Health Patterns across Adulthood: An Age-Based Investigation of the Nutritional Status, Homocysteine, and CoQ10 of Bank Staff
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurements and Classifications
2.3. Blood Sampling and Laboratory Methods
2.4. Physical Activity
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Micronutrients
4.2. Homocysteine and CoQ10
4.3. PA Levels and Sedentary Time
4.4. Anthropometry
4.5. Limitations and Strengths
4.6. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Non-Communicable Diseases (NCDs). Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 7 January 2024).
- Hajat, C.; Stein, E. The global burden of multiple chronic conditions: A narrative review. Prev. Med. Rep. 2018, 12, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Elmadfa, I. Österreichischer Ernährungsbericht 2012. Available online: https://ernaehrungsbericht.univie.ac.at/fileadmin/user_upload/dep_ernaehrung/forschung/ernaehrungsberichte/oesterr_ernaehrungsbericht_2012.pdf (accessed on 7 January 2024).
- Elmadfa, I. Österreichischer Ernährungsbericht 2017. Available online: https://ernaehrungsbericht.univie.ac.at/fileadmin/user_upload/dep_ernaehrung/forschung/ernaehrungsberichte/erna_hrungsbericht2017_web_20171018.pdf (accessed on 7 January 2024).
- World Health Organization (Regional Office for Europe). Healthy Living: What Is a Healthy Lifestyle? WHO Regional Office for Europe: Copenhagen, Denmark, 1999; Available online: https://apps.who.int/iris/handle/10665/108180 (accessed on 7 January 2024).
- Erema, V.V.; Yakovchik, A.Y.; Kashtanova, D.A.; Bochkaeva, Z.V.; Ivanov, M.V.; Sosin, D.V.; Matkava, L.R.; Yudin, V.S.; Makarov, V.V.; Keskinov, A.A.; et al. Biological Age Predictors: The Status Quo and Future Trends. Int. J. Mol. Sci. 2022, 23, 15103. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Šoštarič, A.; Jenko, B.; Kozjek, N.R.; Ovijač, D.; Šuput, D.; Milisav, I.; Dolžan, V. Detection of metabolic syndrome burden in healthy young adults may enable timely introduction of disease prevention. Arch. Med. Sci. 2019, 15, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.Y.; Ravussin, E. Analysis of energy metabolism in humans: A review of methodologies. Mol. Metab. 2016, 5, 1057–1071. [Google Scholar] [CrossRef]
- Committee on Improving the Health, Safety, and Well-Being of Young Adults; Board on Children, Youth, and Families; Institute of Medicine; National Research Council. Investing in the Health and Well-Being of Young Adults; Public Health; Bonnie, R.J., Stroud, C., Breiner, H., Eds.; National Academies Press (US): Washington, DC, USA, 2015; p. 6. Available online: https://www.ncbi.nlm.nih.gov/books/NBK284781/ (accessed on 7 January 2024).
- Tian, W.H.; Tien, J.J. Health Behaviors and Health Status among Middle-Aged and Older Adults with Chronic Diseases in Taiwan. Int. J. Environ. Res. Public. Health 2020, 17, 7196. [Google Scholar] [CrossRef] [PubMed]
- Renzaho, A.M.; Houng, B.; Oldroyd, J.; Nicholson, J.M.; D’Esposito, F.; Oldenburg, B. Stressful life events and the onset of chronic diseases among Australian adults: Findings from a longitudinal survey. Eur. J. Public. Health 2014, 24, 57–62. [Google Scholar] [CrossRef]
- Novotny, S.A.; Warren, G.L.; Hamrick, M.W. Aging and the muscle-bone relationship. Physiology 2015, 30, 8–16. [Google Scholar] [CrossRef]
- Abud, T.; Kounidas, G.; Martin, K.R.; Werth, M.; Cooper, K.; Myint, P.K. Determinants of healthy ageing: A systematic review of contemporary literature. Aging Clin. Exp. Res. 2022, 34, 1215–1223. [Google Scholar] [CrossRef]
- Harrison, J.; Dawson, L. Occupational Health: Meeting the Challenges of the Next 20 Years. Saf. Health Work. 2016, 7, 143–149. [Google Scholar] [CrossRef]
- Schulte, P.A.; Wagner, G.R.; Ostry, A.; Blanciforti, L.A.; Cutlip, R.G.; Krajnak, K.M.; Luster, M.; Munson, A.E.; O’Callaghan, J.P.; Parks, C.G.; et al. Work, obesity, and occupational safety and health. Am. J. Public Health 2007, 97, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Statistics Austria. Österreichische Gesundheitsbefragung 2019—Hauptergebnisse; Statistics Austria: Vienna, Austria, 2019; Available online: https://www.statistik.at/fileadmin/publications/Oesterreichische-Gesundheitsbefragung2019_Hauptergebnisse.pdf (accessed on 7 January 2024).
