Five-Year Retrospective Study of Uterine STUMP and Leiomyosarcoma
Abstract
1. Introduction
2. Materials and Methods
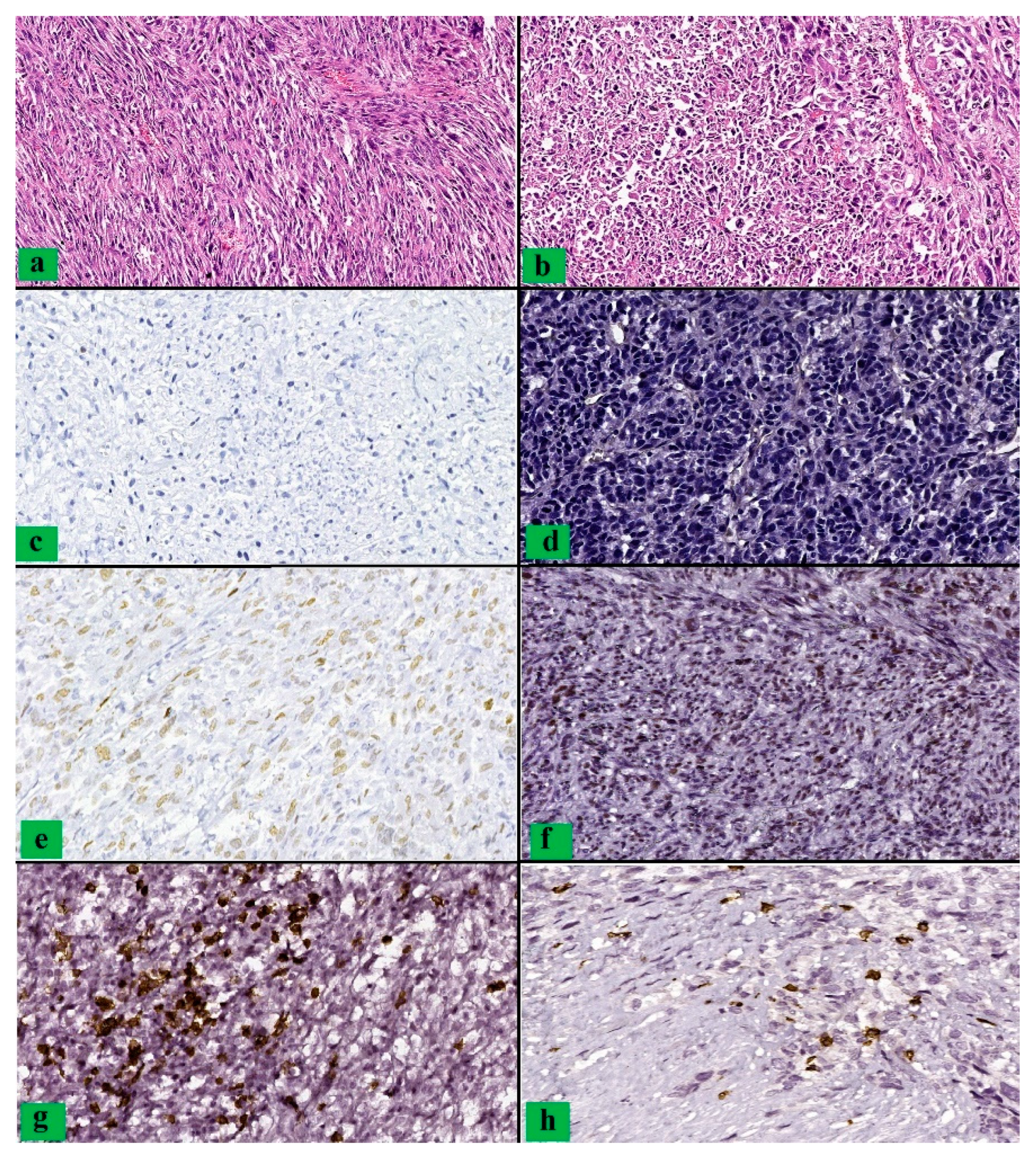
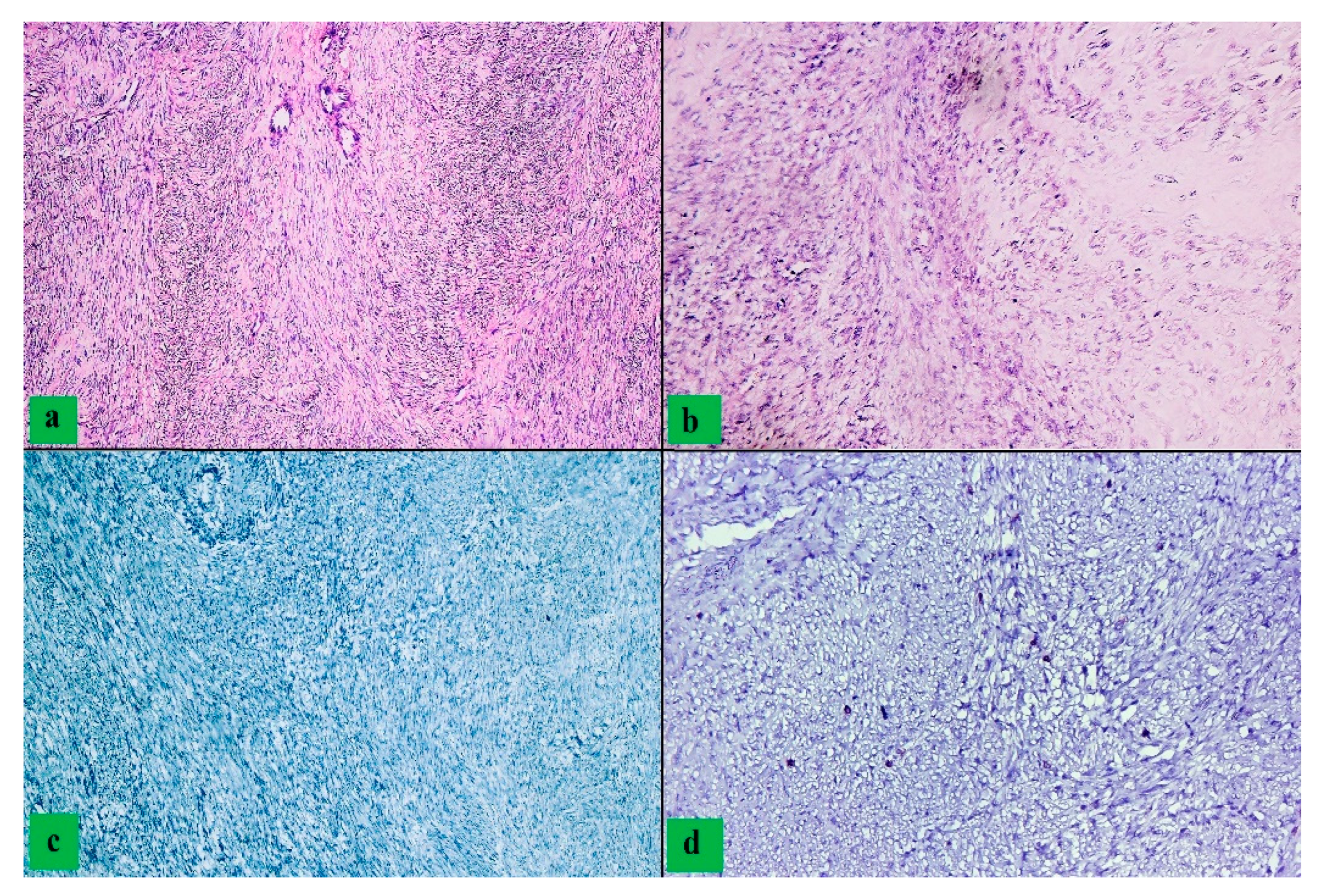
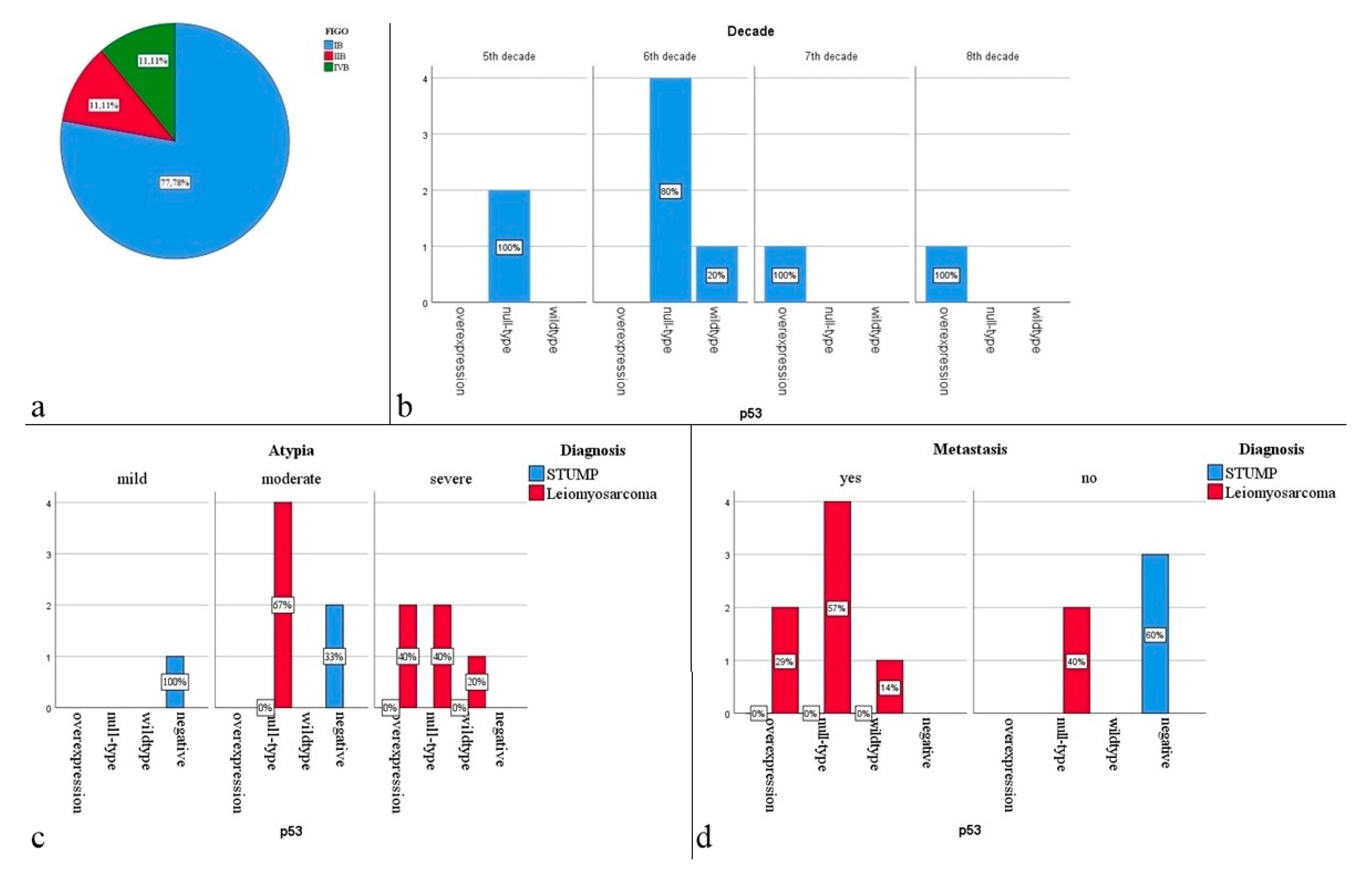
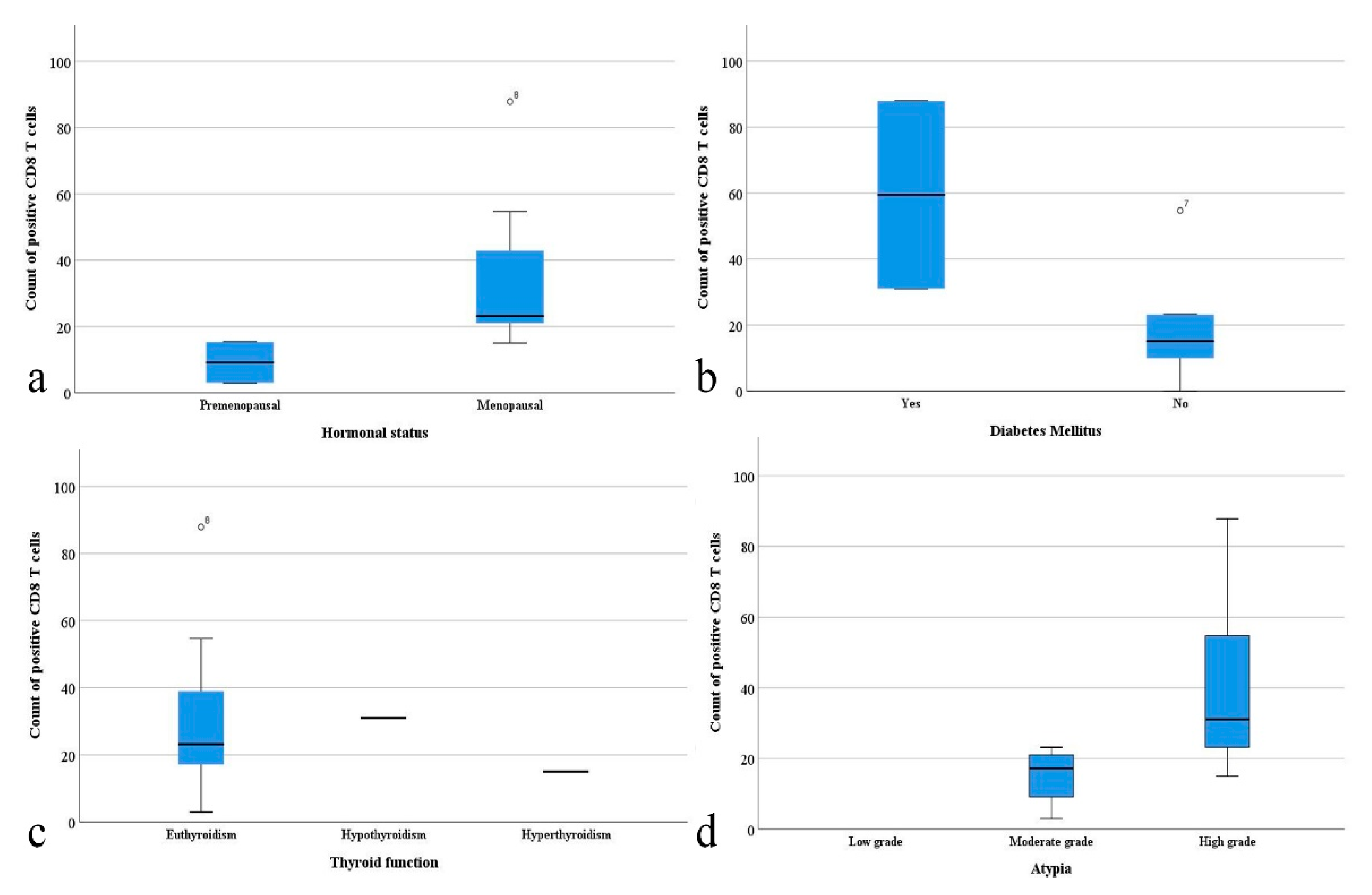
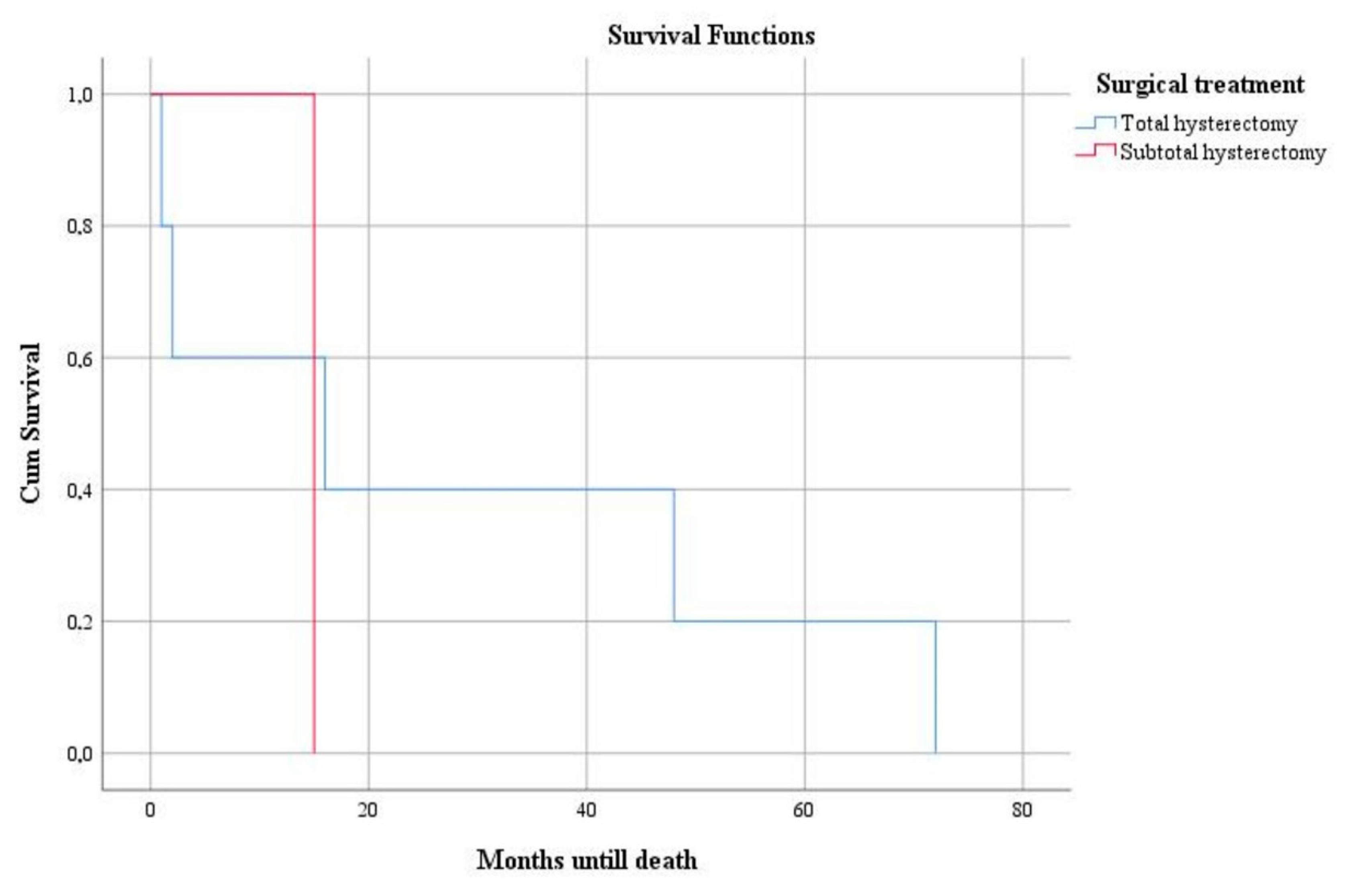
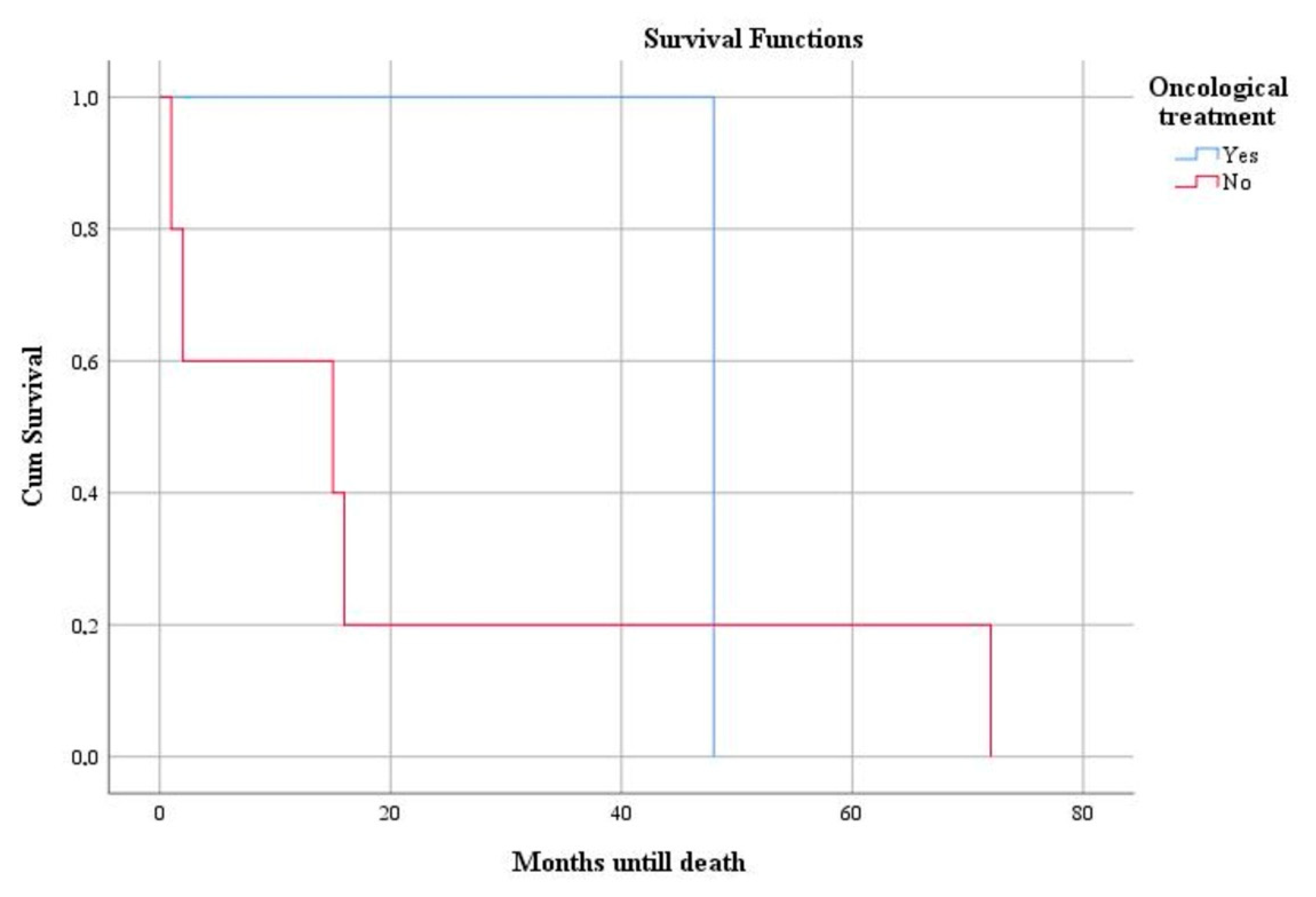
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Classification of Tumors Editorial Board. Female Genital Tumors, 5th ed.; IARC: Lyon, France, 2020; p. 247. [Google Scholar]
- Olga, T.; Lila, K.S.; Kounidas, G.; Maria, P.; Nikolaos, V. Uterine smooth muscle tumour of uncertain malignant potential and in vitro fertilization treatment in an infertile patient. SAGE Open Med. Case Rep. 2021, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Akad, F.; Filip, B.; Mocanu, V.; Akad, M.; Acatrinei, C.; Scripcariu, V. Rare Case of Smooth Muscle Tumor of Uncertain Malignant Potential—Clinical Case. Maedica 2021, 16, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, A.; Gizzo, S.; Musarò, A.; Quaranta, M.; Noventa, M.; Migliavacca, C.; Sozzi, G.; Monica, M.; Mautone, D.; Berretta, R. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): Pathology, follow-up and recurrence. Int. J. Clin. Exp. Pathol. 2014, 7, 8136–8142. [Google Scholar]
- Yordanov, A.D.; Tantchev, L.; Vasileva, P.; Strashilov, S.; Vasileva-Slaveva, M.; Konsoulova, A. Uterine smooth muscle tumours of uncertain malignant potential: Single-centre experience and review of the literature. Menopausal Rev. 2020, 19, 30–34. [Google Scholar] [CrossRef]
