Cattle Diets Strongly Affect Nitrous Oxide in the Rumen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Sampling and Measurements of Gaseous Emissions
2.3. Laboratory Analyses of the Forages
- Alfalfa silage [32]:DMcor = DM + (1.05 − 0.059 × pH) × total volatile fatty acids (VFA, C2 − C6) + 0.08 × lactic acid + 0.77 × 1,2-propanediol + 0.87 × 2,3-butanediol + 1.00 × total of other alcohols.
- Corn silage [33]:DMcor = DM + 0.95 × VFA (C2 − C6) + 0.08 × lactic acid + 0.77 × 1,2-propanediol + 1.00 × other alcohols.
- Corn silage [36]:ME = 0.136 × GP + 0.0057 × CP + 0.000286 × EE2 + 2.20.
- Alfalfa silage [37]:ME [MJ/kg organic matter] = 11.09 − 0.01040 × ADFom + 0.00497 × CP + 0.00750 × EE + 0.0351 × GP; ME [MJ/kg DM] = ME (MJ/kg organic matter) × [1000 − ash (g/kg DM)]/1000.
- Grass hay [38]:ME = 7.81 + 0.07559 × GP + 0.00384 × ash + 0.00565 × CP + 0.01898 × EE − 0.00831 × ADFom.
2.4. Statistical Analyses
3. Results
3.1. Gas Production from Forages
3.2. Gas Composition in the Rumen
4. Discussion
4.1. Emissions from Forages
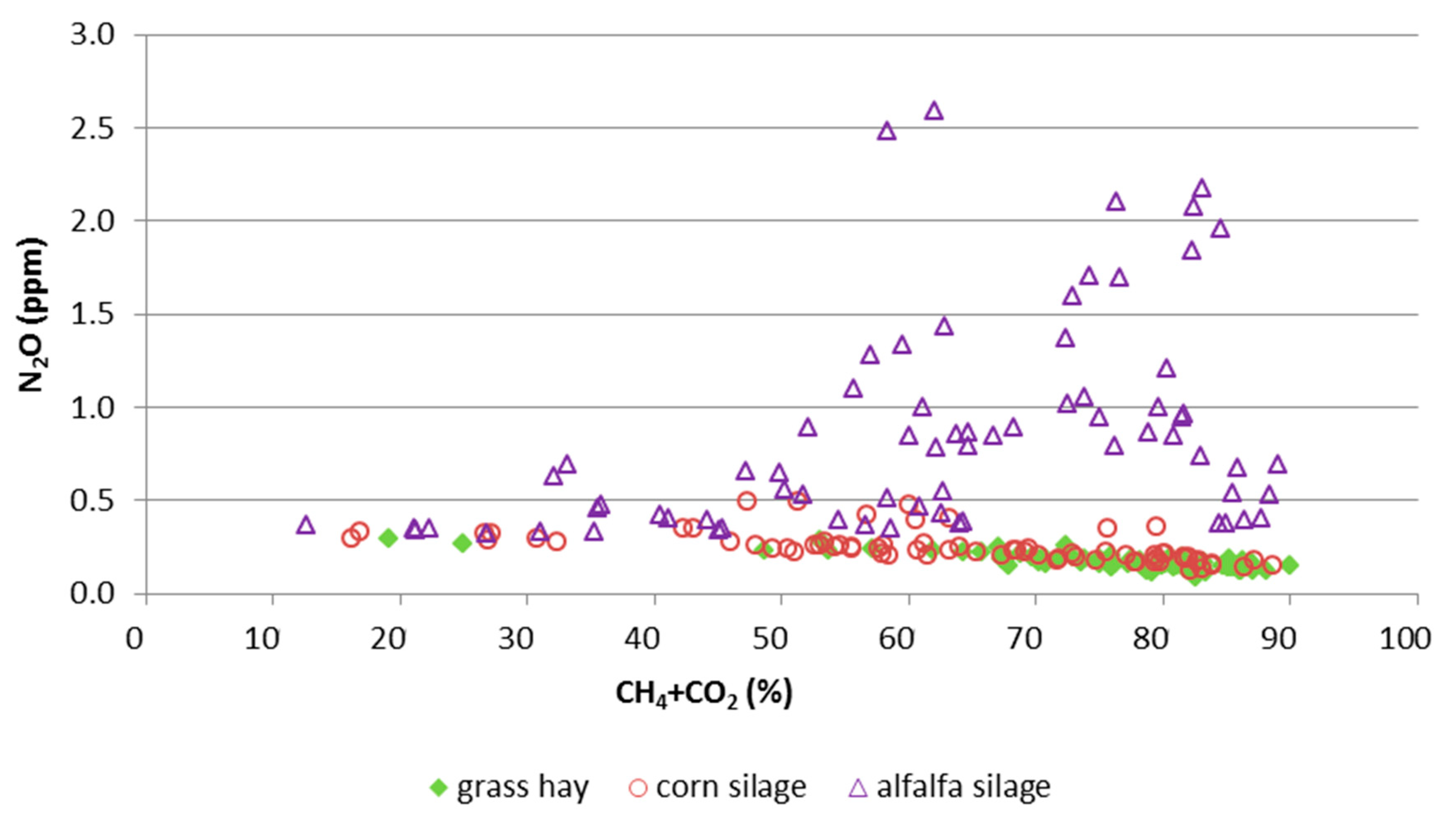
4.2. Concentration Ratios in the Rumen
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Hristov, A.; Oh, J.; Lee, C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J.; Rotz, A.; Dell, C.; Adesogan, A. Mitigation of Greenhouse gas Emissions in Livestock Production: A Review of Technical Options for Non-CO2 Emissions; FAO Anim. Produon and Health Paper; FAO: Rome, Italy, 2013; Volume 177, pp. 1–206. [Google Scholar]
- IPCC. The Physical Science Basis. Contribution of working group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- McAllister, T.; Newbold, C. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 2008, 48, 7–13. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, W.; Schweigel, M.; Weissbach, F.; Scholze, H.; Pitroff, W.; Derno, M. Methane production in cattle calculated by the nutrient composition of the diet. Arch. Anim. Nutr. 2007, 61, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kirchgessner, M.; Windisch, W.; Müller, H.L. Nutritional Factors for the Quantification of Methane Production. In Proceedings of the 8th Symposium on Ruminant Physiology, 1995; Engelhart, W., Leonhard-Marek, S., Breves, G., Giesecke, D., Eds.; Ferdinand Enke: Stuttgart, Germany, 1995; pp. 333–348. [Google Scholar]