- Ganesh Kumar, S.; Deivanai Sundaram, N. Prevalence and risk factors of hypertension among bank employees in urban Puducherry, India. Int. J. Occup. Environ. Med. 2014, 5, 94–100. [Google Scholar] [PubMed]
- Lamers, Y. Approaches to improving micronutrient status assessment at the population level. Proc. Nutr. Soc. 2019, 78, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E. Global Distribution and Disease Burden Related to Micronutrient Deficiencies; Nestlé Nutrition Institute Workshop Series; Karger Publishers: Basel, Switzerland, 2014; pp. 21–28. [Google Scholar]
- Hooper, L.; Ashton, K.; Harvey, L.J.; Decsi, T.; Fairweather-Tait, S.J. Assessing potential biomarkers of micronutrient status by using a systematic review methodology: Methods. Am. J. Clin. Nutr. 2009, 89, 1953S–1959S. [Google Scholar] [CrossRef] [PubMed]
- Shenkin, A. Micronutrients in health and disease. Postgrad. Med. J. 2006, 82, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Overweight and Obesity. Available online: https://www.paho.org/en/enlace/overweight-and-obesity (accessed on 7 January 2024).
- Wimalawansa, S.J. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J. Steroid Biochem. Mol. Biol. 2018, 175, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Strange, R.C.; Shipman, K.E.; Ramachandran, S. Metabolic syndrome: A review of the role of vitamin D in mediating susceptibility and outcome. World J. Diabetes 2015, 6, 896–911. [Google Scholar] [CrossRef]
- Houston, M.C.; Harper, K.J. Potassium, magnesium, and calcium: Their role in both the cause and treatment of hypertension. J. Clin. Hypertens. 2008, 10, 3–11. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef]
- Peng, H.; Man, C.; Xu, J.; Fan, Y. Elevated homocysteine levels and risk of cardiovascular and all-cause mortality: A meta-analysis of prospective studies. J. Zhejiang Univ. Sci. B 2015, 16, 78–86. [Google Scholar] [CrossRef]
- Pallotti, F.; Bergamini, C.; Lamperti, C.; Fato, R. The Roles of Coenzyme Q in Disease: Direct and Indirect Involvement in Cellular Functions. Int. J. Mol. Sci. 2021, 23, 128. [Google Scholar] [CrossRef] [PubMed]
- Saini, R. Coenzyme Q10: The essential nutrient. J. Pharm. Bioallied Sci. 2011, 3, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Lopez-Lluch, G.; Hargreaves, I.P. Coenzyme Q10 Metabolism: A Review of Unresolved Issues. Int. J. Mol. Sci. 2023, 24, 2585. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. (Ed.) Das Standardlabor in der Naturheilkundlichen Praxis [The Standard Laboratory in Naturopathic Practice]; Urban & Fischer in Elsevier: Amsterdam, Netherlands, 2016; ISBN 978-3-437-56303-4. [Google Scholar]
- World Health Organization. Body Mass Index (BMI). Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index (accessed on 7 January 2024).
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Wirnitzer, K.; Motevalli, M.; Tanous, D.; Wirnitzer, G.; Leitzmann, C.; Wagner, K.-H.; Rosemann, T.; Knechtle, B. Training and Racing Behaviors of Omnivorous, Vegetarian, and Vegan Endurance Runners—Results from the NURMI Study (Step 1). Nutrients 2021, 13, 3521. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ruhtenberg, N. Mikronährstoffe und Aminosäuren—Einsatz in der Sporternährung; Sportsnutrition; Sportaerztezeitung: Mainz, Germany, 2014; pp. 36–38. [Google Scholar]
- Mikronährstoffdefizite. 2019. Available online: https://www.sportaerztezeitung.de/sportkardiologie-abo/articles/mikronaehrstoffdefizite (accessed on 7 January 2024).
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Niklowitz, P.; Onur, S.; Fischer, A.; Laudes, M.; Palussen, M.; Menke, T.; Döring, F. Coenzyme Q10 serum concentration and redox status in European adults: Influence of age, sex, and lipoprotein concentration. J. Clin. Biochem. Nutr. 2016, 58, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Refsum, H.; Smith, A.D.; Ueland, P.M.; Nexo, E.; Clarke, R.; McPartlin, J.; Johnston, C.; Engbaek, F.; Schneede, J.; McPartlin, C.; et al. Facts and recommendations about total homocysteine determinations: An expert opinion. Clin. Chem. 2004, 50, 3–32. [Google Scholar] [CrossRef]
- World Health Organization. Global Physical Activity Questionnaire (GPAQ). Available online: https://www.who.int/publications/m/item/global-physical-activity-questionnaire (accessed on 7 January 2024).
- Wanner, M.; Hartmann, C.; Pestoni, G.; Martin, B.W.; Siegrist, M.; Martin-Diener, E. Validation of the Global Physical Activity Questionnaire for self-administration in a European context. BMJ Open Sport. Exerc. Med. 2017, 3, e000206. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health. Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 7 January 2024).
- DGE Nutrition Report. Available online: https://www.dge.de/english/nutrition-reports/ (accessed on 7 January 2024).