- Di Giuseppe, J.; Grelloni, C.; Giuliani, L.; Carpini, G.D.; Giannella, L.; Ciavattini, A. Recurrence of Uterine Smooth Muscle Tumor of Uncertain Malignant Potential: A Systematic Review of the Literature. Cancers 2022, 14, 2323. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Roex, A.; Parange, A. STUMP, a surprise finding in a large fibroid uterus in a 20-year-old woman. Int. J. Women Health 2018, 10, 211–214. [Google Scholar] [CrossRef]
- Kyriazoglou, A.; Liontos, M.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M. The systemic treatment of uterine leiomyosarcomas. Medicine 2021, 100, e25309. [Google Scholar] [CrossRef]
- Cui, R.R.; Wright, J.D.; Hou, J.Y. Uterine leiomyosarcoma: A review of recent advances in molecular biology, clinical management and outcome. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1028–1037. [Google Scholar] [CrossRef]
- Mäkinen, N.; Kämpjärvi, K.; Frizzell, N.; Bützow, R.; Vahteristo, P. Characterization of MED12, HMGA2, and FH alterations reveals molecular variability in uterine smooth muscle tumors. Mol. Cancer 2017, 16, 1–8. [Google Scholar] [CrossRef]
- Croce, S.S.; Ribeiro, A.A.; Brulard, C.C.; Noel, J.-C.; Amant, F.; Stoeckle, E.E.; Devouassoux-Shisheborah, M.M.; Floquet, A.A.; Arnould, L.L.; Guyon, F.F.; et al. Uterine smooth muscle tumor analysis by comparative genomic hybridization: A useful diagnostic tool in challenging lesions. Mod. Pathol. 2015, 28, 1001–1010. [Google Scholar] [CrossRef]
- Alpha, B.C.; Elhaoudani, J.; Yessoufou, M.; Chaara, H.; Melhouf, M.A. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): Management, follow up and prognosis. PAMJ Clin. Med. 2020, 3, 82. [Google Scholar] [CrossRef]
