, S., Xu, C., Li, J., & Zhang, Y. (2018). Adsorption of Hexavalent Chromium Using Banana Pseudostem Biochar and Its Mechanism. Sustainability, 10(11), 4250. https://doi.org/10.3390/su10114250