- Elmadfa, I.; Meyer, A.L.; Wottawa, D.; Wagner, K.; Hasenegger, V. Vitamin D Intake and Status in Austria and Its Effects on Some Health Indicators. Austin J. Nutr. Metab. 2017, 4, 1050. [Google Scholar]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D status and nutrition in Europe and Asia. J. Steroid Biochem. Mol. Biol. 2007, 103, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Siddiqee, M.H.; Bhattacharjee, B.; Siddiqi, U.R.; MeshbahurRahman, M. High prevalence of vitamin D deficiency among the South Asian adults: A systematic review and meta-analysis. BMC Public Health 2021, 21, 1823. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Grant, W.B.; Bhattoa, H.P.; Bayer, M.; Povoroznyuk, V.; Rudenka, E.; Ramanau, H.; Varbiro, S.; Rudenka, A.; Karczmarewicz, E.; et al. Vitamin D status in central Europe. Int. J. Endocrinol. 2014, 2014, 589587. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Brett, A.S. Standard Definition of Vitamin D Deficiency Is Challenged. Available online: https://www.jwatch.org/na44769/2017/08/15/standard-definition-vitamin-d-deficiency-challenged (accessed on 7 January 2024).
- Kim, J.; Kim, H.; Roh, H.; Kwon, Y. Causes of hyperhomocysteinemia and its pathological significance. Arch. Pharm. Res. 2018, 41, 372–383. [Google Scholar] [CrossRef]
- Al Mutairi, F. Hyperhomocysteinemia: Clinical Insights. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520962230. [Google Scholar] [CrossRef]
- Rauh, M.; Verwied, S.; Knerr, I.; Dörr, H.G.; Sönnichsen, A.; Koletzko, B. Homocysteine concentrations in a German cohort of 500 individuals: Reference ranges and determinants of plasma levels in healthy children and their parents. Amino Acids 2001, 20, 409–418. [Google Scholar] [CrossRef]
- Xu, R.; Huang, F.; Wang, Y.; Liu, Q.; Lv, Y.; Zhang, Q. Gender- and age-related differences in homocysteine concentration: A cross-sectional study of the general population of China. Sci. Rep. 2020, 10, 17401. [Google Scholar] [CrossRef]
- Fakhrzadeh, H.; Ghotbi, S.; Pourebrahim, R.; Nouri, M.; Heshmat, R.; Bandarian, F.; Shafaee, A.; Larijani, B. Total plasma homocysteine, folate, and vitamin B12 status in healthy Iranian adults: The Tehran homocysteine survey (2003–2004)/a cross-sectional population based study. BMC Public Health 2006, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Nygrad, O.; Vollset, S.E.; Refsum, H.M. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA 1995, 274, 1536–1543. [Google Scholar]
- Brattstrom, L.; Lindgren, A.; Israelsson, B.; Andersson, A.; Hultberg, B. Homocysteine and cysteine: Determinants of plasma levels in middle-aged and elderly subjects. J. Intern. Med. 1994, 36, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; Rogers, G.; Bowman, B.A.; Gunter, E.W.; Wright, J.D.; Johnson, C.L. Serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey (1991–1994): Population reference ranges and contribution of vitamin status to high serum concentrations. Ann. Intern. Med. 1999, 131, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Miyaki, K. Genetic polymorphisms in homocysteine metabolism and response to folate intake: A comprehensive strategy to elucidate useful genetic information. J. Epidemiol. 2010, 20, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Salaroli, L.B.; Saliba, R.A.; Zandonade, E.; Molina, M.C.; Bissoli, N.S. Prevalence of metabolic syndrome and related factors in bank employees according to different defining criteria, Vitoria/ES, Brazil. Clinics 2013, 68, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Kumar, S.G.; Unnikrishnan, B.; Nagaraj, K. Self-reported Chronic Diseases and Occupational Health Risks among Bank Employees of Southern Karnataka City, India. Indian J. Community Med. 2013, 38, 61–62. [Google Scholar] [CrossRef]
- Łopuszańska-Dawid, M.; Szklarska, A.; Kołodziej, H.; Lipowicz, A.; Jankowska, E.A. The relationship between: Occupational status, biological condition and androgen hormone level among Polish adult men: The Wroclaw Male Study. Aging Male 2016, 19, 231–238. [Google Scholar] [CrossRef]
- Ford, E.S.; Caspersen, C.J. Sedentary behaviour and cardiovascular disease: A review of prospective studies. Int. J. Epidemiol. 2012, 41, 1338–1353. [Google Scholar] [CrossRef]
- Ekelund, U. Infographic: Physical activity, sitting time and mortality. Br. J. Sports Med. 2018, 52, 1164–1165. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Physical Activity in the European Union: Facts and Figures. 2021. Available online: https://sport.ec.europa.eu/sites/default/files/2021-11/PA%20European%20Report%202021%20Web_v1_1.pdf (accessed on 7 January 2024).