- McAllister, T.; Cheng, K.-J.; Okine, E.; Mathison, G. Dietary, environmental and microbiological aspects of methane production in ruminants. Can. J. Anim. Sci. 1996, 76, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Eckard, R.J.; Grainger, C.; de Klein, C.A.M. Options for the abatement of methane and nitrous oxide from ruminant production: A review. Livest. Sci. 2010, 130, 47–56. [Google Scholar] [CrossRef]
- Prusty, S.; Sontakke, U.; Kundu, S. Methane and nitrous oxide emission from livestock manure. Afr. J. Biotechnol. 2014, 13, 4200–4207. [Google Scholar]
- Lee, C.; Araujo, R.C.; Koenig, K.M.; Beauchemin, K.A. In situ and in vitro evaluations of a slow release form of nitrate for ruminants: Nitrate release rate, rumen nitrate metabolism and the production of methane, hydrogen, and nitrous oxide. Anim. Feed Sci. Technol. 2017, 231, 97–106. [Google Scholar] [CrossRef]
- Macome, F.M.; Pellikaan, W.F.; Schonewille, J.T.; Bannink, A.; van Laar, H.; Hendriks, W.H.; Warner, D.; Cone, J.W. In vitro rumen gas and methane production of grass silages differing in plant maturity and nitrogen fertilisation, compared to in vivo enteric methane production. Anim. Feed Sci. Technol. 2017, 230, 96–102. [Google Scholar] [CrossRef]
- Hippenstiel, F.; Pries, M.; Büscher, W.; Südekum, K.-H. Comparative evaluation of equations predicting methane production of dairy cattle from feed characteristics. Arch. Anim. Nutr. 2013, 67, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Storm, I.M.L.D.; Hellwing, A.L.F.; Nielsen, N.I.; Madsen, J. Methods for measuring and estimating methane emission from ruminants. Animals 2012, 2, 160–183. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; López, M.; Lachica, M. Low-cost mobile open-circuit hood system for measuring gas exchange in small ruminants: From manual to automatic recording. J. Agric. Sci. 2015, 153, 1302–1309. [Google Scholar] [CrossRef]
- Place, S.E.; Pan, Y.; Zhao, Y.; Mitloehner, F.M. Construction and operation of a ventilated hood system for measuring greenhouse gas and volatile organic compound emissions from cattle. Animals 2011, 1, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Hassanat, F.; Gervais, R.; Julien, C.; Massé, D.I.; Lettat, A.; Chouinard, P.Y.; Petit, H.V.; Benchaar, C. Replacing alfalfa silage with corn silage in dairy cow diets: Effects on enteric methane production, ruminal fermentation, digestion, N balance, and milk production. J. Dairy Sci. 2013, 96, 4553–4567. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N.; Hansen, H.H.; Storm, I.M.; Madsen, J. Comparative methane estimation from cattle based on total CO2 production using different techniques. Anim. Nutr. 2017, 3, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Rotz, C.A.; Thoma, G. Assessing carbon footprints of dairy production systems. In Large Dairy Herd Management, 3rd. ed.; Beede, D.K., Ed.; American Dairy Science Association: Champaign, IL, USA, 2017; pp. 19–31. [Google Scholar]
- Petersen, S.O.; Hellwing, A.L.F.; Brask, M.; Højberg, O.; Poulsen, M.; Zhu, Z.; Baral, K.R.; Lund, P. Dietary nitrate for methane mitigation leads to nitrous oxide emissions from dairy cows. J. Environ. Qual. 2015, 44, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Emery, I.; Mosier, N. Direct emission of methane and nitrous oxide from switchgrass and corn stover: Implications for large-scale biomass storage. GCB Bioenergy 2015, 7, 865–876. [Google Scholar] [CrossRef]
- Wang, L.C.; Burris, R. Toxic gases in silage, mass spectrometric study of nitrogenous gases produced by silage. J. Agric. Food Chem. 1960, 8, 239–242. [Google Scholar] [CrossRef]
- Spoelstra, S.F. Nitrate in silage. Grass Forage Sci. 1985, 40, 1–11. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Oude Elferink, S.J.W.H.; Spoelstra, S.F. Microbiology of ensiling. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; ASA, CSSA, SSSA: Madison, WI, USA, 2003; pp. 31–93. [Google Scholar]
- Zhao, Y.; Wexler, A.S.; Hase, F.; Pan, Y.; Mitloehner, F.M. Detecting nitrous oxide in complex mixtures using FTIR spectroscopy: Silage gas. J. Environ. Prot. 2016, 7, 1719–1729. [Google Scholar] [CrossRef]
- Franco, R. Measuring Emissions and Developing Strategies to Mitigate Volatile Organic Compounds and Oxides of Nitrogen from Silage. Ph.D. Thesis, University of California, Davis, CA, USA, 2016. [Google Scholar]
- Schmithausen, A.J.; Trimborn, M.; Büscher, W. Sources of nitrous oxide and other climate relevant gases on surface area in a dairy free stall barn with solid floor and outside slurry storage. Atmos. Environ. 2018, 178, 41–48. [Google Scholar] [CrossRef]
- Wulf, S.; Maeting, M.; Clemens, J. Application technique and slurry co-fermentation effects on ammonia, nitrous oxide, and methane emissions after spreading. J. Environ. Qual. 2002, 31, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.; Trimborn, M.; Weiland, P.; Amon, B. Mitigation of greenhouse gas emissions by anaerobic digestion of cattle slurry. Agric. Ecosyst. Environ. 2006, 112, 171–177. [Google Scholar] [CrossRef]
