Abstract
This systematic review and meta-analysis aimed to evaluate the analgesic efficacy and adverse effects of celecoxib after total knee arthroplasty. Keywords in the PubMed and Scopus databases were used to find article abstracts. Each included clinical trial was assessed using the Cochrane Collaboration risk of bias tool, and we extracted data on postoperative pain assessment using the Visual Analogue Scale (VAS) at rest, ambulation, and active range of motion, rescue analgesic intake, and adverse effects. Inverse variance tests with mean differences were used to analyze the numerical variables. The Mantel–Haenszel statistical method and the odds ratio were used to evaluate the dichotomous data. According to this qualitative assessment (n = 482), two studies presented conclusions in favor of celecoxib (n = 187), one showed similar results between celecoxib and the placebo (n = 44), and three clinical trials did not draw conclusions as to the effectiveness of celecoxib versus the placebo (n = 251). Moreover, the evaluation of the rescue analgesic intake showed that the patients receiving celecoxib had a lower intake compared to patients receiving a placebo (n = 278, I2 = 82%, p = 0.006, mean difference = −6.89, 95% IC = −11.76 to −2.02). In conclusion, the pooled analysis shows that administration of celecoxib alone results in a decrease in rescue analgesic consumption compared to a placebo after total knee surgery.
1. Introduction
Acute pain after total knee arthroplasty is very intense and disabling for patients undergoing this surgical procedure [1,2,3]. This can hinder the patient’s mobility in terms of both passive and active movement, support while walking or resting, and stiffness in the joints. This lack of mobility, consequently, delays the patient’s recovery, affecting the quality of life [4,5,6,7,8].
Advances in clinical, surgical, and pharmacological procedures have allowed better pain management in patients undergoing total knee arthroplasty [2,8,9,10]. Recommendations include the use of a multimodal approach using different types of pain management medications, such as opioid analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticosteroids, gabapentinoids, and anesthetics—i.e., bupivacaine hydrochloride—[8,9,10,11]. On the other hand, the use of pharmacological monotherapy for pain management after this type of surgical procedure is questionable [12].
The use of COX-2 selective NSAIDs before, during, and after total knee arthroplasty is a relatively common choice that would imply advantages due to the nature of the type of pain suffered by the patient [13]. However, high concentrations of this drug also produce inhibition of the COX-1 enzyme using in vitro assays. Preclinical studies have shown that the therapeutic plasma concentration of celecoxib should be approximately 300 ng/mL, and single-dose pharmacokinetic studies in humans have suggested that doses as low as 100 mg of celecoxib would achieve this concentration [14]. Currently, there is no systematic review with meta-analysis that evaluates the individual effect of celecoxib in total knee arthroplasty; so, this study aims to compile the best scientific evidence available to provide the clinician with a real view of the analgesic efficacy and adverse effects of this drug after total knee arthroplasty.
2. Materials and Methods
2.1. Population, Interventions, Control, and Outcome Strategy [15]
2.1.1. Inclusion Criteria
- Population: clinical trials included patients undergoing total knee arthroplasty;
- Interventions: patients received celecoxib;
- Control: patients received a placebo;
- Outcome: evaluation of postoperative pain using the Visual Analogue Scale (VAS) at rest, ambulation, and active range of motion, rescue analgesic intake, and adverse effects.
2.1.2. Exclusion Criteria
RCT with a loss to follow-up greater than 20%.
2.2. Research Question
What are the analgesic and adverse effects of celecoxib and placebo after total knee arthroplasty?
2.3. Information Search
Studies published from 2000 to July 2023 were considered. The following terms were used in the PubMed and Scopus databases to find abstracts of clinical trials related to the keywords: “celecoxib” AND “total knee arthroplasty”; “celecoxib” AND “orthopedic surgery”; “COX-2 inhibitor” AND “total knee arthroplasty”; “COX-2 inhibitor” AND “orthopedic surgery”; “NSAIDs” AND “total knee arthroplasty”; and “NSAIDs” AND “orthopedic surgery”. PubMed filters for study type/design and language (“English” and “Spanish”) were used. The running protocol was sent to and accepted by the PROSPERO system from the University of York (ID CRD42023486909).
2.4. Assessment of Bias
Each clinical trial was assessed using the Cochrane Collaboration risk of bias tool [16,17,18,19]. The evaluations were carried out by two independent evaluators [16,17,18,19]. The decision on the qualification of each evaluating study was made by consensus between both participants, and when there was a difference between them, a third evaluator participated to reach a majority decision [16,17,18,19].