- Krug, S.; Jordan, S.; Mensink, G.B.M.; Mu¨ters, S.; Finger, J.D.; Lampert, T. English version of “Körperliche Aktivität. Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1)”. Bundesgesundheitsblatt 2013, 56, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Luzak, A.; Heier, M.; Thorand, B.; Laxy, M.; Nowak, D.; Peters, A.; Schulz, H.; KORA-Study Group. Physical activity levels, duration pattern and adherence to WHO recommendations in German adults. PLoS ONE 2017, 12, e0172503. [Google Scholar] [CrossRef]
- Statistics Austria. Bevölkerungsbewegung (Wanderungen) 2020. Available online: https://www.statistik.at/services/tools/services/publikationen/detail/848 (accessed on 7 January 2024).
- Cattafesta, M.; Bissoli, N.S.; Salaroli, L.B. Metabolic syndrome and C-reactive protein in bank employees. Diabetes Metab. Syndr. Obes. 2016, 9, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Agostino, D.; Daskalopoulou, C.; Wu, Y.T.; Koukounari, A.; Haro, J.M.; Tyrovolas, S.; Panagiotakos, D.B.; Prince, M.; Prina, A.M. The impact of physical activity on healthy ageing trajectories: Evidence from eight cohort studies. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, S.A.; Sule, G.O.; Olatona, F.A.; Goodman, O.O.; Sekoni, O.O. Knowledge and practice of sedentary lifestyle among bankers in Abuja, North-Central Nigeria. Res. J. Health Sci. 2017, 5, 167. [Google Scholar] [CrossRef]
- Nketiah, G.B.; Odoi-Agyarko, K.; Ndanu, T.A.; Hayford, F.E.A.; Amoh, G.; Lawson, H. Physical inactivity among corporate bank workers in Accra, Ghana: Implications for health promotion. PLoS ONE 2023, 18, e0277994. [Google Scholar] [CrossRef]
- Gichu, M.; Asiki, G.; Juma, P.; Kibachio, J.; Kyobutungi, C.; Ogola, E. Prevalence and predictors of physical inactivity levels among Kenyan adults (18–69 years): An analysis of STEPS survey 2015. BMC Public Health 2018, 18, 1217. [Google Scholar] [CrossRef]
- Kelly, S.; Martin, S.; Kuhn, I.; Cowan, A.; Brayne, C.; Lafortune, L. Barriers and facilitators to the uptake and maintenance of healthy behaviors by people at mid-life: A rapid systematic review. PLoS ONE 2016, 11, e0145074. [Google Scholar] [CrossRef]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.M. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonized meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef]
