The Antimicrobial and Anti-Biofilm Effects of Hypericum perforatum Oil on Common Pathogens of Periodontitis: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Disk Diffusion Method
2.3. Minimum Inhibitory Concentration (MIC)
2.4. Minimum Bactericidal Concentration (MBC)
2.5. The Ability of Biofilm Formation
2.6. Minimum Biofilm Inhibitory Concentration (MBIC)
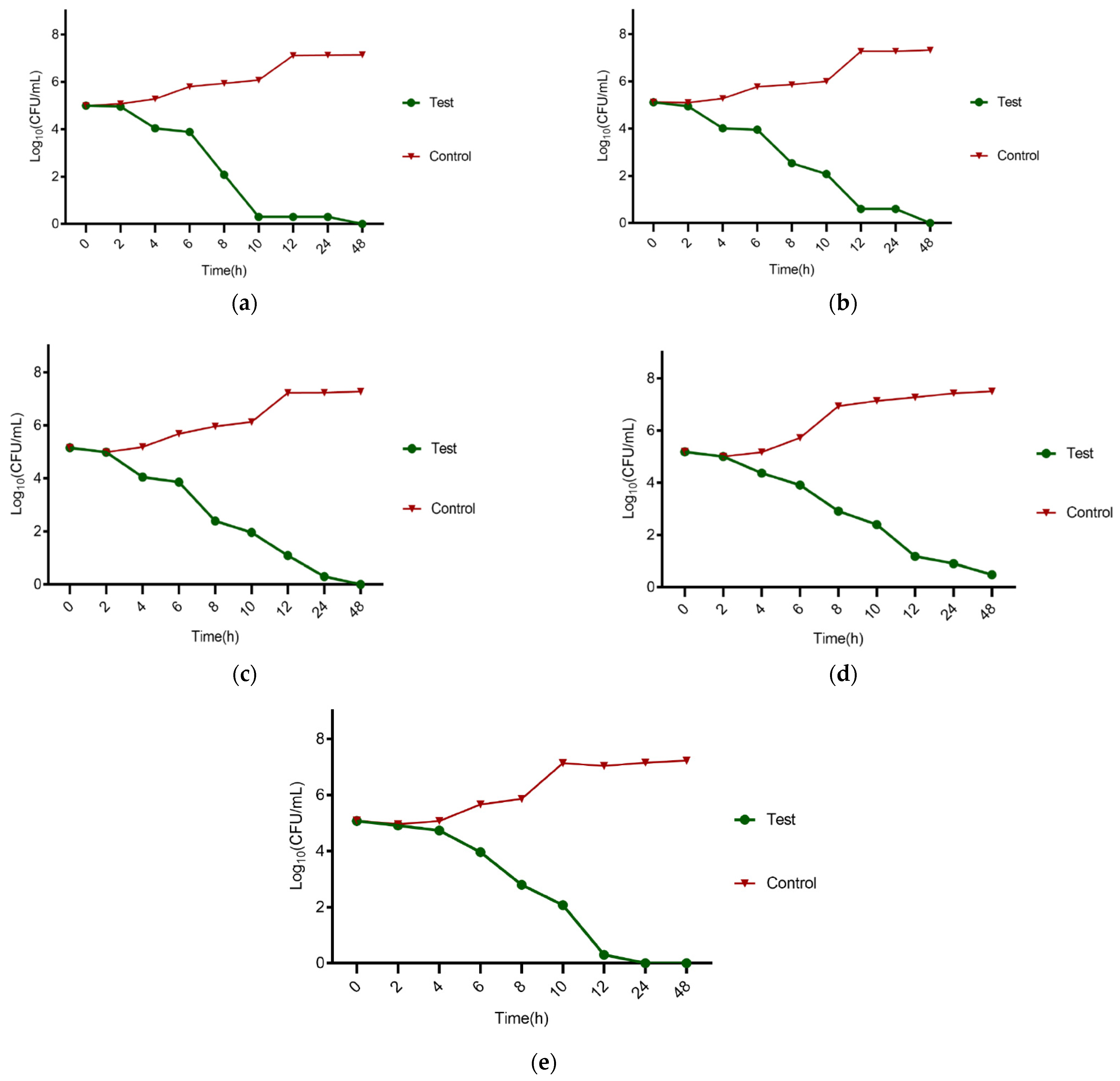
2.7. Time Kill Kinetics
2.8. Statistical Analysis
3. Results
3.1. Disk Diffusion
3.2. MIC Results
3.3. MBC Results
3.4. The Ability of Biofilm Formation Results
3.5. MBIC Results
3.6. Time Kill Kinetics
4. Discussion
5. The Strengths and Limitations
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Di, S.F.; Toti, P.; Brevi, B.; Martuscelli, R.; Sbordone, L.; Sbordone, C. Computed tomography evaluation of jaw atrophies before and after surgical bone augmentation. Int. J. Clin. Dent. 2019, 12, 259–270. [Google Scholar]
- Di, S.F.; Iacono, V.J.; Alfredo, I.; Alessandra, A.; Sbordone, L.; Lanza, A. Evidence-based recommendations on periodontal practice and the management of periodontal patients during and after the COVID-19 era: Challenging infectious diseases spread by airborne transmission. Open Dent. J. 2021, 15, 325–336. [Google Scholar]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Datta, H.; Ng, W.; Walker, J.; Tuck, S.; Varanasi, S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef]
- Bodet, C.; Chandad, F.; Grenier, D. Pathogenic potential of Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, the red bacterial complex associated with periodontitis. Pathologie-Biologie 2006, 55, 154–162. [Google Scholar] [CrossRef]
- Schmidt, J.; Jentsch, H.; Stingu, C.-S.; Sack, U. General immune status and oral microbiology in patients with different forms of periodontitis and healthy control subjects. PLoS ONE 2014, 9, e109187. [Google Scholar] [CrossRef]
- Casarin, R.; Del Peloso Ribeiro, É.; Mariano, F.; Nociti, F., Jr.; Casati, M.; Gonçalves, R. Levels of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, inflammatory cytokines and species-specific immunoglobulin G in generalized aggressive and chronic periodontitis. J. Periodontal Res. 2010, 45, 635–642. [Google Scholar] [CrossRef]
- Mahanonda, R.; Seymour, G.; Powell, L.; Good, M.; Halliday, J. Effect of initial treatment of chronic inflammatory periodontal disease on the frequency of peripheral blood T-lymphocytes specific to periodontopathic bacteria. Oral Microbiol. Immunol. 1991, 6, 221–227. [Google Scholar] [CrossRef]
- Rams, T.E.; Feik, D.; Mortensen, J.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic susceptibility of periodontal Enterococcus faecalis. J. Periodontol. 2013, 84, 1026–1033. [Google Scholar] [CrossRef]