2.5. Data Extraction
The data were recorded in an Excel database and subsequently moved to a statistical program. The data included the author, study design, treatment groups, sample size (n), dose, evaluation of postoperative pain with the VAS in rest, ambulation, and active range of motion, rescue analgesic intake, and adverse effects.
2.6. Statistical Analysis
The inverse variance test with means difference was employed to analyze the numerical variables. The Mantel–Haenszel statistical method and odds ratio (OR) were used to evaluate the dichotomous data. Moreover, the heterogeneity was evaluated as previously reported in a published article [20]. All meta-analyses were conducted using a random effect model with the Review Manager Software 5.3 for Windows. A p value overall statistical test <0.05 and an OR > 1 with 95% confidence intervals (CI) in each meta-analysis were considered as statistical differences [16,21,22].
Sensitivity analysis was performed to observe variations in the statistical analysis when statistical differences were obtained in the meta-analyses and to understand the influence that each study had on the results of the pooled data [23].
3. Results
3.1. Information Search
The search in the databases used in this systematic review resulted in 173 articles related to the different groups of keywords used. Fifteen duplicate reports were removed, and 146 articles were removed for other reasons. As Figure 1 and Table 1 show, six clinical trials [24,25,26,27,28,29] were included in the qualitative analysis of this systematic review.
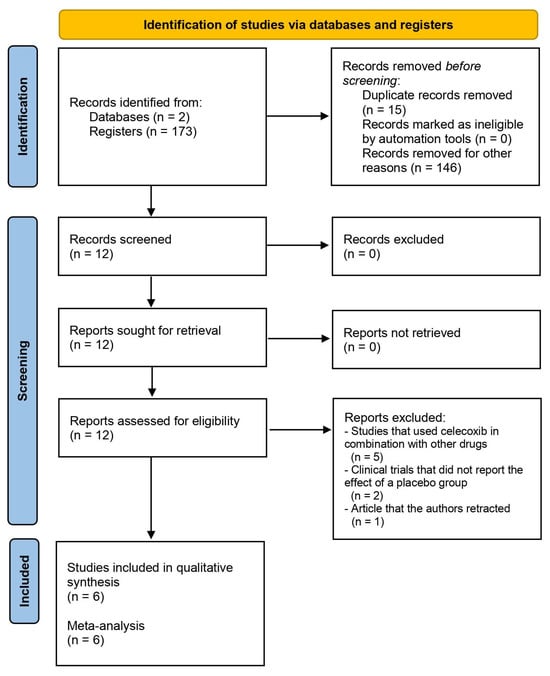
Figure 1.
Study flow diagram.

Table 1.
Details of the included clinical trials.
3.2. Bias Assessment
The risk of bias assessment included a total of six scientific reports evaluating the efficacy of celecoxib compared to a placebo in total knee arthroplasty. The results of the risk of bias assessment showed that four articles [24,27,28,29] had a low to moderate risk because they did not obtain red circles in their evaluations. However, two of those clinical trials had a high risk of bias [25,26]. The reason for this high risk of bias was the lack of blinding of participants, staff, and the clinical evaluator who collected the data (Figure 2).
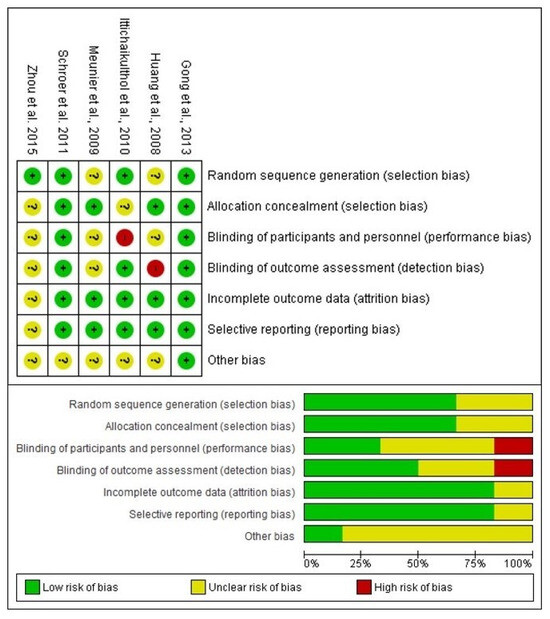
Figure 2.
Assessment of risk of bias [24,25,26,27,28,29].