- Statistics Austria. Overweight and Obesity. Available online: https://www.statistik.at/en/statistics/population-and-society/health/health-determinants/overweight-and-obesity (accessed on 7 January 2024).
- Sanchi, G.R.; Borges, L.R. Lifestyle and nutritional status of employees of a chain of banks in Pelotas, Rio Grande do Sul, Brazil. Rev. Bras. Med. Trab. 2020, 17, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Cattafesta, M.; Salaroli, L.B. Diets high in vegetables, fruits, cereals, and tubers as a protective factor for metabolic syndrome in bank employees. Diabetes Metab. Syndr. Obes. 2018, 11, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Addo, P.N.; Nyarko, K.M.; Sackey, S.O.; Akweongo, P.; Sarfo, B. Prevalence of obesity and overweight and associated factors among financial institution workers in Accra Metropolis, Ghana: A cross-sectional study. BMC Res. Notes 2015, 8, 599. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Motevalli, M.; Wagner, K.-H.; Leitzmann, C.; Tanous, D.; Wirnitzer, G.; Knechtle, B.; Wirnitzer, K. Female Endurance Runners Have a Healthier Diet than Males—Results from the NURMI Study (Step 2). Nutrients 2022, 14, 2590. [Google Scholar] [CrossRef] [PubMed]
- Rennie, K.L.; Johnson, L.; Jebb, S.A. Behavioural determinants of obesity. Best. Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 343–358. [Google Scholar] [CrossRef]
- Newton, S.; Braithwaite, D.; Akinyemiju, T.F. Socio-economic status over the life course and obesity: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0177151. [Google Scholar] [CrossRef]
- Wirnitzer, K.C.; Drenowatz, C.; Cocca, A.; Tanous, D.R.; Motevalli, M.; Wirnitzer, G.; Schätzer, M.; Ruedl, G.; Kirschner, W. Health Behaviors of Austrian Secondary School Teachers and Principals at a Glance: First Results of the from Science 2 School Study Focusing on Sports Linked to Mixed, Vegetarian, and Vegan Diets. Nutrients 2022, 14, 1065. [Google Scholar] [CrossRef]
- Wirnitzer, K.C.; Motevalli, M.; Cocca, A.; Tanous, D.R.; Wirnitzer, G.; Wagner, K.H.; Drenowatz, C.; Ruedl, G. Health behavior of Austrian tertiary students focusing on diet type linked to sports and exercise-first glimpse of results from the “sustainably healthy-from science 2 high school and university” study. Front. Public Health 2023, 11, 1129004. [Google Scholar] [CrossRef]
