Brassica napus Roots Use Different Strategies to Respond to Warm Temperatures
Abstract
:1. Introduction
2. Results
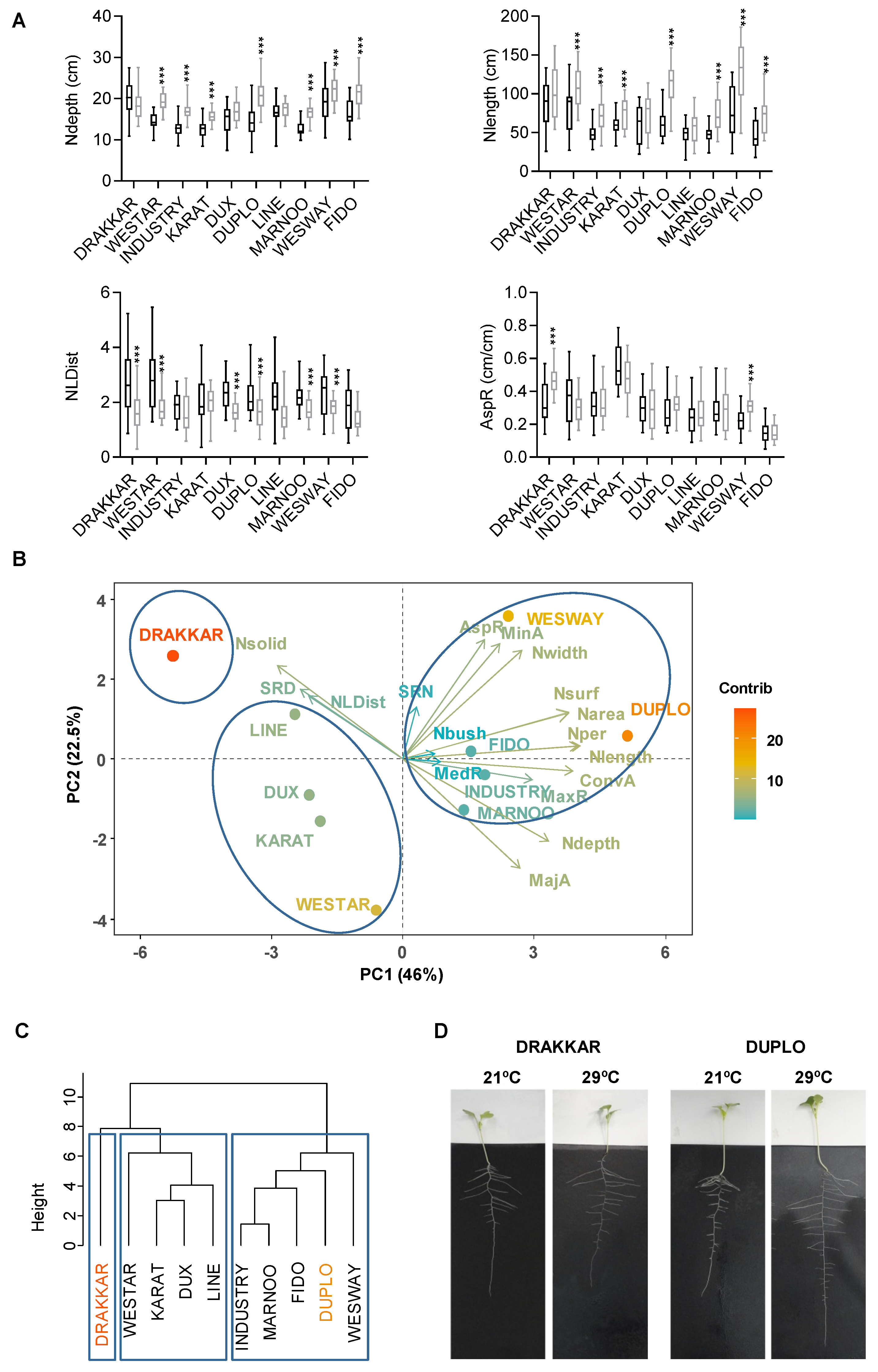
2.1. Brassica napus Roots Adopt Different RSA Configurations to Increase Their Ability to Explore the Soil in Response to Warm Temperatures
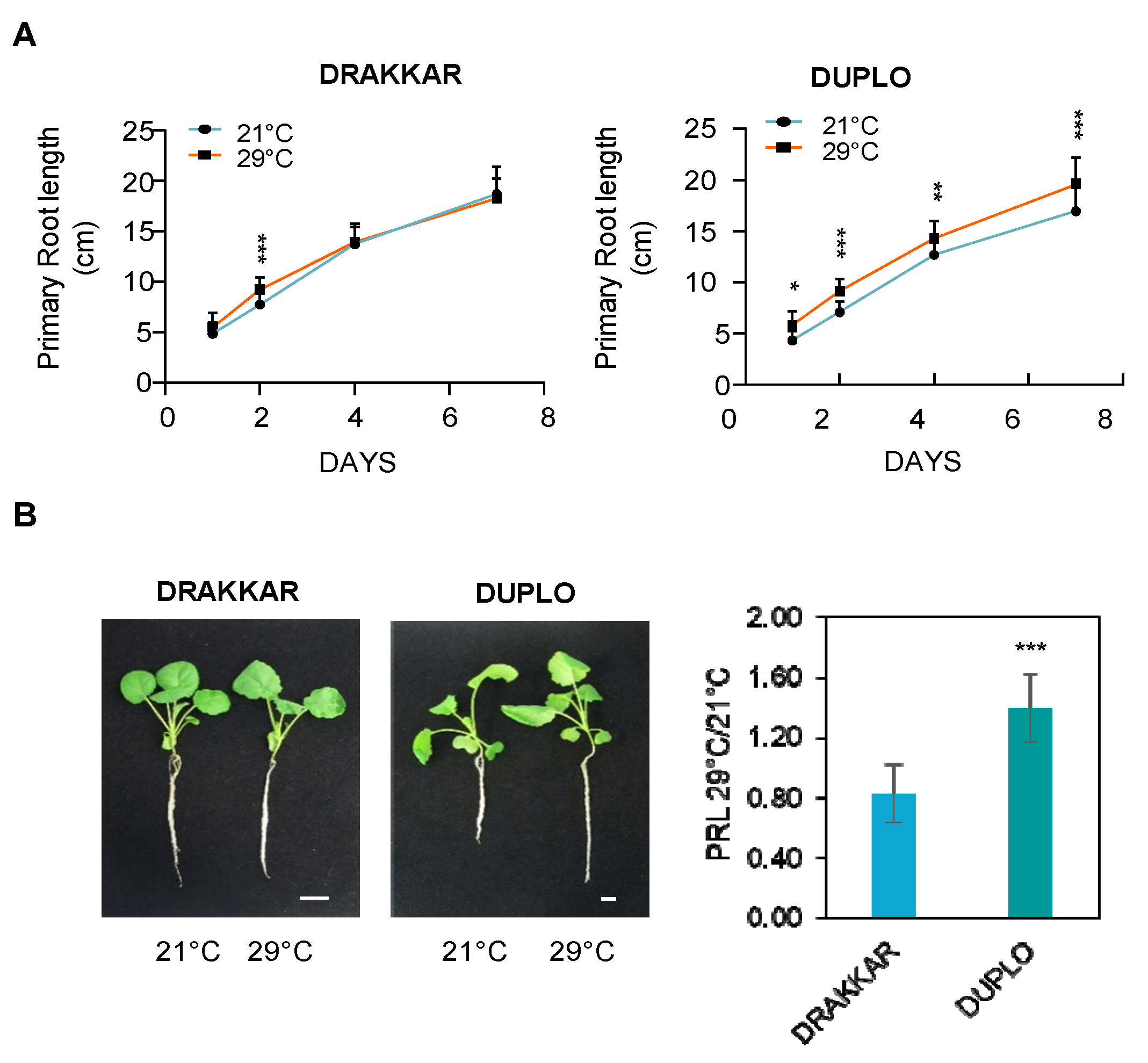
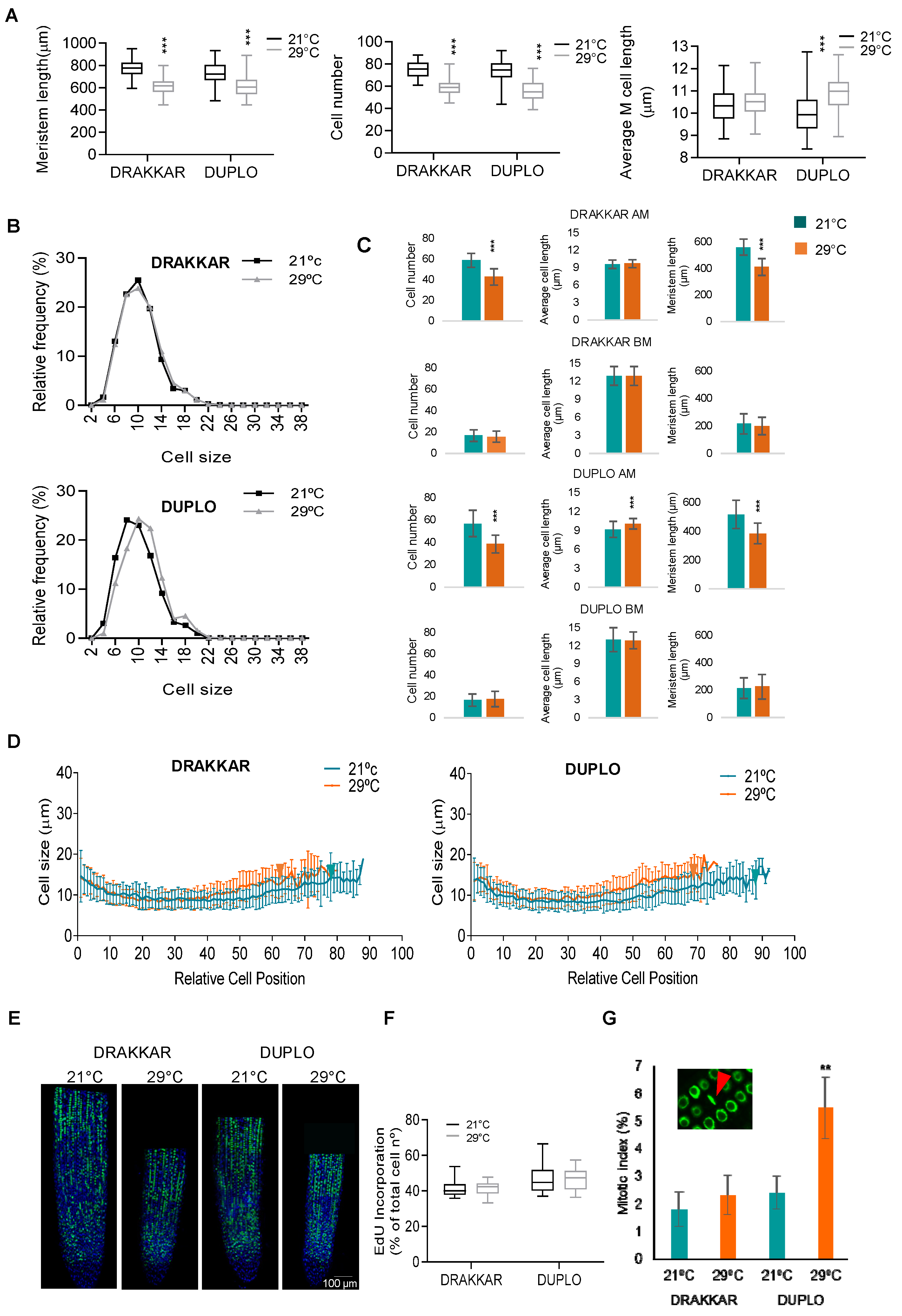
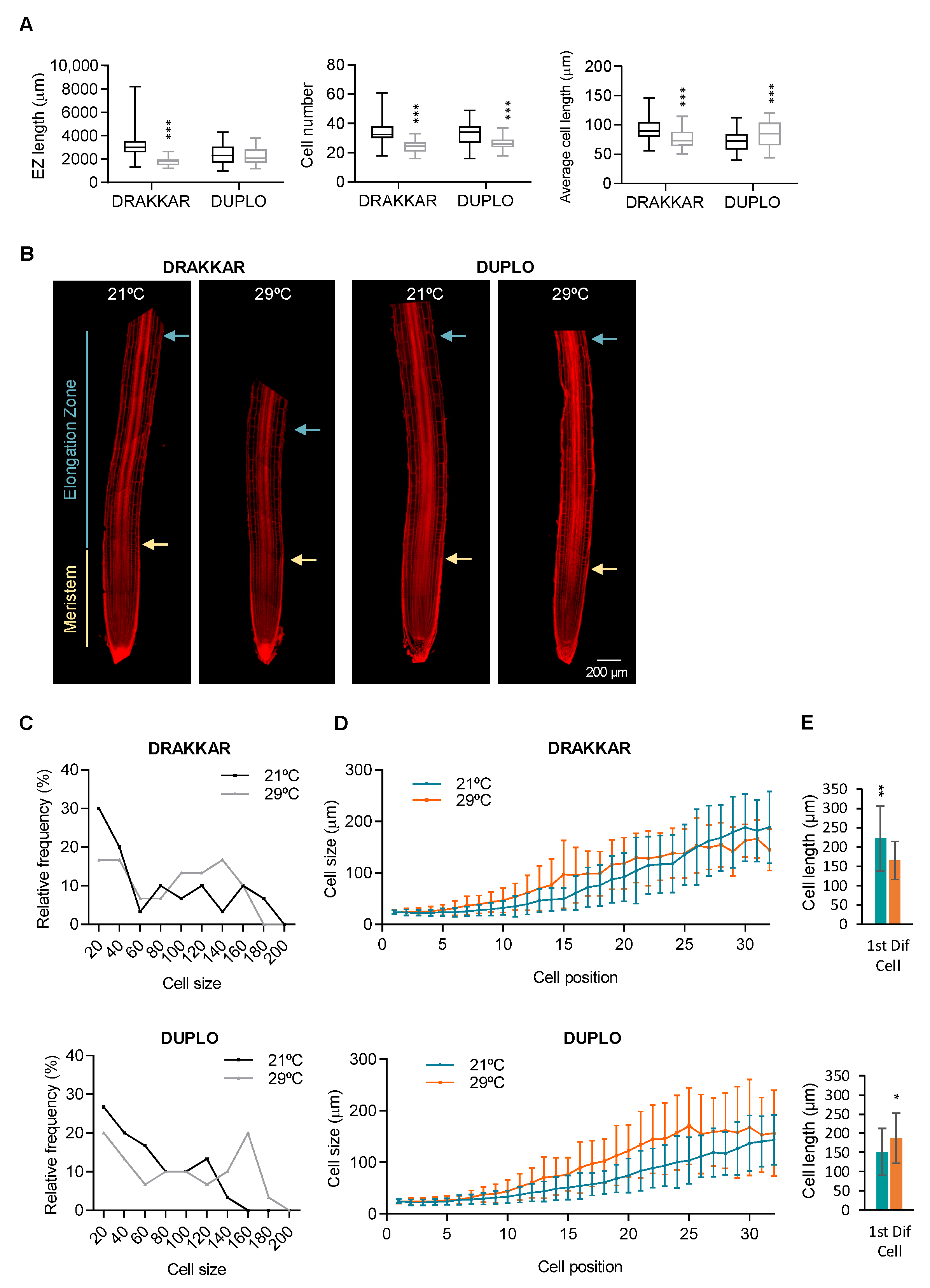
2.2. Combinatory Changes in Cell Elongation and Cell Division Drive Differential Root Response to Warm Temperatures
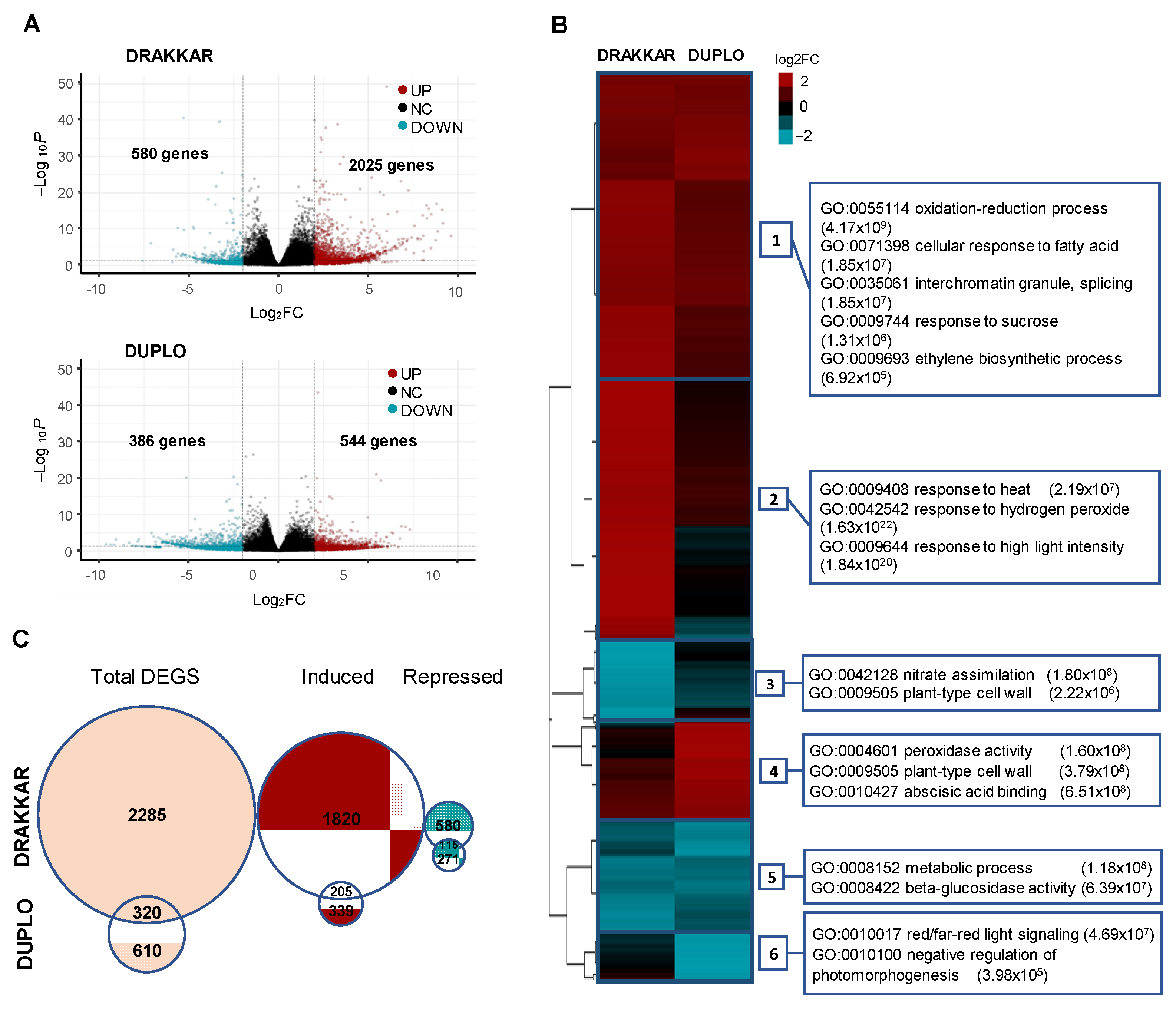
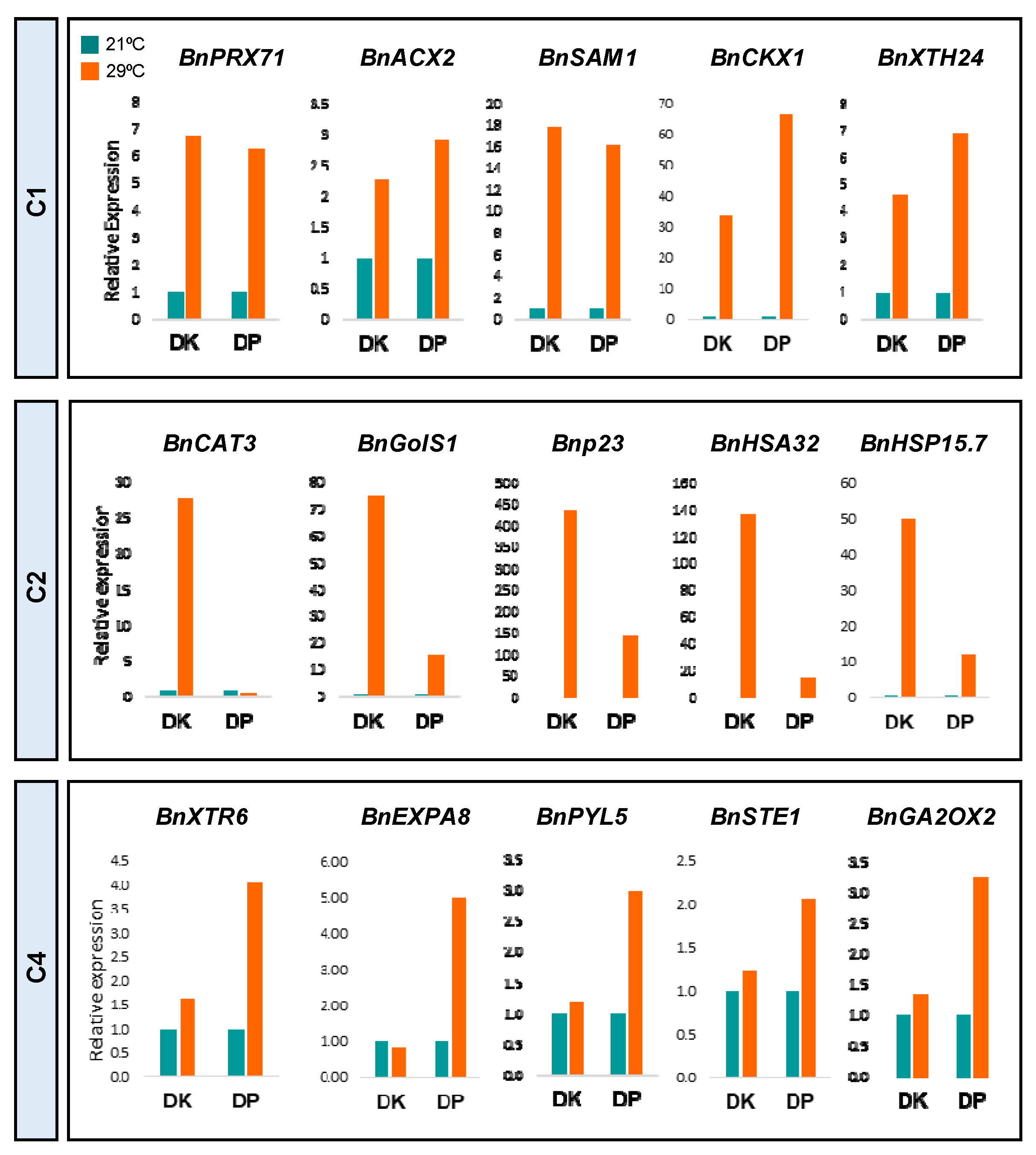
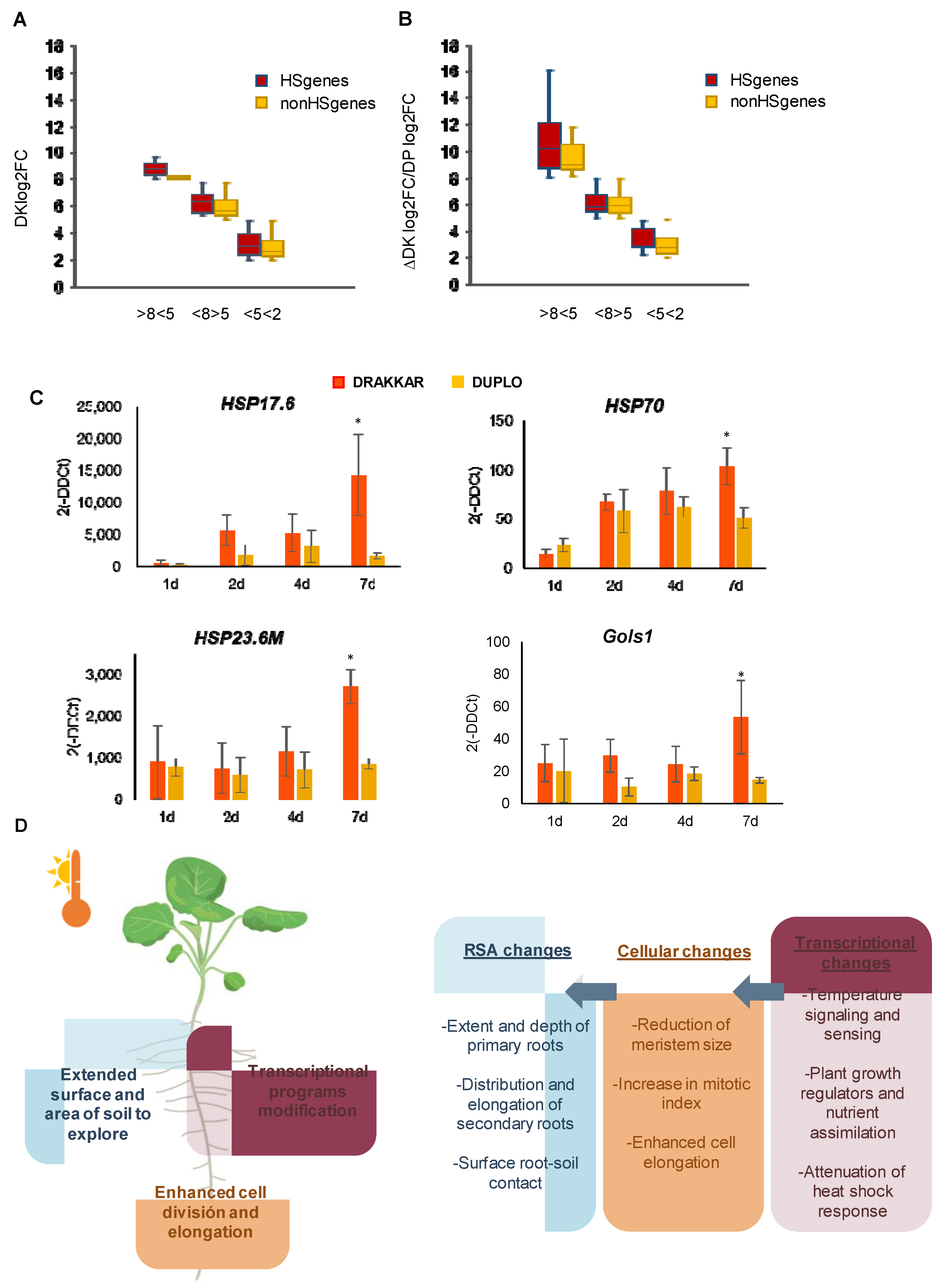
2.3. Balanced Regulation of Transcriptional Temperature Response Is Crucial to Adjusting Root Growth to Warming Conditions
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Root Trait Analysis
4.3. Cellular Parameter Analysis
4.4. RNA Extraction and Sequencing Analysis
4.5. Expression Analysis by qRT-PCR
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Khoury, C.K.; Mller, J.V.; Toll, J. Adapting Agriculture to Climate Change: A Global Initiative to Collect, Conserve, and Use Crop Wild Relatives. Agroecol. Sustain. Food Syst. 2014, 38, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Tai, A.P.K.; Martin, M.V.; Heald, C.L. Threat to future global food security from climate change and ozone air pollution. Nat. Clim. Chang. 2014, 4, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidi, S.S.; Vanderschuren, H.; Qaim, M.; Mahfouz, M.M.; Kohli, A.; Mansoor, S.; Tester, M. New plant breeding technologies for food security. Science 2019, 363, 1390–1391. [Google Scholar] [CrossRef] [Green Version]
- Foster, G.; Rahmstorf, S. Global temperature evolution 1979–2010. Environ. Res. Lett. 2011, 6, 044022. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Lean, J.L.; Rind, D.H. How will Earth’s surface temperature change in future decades? Geophys. Res. Lett. 2009, 36, L15708. [Google Scholar] [CrossRef] [Green Version]
- Vu, L.D.; Xu, X.; Gevaert, K.; De Smet, I. Developmental Plasticity at High Temperature. Plant Physiol. 2019, 181, 399–411. [Google Scholar] [CrossRef] [Green Version]
- Ayenan, M.A.T.; Danquah, A.; Hanson, P.; Ampomah-Dwamena, C.; Sodedji, F.A.K.; Asante, I.K.; Danquah, E.Y. Accelerating breeding for heat tolerance in tomato (Solanum lycopersicum L.): An integrated approach. Agronomy 2019, 9, 720. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiault, L.; Mora, C.; Cinner, J.E.; Cheung, W.W.L.; Graham, N.A.J.; Januchowski-Hartley, F.A.; Mouillot, D.; Sumaila, U.R.; Claudet, J. Escaping the perfect storm of simultaneous climate change impacts on agriculture and marine fisheries. Sci. Adv. 2019, 5, eaaw9976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Babar, M.A.; et al. Climate change impact and adaptation for wheat protein. Glob. Chang. Biol. 2019, 25, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of Pre-Anthesis Drought, Heat and Their Combination on the Growth, Yield and Physiology of diverse Wheat (Triticum aestivum L.) Genotypes Varying in Sensitivity to Heat and drought stress. Sci. Rep. 2019, 9, 6955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quint, M.; Delker, C.; Franklin, K.A.; Wigge, P.A.; Halliday, K.J.; Van Zanten, M. Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2016, 2, 15190. [Google Scholar] [CrossRef] [Green Version]