3.3. Qualitative Assessment
The qualitative evaluation of the studies was carried out considering the conclusion of each of the articles (n = 482) [24,25,26,27,28,29]. According to this section, two studies presented conclusions in favor of celecoxib (n = 187) [25], one showed similar results between celecoxib and the placebo (n = 44) [27], and three clinical trials did not conclude as to the effectiveness of celecoxib versus the placebo (n = 251) [24,26,29] because they had another objective, i.e., two studies had as their main objective to compare the effectiveness of drug combinations in total knee arthroplasty, and one concluded that another analgesic was superior to celecoxib and the placebo.
3.4. Quantitative Evaluation
The assessment of pain intensity with the VAS at rest was performed using data from two clinical trials [24,29]. Analysis of the data showed no statistical differences between celecoxib and the placebo (n = 171, I2 = 91%, p = 0.3, mean difference = −0.79, 95% IC = −2.27 to 0.69; Figure 3). On the other hand, the evaluation of the intake of rescue analgesics was carried out with four clinical trials [25,26,27,29]. The statistical evaluation showed that the patients receiving celecoxib had a lower rescue analgesic intake compared to patients receiving a placebo (n = 278, I2 = 82%, p = 0.006, mean difference = −6.89, 95% IC = −11.76 to −2.02; Figure 4). Finally, the adverse reactions were evaluated using information from three clinical trials (n = 234) [25,26,29]. Both nausea (n = 234, I2 = 0%, p = 0.08, OR = 0.57, 95% IC = 0.3 to 1.06; Figure 5) and vomiting (n = 234, I2 = 0%, p = 0.13, OR = 0.56, 95% IC = 0.27 to 1.17; Figure 5) were similar between celecoxib and a placebo.
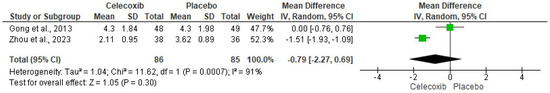
Figure 3.
Evaluation of postoperative pain with the limb at rest [24,29].
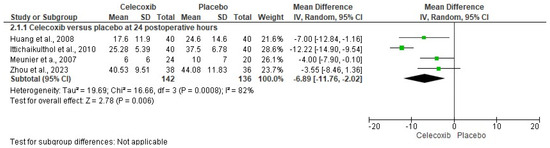
Figure 4.
Analysis of pooled data on rescue analgesic intake in patients receiving celecoxib versus a placebo after total knee arthroplasty [25,26,27,29].
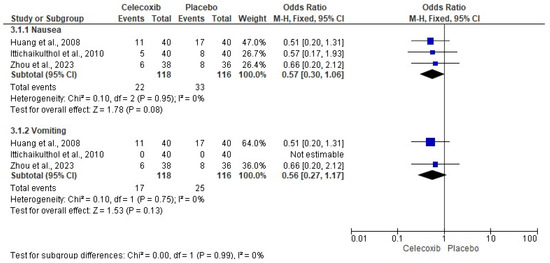
Figure 5.
Meta-analysis of the adverse effects of celecoxib [25,26,29].
3.5. The Sensitivity Assessment and Publication Bias
The sensitivity analysis was carried out only for the consumption of rescue analgesics, which did not show variability in the results. That is, despite having performed this sensitivity analysis, the results maintained the statistical difference (Figure 6) [25,26,27,29].
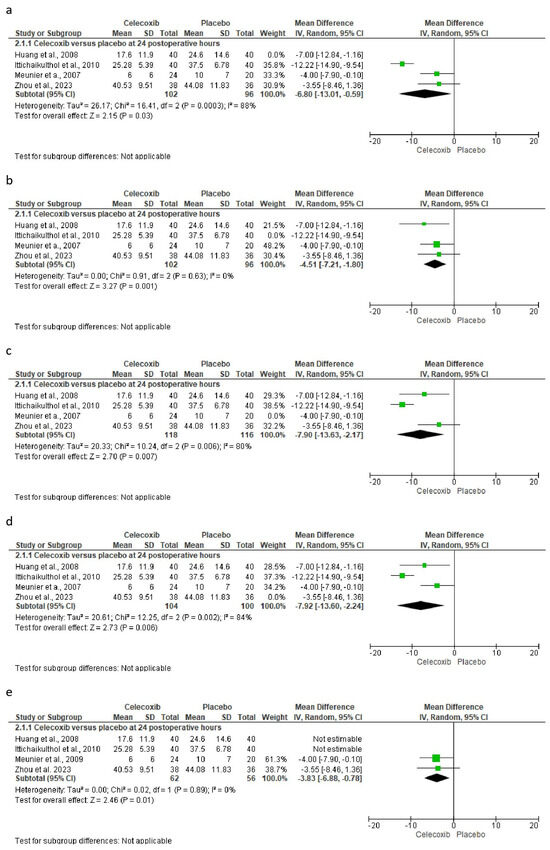
Figure 6.