- Wirnitzer, K.C.; Motevalli, M.; Tanous, D.R.; Wirnitzer, G.; Wagner, K.H.; Drenowatz, C.; Cocca, A.; Ruedl, G. A glimpse of academic staff health behavior on diet type and physical activity at Austrian universities: First findings from the “Sustainably Healthy—From Science 2 Highschool & University” study. Front. Public Health 2023, 11, 1194602. [Google Scholar] [CrossRef]
- Mizuno, T.; Shu, I.; Makimura, H.; Mobbs, C. Obesity over the Life Course. Sci. Aging Knowl. Environ. 2004, 2004, re4. [Google Scholar] [CrossRef]

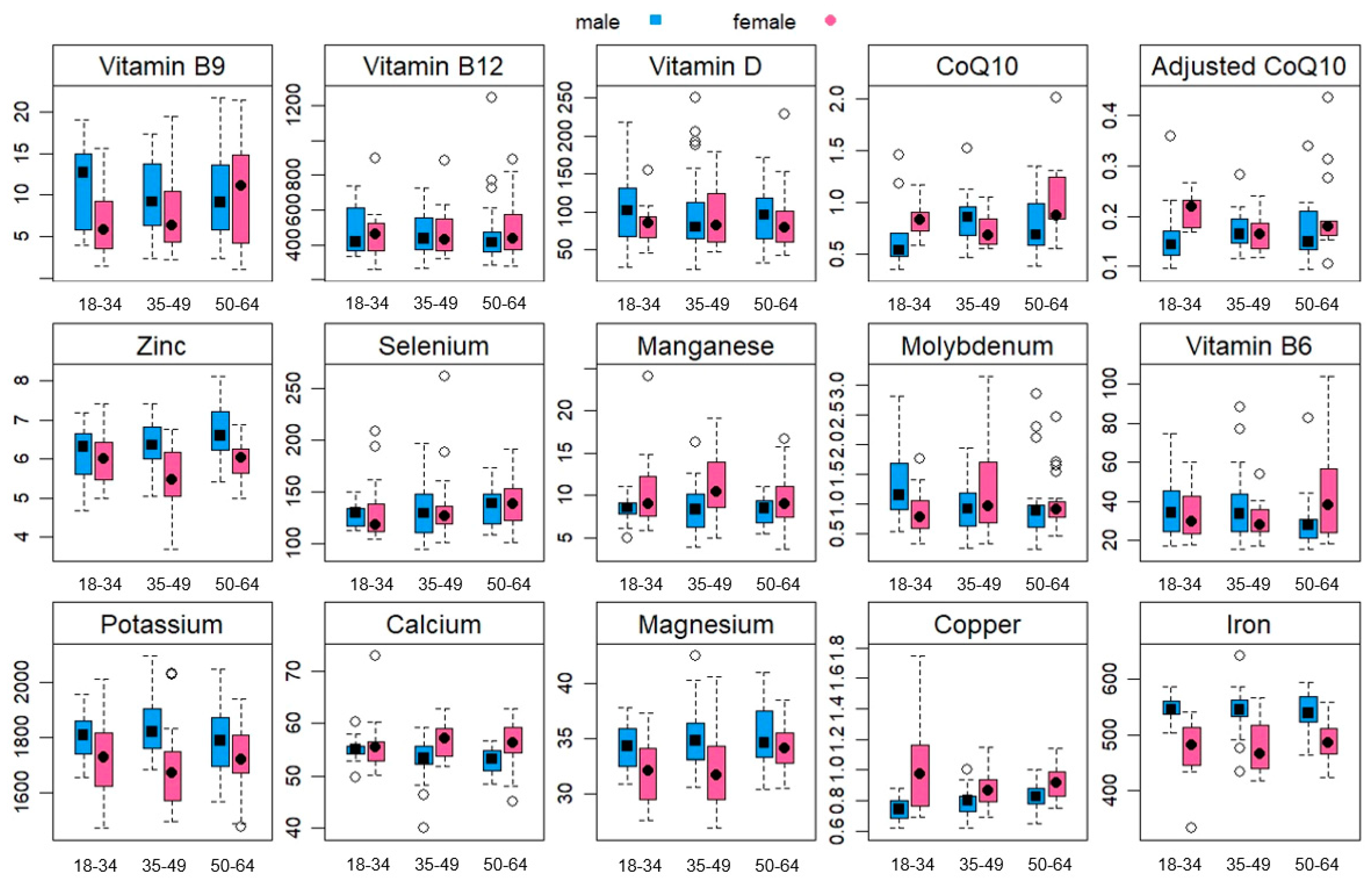
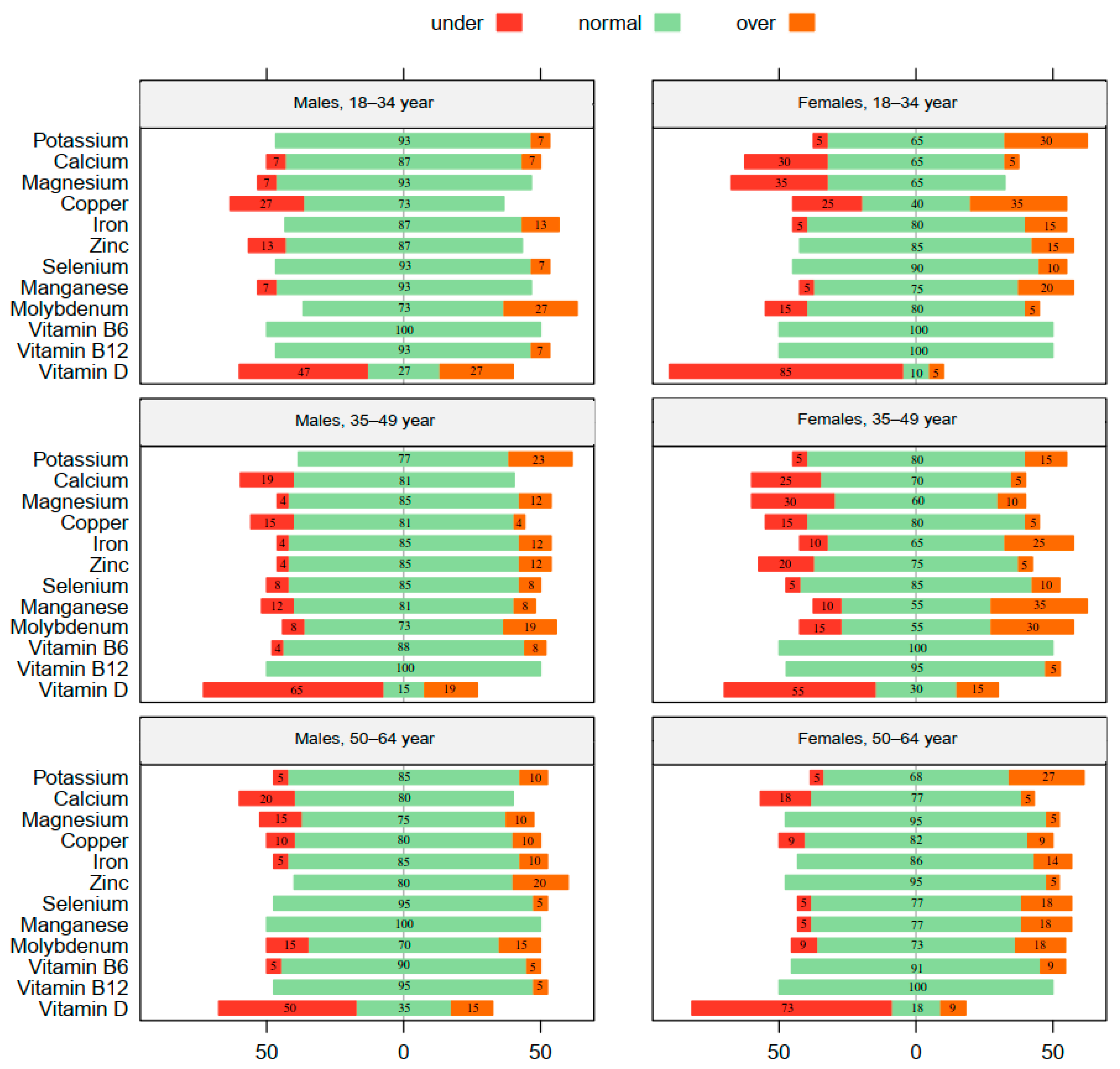
| Total (n = 123) | 18–34 Year (n = 35) | 35–49 Year (n = 46) | 50–64 Year (n = 42) | Statistics and p-Values | ||
|---|---|---|---|---|---|---|
| Sex | male female | 50% 50% | 43% 57% | 57% 43% | 48% 52% | χ2(2) = 1.58, p = 0.453 |
| Body Weight (kg) | 71 (43–114) | 65 (43–114) | 74 (50–99) | 72 (45–114) | F(2, 120) = 2.10, p = 0.126 | |
| Height (cm) | 173 (154–190) | 170 (157–190) | 175 (156–190) | 172 (154–186) | F(2, 120) = 1.51, p = 0.224 | |
| BMI (kg/m2) | 23.6 (16.9–40.4) | 22.7 (16.9–40.4) | 23.8 (17.3–32.3) | 24.2 (17.6–36.0) | F(2, 120) = 3.00, p = 0.053 | |
| BMI Levels | <18.5 | 6% | 9% | 4% | 5% | χ2(6) = 11.51, p = 0.074 |
| 18.5–24.9 | 61% | 77% | 61% | 48% | ||
| 25.0–29.9 | 28% | 11% | 33% | 38% | ||
| ≥30.0 | 5% | 3% | 2% | 10% | ||
| Homocysteine (µmol/L) | 10.57 ± 4.30 | 9.73 ± 2.59 | 10.11 ± 4.10 | 11.77 ± 5.36 | F(2, 120) = 3.35, p = 0.039 | |
| Homocysteine Levels | <10 | 52% | 60% | 63% | 33% | χ2(4) = 10.43, p = 0.034 |
| 10–15 | 39% | 37% | 28% | 52% | ||
| >15 | 9% | 3% | 9% | 14% | ||
| CoQ10 (mg/L) | 0.82 ± 0.28 * | 0.73 ± 0.28 | 0.81 ± 0.22 | 0.89 ± 0.34 | F(2, 79) = 1.92, p = 0.153 | |
| Adjusted CoQ10 (µmol/mmol Chol) | 0.18 ± 0.06 * | 0.19 ± 0.06 | 0.17 ± 0.04 | 0.19 ± 0.07 | F(2, 79) = 0.14, p = 0.866 | |
| Diet Type | mixed | 93% | 91% | 91% | 95% | χ2(2) = 0.61, p = 0.735 |
| vegetarian/vegan | 7% | 9% | 9% | 5% | ||
| PA Levels | low | 18% | 11% | 13% | 29% | χ2(4) = 5.43, p = 0.246 |
| moderate | 21% | 20% | 22% | 21% | ||
| high | 61% | 69% | 65% | 50% | ||
| Sedentary Time (h/week) | 42.5 ± 23.3 | 43.9 ± 22.5 | 38.8 ± 24.6 | 45.3 ± 22.5 | F(2, 120) = 1.52, p = 0.222 | |
| Work MET | 4889 ± 9949 | 5114 ± 7306 | 4980 ± 10481 | 4602 ± 11382 | F(2, 120) = 1.83, p = 0.165 | |
| Transport MET | 313 ± 1866 | 62 ± 234 | 703 ± 3005 | 97 ± 356 | F(2, 120) = 1.03, p = 0.361 | |