- Jang, T.-K.; Kwon, S.-H.; Cho, C.-H.; Lee, H.-W.; Shin, S.-J. Giant uterine mass with uterine smooth muscle tumor of uncertain malignant potential: A case report. Gynecol. Oncol. Rep. 2020, 34, 100663. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Aynardi, J.T.; Chu, C.S. Uterine leiomyosarcoma: A review of the literature and update on management options. Gynecol. Oncol. 2018, 151, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Stone, R.L.; Fader, A.N. Uterine leiomyosarcoma: Epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol. Oncol. 2017, 145, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Zhang, Z.; Jia, J.; Shan, B. Prevalence and occult rates of uterine leiomyosarcoma. Medicine 2020, 99, e21766. [Google Scholar] [CrossRef]
- Suh, D.S.; Kim, Y.H.; Yun, K.Y.; Lee, N.K.; Choi, K.U.; Kim, K.H.; Yoon, M.S. An unusual case of pedunculated subserosal leiomyosarcoma of the uterus mimicking ovarian carcinoma. J. Ovarian Res. 2016, 9, 2. [Google Scholar] [CrossRef]
- Santos, P.; Cunha, T.M. Uterine sarcomas: Clinical presentation and MRI features. Diagn. Interv. Radiol. 2015, 21, 4–9. [Google Scholar] [CrossRef]
- George, S.; Serrano, C.; Hensley, M.L.; Ray-Coquard, I. Soft Tissue and Uterine Leiomyosarcoma. J. Clin. Oncol. 2018, 36, 144–150. [Google Scholar] [CrossRef]
- Lim, D.; Alvarez, T.; Nucci, M.R.; Gilks, B.; Longacre, T.; Soslow, R.A.; Oliva, E. Interobserver Variability in the Interpretation of Tumor Cell Necrosis in Uterine Leiomyosarcoma. Am. J. Surg. Pathol. 2013, 37, 650–658. [Google Scholar] [CrossRef]
- Juhasz-Böss, I.; Gabriel, L.; Bohle, R.M.; Horn, L.C.; Solomayer, E.-F.; Breitbach, G.-P. Uterine Leiomyosarcoma. Oncol. Res. Treat. 2018, 41, 680–686. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, S.; Young, R.H. WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; IARC: Lyon, France, 2014; p. 307. [Google Scholar]