- Souto, R.; Colombo, A.P.V. Prevalence of Enterococcus faecalis in subgingival biofilm and saliva of subjects with chronic periodontal infection. Arch. Oral Biol. 2008, 53, 155–160. [Google Scholar] [CrossRef]
- Balaei-Gajan, E.; Shirmohammadi, A.; Abashov, R.; Agazadeh, M.; Faramarzie, M. Detection of Enterococcus faecalis in subgingival biofilm of patients with chronic refractory periodontitis. Med. Oral Patol. Oral Cir. Bucal. 2010, 15, e667–e670. [Google Scholar] [CrossRef]
- Sun, J.; Sundsfjord, A.; Song, X. Enterococcus faecalis from patients with chronic periodontitis: Virulence and antimicrobial resistance traits and determinants. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Picozzi, S.C.; Casellato, S.; Rossini, M.; Paola, G.; Tejada, M.; Costa, E.; Carmignani, L. Extended-spectrum beta-lactamase-positive Escherichia coli causing complicated upper urinary tract infection: Urologist should act in time. Urol. Ann. 2014, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S. Preliminary studies on the inhibition potential of Indian domestic curd against coliforms, an emerging periodontal pathogen. J. Indian Soc. Periodontol. 2017, 21, 357. [Google Scholar]
- Barksby, H.; Nile, C.; Jaedicke, K.M.; Taylor, J.; Preshaw, P. Differential expression of immunoregulatory genes in monocytes in response to Porphyromonas gingivalis and Escherichia coli lipopolysaccharide. Clin. Exp. Immunol. 2009, 156, 479–487. [Google Scholar] [CrossRef]
- Nebel, D.; Arvidsson, J.; Lillqvist, J.; Holm, A.; Nilsson, B.-O. Differential effects of LPS from Escherichia coli and Porphyromonas gingivalis on IL-6 production in human periodontal ligament cells. Acta Odontol. Scand. 2013, 71, 892–898. [Google Scholar] [CrossRef]
- Damgaard, C.; Kantarci, A.; Holmstrup, P.; Hasturk, H.; Nielsen, C.H.; Van Dyke, T.E. Porphyromonas gingivalis-induced production of reactive oxygen species, tumor necrosis factor-α, interleukin-6, CXCL 8 and CCL 2 by neutrophils from localized aggressive periodontitis and healthy donors: Modulating actions of red blood cells and resolvin E1. J. Periodontal Res. 2017, 52, 246–254. [Google Scholar]
- Liu, R.; Desta, T.; Raptis, M.; Darveau, R.P.; Graves, D.T. P. gingivalis and E. coli lipopolysaccharides exhibit different systemic but similar local induction of inflammatory markers. J. Periodontol. 2008, 79, 1241–1247. [Google Scholar] [CrossRef]
- Pérez-Chaparro, P.J.; Gonçalves, C.; Figueiredo, L.C.; Faveri, M.; Lobão, E.; Tamashiro, N.; Duarte, P.; Feres, M. Newly identified pathogens associated with periodontitis: A systematic review. J. Dent. Res. 2014, 93, 846–858. [Google Scholar] [CrossRef]