Sensitivity analysis shows how the statistical difference is preserved even when data from different studies are excluded. Excluded study: (a) = Huang et al., 2008; (b) = Ittichaikulthol et al., 2010; (c) = Meunier et al., 2009; (d) = Zhou et al., 2023; and (e) = Huang et al., 2008 and Ittichaikulthol et al., 2010 [25,26,27,29].
4. Discussion
This is the first systematic review and meta-analysis that evaluates the analgesic efficacy as well as the safety of only celecoxib following total knee surgery. The most important result of this systematic review is the decreased consumption of postoperative analgesics in patients who received celecoxib compared to those who received a placebo. It is important to highlight that this same variable was used to carry out the sensitivity analysis in which it was observed that despite extracting the different trials to carry out the statistical analysis and even having excluded from the analysis those studies with a high risk of bias, the statistical difference was maintained during all executions.
During the full reading of the articles, several clinical trials were excluded for different reasons: studies that used celecoxib in combination with other drugs [30,31,32,33,34], clinical trials that did not report the effect of a placebo group [35,36], and an article that the authors retracted [37]. That is, they did not report the therapeutic effect of celecoxib alone after total knee arthroplasty. Moreover, the assessment of the risks of bias in the clinical trials included in this systematic review and meta-analysis showed a moderate risk of bias in four studies [24,27,28,29], and two clinical trials had a high risk of bias [25,26]. Blinding of participants and personnel, as well as blinding of outcome assessment, were the reasons why these last two clinical trials were considered at high risk of bias.
The qualitative evaluation of the results showed that celecoxib produced a better analgesic effect compared with a placebo after total knee surgery. Two studies showed favorable results for celecoxib [25,28], one reported similar analgesic efficacy to a placebo [27], and in the remaining three, although they did not conclude in terms of the use of celecoxib, the detailed analysis of the information clearly showed that celecoxib was better than a placebo after total knee surgery [24,26,29].
The quantitative evaluation showed that celecoxib presented a statistically significant decrease in postoperative rescue analgesic consumption compared to a placebo after this type of surgery. It is important to note that the heterogeneity of this meta-analysis was high, and to be conservative in our statistical analysis, the random effects model was used. The decreased consumption of rescue analgesics in the postoperative period is an important finding that has been observed in many surgical areas [38,39,40,41,42,43,44,45].
Jiang et al., 2020 performed a systematic review and meta-analysis on the analgesic efficacy and adverse effects of COX-2 enzyme inhibitors in total knee and hip arthroplasty [46]. The authors observed a statistical difference in several indicators of clinical effectiveness, such as pain perception at rest and while walking, a decrease in postoperative opioid analgesic consumption, and the incidence of nausea and fever. When comparing our systematic review with that conducted by Jiang et al., 2020 [46], we observed some important differences. The first difference is that our study reports the analgesic efficacy and adverse effects of celecoxib alone compared with a placebo, and the study by Jiang et al., 2020 [46] showed the overall effect of COX-2 enzyme inhibitors after total knee surgery. In our study, we only observed that a single variable obtained a statistical difference; we observed a decrease in the consumption of rescue analgesics in the postoperative period. Jiang et al., 2020 [46] found differences in five variables for which it was possible to combine the data to perform a statistical analysis. Furthermore, the sample size of our study was smaller than that of the aforementioned study [46]. Hong et al. performed a systematic review and meta-analysis to determine the analgesic efficacy and adverse effects of parecoxib compared with a placebo. The authors reported that parecoxib produced better pain relief 24 h postoperatively compared with a placebo, while adverse effects such as nausea and vomiting were similar between both groups [47]. Moreover, Geng et al., 2022 performed a systematic review and meta-analysis on the use of celecoxib in total knee arthroplasty, and their results showed statistical differences in the intensity of extremity pain at rest, a decrease in the consumption of opioid analgesics, and a greater range of active motion. However, several of the studies included in their statistical analysis used a combination of celecoxib with another drug [30,33,34]; that is, the analgesic effect and adverse effects of celecoxib alone in total knee arthroplasty were not evaluated [48].