- Ibañez, C.; Poeschl, Y.; Peterson, T.; Bellstadt, J.; Denk, K.; Gogol-Döring, A.; Quint, M.; Delker, C. Ambient temperature and genotype differentially affect developmental and phenotypic plasticity in Arabidopsis thaliana. BMC Plant Biol. 2017, 17, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvi, M.P.; Burton, A.J. Root respiration and biomass responses to experimental soil warming vary with root diameter and soil depth. Plant Soil 2020, 451, 435–446. [Google Scholar] [CrossRef]
- Klein, S.P.; Schneider, H.M.; Perkins, A.C.; Brown, K.M.; Lynch, J.P. Multiple Integrated Root Phenotypes Are Associated with Improved Drought Tolerance. Plant Physiol. 2020, 183, 1011–1025. [Google Scholar] [CrossRef] [Green Version]
- Borniego, M.B.; Costigliolo-Rojas, C.; Casal, J.J. Shoot thermosensors do not fulfil the same function in the root. New Phytol. 2020, 236, 9–14. [Google Scholar] [CrossRef]
- Calleja-Cabrera, J.; Boter, M.; Oñate-Sánchez, L.; Pernas, M. Root Growth Adaptation to Climate Change in Crops. Front. Plant Sci. 2020, 11, 544. [Google Scholar] [CrossRef]
- Göbel, L.; Coners, H.; Hertel, D.; Willinghöfer, S.; Leuschner, C. The Role of Low Soil Temperature for Photosynthesis and Stomatal Conductance of Three Graminoids From Different Elevations. Front. Plant Sci. 2019, 10, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Pfordt, A.; Khatri, L.; Eseola, A.B.; Wilch, A.; Koopmann, B.; Von Tiedemann, A. Contrasting Patterns of Colonization with Verticillium longisporum in Winter- and Spring-Type Oilseed Rape (Brassica napus) in the Field and Greenhouse and the Role of Soil Temperature. Plant Dis. 2019, 103, 2090–2099. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xu, H.; Chu, C.; He, F.; Fang, S. High Temperature can Change Root System Architecture and Intensify Root Interactions of Plant Seedlings. Front. Plant Sci. 2020, 11, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.; Testerink, C. Roots Withstanding their Environment: Exploiting Root System Architecture Responses to Abiotic Stress to Improve Crop Tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, H.; Kelly, A.; Fox, G.; Franckowiak, J.; Borrell, A.; Hickey, L. Root architectural traits and yield: Exploring the relationship in barley breeding trials. Euphytica 2018, 214, 151. [Google Scholar] [CrossRef]
- Bray, A.L.; Topp, C.N. The quantitative genetic control of root architecture in Maize. Plant Cell Physiol. 2018, 59, 1919–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.; Liu, Y.; Gruber, B.D.; Neumann, K.; Kilian, B.; Graner, A.; Von Wirn, N. Genetic dissection of root system architectural traits in spring barley. Front. Plant Sci. 2019, 10, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, H.M.; Lynch, J.P. Should Root Plasticity Be a Crop Breeding Target? Front. Plant Sci. 2020, 11, 546. [Google Scholar] [CrossRef]
- Rangarajan, H.; Lynch, J.P. A Comparative Analysis of Quantitative Metrics of Root Architecture. Plant Phenomics 2021, 2021, 6953197. [Google Scholar] [CrossRef]