- Dani, S.; Prabhu, A.; Chaitra, K.; Desai, N.; Patil, S.R.; Rajeev, R. Assessment of Streptococcus mutans in healthy versus gingivitis and chronic periodontitis: A clinico-microbiological study. Contemp. Clin. Dent. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Van der Reijden, W.; Dellemijn-Kippuw, N.; Stijne-van Nes, A.; De Soet, J.; Van Winkelhoff, A. Mutans streptococci in subgingival plaque of treated and untreated patients with periodontitis. J. Clin. Periodontol. 2001, 28, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Mazur, M.; Ndokaj, A.; Corridore, D.; La Torre, G.; Polimeni, A.; Ottolenghi, L. Periodontitis and the microbiome: A systematic review and meta-analysis. Minerva Stomatol. 2018, 67, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Contardo, M.; Díaz, N.; Lobos, O.; Padilla, C.; Giacaman, R. Oral colonization by Streptococcus mutans and its association with the severity of periodontal disease in adults. Rev. Clínica Periodoncia Implantol. Rehabil. Oral 2011, 4, 9–12. [Google Scholar] [CrossRef]
- Masalha, M.; Borovok, I.; Schreiber, R.; Aharonowitz, Y.; Cohen, G. Analysis of transcription of the Staphylococcus aureus aerobic class Ib and anaerobic class III ribonucleotide reductase genes in response to oxygen. J. Bacteriol. 2001, 183, 7260–7272. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, B.Z.; Albert-Kiszely, A.; Persson, G.R. Staphylococcus aureus and other bacteria in untreated periodontitis. J. Dent. Res. 2008, 87, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Uribe-García, A.; Paniagua-Contreras, G.L.; Monroy-Pérez, E.; Bustos-Martínez, J.; Hamdan-Partida, A.; Garzón, J.; Alanís, J.; Quezada, R.; Vaca-Paniagua, F.; Vaca, S. Frequency and expression of genes involved in adhesion and biofilm formation in Staphylococcus aureus strains isolated from periodontal lesions. J. Microbiol. Immunol. Infect. 2021, 54, 267–275. [Google Scholar] [CrossRef]
- Passariello, C.; Puttini, M.; Iebba, V.; Pera, P.; Gigola, P. Influence of oral conditions on colonization by highly toxigenic Staphylococcus aureus strains. Oral Dis. 2012, 18, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Passariello, C.; Lucchese, A.; Virga, A.; Pera, F.; Gigola, P. Isolation of Staphylococcus aureus and progression of periodontal lesions in aggressive periodontitis. Eur. J. Inflamm. 2012, 10, 501–513. [Google Scholar] [CrossRef]
- Zhuang, L.F.; Watt, R.M.; Mattheos, N.; Si, M.S.; Lai, H.C.; Lang, N.P. Periodontal and peri-implant microbiota in patients with healthy and inflamed periodontal and peri-implant tissues. Clin. Oral Implant. Res. 2016, 27, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kamma, J.; Nakou, M.; Gmür, R.; Baehni, P. Microbiological profile of early onset/aggressive periodontitis patients. Oral Microbiol. Immunol. 2004, 19, 314–321. [Google Scholar] [CrossRef]
