Abstract
Cancer research and clinical trials are essential to improve cancer patients’ outcomes and advance the oncology field. The United Arab Emirates (UAE) has been lagging in cancer research with many barriers, including healthcare, institutional, regulatory, patient and community, the global oncology community, and the pharmaceutical industry. In this report, we try to address these challenges from our perspective. Making clinical trials accessible for cancer patients in the UAE requires a collaborative approach from all stakeholders and serious consideration for the greater cause to improve the patient’s outcome and contribute to the advancement of the cancer field worldwide. There has been significant support from the UAE government and the regulators in the UAE to facilitate and encourage research in general and cancer research in particular with recent initiatives and international collaborations. Private and public institutions must overcome their competitive moods and work together to strengthen the research network across the UAE and improve accrual for potential clinical trials. Public awareness and education must overcome long-standing perceptions about research and clinical trials in the UAE. The pharmaceutical industry must work closely with institutions across the UAE and support them in establishing accredited research programs and clinical trial units. The Emirates Oncology Society is establishing the Oncology Research Working Group to advocate and advance cancer research in the UAE. All stakeholders must be engaged to successfully implement impactful clinical trials in the UAE and the region.
1. Background
Between 1 January–31 December 2019, the total number of newly diagnosed cancer cases (malignant and in situ) reported to the United Arab Emirates National Cancer Registry (UAE-NCR) was 4633, of which 4381 (94.56%) were malignant and 252 (5.44%) were in situ cases. Overall, cancer was more prevalent among women than men; it affected 2604 (56.2%) females and 2029 (43.8%) males. Among UAE citizens, a total of 1193 cases were newly diagnosed with cancer, out of which 1117 (93.6%) were malignant and 76 (6.4%) were in situ cases. Similarly, in non-UAE citizens, 3440 cases were newly diagnosed with cancer, of which 3264 (94.9%) were malignant, and 176 (5.1%) were in situ cases, representing an overall crude incidence rate of 46.1/100,000 for both genders. There is a clear female predominance in cancer incidence. The crude incidence rate was higher for females (75.8/100,000) than for males (31.0/100,000). The overall age-standardized incidence rate (ASR) was 78.4/100,000. Breast, thyroid, colorectal, skin, and leukemia were the top-ranked cancers among all new cancer cases in both genders. Colorectal, skin, prostate, leukemia, and non-Hodgkin’s lymphoma were the top-ranked cancers among males. Among females, breast, thyroid, colorectal, uterus, and ovary were the top-ranked cancers. In the year 2019, there were 125 children in the age group of 0–14 years diagnosed with new cancer in the UAE (54% were females and 46% were males). This constitutes about 2.9% of all registered malignant cases. Leukemia, brain and CNS, connective and soft tissue, non-Hodgkin’s lymphoma, and bone and articular were the most common cancers in boys and girls. The third leading cause of death in the UAE, after diseases of the circulatory system and injuries, was found to be cancer. The number of deaths from cancer totaled 1181 (629 in males and 552 in females) and accounted for 13.11% of all deaths regardless of nationality, type of cancer, or gender. This represents an estimated age-standardized mortality rate of 33.3 deaths per 100,000 for both genders. Breast cancer was the leading cause of cancer death in 2019, with an estimated average of 11.6% of cancer deaths per year; colon cancer was the second most common cause of cancer death in both sexes, and lung cancer was the third most common cause of cancer death in both sexes [1].