NSAIDs selective for the COX-2 enzyme reduce the risk of gastropathies, as well as kidney and cardiovascular damage—cerebrovascular or myocardial infarction—because they do not inhibit COX-1 [49,50,51,52,53,54]. Selective inhibition of COX-2 produces an analgesic and anti-inflammatory effect, because this COX-2 enzyme produces prostaglandins and other byproducts of arachidonic acid [49,50,51,52,53].
Pain is a subjective phenomenon that varies greatly between each subject. Making a comparison between a pharmacological treatment and a placebo is key for many studies that evaluate analgesia since it offers many advantages, from methodological to statistical, in randomized clinical trials. In this particular case, the comparison with a placebo gives us the possibility to calculate the NNT and CI for this particular drug, which would have allowed valid indirect comparisons with other analgesic treatments using the NNT and CI [54,55,56,57,58,59,60]. Unfortunately, none of the variables including the number of patients presented statistical differences in the meta-analysis; so, it was not possible to calculate these analgesic efficacy estimators that would have been of great interest to physicians.
The main advantage of this study is that it reports the analgesic efficacy and adverse effects of celecoxib alone compared to a placebo. The strengths of this systematic review are the statistics, which were performed conservatively, always considering the result of heterogeneity, as well as the sensitivity analysis [16,20,21,22,58,59]. The main disadvantage of this study is the limited number of studies that met the selection criteria, which allowed for a relatively small sample size, as well as the design of this type of study—retrospective—[60]. Another limitation of this systematic review was the language since only studies in Spanish and English were included. The study by Jiang et al. included some Chinese language studies that were not found by our electronic search team and that would have allowed a larger sample size [46]. In addition, another important limitation of this study was the calculation of clinical effectiveness estimators, such as the number needed to treat and confidence intervals, which could not be calculated because the data were not presented as frequencies.
In conclusion, we can highlight that there is evidence of moderate quality from the pooled analysis of data from the studies included in this systematic review and meta-analysis that shows that administration of celecoxib alone results in a decrease in rescue analgesic consumption compared to a placebo after total knee surgery.
Author Contributions
The conception and design of the study were carried out by A.J.A.-C. and M.A.I.-E. Acquisition, analysis, and interpretation of data, critical review for important intellectual content, and final approval of the version to be published were performed by all authors of the manuscript. Also, we agree to be responsible for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data included in this study were extracted from articles that met the selection criteria of our study. Data can be found available in these clinical trials and in the files generated to perform meta-analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhao, J.; Davis, S.P. An integrative review of multimodal pain management on patient recovery after total hip and knee arthroplasty. Int. J. Nurs. Stud. 2019, 98, 94–106. [Google Scholar] [CrossRef]
- Li, J.W.; Ma, Y.S.; Xiao, L.K. Postoperative Pain Management in Total Knee Arthroplasty. Orthop. Surg. 2019, 11, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Lavand’homme, P.M.; Kehlet, H.; Rawal, N.; Joshi, G.P.; PROSPECT Working Group of the European Society of Regional Anaesthesia and Pain Therapy (ESRA). Pain management after total knee arthroplasty: Procedure Specific Postoperative Pain Management recommendations. Eur. J. Anaesthesiol. 2022, 39, 743–757. [Google Scholar] [CrossRef]
- Corap, Y.; Brix, M.; Brandt, J.R.; Emmeluth, C.; Lindberg-Larsen, M. Knee function, quality of life, pain, and living conditions after distal femoral resection knee arthroplasty for non-tumor indications. BMC Musculoskelet. Disord. 2023, 24, 9. [Google Scholar] [CrossRef]