- Raboanatahiry, N.; Li, H.; Yu, L.; Li, M. Rapeseed (Brassica napus): Processing, Utilization, and Genetic Improvement. Agronomy 2021, 11, 1776. [Google Scholar] [CrossRef]
- Brown, J.K.M.; Beeby, R.; Penfield, S. Yield instability of winter oilseed rape modulated by early winter temperature. Sci. Rep. 2019, 9, 6953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Shah, F.; Duncan, R.W.; Ma, B.L. Grain yield, root growth habit and lodging of eight oilseed rape genotypes in response to a short period of heat stress during flowering. Agric. For. Meteorol. 2020, 287, 107954. [Google Scholar] [CrossRef]
- Weymann, W.; Böttcher, U.; Sieling, K.; Kage, H. Effects of weather conditions during different growth phases on yield formation of winter oilseed rape. Field Crops Res. 2015, 173, 41–48. [Google Scholar] [CrossRef]
- Marjanović-Jeromela, A.; Terzić, S.; Jankulovska, M.; Zorić, M.; Kondić-Špika, A.; Jocković, M.; Hristov, N.; Crnobarac, J.; Nagl, N. Dissection of Year Related Climatic Variables and Their Effect on Winter Rapeseed (Brassica Napus L.) Development and Yield. Agronomy 2019, 9, 517. [Google Scholar] [CrossRef] [Green Version]
- Fonseca de Lima, C.F.; Kleine-Vehn, J.; De Smet, I.; Feraru, E. Getting to the root of belowground high temperature responses in plants. J. Exp. Bot. 2021, 72, 7404–7413. [Google Scholar] [CrossRef]
- Robertson, M.J.; Lilley, J.M. Simulation of growth, development and yield of canola (Brassica napus) in APSIM. Crop Pasture Sci. 2016, 67, 332–344. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, F.; Yang, P.; Li, J.; Yan, G.; Hu, L. Responses of canola (Brassica napus L.) cultivars under contrasting temperature regimes during early seedling growth stage as revealed by multiple physiological criteria. Acta Physiol. Plant 2015, 37, 7. [Google Scholar] [CrossRef]
- Butkevičienė, L.M.; Kriaučiūnienė, Z.; Pupalienė, R.; Velička, R.; Kosteckienė, S.; Kosteckas, R.; Klimas, E. Influence of Sowing Time on Yield and Yield Components of Spring Rapeseed in Lithuania. Agronomy 2021, 11, 2170. [Google Scholar] [CrossRef]
- Atkinson, J.A.; Wingen, L.U.; Griffiths, M.; Pound, M.P.; Gaju, O.; Foulkes, M.J.; Le Gouis, J.; Griffiths, S.; Bennett, M.J.; King, J.; et al. Phenotyping pipeline reveals major seedling root growth QTL in hexaploid wheat. J. Exp. Bot. 2015, 66, 2283–2292. [Google Scholar] [CrossRef] [Green Version]
- McClung, C.R.; Davis, S.J. Ambient thermometers in plants: From physiological outputs towards mechanisms of thermal sensing. Curr. Biol. 2010, 20, R1086–R1092. [Google Scholar] [CrossRef]
- Fei, Q.; Zhang, J.; Zhang, Z.; Wang, Y.; Liang, L.; Wu, L.; Gao, H.; Sun, Y.; Niu, B.; Li, X. Effects of auxin and ethylene on root growth adaptation to different ambient temperatures in Arabidopsis. Plant Sci. 2016, 281, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yi, H.; Lee, J.; Nou, I.S.; Han, C.T.; Hur, Y. Global Gene-Expression Analysis to Identify Differentially Expressed Genes Critical for the Heat Stress Response in Brassica rapa. PLoS ONE 2015, 10, e0130451. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Z.; Zhou, M.; Ding, Y.-F.; Zhu, C. Gene Networks Involved in Plant Heat Stress Response and Tolerance. Int. J. Mol. Sci. 2022, 23, 11970. [Google Scholar] [CrossRef] [PubMed]