Cancer care in the UAE has considerably evolved over the last two decades. However, advances in cancer research have notably lagged behind [2]. The USA and Europe are leading the cancer research world with significant research and development (R&D) programs; these programs have been long-established, well structured, and have received global recognition for their contribution to cancer research and improving patient outcomes. Oncology trials worldwide reached historically high levels in 2021, up 56% from 2016, and mostly focused on rare cancer indications [3,4]. No previous publications have addressed the barriers and facilitators to conducting cancer research in general and clinical trials in the United Arab Emirates (UAE). For perspective and to take breast cancer as an example, we conducted a dedicated and systematic literature search to identify publications related to breast cancer from the UAE. We searched PubMed using the terms “breast” AND “Cancer* OR Oncol* OR malignant* OR tumor OR tumor” AND “emirates OR UAE” on 8 August 2022. A total of 203 journal publications by authors from the UAE were retrieved, with the earliest publication being from 2001. The majority were basic science/translational (45.8%) or observational (26.1%) studies, while 40 (19.1%) were non-data-driven publications (e.g., reviews, consensus statements, editorials), and only five clinical trials were identified. Of note, among the 163 data-driven publications, only 62 (38%) were performed in the UAE, while the remaining were conducted abroad with authors having a UAE affiliation. Only seven clinical trials were identified by expanding the search to all cancers (Table 1).

Table 1.
Clinical trials in cancer indications conducted in the UAE. RCT, randomized, controlled trial.
We conducted a further search on clinicaltrials.gov to identify clinical trials that may not have been published; this search was done on 21 October 2022, and we identified 25 studies. There were 15 completed trials, 6 actively recruiting, and 3 of unknown status (Table 2).

Table 2.
Clinical trials in the UAE registered at clinicaltrials.gov as of October 2022.
Cancer research activities varied between the Gulf Cooperation Council Countries (UAE, Saudi Arabia, Oman, Bahrain, Qatar, and Kuwait). We conducted a search for oncology clinical trials in each country using clinicaltrials.gov. Saudi Arabia had the highest number of registered clinical trials with 175 trials, followed by the UAE with 25 registered clinical trials (Figure 1).
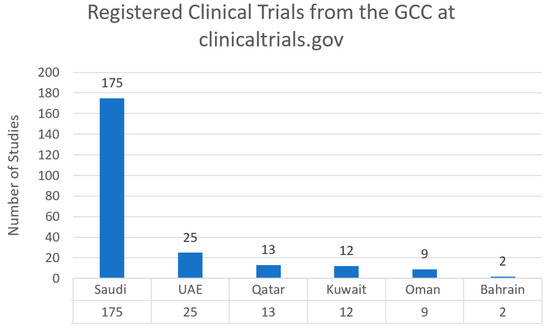
Figure 1.
Registered clinical trials from the GCC countries at clinicaltrials.gov as of 21 October 2022.
We herein provide perspectives on some of the reasons for such limited participation and conduct of clinical trials in the UAE while highlighting some unique opportunities.
2. Healthcare Provider Barriers
Healthcare providers are the cornerstone for clinical research; they identify the clinical question in their patient population that needs to be addressed in clinical trials and are responsible for the accrual of patients, the clinical assessment of treatment toxicity, and eventually the implementation of the clinical trial results in their patients. Many studies have assessed barriers to clinical research from healthcare providers’ perspectives. The following factors were identified: excessive clinical work, lack of personal motivation, lack of research experience, lack of research mentorship, lack of financial incentive, lack of protected time, and lack of understanding of the importance of research [12,13,14,15] (Figure 2). Most of these barriers related to healthcare providers in the UAE were also shared and highlighted in a survey to clarify UAE nurses’ perceptions of barriers to implementing research in clinical practice in the UAE. The two highest-ranked barriers to nurses conducting research in the UAE were a lack of time and competing demands for time [16] (Figure 2). Due to the lack of clear clinical oncology research programs in the UAE, skilled researchers and scholars are potentially migrating to Western countries for better opportunities; with more research programs, more opportunities will attract and retain researchers in the UAE and the wider region. Lastly, the medical school curriculum, residency, and fellowship training must include specific training and education for medical students and health care trainees to obtain research skills and experience.