- Zivanovic, O.; Leitao, M.M.; Iasonos, A.; Jacks, L.M.; Zhou, Q.; Abu-Rustum, N.R.; Soslow, R.A.; Juretzka, M.M.; Chi, D.S.; Barakat, R.R.; et al. Stage-Specific Outcomes of Patients With Uterine Leiomyosarcoma: A Comparison of the International Federation of Gynecology and Obstetrics and American Joint Committee on Cancer Staging Systems. J. Clin. Oncol. 2009, 27, 2066–2072. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.-S.; Koh, E.; Pang, C.; Ong, W.-S.; Ngo, L.; Soh, L.-T.; Quek, R.; Chay, W.-Y.; Ho, T.-H.; Tay, S.-K.; et al. Uterine Leiomyosarcoma in Asian Patients: Validation of the Revised Federation of Gynecology and Obstetrics Staging System and Identification of Prognostic Classifiers. Oncology 2012, 17, 1286–1293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huo, L.; Wang, D.; Wang, W.; Cao, D.; Yang, J.; Wu, M.; Yang, J.; Xiang, Y. Oncologic and Reproductive Outcomes of Uterine Smooth Muscle Tumor of Uncertain Malignant Potential: A Single Center Retrospective Study of 67 Cases. Front. Oncol. 2020, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Nassif, E.; Auclin, E.; Bahleda, R.; Honoré, C.; Mir, O.; Dumont, S.; Mery, B.; Hodroj, K.; Brahmi, M.; Trédan, O.; et al. TP53 Mutation as a Prognostic and Predictive Marker in Sarcoma: Pooled Analysis of MOSCATO and ProfiLER Precision Medicine Trials. Cancers 2021, 13, 3362. [Google Scholar] [CrossRef]
- Choi, J.; Manzano, A.; Dong, W.; Bellone, S.; Bonazzoli, E.; Zammataro, L.; Yao, X.; Deshpande, A.; Zaidi, S.; Guglielmi, A.; et al. Integrated mutational landscape analysis of uterine leiomyosarcomas. Proc. Natl. Acad. Sci. USA 2021, 118, e2025182118. [Google Scholar] [CrossRef]