- Pahlavanian, A.M.; Silk, W.K. Effect of Temperature on Spatial and Temporal Aspects of Growth in the Primary Maize Root. Plant Physiol. 1988, 87, 529–532. [Google Scholar] [CrossRef] [Green Version]
- Pardales, J.R.; Kono, Y.; Yamauchi, A. Epidermal cell elongation in sorghum seminal roots exposed to high root-zone temperature. Plant Sci. 1992, 81, 143–146. [Google Scholar] [CrossRef]
- Sattelmacher, B.; Marschner, H.; Kühne, R. Effects of the temperature of the rooting zone on the growth and development of roots of potato (Solanum tuberosum). Ann. Bot. 1990, 65, 27–36. [Google Scholar] [CrossRef]
- Shanmugavadivel, P.S.; Amitha Mithra, S.V.; Prakash, C.; Mk, R.; Tiwari, R.; Mohapatra, T.; Singh, N.K. High resolution mapping of QTLs for heat tolerance in rice using a 5K SNP Array. Rice 2017, 10, 167. [Google Scholar]
- Joshi, M.; Fogelman, E.; Belausov, E.; Ginzberg, I. Potato root system development and factors that determine its architecture. J. Plant Physiol. 2016, 205, 113–123. [Google Scholar] [CrossRef]
- Pardales, J.R.; Banoc, D.M.; Yamauchi, A.; Iijima, M.; Kono, Y. Root system development of cassava and sweetpotato during early growth stage as affected by high root zone temperature. Plant Prod. Sci. 1999, 2, 247–251. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, M.; Zheng, X.; Gautam, M.; He, S.; Li, L. The role of promoter-associated histone acetylation of haem oxygenase-1 (HO-1) and giberellic acid-stimulated Like-1 (GSL-1) genes in heat-induced lateral root primordium inhibition in maize. Front. Plant Sci. 2018, 9, 1520. [Google Scholar] [CrossRef] [Green Version]
- Acuña-Galindo, M.A.; Mason, R.E.; Subramanian, N.K.; Hays, D.B. Meta-analysis of wheat QTL regions associated with adaptation to drought and heat stress. Crop Sci. 2015, 55, 477–492. [Google Scholar] [CrossRef]
- Pinto, R.S.; Reynolds, M.P. Common genetic basis for canopy temperature depression under heat and drought stress associated with optimized root distribution in bread wheat. Theor. Appl. Genet. 2015, 128, 575–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gous, P.W.; Hickey, L.; Christopher, J.T.; Franckowiak, J.; Fox, G.P. Discovery of QTL for stay-green and heat-stress in barley (Hordeum vulgare) grown under simulated abiotic stress conditions. Euphytica 2016, 207, 305–317. [Google Scholar] [CrossRef]
- Kilasi, N.L.; Singh, J.; Vallejos, C.E.; Ye, C.; Jagadish, S.K.; Kusolwa, P.; Rathinasabapathi, B. Heat Stress Tolerance in rice (Oryza sativa L.): Identification of quantitative trait loci and candidate genes for seedling growth under heat stress. Front. Plant Sci. 2018, 9, 1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagel, K.A.; Kastenholz, B.; Jahnke, S.; Van Dusschoten, D.; Aach, T.; Mühlich, M.; Truhn, D.; Scharr, H.; Terjung, S.; Walter, A.; et al. Temperature responses of roots: Impact on growth, root system architecture and implications for phenotyping. Funct. Plant Biol. 2009, 36, 947–959. [Google Scholar] [CrossRef]