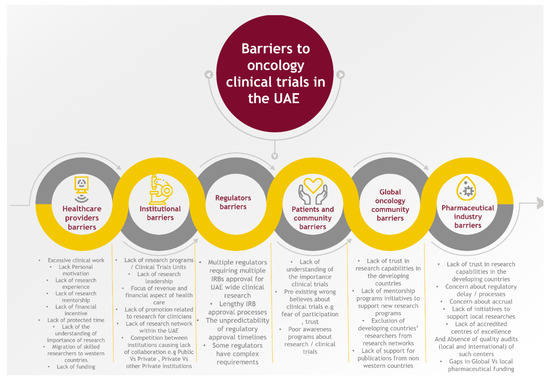
Figure 2.
Barriers to oncology clinical trials in the UAE.
3. Institutional Barriers
Institutions play a critical role in the ecosystem of oncology research and clinical trials. The lack of research programs and clinical trials units (CTUs) in health care institutions contributes immensely to the progress of cancer research in the UAE. Only a few cancer centers (5 out of 33) in the UAE have ongoing research and clinical trials. Establishing and activating such units is a limiting factor for cancer research. Lack of research leadership is another limitation. With the focus of institutions on meeting financial targets, less attention is given to clinical research. Furthermore, the competition between institutions may lead to poor collaboration between private institutions or between private and public institutions. To overcome this, institutions should see the value and potential of research networking and collaboration within the UAE (Figure 2). Lastly, the lack of promotion related to research productivity for clinicians must be evaluated and readdressed to increase the clinician’s interest and involvement.
4. Regulatory Barriers
In the UAE, multiple regulatory agencies exist (for the whole country and by Emirate), which means several approvals may need to be sought to conduct clinical research. In some instances, approvals may be lengthy or come with unpredictable approval timelines, although this is changing with the expansion of medical research teams in regulatory agencies and with optimized and more efficient procedures. The Dubai Health Authority, through the Dubai Scientific Research Ethics Committee (DSREC), follows the following general guidelines for research approvals in Dubai: Phase 1—not approved to be conducted in DHA; Phase 2—requires review and approval by higher management of the Dubai Health Authority; Phase 3 and Phase 4—DSREC review and a favorable opinion for the study submitted [17]. The Department of Health (DOH) in Abu Dhabi does allow the conduct of phases I and II trials in addition to phases III and IV [18,19]. The DOH has been leading research efforts at the regulatory level to enhance and improve the research capabilities in Abu Dhabi [20,21]. The Statistics and Research Center at the Ministry of Health and Prevention allows all types of clinical research with a more in-depth review before approval for phase I and II trials [22].
5. Patients and Community Barriers
With the lack of research and clinical trial culture in the medical community in the UAE, a lack of understanding of the importance of clinical trials is the norm among patients and the community. This leads to lower acceptance rates for enrollment in clinical trials, even if trials are accessible in the UAE. This is usually coupled with pre-existing wrong beliefs about clinical trials, e.g., fear of experimentation and trust issues. To change this, a strong awareness program about research/clinical trials is necessary (Figure 2).
6. Global Oncology Community Barriers
The global oncology community has an important role in supporting knowledge transfer and the involvement of researchers from the UAE and the region in designing and conducting clinical trials. However, the lack of trust in research capabilities in developing countries and the exclusion of researchers from those countries from research networks is another significant barrier. The lack of support for publications from developing countries in reputable journals is also a primary challenge [23]. The global community should continue to support and create mentorship programs and initiatives to support new research programs in the UAE and other countries (Figure 2).
7. Pharmaceutical Industry Barriers
Pharmaceutical research and development funding play a significant role in advancing cancer research and drug discovery [24]. The pharmaceutical industry’s support for research in some regions and countries, including the UAE, faces many challenges and barriers. This mainly stems from a lack of trust in research capabilities, concern about regulatory delays/processes, a limited/lack of accredited centers of excellence, and the absence of quality audits (local and international) of such centers. There is also a concern about successful accrual if clinical trials open in the UAE. The above challenges led to a lack of initiatives to support local research [18] and gaps in global vs. local pharmaceutical funding for research and investigator-initiated trials [25].