- Schmithausen, A.J.; Trimborn, M.; Büscher, W. Methodological comparison between a novel automatic sampling system for gas chromatography versus photoacoustic spectroscopy for measuring greenhouse gas emissions under field conditions. Sensors 2016, 16, 1638. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.; Bjerg, B.S.; Hvelplund, T.; Weisbjerg, M.R.; Lund, P. Methane and carbon dioxide ratio in excreted air for quantification of the methane production from ruminants. Livest. Sci. 2010, 129, 223–227. [Google Scholar] [CrossRef]
- Weißbach, F.; Strubelt, C. Correcting the dry matter content of grass silages as a substrate for biogas production. Landtechnik 2008, 63, 210–246. [Google Scholar]
- Weißbach, F.; Strubelt, C. Correcting the dry matter content of maize silages as a substrate for biogas production. Landtechnik 2008, 63, 82–83. [Google Scholar]
- VDLUFA. VDLUFA-Methodenbuch, Bd. III. Die Chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- Brandt, M.; Schuldt, A.; Mannerkorpi, P.; Vearasilp, T. Zur enzymatischen Stärkebestimmung im Darminhalt und Kot von Kühen mit hitzestabiler Amylase. Arch. Anim. Nutr. 1987, 37, 455. [Google Scholar]
- Menke, K.H.; Steingass, H. Schätzung des energetischen Futterwerts aus der in vitro mit Pansensaft bestimmten Gasbildung und der chemischen Analyse. II. Regressionsgleichungen. Übers Tierernährg 1987, 15, 59–94. [Google Scholar]
- GfE. Equations for predicting metabolisable energy and digestibility of organic matter in forage legumes for ruminants. Proc. Soc. Nutr. Physiol. 2017, 26, 186–193. [Google Scholar]
- GfE. New equations for predicting metabolisable energy of grass and maize products for ruminants. Proc. Soc. Nutr. Physiol. 2008, 17, 191–197. [Google Scholar]
- Weiß, K.; Kaiser, E. Milchsäurebestimmung in Silageextrakten mit Hilfe der HPLC. Wirtschaftseig. Futter 1995, 41, 69–80. [Google Scholar]
- von Lengerken, J.; Zimmermann, K. Handbuch Futtermittelprüfung; Deutscher Landwirtschaftsverlag: Berlin, Germany, 1991. [Google Scholar]
- Barry, T.; Thompson, A.; Armstrong, D. Rumen fermentation studies on two contrasting diets. 1. Some characteristics of the in vivo fermentation, with special reference to the composition of the gas phase, oxidation/reduction state and volatile fatty acid proportions. J. Agric. Sci. 1977, 89, 183–195. [Google Scholar] [CrossRef]