- Battisti, D.S.; Naylor, R.L. Historical warnings of future food insecurity with unprecedented seasonal heat. Science 2009, 323, 240–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waidmann, S.; Ruiz Rosquete, M.; Schöller, M.; Sarkel, E.; Lindner, H.; LaRue, T.; Petřík, I.; Dünser, K.; Martopawiro, S.; Sasidharan, R.; et al. Cytokinin functions as an asymmetric and anti-gravitropic signal in lateral roots. Nat. Commun. 2019, 10, 3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Feraru, E.; Feraru, M.I.; Waidmann, S.; Wang, W.; Passaia, G.; Wang, Z.Y.; Wabnik, K.; Kleine-Vehn, J. PIN-LIKES coordinate brassinosteroid signaling with nuclear auxin input in Arabidopsis thaliana. Curr. Biol. 2020, 30, 1579–1588.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.J.; Kim, W.Y. Plant Hormone-Mediated Regulation of Heat Tolerance in Response to Global Climate Change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, P.; Hou, H.; Zhang, H.; Wang, Y.; Yan, S.; Huang, Y.; Li, H.; Tan, J.; Hu, A.; et al. Transcriptional Regulation of Cell Cycle Genes in Response to Abiotic Stresses Correlates with Dynamic Changes in Histone Modifications in Maize. PLoS ONE 2014, 9, e106070. [Google Scholar] [CrossRef]
- Martins, S.; Montiel-Jorda, A.; Cayrel, A.; Huguet, S.; Roux, C.P.; Ljung, K.; Vert, G. Brassinosteroid signaling-dependent root responses to prolonged elevated ambient temperature. Nat. Commun. 2017, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dong, G.; Palaniappan, K.; Mi, G.; Baskin, T.I. Temperature-compensated cell production rate and elongation zone length in the root of Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Beemster, G.T.S.; Fiorani, F.; Inzé, D. Cell cycle: The key to plant growth control? Trends Plant Sci. 2003, 8, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Mislata, A.; Schiessl, K.; Sablowski, R. Active Control of Cell Size Generates Spatial Detail during Plant Organogenesis. Curr. Biol. 2015, 25, 2991–2996. [Google Scholar] [CrossRef] [Green Version]
- Beemster, G.T.S.; Baskin, T.I. Analysis of Cell Division and Elongation Underlying the Developmental Acceleration of Root Growth in Arabidopsis thaliana. Plant Physiol. 1998, 116, 1515–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.R.; Forero-Vargas, M.; Withers, S.P.; Smith, R.S.; Traas, J.; Dewitte, W.; Murray, J.A.H. Cell-size dependent progression of the cell cycle creates homeostasis and flexibility of plant cell size. Nat. Commun. 2017, 8, 15060. [Google Scholar] [CrossRef] [Green Version]
- Qi, F.; Zhang, F. Cell Cycle Regulation in the Plant Response to Stress. Front. Plant Sci. 2020, 10, 1765. [Google Scholar] [CrossRef] [Green Version]
- Alarcon, M.V.; Salguero, J. Transition zone cells reach G2 phase before initiating elongation in maize root apex. Biol. Open 2017, 6, 909–913. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Ogita, N.; Takahashi, T.; Taniguchi, S.; Tanaka, M.; Seki, M.; Umeda, M. A regulatory module controlling stress-induced cell cycle arrest in Arabidopsis. eLife 2019, 8, e43944. [Google Scholar] [CrossRef]
- Rahni, R.; Birnbaum, K.D. Week-long imaging of cell divisions in the Arabidopsis root meristem. Plant Methods 2019, 15, 30. [Google Scholar] [CrossRef] [Green Version]
- Desvoyes, B.; Arana-Echarri, A.; Barea, M.D.; Gutierrez, C. A comprehensive fluorescent sensor for spatiotemporal cell cycle analysis in Arabidopsis. Nat. Plants 2020, 6, 1330–1334. [Google Scholar] [CrossRef]