- Ning, C.; Zhang, L.; Zhao, C.; Chen, X.; Liu, X.; Gu, C. Clinical and reproductive outcomes of uterine smooth muscle tumor of uncertain malignant potential: A single-center retrospective study. J. Int. Med. Res. 2021, 49, 4. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Liu, X.-B.; Mao, Y.-Y.; Lin, M.-H. Smooth muscle tumor of uncertain malignant potential (STUMP): A clinicopathologic analysis of 26 cases. Int. J. Clin. Exp. Pathol. 2020, 13, 818–826. [Google Scholar]
- Şahin, H.; Karatas, F.; Coban, G.; Özen, Ö.; Erdem, Ö.; Onan, M.A.; Ayhan, A. Uterine smooth muscle tumor of uncertain malignant potential: Fertility and clinical outcomes. J. Gynecol. Oncol. 2019, 30, e54. [Google Scholar] [CrossRef]
- Ha, H.I.; Choi, M.C.; Heo, J.H.; Kim, K.A.; Jung, S.G.; Park, H.; Joo, W.D.; Song, S.H.; Kim, T.H.; Lee, C. A clinicopathologic review and obstetric outcome of uterine smooth muscle tumor of uncertain malignant potential (STUMP) in a single institution. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 1–5. [Google Scholar] [CrossRef]
- Powell, E.; Piwnica-Worms, D.; Piwnica-Worms, H. Contribution of p53 to Metastasis. Cancer Discov. 2014, 4, 405–414. [Google Scholar] [CrossRef]
- Baek, M.-H.; Park, J.-Y.; Park, Y.; Kim, K.-R.; Kim, D.-Y.; Suh, D.-S.; Kim, J.-H.; Kim, Y.-M.; Kim, Y.-T.; Nam, J.-H. The combination of histone deacetylase and p53 expressions and histological subtype has prognostic implication in uterine leiomyosarcoma. Jpn. J. Clin. Oncol. 2019, 49, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kanis, M.J.; Ubago, J.; Liu, D.; Scholtens, D.M.; Strohl, A.E.; Lurain, J.R.; Shahabi, S.; Kong, B.; Wei, J.-J. The selected biomarker analysis in 5 types of uterine smooth muscle tumors. Hum. Pathol. 2018, 76, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Cuppens, T.; Moisse, M.; Depreeuw, J.; Annibali, D.; Colas, E.; Gil-Moreno, A.; Huvila, J.; Carpén, O.; Zikán, M.; Matias-Guiu, X.; et al. Integrated genome analysis of uterine leiomyosarcoma to identify novel driver genes and targetable pathways. Int. J. Cancer 2017, 142, 1230–1243. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, N.; Aavikko, M.; Heikkinen, T.; Taipale, M.; Taipale, J.; Koivisto-Korander, R.; Bützow, R.; Vahteristo, P. Exome Sequencing of Uterine Leiomyosarcomas Identifies Frequent Mutations in TP53, ATRX, and MED12. PLoS Genet. 2016, 12, e1005850. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, H.; Yuan, L.-J.; Xiong, Y.; Huang, X.; Lin, J.-X.; Zheng, M. CD146 as an adverse prognostic factor in uterine sarcoma. Eur. J. Med. Res. 2015, 20, 67. [Google Scholar] [CrossRef]
- Zhang, Q.; Ubago, J.; Li, L.; Guo, H.; Liu, Y.; Qiang, W.; Kim, J.J.; Kong, B.; Wei, J.-J. Molecular analyses of 6 different types of uterine smooth muscle tumors: Emphasis in atypical leiomyoma. Cancer 2014, 120, 3165–3177. [Google Scholar] [CrossRef]
- Lin, B.; Du, L.; Li, H.; Zhu, X.; Cui, L.; Li, X. Tumor-infiltrating lymphocytes: Warriors fight against tumors powerfully. Biomed. Pharmacother. 2020, 132, 110873. [Google Scholar] [CrossRef]
- Patel, M.V.; Shen, Z.; Rodriguez-Garcia, M.; Usherwood, E.J.; Tafe, L.J.; Wira, C.R. Endometrial Cancer Suppresses CD8+ T Cell-Mediated Cytotoxicity in Postmenopausal Women. Front. Immunol. 2021, 12, 657326. [Google Scholar] [CrossRef]
- Asano, H.; Isoe, T.; Ito, Y.M.; Nishimoto, N.; Watanabe, Y.; Yokoshiki, S.; Watari, H. Status of the Current Treatment Options and Potential Future Targets in Uterine Leiomyosarcoma: A Review. Cancers 2022, 14, 1180. [Google Scholar] [CrossRef]
- Ip, P.P.C.; Tse, K.Y.; Tam, K.F. Uterine Smooth Muscle Tumors Other Than the Ordinary Leiomyomas and Leiomyosarcomas: A Review of Selected Variants With Emphasis on Recent Advances and Unusual Morphology That May Cause Concern for Malignancy. Adv. Anat. Pathol. 2010, 17, 91–112. [Google Scholar] [CrossRef]