- Lindgren, S.; Pettersson, K.; Kaspersson, A.; Jonsson, A.; Lingvall, P. Microbial dynamics during aerobic deterioration of silages. J. Sci. Food Agric. 1985, 36, 765–774. [Google Scholar] [CrossRef]
- McAllister, T.A.; Feniuk, R.; Mir, Z.; Mir, P.; Selinger, L.B.; Cheng, K.J. Inoculants for alfalfa silage: Effects on aerobic stability, digestibility and the growth performance of feedlot steers. Livest. Prod. Sci. 1998, 53, 171–181. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Ashbell, G. Changes in gas composition in corn silages in bunker silos during storage and feedout. Can. Agric. Eng. 1994, 36, 155–158. [Google Scholar]
- Kaspar, H.F.; Tiedje, J.M. Dissimilatory reduction of nitrate and nitrite in the bovine rumen: Nitrous oxide production and effect of acetylene. Appl. Environ. Microbiol. 1981, 41, 705–709. [Google Scholar] [PubMed]
- De Raphélis-Soissan, V.; Li, L.; Godwin, I.R.; Barnett, M.C.; Perdok, H.B.; Hegarty, R.S. Use of nitrate and Propionibacterium acidipropionici to reduce methane emissions and increase wool growth of Merino sheep. Anim. Prod. Sci. 2014, 54, 1860–1866. [Google Scholar] [CrossRef]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef] [PubMed]
- Bjerg, B.; Zhang, G.; Madsen, J.; Rom, H.B. Methane emission from naturally ventilated livestock buildings can be determined from gas concentration measurements. Environ. Monit. Assess. 2012, 184, 5989–6000. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, K.A.; Kreuzer, M.; O’Mara, F.; McAllister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Hegarty, R.; Gerdes, R. Hydrogen production and transfer in the rumen. Rec. Adv. Anim. Nutr. Aust. 1999, 12, 37–44. [Google Scholar]
- Dittmann, M.T.; Hammond, K.J.; Kirton, P.; Humphries, D.J.; Crompton, L.A.; Ortmann, S.; Misselbrook, T.H.; Südekum, K.-H.; Schwarm, A.; Kreuzer, M.; et al. Influence of ruminal methane on digesta retention and digestive physiology in non-lactating dairy cattle. Br. J. Nutr. 2016, 116, 763–773. [Google Scholar] [CrossRef] [PubMed]
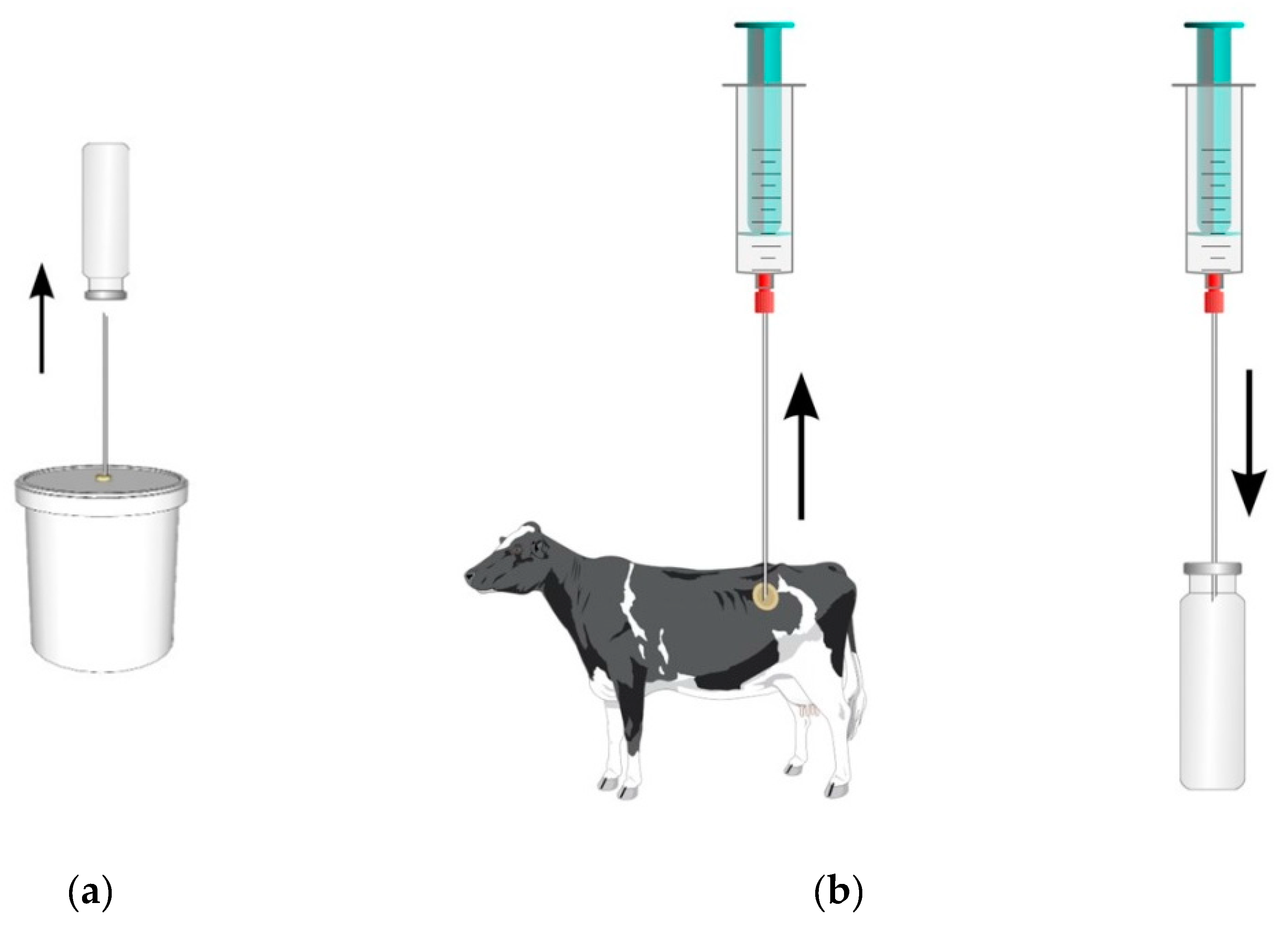
| Corn Silage (CS) | Alfalfa Silage (AS) | Grass Hay (GH) | |
|---|---|---|---|
| DM [g/kg] | 366 | 415 | 881 |
| Ash | 34.9 | 124 | 70.5 |
| Crude protein | 70.7 | 246 | 79.2 |