- Somssich, M.; Khan, G.A.; Staffan, S.P. Cell wall heterogeneity in root development of arabidopsis. Front. Plant Sci. 2016, 7, 1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, X.; Dixon, R.A. Brassinosteroid mediated cell wall remodeling in grasses under abiotic stress. Front. Plant Sci. 2017, 8, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, T.M.; Vukasinovic, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [Green Version]
- Goda, H.; Shimada, Y.; Asami, T.; Fujioka, S.; Yoshida, S. Microarray analysis of brassinosteroid-regulated genes in arabidopsis. Plant Physiol. 2002, 130, 1319–1334. [Google Scholar] [CrossRef] [Green Version]
- Takatsuka, H.; Umeda, M. Hormonal control of cell division and elongation along differentiation trajectories in roots. J. Exp. Bot. 2014, 65, 2633–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaillochet, C.; Burko, Y.; Platre, M.P.; Zhang, L.; Simura, J.; Willige, B.C.; Kumar, S.V.; Ljung, K.; Chory, J.; Busch, W. HY5 and phytochrome activity modulate shoot-to-root coordination during thermomorphogenesis in Arabidopsis. Development 2020, 147, dev192625. [Google Scholar] [CrossRef]
- Lee, S.; Wang, W.; Huq, E. Spatial regulation of thermomorphogenesis by HY5 and PIF4 in Arabidopsis. Nat. Commun. 2021, 12, 3656. [Google Scholar] [CrossRef]
- Talanova, V.V.; Akimova, T.V.; Titov, A.F. Effect of Whole Plant and Local Heating on the ABA Content in Cucumber Seedling Leaves and Roots and on Their Heat Tolerance. Russ. J. Plant Physiol. 2003, 50, 90–94. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.S.; Yang, C.Y. Ethylene-mediated signaling confers thermotolerance and regulates transcript levels of heat shock factors in rice seedlings under heat stress. Bot. Stud. 2019, 60, 23. [Google Scholar] [CrossRef] [PubMed]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Cortijo, S.; Charoensawan, V.; Brestovitsky, A.; Buning, R.; Ravarani, C.; Rhodes, D.; Van Noort, J.; Jaeger, K.E.; Wigge, P.A. Transcriptional Regulation of the Ambient Temperature Response by H2A.Z Nucleosomes and HSF1 Transcription Factors in Arabidopsis. Mol. Plant 2017, 10, 1258–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, M.; Mitsuda, N.; Ohme-Takagi, M. Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance. Plant Physiol. 2011, 157, 1243–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrási, N.; Pettk-Szandtner, A.; Szabados, L. Diversity of plant heat shock factors: Regulation, interactions, and functions. J. Exp. Bot. 2021, 72, 1558–1575. [Google Scholar] [CrossRef]
- Wigge, P.A. Ambient temperature signalling in plants. Curr. Opin. Plant Biol. 2013, 16, 661–666. [Google Scholar] [CrossRef]
- Sánchez-Bermúdez, M.; Del Pozo, J.C.; Pernas, M. Effects of Combined Abiotic Stresses Related to Climate Change on Root Growth in Crops. Front. Plant Sci. 2022, 13, 918537. [Google Scholar] [CrossRef]
- Dinneny, J.R. Developmental responses to water and salinity in root systems. Annu. Rev. Cell Dev. Biol. 2019, 35, 239–257. [Google Scholar] [CrossRef]