| Recreation MET | 3395 ± 4448 | 3514 ± 3051 | 3235 ± 5113 | 3472 ± 4733 | F(2, 120) = 0.90, p = 0.409 | |
| Total MET | 8598 ± 13526 | 8690 ± 7866 | 8918 ± 17276 | 8171 ± 12853 | F(2, 120) = 2.25, p = 0.110 | |
| Reference Range for Female Adults * | Females (n = 62) | Statistics and p-Values | ||||
|---|---|---|---|---|---|---|
| Total | 18–34 Year | 35–49 Year | 50–64 Year | |||
| Potassium (mg/L) | 1484–1794 | 1687 ± 234 | 1724 ± 134 | 1616 ± 366 | 1718 ± 119 | F(2, 59) = 1.15, p = 0.323 |
| Calcium (mg/L) | 53.8–62.7 | 56.3 ± 4.1 | 55.8 ± 4.7 | 56.7 ± 3.2 | 56.4 ± 4.3 | F(2, 59) = 1.22, p = 0.301 |
| Magnesium (mg/L) | 29.8–37.5 | 32.8 ± 3.0 | 31.9 ± 3.1 | 32.1 ± 3.5 | 34.2 ± 1.9 | F(2, 59) = 5.24, p = 0.008 |
| Copper (mg/L) | 0.76–1.12 | 0.93 ± 0.20 | 1.01 ± 0.29 | 0.88 ± 0.12 | 0.92 ± 0.12 | F(2, 59) = 0.88, p = 0.419 |
| Iron (mg/L) | 423–520 | 482 ± 43 | 477 ± 48 | 479 ± 49 | 489 ± 34 | F(2, 59) = 0.75, p = 0.476 |
| Zinc (mg/L) | 4.88–6.67 | 5.80 ± 0.69 | 5.98 ± 0.63 | 5.47 ± 0.83 | 5.93 ± 0.51 | F(2, 59) = 1.99, p = 0.146 |
| Selenium (µg/L) | 101–170 | 138 ± 42 | 130 ± 29 | 135 ± 36 | 149 ± 54 | F(2, 59) = 2.06, p = 0.137 |
| Manganese (µg/L) | 5.91–12.7 | 10.33 ± 3.70 | 10.32 ± 4.20 | 11.17 ± 3.67 | 9.57 ± 3.20 | F(2, 59) = 1.38, p = 0.259 |
| Molybdenum (µg/L) | 0.5–1.6 | 1.12 ± 0.83 | 0.85 ± 0.37 | 1.27 ± 0.83 | 1.22 ± 1.06 | F(2, 59) = 1.67, p = 0.197 |
| Vitamin B6 (µg/L) | 16.4–80.4 | 38.2 ± 30.8 | 33.3 ± 13.3 | 29.6 ± 8.7 | 50.6 ± 47.6 | F(2, 59) = 1.73, p = 0.187 |
| Vitamin B9 (ng/mL) | >5.38 | 8.88 ± 5.70 | 7.37 ± 4.61 | 8.04 ± 5.28 | 10.91 ± 6.50 | F(2, 57) = 1.67, p = 0.197 |
| Vitamin B12 (pg/mL) | 211–911 | 579 ± 877 | 451 ± 143 | 831 ± 1554 | 478 ± 158 | F(2, 58) = 0.24, p = 0.785 |
| Vitamin D (nmol/L) | 100–150 | 89.9 ± 37.2 | 84.1 ± 24.4 | 96.7 ± 42.2 | 89.1 ± 42.3 | F(2, 59) = 0.32, p = 0.728 |
| Reference Range for Male Adults * | Males (n = 61) | Statistics and p-Values | ||||
|---|---|---|---|---|---|---|
| Total | 18–34 Year | 35–49 Year | 50–64 Year | |||
| Potassium (mg/L) | 1568–1908 | 1811 ± 113 | 1800 ± 86 | 1839 ± 108 | 1782 ± 131 | F(2, 58) = 1.10, p = 0.340 |
| Calcium (mg/L) | 50.3–59.8 | 53.5 ± 3.4 | 55.0 ± 2.4 | 53.1 ± 4.3 | 52.8 ± 2.4 | F(2, 58) = 3.02, p = 0.057 |
| Magnesium (mg/L) | 31.2–39.1 | 34.9 ± 2.8 | 34.3 ± 2.3 | 35.1 ± 2.8 | 35.0 ± 3.1 | F(2, 58) = 0.26, p = 0.773 |