| Ether extract | 35.9 | 30.2 | 20.2 |
| aNDFom 1 | 314 | 396 | 599 |
| ADFom 2 | 175 | 300 | 340 |
| Acid detergent lignin | 17.4 | 98.3 | 36.8 |
| Starch | 438 | n.a. | n.a. |
| In vitro gas production [mL/200 mg DM] | 64.1 | 39.8 | 50.5 |
| Metabolizable energy [MJ/kg DM] | 11.7 | 8.78 | 9.40 |
| pH | 3.9 | 5.77 | n.a. |
| Lactic acid | 40.7 | 8.2 | n.a. |
| Acetic acid | 9.9 | 6.3 | n.a. |
| Butyric acid | n.d. 3 | n.d. | n.a. |
| Methanol | 0.3 | 1.5 | n.a. |
| Ethanol | 1.7 | 1.6 | n.a. |
| Water-soluble carbohydrates | 13.4 | 49.8 | n.a. |
| NH3-N [g/kg total N] | 109 | 96.7 | n.a. |
| Ethyl acetate [mg/kg DM] | 54.4 | 19.3 | n.a. |
| Ethyl lactate [mg/kg DM] | 105 | n.d. | n.a. |
| Least Square Means | Effect | |||||||
|---|---|---|---|---|---|---|---|---|
| CS | AS | GH | SEM | F | P | F·P | ||
| N2O [μg/(kg dry matter × h)] | 8 a.m. | 0.109 b | 24.1 a | 0.233 b | 3.81 | 0.02 | n.s. | n.s. |
| 11 a.m. | 0.140 b | 2.46 a | 0.176 b | 0.172 | <0.01 | 0.01 | <0.01 | |
| CO2 [mg/(kg dry matter × h)] | 8 a.m. | 391 a | 141 b | 8.13 c | 32.0 | <0.01 | n.s. | n.s. |
| 11 a.m. | 170 a | 19.0 b | 9.38 b | 14.4 | <0.01 | n.s. | n.s. | |
| Least Square Means | Effect | ||||||
|---|---|---|---|---|---|---|---|
| Corn Silage (CS) | Alfalfa Silage (AS) | Grass Hay (GH) | SEM | F | P | F·P | |
| DMI [kg/180 min] | 4.60 b | 3.22 b | 6.64 a | 0.473 | <0.01 | 0.02 | <0.01 |
| N2O [ppm] | 0.246 b | 0.857 a | 0.171 b | 0.068 | <0.01 | n.s. | 0.02 |
| CH4 [%] | 16.9 b | 20.6 a | 20.3 a | 0.890 | 0.01 | <0.01 | 0.03 |
| CO2 [%] | 46.1 b | 41.1 b | 54.8 a | 2.21 | <0.01 | n.s. | 0.03 |
| CH4:CO2 | 0.358 b | 0.501 a | 0.372 b | 0.010 | <0.01 | 0.02 | <0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerlach, K.; Schmithausen, A.J.; Sommer, A.C.H.; Trimborn, M.; Büscher, W.; Südekum, K.-H. Cattle Diets Strongly Affect Nitrous Oxide in the Rumen. Sustainability 2018, 10, 3679. https://doi.org/10.3390/su10103679
Gerlach K, Schmithausen AJ, Sommer ACH, Trimborn M, Büscher W, Südekum K-H. Cattle Diets Strongly Affect Nitrous Oxide in the Rumen. Sustainability. 2018; 10(10):3679. https://doi.org/10.3390/su10103679
Chicago/Turabian StyleGerlach, Katrin, Alexander J. Schmithausen, Ansgar C. H. Sommer, Manfred Trimborn, Wolfgang Büscher, and Karl-Heinz Südekum. 2018. "Cattle Diets Strongly Affect Nitrous Oxide in the Rumen" Sustainability 10, no. 10: 3679. https://doi.org/10.3390/su10103679