- Adu, M.O.; Chatot, A.; Wiesel, L.; Bennett, M.J.; Broadley, M.R.; White, P.J.; Dupuy, L.X. A scanner system for high-resolution quantification of variation in root growth dynamics of Brassica rapa genotypes. J. Exp. Bot. 2014, 65, 2039–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.L.; Graham, N.S.; Hayden, R.; Meacham, M.C.; Neugebauer, K.; Nightingale, M.; Dupuy, L.X.; Hammond, J.P.; White, P.J.; Broadley, M.R. High-throughput phenotyping (HTP) identifies seedling root traits linked to variation in seed yield and nutrient capture in field-grown oilseed rape (Brassica napus L.). Ann. Bot. 2016, 118, 655–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, A.; Dietz, K.-J.; Gifford, M.L.; Lunn, J.E. The importance of independent replication of treatments in plant science. J. Exp. Bot. 2021, 72, 5270–5274. [Google Scholar] [CrossRef]
- Galkovskyi, T.; Mileyko, Y.; Bucksch, A.; Moore, B.; Symonova, O.; Price, C.A.; Topp, C.; Iyer-Pascuzzi, A.; Zurek, P.; Fang, S.; et al. GiA Roots: Software for the high throughput analysis of plant root system architecture. BMC Plant Biol. 2012, 12, 116. [Google Scholar] [CrossRef] [Green Version]
- Narisetti, N.; Henke, M.; Seiler, C.; Shi, R.; Junker, A.; Altmann, T.; Gladilin, E. Semi-automated Root Image Analysis (saRIA). Sci. Rep. 2019, 9, 19674. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truernit, E.; Bauby, H.; Dubreucq, B.; Grandjean, O.; Runions, J.; Barthelemy, J.; Palauqui, J.C. High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of Phloem development and structure in Arabidopsis. Plant Cell 2008, 20, 1494–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, A.P.; Wilson, M.H.; Kenobi, K.; Dietrich, D.; Voss, U.; Ubeda-Tomas, S.; Pridmore, T.P.; Wells, D.M. Identifying biological landmarks using a novel cell measuring image analysis tool: Cell-o-Tape. Plant Methods 2012, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazda, A.; Akimcheva, S.; Watson, J.M.; Riha, K. Cell Proliferation Analysis Using EdU Labeling in Whole Plant and Histological Samples of Arabidopsis. Methods Mol. Biol. 2016, 1370, 169–182. [Google Scholar] [PubMed]
- Lamprecht, M.R.; Sabatini, D.M.; Carpenter, A.E. CellProfiler: Free, versatile software for automated biological image analysis. Biotechniques 2007, 42, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.P.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Early allopolyploid evolution in the post-neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- De Hoon, M.J.L.; Imoto, S.; Nolan, J.; Miyano, S. Open source clustering software. Bioinformatics 2004, 20, 1453–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, M.G.; Walker, P.L.; Pulgar-Vidal, N.C.; Belmonte, M.F. SeqEnrich: A tool to predict transcription factor networks from co-expressed Arabidopsis and Brassica napus gene sets. PLoS ONE 2017, 12, e0178256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Truksa, M.; Shah, S.; Weselake, R.J. A survey of quantitative real-time polymerase chain reaction internal reference genes for expression studies in Brassica napus. Anal. Biochem. 2010, 405, 138–140. [Google Scholar] [CrossRef] [PubMed]
| Trait | Category | 21 °C | 29 °C | Change | ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d | 95% CI of the Mean | Coefficient of Variation | Mean | s.d | 95% CI of the Mean | Coefficent of Variation | G | T | GxT | |||