- Connolly, P.; Coombs, S.; Schwarzkopf, R. Mechanical complications after total knee arthroplasty. Expert. Rev. Med. Devices 2023, 20, 1105–1117. [Google Scholar] [CrossRef]
- Canovas, F.; Dagneaux, L. Quality of life after total knee arthroplasty. Orthop. Traumatol. Surg. Res. 2018, 104, S41–S46. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, C.J.; Pelt, C.E.; Gililland, J.M.; Peters, C.L. Perioperative Pain Management in Hip and Knee Arthroplasty. Orthop. Clin. N. Am. 2017, 48, 407–419. [Google Scholar] [CrossRef]
- Derogatis, M.J.; Sodhi, N.; Anis, H.K.; Ehiorobo, J.O.; Bhave, A.; Mont, M.A. Pain Management Strategies to Reduce Opioid Use Following Total Knee Arthroplasty. Surg. Technol. Int. 2019, 35, 301–310. [Google Scholar] [PubMed]
- Elmallah, R.K.; Chughtai, M.; Khlopas, A.; Newman, J.M.; Stearns, K.L.; Roche, M.; Kelly, M.A.; Harwin, S.F.; Mont, M.A. Pain Control in Total Knee Arthroplasty. J. Knee Surg. 2018, 31, 504–513. [Google Scholar] [CrossRef]
- Soffin, E.M.; Memtsoudis, S.G. Anesthesia and analgesia for total knee arthroplasty. Minerva Anestesiol. 2018, 84, 1406–1412. [Google Scholar] [CrossRef]
- O’Neill, A.; Lirk, P. Multimodal Analgesia. Anesthesiol. Clin. 2022, 40, 455–468. [Google Scholar] [CrossRef]
- Xu, C.P.; Li, X.; Wang, Z.Z.; Song, J.Q.; Yu, B. Efficacy and safety of single-dose local infiltration of analgesia in total knee arthroplasty: A meta-analysis of randomized controlled trials. Knee 2014, 21, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Moore, R.A. Single dose oral celecoxib for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2013, 2013, CD004233. [Google Scholar] [CrossRef]
- Lefkowith, J.B. Cyclooxygenase-2 specificity and its clinical implications. Am. J. Med. 1999, 106, 43S–50S. [Google Scholar] [CrossRef]
- Leonardo, R. PICO: Model for clinical questions. Evid. Based Med. Pract. 2008, 3, 2. [Google Scholar]
- Higgins, P.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0 [Updated March 2011]; The Cochrane Collaboration: Oxford, UK, 2011; Available online: http://www.cochrane-handbook.org/ (accessed on 22 September 2023).
- Jones, A.; Steel, D. Evaluating the quality of medical evidence in real-world contexts. J. Eval. Clin. Pract. 2018, 24, 950–956. [Google Scholar] [CrossRef]
- Atkins, D.; Eccles, M.; Flottorp, S.; Guyatt, G.H.; Henry, D.; Hill, S.; Liberati, A.; O’Connell, D.; Oxman, A.D.; Phillips, B.; et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv. Res. 2004, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Franco-de la Torre, L.; Gómez-Sánchez, E.; Aragon-Martinez, O.H.; Hernández-Gómez, A.; Franco-González, D.L.; Guzmán-Flores, J.M.; Alonso-Castro, A.J.; Granados-Soto, V.; Isiordia-Espinoza, M.A. Analgesic Efficacy and Safety of Tapentadol Immediate Release in Bunionectomy: A Meta-Analysis. Pharmaceuticals 2023, 16, 1287. [Google Scholar] [CrossRef]
- Whitley, E.; Ball, J. Statistics review 3: Hypothesis testing and P values. Crit. Care 2002, 6, 222–225. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Franco-de la Torre, L.; Gómez-Sánchez, E.; Serafín-Higuera, N.A.; Alonso-Castro, Á.J.; López-Verdín, S.; Molina-Frechero, N.; Granados-Soto, V.; Isiordia-Espinoza, M.A. Dexamethasone Increases the Anesthetic Success in Patients with Symptomatic Irreversible Pulpitis: A Meta-Analysis. Pharmaceuticals 2022, 15, 878. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Dong, J.Y.; Li, Z.R. Effects of combined application of muscle relaxants and celecoxib administration after total knee arthroplasty (TKA) on early recovery: A randomized, double-blind, controlled study. J. Arthroplast. 2013, 28, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Wang, C.M.; Wang, C.T.; Lin, W.P.; Horng, L.C.; Jiang, C.C. Perioperative celecoxib administration for pain management after total knee arthroplasty—A randomized, controlled study. BMC Musculoskelet. Disord. 2008, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Ittichaikulthol, W.; Prachanpanich, N.; Kositchaiwat, C.; Intapan, T. The post-operative analgesic efficacy of celecoxib compared with placebo and parecoxib after total hip or knee arthroplasty. J. Med. Assoc. Thai. 2010, 93, 937–942. [Google Scholar] [PubMed]