- O’Neill, C.J.; McBride, H.; Connolly, L.; McCluggage, W.G. Uterine leiomyosarcomas are characterized by high p16, p53 and MIB1 expression in comparison with usual leiomyomas, leiomyoma variants and smooth muscle tumours of uncertain malignant potential. Histopathology 2007, 50, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Vilos, G.A.; Marks, J.; Ettler, H.C.; Vilos, A.G.; Prefontaine, M.; Abu-Rafea, B. Uterine Smooth Muscle Tumors of Uncertain Malignant Potential: Diagnostic Challenges and Therapeutic Dilemmas. Report of 2 Cases and Review of the Literature. J. Minim. Invasive Gynecol. 2012, 19, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Saponara, M.; De Leo, A.; Perrone, A.M.; DE Iaco, P.; Pantaleo, M.A.; Nannini, M. Recurrent Uterine Smooth-Muscle Tumors of Uncertain Malignant Potential (STUMP): State of The Art. Anticancer Res. 2020, 40, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
| Diagnosis | Author (Year) | Number of Cases | Average Age | Average of Maximum Diameter (cm) | p53 Positive Reaction | Myomectomy (%)/Hysterectomy (%) | Recurrences | Menopausal/Premenopausal |
|---|---|---|---|---|---|---|---|---|
| STUMP | Ning C et al. (2021) [28] | 16 | 45 | NS ** | 0% | 37.5%/62.5% | 6.3% (STUMP) | 25%/75% |
| Huo L et al. (2020) [25] | 26 */67 | 42 | 7 | 27% (26 cases) | 56.7%/43.3% | 15% (leiomyosarcoma, STUMP) | 8.9%/91.1% | |
| Zheng YY et al. (2020) [29] | 26 | 42.96 | 8.2 | 42.3% | 26.9%/73.1% | 23% (STUMP) | NS ** | |
| Yordanov AD et al. (2020) [5] | 14 | 45.4 | 7.5 | NE *** | 16.7%/85.7% | 0% | 7.1%/92.9% | |
| Şahin H et al. (2019) [30] | 57 | 42 | 6 | 0% | 47.3%/52.7% | 14% (leiomyosarcoma, STUMP) | NS ** | |