| Ndepth (cm) | Extent | 15.43 | 2.6 | [13.57–17.29] | 16.85 | 18.52 | 2.26 | [16.9–20.13] | 12.19 | ↑ | *** | *** | *** |
| Nwidth (cm) | Extent | 4.91 | 1.08 | [4.14–5.69] | 22.05 | 6.24 | 1.25 | [5.35–7.13] | 20.01 | ↑ | *** | *** | ns |
| ConvA (cm2) | Extent | 47.23 | 14.53 | [36.84–57.63] | 30.77 | 72.62 | 18.25 | [59.56–85.67] | 25.13 | ↑ | *** | *** | *** |
| MajA (cm) | Extent | 14.12 | 2.56 | [12.28–15.95] | 18.17 | 16.45 | 2.2 | [14.88–18.02] | 13.35 | ↑ | *** | *** | *** |
| MinA (cm) | Extent | 4.05 | 0.90 | [3.38–4.71] | 22.98 | 5.08 | 1.1 | [4.3–5.86] | 21.59 | ↑ | *** | *** | ns |
| Nlength (cm) | Size | 61.85 | 15.35 | [50.86–72.83] | 24.82 | 87.37 | 22.97 | [70.94103.8] | 26.29 | ↑ | *** | *** | *** |
| Narea (cm2) | Size | 3.15 | 0.83 | [2.56–3.75] | 26.4 | 4.26 | 1.17 | [3.39–5.06] | 27.64 | ↑ | *** | *** | ** |
| Nper (cm) | Size | 128.5 | 31.27 | [106.1150.8] | 24.34 | 183.1 | 47.73 | [148.9217.2] | 26.07 | ↑ | *** | *** | *** |
| Nsurf (cm2) | Size | 11.46 | 3.04 | [9.28–13.64] | 25.55 | 15.34 | 4.26 | [12.29–18.39] | 27.78 | ↑ | *** | *** | ** |
| Nbush | Distribution | 3.21 | 0.55 | [2.81–3.61] | 17.3 | 3.36 | 0.34 | [3.11–3.6] | 10.23 | ≈ | *** | ns | * |
| NLdist | Distribution | 2.26 | 0.33 | [2.02–2.5] | 14.63 | 1.68 | 0.17 | [1.56–1.8] | 9.88 | ↓ | *** | *** | * |
| Nsolid (cm2 cm−2) | Distribution | 0.07 | 0.01 | [0.066–0.079] | 12.53 | 0.06 | 0.01 | [0.056–0.064] | 10.26 | ↓ | *** | *** | *** |
| MaxR | Distribution | 3.31 | 0.63 | [2.86–3.76] | 19.14 | 4.01 | 0.63 | [3.56–4.46] | 15.74 | ↑ | *** | *** | ns |
| MedR | Distribution | 1.04 | 0.05 | [1–1.07] | 4.56 | 1.28 | 0.21 | [1.13–1.43] | 16.1 | ↑ | *** | *** | ** |
| SRN | Secondary roots | 24.68 | 4. 46 | [21.49–27.87] | 18.07 | 34.11 | 6.48 | [29.48–38.75] | 18.99 | ↑ | *** | *** | * |
| SRD (n cm−1) | Secondary roots | 1.64 | 0.32 | [1.41–1.86] | 19.27 | 1.84 | 0.21 | [1.69–1.99] | 11.66 | ↑ | *** | *** | * |
| AspR (cm cm−1) | Shape | 0.30 | 0.10 | [0.23–0.38] | 34.87 | 0.32 | 0.09 | [0.25–0.39] | 28.92 | ↑ | *** | ns | *** |
| Nw/d (cm cm−1) | Shape | 0.32 | 0.08 | [0.26–0.38] | 25.28 | 0.34 | 0.08 | [0.29–0.39] | 22.44 | ↑ | *** | ns | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boter, M.; Pozas, J.; Jarillo, J.A.; Piñeiro, M.; Pernas, M. Brassica napus Roots Use Different Strategies to Respond to Warm Temperatures. Int. J. Mol. Sci. 2023, 24, 1143. https://doi.org/10.3390/ijms24021143
Boter M, Pozas J, Jarillo JA, Piñeiro M, Pernas M. Brassica napus Roots Use Different Strategies to Respond to Warm Temperatures. International Journal of Molecular Sciences. 2023; 24(2):1143. https://doi.org/10.3390/ijms24021143
Chicago/Turabian StyleBoter, Marta, Jenifer Pozas, Jose A. Jarillo, Manuel Piñeiro, and Mónica Pernas. 2023. "Brassica napus Roots Use Different Strategies to Respond to Warm Temperatures" International Journal of Molecular Sciences 24, no. 2: 1143. https://doi.org/10.3390/ijms24021143
APA StyleBoter, M., Pozas, J., Jarillo, J. A., Piñeiro, M., & Pernas, M. (2023). Brassica napus Roots Use Different Strategies to Respond to Warm Temperatures. International Journal of Molecular Sciences, 24(2), 1143. https://doi.org/10.3390/ijms24021143