| Copper (mg/L) | 0.7–0.94 | 0.79 ± 0.09 | 0.75 ± 0.09 | 0.79 ± 0.08 | 0.82 ± 0.09 | F(2, 58) = 3.36, p = 0.042 |
| Iron (mg/L) | 465–577 | 543 ± 34 | 546 ± 22 | 542 ± 40 | 541 ± 33 | F(2, 58) = 0.13, p = 0.874 |
| Zinc (mg/L) | 5.36–7.29 | 6.43 ± 0.68 | 6.12 ± 0.68 | 6.42 ± 0.62 | 6.69 ± 0.69 | F(2, 58) = 2.23, p = 0.117 |
| Selenium (µg/L) | 101–168 | 136 ± 35 | 144 ± 61 | 131 ± 25 | 137 ± 18 | F(2, 58) = 0.93, p = 0.402 |
| Manganese (µg/L) | 5.39–11.2 | 8.29 ± 2.13 | 8.37 ± 1.64 | 8.38 ± 2.74 | 8.13 ± 1.56 | F(2, 58) = 0.04, p = 0.958 |
| Molybdenum (µg/L) | 0.45–1.56 | 1.60 ± 4.23 | 1.33 ± 0.66 | 2.23 ± 6.45 | 0.98 ± 0.67 | F(2, 58) = 2.24, p = 0.115 |
| Vitamin B6 (µg/L) | 16.4–80.4 | 36.5 ± 22.3 | 37.2 ± 16.7 | 41.4 ± 28.6 | 29.7 ± 14.5 | F(2, 58) = 1.88, p = 0.161 |
| Vitamin B9 (ng/mL) | >5.38 | 10.02 ± 4.92 | 10.95 ± 5.30 | 9.47 ± 4.53 | 10.04 ± 5.26 | F(2, 58) = 0.29, p = 0.746 |
| Vitamin B12 (pg/mL) | 211–911 | 491 ± 247 | 567 ± 402 | 458 ± 120 | 478 ± 222 | F(2, 58) = 0.27, p = 0.767 |
| Vitamin D (nmol/L) | 100–150 | 99.8 ± 49.9 | 106.1 ± 50.2 | 98.4 ± 57.1 | 97.0 ± 40.9 | F(2, 58) = 0.21, p = 0.810 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schauer, M.; Mair, S.; Motevalli, M.; Tanous, D.; Burtscher, M.; Wirnitzer, K. Health Patterns across Adulthood: An Age-Based Investigation of the Nutritional Status, Homocysteine, and CoQ10 of Bank Staff. Clin. Pract. 2024, 14, 443-460. https://doi.org/10.3390/clinpract14020034
Schauer M, Mair S, Motevalli M, Tanous D, Burtscher M, Wirnitzer K. Health Patterns across Adulthood: An Age-Based Investigation of the Nutritional Status, Homocysteine, and CoQ10 of Bank Staff. Clinics and Practice. 2024; 14(2):443-460. https://doi.org/10.3390/clinpract14020034
Chicago/Turabian StyleSchauer, Markus, Susanne Mair, Mohamad Motevalli, Derrick Tanous, Martin Burtscher, and Katharina Wirnitzer. 2024. "Health Patterns across Adulthood: An Age-Based Investigation of the Nutritional Status, Homocysteine, and CoQ10 of Bank Staff" Clinics and Practice 14, no. 2: 443-460. https://doi.org/10.3390/clinpract14020034
APA StyleSchauer, M., Mair, S., Motevalli, M., Tanous, D., Burtscher, M., & Wirnitzer, K. (2024). Health Patterns across Adulthood: An Age-Based Investigation of the Nutritional Status, Homocysteine, and CoQ10 of Bank Staff. Clinics and Practice, 14(2), 443-460. https://doi.org/10.3390/clinpract14020034