- Meunier, A.; Lisander, B.; Good, L. Effects of celecoxib on blood loss, pain, and recovery of function after total knee replacement: A randomized placebo-controlled trial. Acta Orthop. 2007, 78, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Schroer, W.C.; Diesfeld, P.J.; LeMarr, A.R.; Reedy, M.E. Benefits of prolonged postoperative cyclooxygenase-2 inhibitor administration on total knee arthroplasty recovery: A double-blind, placebo-controlled study. J. Arthroplast. 2011, 26 (Suppl. S6), 2–7. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Ding, C.; Xiang, B.; Yan, L. Positive Preemptive Analgesia Effectiveness of Pregabalin Combined with Celecoxib in Total Knee Arthroplasty: A Prospective Controlled Randomized Study. Pain Res. Manag. 2023, 2023, 7088004. [Google Scholar] [CrossRef]
- Mammoto, T.; Fujie, K.; Taguchi, N.; Ma, E.; Shimizu, T.; Hashimoto, K. Short-Term Effects of Early Postoperative Celecoxib Administration for Pain, Sleep Quality, and Range of Motion After Total Knee Arthroplasty: A Randomized Controlled Trial. J. Arthroplast. 2021, 36, 526–531. [Google Scholar] [CrossRef]
- Mammoto, T.; Fujie, K.; Mamizuka, N.; Taguchi, N.; Hirano, A.; Yamazaki, M.; Ueno, S.; Ma, E.; Hashimoto, K. Effects of postoperative administration of celecoxib on pain management in patients after total knee arthroplasty: Study protocol for an open-label randomized controlled trial. Trials 2016, 17, 45. [Google Scholar] [CrossRef]
- Xu, X.; Sang, W.; Liu, Y.; Zhu, L.; Lu, H.; Ma, J. Effect of Celecoxib on Surgical Site Inflammation after Total Knee Arthroplasty: A Randomized Controlled Study. Med. Princ. Pract. 2018, 27, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Lubis, A.M.T.; Rawung, R.B.V.; Tantri, A.R. Preemptive Analgesia in Total Knee Arthroplasty: Comparing the Effects of Single Dose Combining Celecoxib with Pregabalin and Repetition Dose Combining Celecoxib with Pregabalin: Double-Blind Controlled Clinical Trial. Pain Res. Treat. 2018, 2018, 3807217. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Tao, L.; Lin, J.; Jin, J.; Qian, W.; Bian, Y.; Li, Y.; Dong, Y.; Peng, H.; Li, Y.; et al. Postoperative intravenous parecoxib sodium followed by oral celecoxib post total knee arthroplasty in osteoarthritis patients (PIPFORCE): A multicentre, double-blind, randomised, placebo-controlled trial. BMJ Open 2020, 10, e030501. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xie, L.; Liu, H.; Hu, Y. Transdermal buprenorphine patch versus oral celecoxib for pain management after total knee arthroplasty: An open- label, randomized controlled trial. Orthop. Traumatol. Surg. Res. 2020, 106, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F. Preoperative celecoxib analgesia is more efficient and equally tolerated compared to postoperative celecoxib analgesia in knee osteoarthritis patients undergoing total knee arthroplasty: A randomized, controlled study. Medicine 2018, 97, e13663. [Google Scholar] [CrossRef] [PubMed]
- Reuben, S.S.; Buvenandran, A.; Katz, B.; Kroin, J.S. A prospective randomized trial on the role of perioperative celecoxib administration for total knee arthroplasty: Improving clinical outcomes. Anesth. Analg. 2008, 106, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.K.; Priye, S.; Singh, S.; Singh, U.; Singh, R.B.; Singh, P.K. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can. J. Anaesth. 2004, 51, 358–363. [Google Scholar] [CrossRef]
- Garcia, J.B.; Barbosa-Neto, J.O.; Vasconcelos, J.W.; Ferro, L.S.; Silva, R.C. Analgesic efficacy of the intra-articular administration of high doses of morphine in patients undergoing total knee arthroplasty. Rev Bras Anestesiol. 2010, 60, 1–12. [Google Scholar] [CrossRef][Green Version]
- Kiefhaber, T.R.; Vyrva, O. Will the Use of Intraoperative Liposomal Bupivacaine During Thumb Carpometacarpal Arthroplasty Decrease Postoperative Use of Opioids? A Prospective Randomized Study. J. Hand Surg. Am. 2022, 47, 586.e1–586.e8. [Google Scholar] [CrossRef]
- Han, S.S.; Do, S.H.; Kim, T.H.; Choi, W.J.; Yun, J.S.; Ryu, J.H. Stepwise tapering of remifentanil at the end of surgery decreased postoperative pain and the need of rescue analgesics after thyroidectomy. BMC Anesthesiol. 2015, 15, 46. [Google Scholar] [CrossRef]