8. Conclusions
We have summarized barriers and potential facilitators for conducting oncology trials in the UAE, which may apply to other countries in the Middle East and Asia. There is a need for collaborative efforts from all stakeholders to address all these challenges. Efforts in cancer research are developing, although numerous evidence gaps remain. With the continuous growth in academic institutions and research programs committed to cellular and molecular research initiatives, the UAE’s scope for basic and translational research is typically improving. However, clinical research in general and clinical trials specifically are far from ideal in quantity and focus. There is an urgent need for a call to action from the local and global communities to optimize the inclusion of the UAE in international clinical trials and to provide local funding opportunities for local studies so that the efficacy and safety of drugs are evaluated in the local population rather than “assumed” from data from Western countries. The Emirates Oncology Society is establishing an oncology research group to advocate and advance cancer research in the UAE. For the success of this initiative, all stakeholders must support each other and work together for better cancer care in the UAE.
Funding
This research received support from Burjeel Medical City, Abu Dhabi, UAE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Available online: https://mohap.gov.ae/assets/download/f3eb10c9/CANCER%20INCIDENCE%20IN%20UNITED%20ARAB%20EMIRATES%20ANNUAL%20REPORT%20OF%20THE%20UAE%20-%202019.pdf.aspx (accessed on 21 October 2022).
- Al-Shamsi, H.; Darr, H.; Abu-Gheida, I.; Ansari, J.; McManus, M.C.; Jaafar, H.; Tirmazy, S.H.; ElKhoury, M.; Azribi, F.; Jelovac, D.; et al. The State of Cancer Care in the United Arab Emirates in 2020: Challenges and Recommendations, A report by the United Arab Emirates Oncology Task Force. Gulf J. Oncol. 2020, 1, 71–87. [Google Scholar]
- Available online: https://www.iqvia.com/newsroom/2022/06/global-oncology-rd-surges-while-cancer-care-disruptions-ease-says-iqvia-institute-for-human-data-sci (accessed on 1 September 2022).
- Eckhouse, S.; Lewison, G.; Sullivan, R. Trends in the global funding and activity of cancer research. Mol. Oncol. 2008, 2, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.; Zwaan, F.; Hedström, U.; Poynton, C.; Kristensen, J.; Jumaa, P.; Wassell, J.; al-Ramadi, B. Recombinant human interleukin 11 and bacterial infection in patients with [correction of] haematological malignant disease undergoing chemotherapy: A double-blind placebo-controlled randomised trial. Lancet 2003, 361, 275–280. [Google Scholar] [CrossRef]
- Ellis, M.; Hedstrom, U.; Frampton, C.; Alizadeh, H.; Kristensen, J.; Shammas, F.V.; Al-Ramadi, B.K. Modulation of the systemic inflammatory response by recombinant human interleukin-11: A prospective randomized placebo controlled clinical study in patients with hematological malignancy. Clin. Immunol. 2006, 120, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Al-Tweigeri, T.; AlSayed, A.; Alawadi, S.; Ibrahim, M.; Ashour, W.; Jaafar, J.; Abulkhair, O.; Al-Abdulkarim, H.; Khalid, H.; Ajarim, D.; et al. A multicenter prospective phase II trial of neoadjuvant epirubicin, cyclophosphamide, and 5-fluorouracil (FEC100) followed by cisplatin-docetaxel with or without trastuzumab in locally advanced breast cancer. Cancer Chemother. Pharm. 