| Ha HI et al. (2018) [31] | 19 | 41 | 9.5 | NE *** | 52.6%/47.4% | 10.5% (leiomyosarcoma, STUMP) | NS ** | |
| Leiomyosarcoma | Baek MH et al. (2018) [33] | 42 | 47 | 7.8 | 38% | NS ** | 54.8% | 64.3%/35.7% |
| Zhang Q et al. (2018) [34] | 38 | 55.3 | 10.5 | 39% | 3%/97% | 57% | NS ** | |
| Cuppens T et al. (2017) [35] | 84 | 57 | 9.7 | 97% | NS ** | NS ** | NS ** | |
| Makinen N et al. (2016) [36] | 52 | 58.55 | 1.5–30 | 66% | NS ** | 53.8% | NS ** | |
| Zhou Y et al. (2015) [37] | 36 | NS ** | ~5 | 44.1% | 0%/100% | 50% | NS ** | |
| Zhang Q et al. (2014) [38] | 38 | 55.3 | 10.5 | 24% | 3%/97% | 60% | NS ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosoteanu, M.; Deacu, M.; Voda, R.I.; Orasanu, C.I.; Aschie, M.; Vlad, S.E.; Penciu, R.C.; Chirila, S.I. Five-Year Retrospective Study of Uterine STUMP and Leiomyosarcoma. Clin. Pract. 2022, 12, 897-907. https://doi.org/10.3390/clinpract12060094
Bosoteanu M, Deacu M, Voda RI, Orasanu CI, Aschie M, Vlad SE, Penciu RC, Chirila SI. Five-Year Retrospective Study of Uterine STUMP and Leiomyosarcoma. Clinics and Practice. 2022; 12(6):897-907. https://doi.org/10.3390/clinpract12060094
Chicago/Turabian StyleBosoteanu, Madalina, Mariana Deacu, Raluca Ioana Voda, Cristian Ionut Orasanu, Mariana Aschie, Sabina Elena Vlad, Roxana Cleopatra Penciu, and Sergiu Ioachim Chirila. 2022. "Five-Year Retrospective Study of Uterine STUMP and Leiomyosarcoma" Clinics and Practice 12, no. 6: 897-907. https://doi.org/10.3390/clinpract12060094
APA StyleBosoteanu, M., Deacu, M., Voda, R. I., Orasanu, C. I., Aschie, M., Vlad, S. E., Penciu, R. C., & Chirila, S. I. (2022). Five-Year Retrospective Study of Uterine STUMP and Leiomyosarcoma. Clinics and Practice, 12(6), 897-907. https://doi.org/10.3390/clinpract12060094