- Yu, L.; Yang, Y.; Wu, H.; Yu, Y.; Wang, Y.; Yan, S.; Li, N.; Li, H.; Chen, C.; Zhang, Z. Pupillary monitoring decreases remifentanil consumption during laparoscopic uterine surgery and improves postoperative recovery. Minerva Anestesiol. 2023, 89, 859–866. [Google Scholar] [CrossRef]
- Bilir, S.; Yurtlu, B.S.; Hancı, V.; Okyay, R.D.; Erdoğan-Kayhan, G.; Ayoğlu, H.P.; Özkoçak-Turan, I. Effects of peroperative intravenous paracetamol and lornoxicam for lumbar disc surgery on postoperative pain and opioid consumption: A randomized, prospective, placebo-controlled study. Agri 2016, 28, 98–105. [Google Scholar] [CrossRef]
- Stepan, J.G.; Sacks, H.A.; Verret, C.I.; Wessel, L.E.; Kumar, K.; Fufa, D.T. Standardized Perioperative Patient Education Decreases Opioid Use after Hand Surgery: A Randomized Controlled Trial. Plast. Reconstr. Surg. 2021, 147, 409–418. [Google Scholar] [CrossRef]
- Gormley, J.; Gouveia, K.; Sakha, S.; Stewart, V.; Emmanuel, U.; Shehata, M.; Tushinski, D.; Shanthanna, H.; Madden, K. Reduction of opioid use after orthopedic surgery: A scoping review. Can. J. Surg. 2022, 65, E695–E715. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Deng, H.; Chen, X.; Lin, Y.; Xie, X.; Bo, Z. The efficacy and safety of selective COX-2 inhibitors for postoperative pain management in patients after total knee/hip arthroplasty: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Xie, H.Y.; Ge, W.K.; Yu, M.; Lin, S.N.; Liu, C.J. The efficacy of parecoxib in improving pain after total knee or total hip arthroplasty: Systematic review and meta-analysis. Medicine 2022, 101, e30748. [Google Scholar] [CrossRef]
- Geng, X.; Zhou, S.; Zhang, X.; Liu, X.; Cheng, X.; Jiang, L.; Zhang, D. The Efficacy and Safety of Celecoxib for Pain Management After Total Knee Arthroplasty: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Surg. 2022, 9, 791513. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.O.; Hjemdahl, P. Lessons from 20 years with COX-2 inhibitors: Importance of dose-response considerations and fair play in comparative trials. J. Intern. Med. 2022, 292, 557–574. [Google Scholar] [CrossRef]
- Coskun Benlidayi, I. Are non-steroidal anti-inflammatory drugs safe and effective in patients with acute gout? A Cochrane review summary with commentary. Int. J. Rheum. Dis. 2023, 26, 1178–1182. [Google Scholar] [CrossRef]
- Oms Arias, M.; Morral Parente, R.M. Are COX-2 inhibitors indicated for patients at risk? Aten. Primaria 2002, 29, 230–232. [Google Scholar] [CrossRef]
- LaForge, J.M.; Urso, K.; Day, J.M.; Bourgeois, C.W.; Ross, M.M.; Ahmadzadeh, S.; Shekoohi, S.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. Non-steroidal Anti-inflammatory Drugs: Clinical Implications, Renal Impairment Risks, and AKI. Adv. Ther. 2023, 40, 2082–2096. [Google Scholar] [CrossRef]
- Giercksky, K.E.; Haglund, U.; Rask-Madsen, J. Selective inhibitors of COX-2 are they safe for the stomach? Scand. J. Gastroenterol. 2000, 35, 1121–1124. [Google Scholar] [CrossRef]
- Vase, L. Can insights from placebo and nocebo mechanisms studies improve the randomized controlled trial? Scand. J. Pain 2020, 20, 451–467. [Google Scholar] [CrossRef]
- Diener, H.C.; Schorn, C.F.; Bingel, U.; Dodick, D.W. The importance of placebo in headache research. Cephalalgia Int. J. Headache 2008, 28, 1003–1011. [Google Scholar] [CrossRef]
- Dobrilla, G.; Scarpignato, C. Placebo and placebo effect: Their impact on the evaluation of drug response in patients. Dig. Dis. 1994, 12, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Vase, L.; Riley, J.L., III; Price, D.D. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain 2002, 99, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Urrútia, G.; Bonfill, X. Declaración PRISMA: Una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis [PRISMA declaration: A proposal to improve the publication of systematic reviews and meta-analyses]. Med. Clin. 2010, 135, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Dawson, B.; Trapp, R.G. Bioestadística Médica, 4th ed.; Editorial El Manual Moderno: Guanajuato, Mexico, 2005. [Google Scholar]
- Argimon-Pallás, J.M.; Jiménez-Villa, J. Métodos de Investigación Clínica y Epidemiológica, 5th ed.; Elsevier: Barcelona, Spain, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).