2016, 77, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Gligorov, J.; Ataseven, B.; Verrillc, M.; De Laurentiis, M.; Jung, K.H.; Azim, H.A.; Al-Sakaff, N.; Lauer, S.; Shing, M.; Pivot, X. Safety and tolerability of subcutaneous trastuzumab for the adjuvant treatment of human epidermal growth factor receptor 2-positive early breast cancer: SafeHer phase III study’s primary analysis of 2573 patients. Eur. J. Cancer 2017, 82, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Miles, D.; Ciruelos, E.; Schneeweiss, A.; Puglisi, F.; Peretz-Yablonski, T.; Campone, M.; Bondarenko, I.; Nowecki, Z.; Errihani, H.; Paluch-Shimon, S.; et al. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Ann. Oncol. 2021, 32, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- AlSaleh, K.; Al Zahwahry, H.; Bounedjar, A.; Oukkal, M.; Saadeddine, A.; Mahfouf, H.; Bouzid, K.; Bensalem, A.; Filali, T.; Abdel-Razeq, H.; et al. Response to Induction Neoadjuvant Hormonal Therapy Using Upfront 21-Gene Breast Recurrence Score Assay-Results from the SAFIA Phase III Trial. JCO Glob. Oncol. 2021, 7, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.L.; Myrtle, R.; Solis, J.M.; Hisserich, J.W.; Oliver, D. Motivational factors for medical oncologists’ participation in cancer treatment protocols. J. Health Hum. Resour. Adm. 1986, 8, 212–230. [Google Scholar] [PubMed]
- Tirupakuzhi Vijayaraghavan, B.K.; Gupta, E.; Ramakrishnan, N.; Beane, A.; Haniffa, R.; Lone, N.; de Keizer, N.; Adhikari, N.K.J. Barriers and facilitators to the conduct of critical care research in low and lower-middle income countries: A scoping review. PLoS ONE 2022, 17, e0266836. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, C.; Mitchell, G.; Nikles, J. Barriers for conducting clinical trials in developing countries—A systematic review. Int. J. Equity Health 2018, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Khoja, A.; Kazim, F.; Ali, N.A. Barriers to Conducting Clinical Trials in Developing Countries. Ochsner J. 2019, 19, 294–295. [Google Scholar] [CrossRef]
- Al-Yateem, N.; Griffiths, J.; McCreaddie, M.; Robertson-Malt, S.; Kuzemski, D.; Anthony, J.M.; Fielding, M.; Al Khatib, F.; Sojka, E.M.; Williams, J.J. A National Scoping Study on Barriers to Conducting and Using Research Among Nurses in the United Arab Emirates. Policy Politics Nurs. Pract. 2019, 20, 216–227. [Google Scholar] [CrossRef]
- Available online: https://www.dha.gov.ae/en/MedicalEducationandResearch/MedicalResearch (accessed on 1 September 2022).
- Available online: https://doh.gov.ae/-/media/40B18D052C3E40B2A079ABD7571E2260.ashx (accessed on 1 September 2022).
- Available online: https://doh.gov.ae/-/media/C07A10ADB6504312A601E3A514D43084.ashx (accessed on 1 September 2022).
- Available online: https://www.zawya.com/en/world/middle-east/abu-dhabi-witnesses-launch-of-real-world-evidence-study-to-assess-astrazenecas-evusheld-o1p3b720 (accessed on 1 September 2022).
- Available online: https://www.arabianbusiness.com/industries/healthcare/abu-dhabi-department-of-health-inks-deal-to-develop-clinical-research-hub-innovative-treatments (accessed on 1 September 2022).
- Available online: https://mohap.gov.ae/en/services/request-for-support-and-coordination-of-medical-research (accessed on 1 September 2022).
- Hamadeh, R.R.; Jahrami, H.; Nazzal, K. Cancer Research in the Arab World. In Cancer in the Arab World; Al-Shamsi, H.O., Abu-Gheida, I.H., Iqbal, F., Al-Awadhi, A., Eds.; Springer: Singapore, 2022; pp. 395–408. [Google Scholar]
- Kanavos, P.; Sullivan, R.; Lewison, G.; Schurer, W.; Eckhouse, S.; Vlachopioti, Z. The role of funding and policies on innovation in cancer drug development. Ecancermedicalscience 2010, 4, 164. [Google Scholar] [CrossRef] [PubMed]
- Benhima, N.; El Fadli, M.; Essâdi, I.; Belbaraka, R. What does it take to conduct clinical trials in African countries? Insights from Morocco ecancer Ecancermedicalscience 2022, 16, 1411. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).