- Cobb, C.M. Non-surgical pocket therapy: Mechanical. Ann. Periodontol. 1996, 1, 443–490. [Google Scholar] [CrossRef]
- Slots, J.; Rams, T.E. Antibiotics in periodontal therapy: Advantages and disadvantages. J. Clin. Periodontol. 1990, 17, 479–493. [Google Scholar] [CrossRef]
- Gillies, M.; Ranakusuma, A.; Hoffmann, T.; Thorning, S.; McGuire, T.; Glasziou, P.; Del Mar, C. Common harms from amoxicillin: A systematic review and meta-analysis of randomized placebo-controlled trials for any indication. Cmaj 2015, 187, E21–E31. [Google Scholar] [CrossRef]
- Kumar, P.; Ansari, S.H.; Ali, J. Herbal remedies for the treatment of periodontal disease-a patent review. Recent Pat. Drug Deliv. Formul. 2009, 3, 221–228. [Google Scholar]
- Di Paola, R.; Mazzon, E.; Muià, C.; Crisafulli, C.; Genovese, T.; Di Bella, P.; Esposito, E.; Menegazzi, M.; Meli, R.; Suzuki, H.; et al. Protective effect of Hypericum perforatum in zymosan-induced multiple organ dysfunction syndrome: Relationship to its inhibitory effect on nitric oxide production and its peroxynitrite scavenging activity. Nitric Oxide 2007, 16, 118–130. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef]
- CLSI. Methods of Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline, M26, V. 19, No. 18; National Committee for Clinical Laboratory Standards Institute: Wayne, PA, USA, 1999. [Google Scholar]
- Kerr, J.E.; Abramian, J.R.; Dao, D.H.; Rigney, T.W.; Fritz, J.; Pham, T.; Gay, I.; Parthasarathy, K.; Wang, B.Y.; Zhang, W.; et al. Genetic exchange of fimbrial alleles exemplifies the adaptive virulence strategy of Porphyromonas gingivalis. PLoS ONE 2014, 9, e91696. [Google Scholar] [CrossRef][Green Version]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Thurnheer, T.; Belibasakis, G.N. Integration of non-oral bacteria into in vitro oral biofilms. Virulence 2015, 6, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, S.K.; Zenouz, A.T.; Aghazadeh, M.; Kafil, H.S. Strong antimicrobial activity of Hypericum perforatum L. against oral isolates of Lactobacillus spp. Cell. Mol. Biol. 2017, 63, 58–62. [Google Scholar] [CrossRef]
- Schempp, C.M.; Pelz, K.; Wittmer, A.; Schöpf, E.; Simon, J.C. Antibacterial activity of hyperforin from St John’s wort, against multiresistant Staphylococcus aureus and gram-positive bacteria. Lancet 1999, 353, 2129. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Briguglio, E.; Mazzon, E.; Galuppo, M.; Oteri, G.; Cordasco, G.; Cuzzocrea, S. Effects of Hypericum perforatum, in a rodent model of periodontitis. BMC Complement. Altern. Med. 2010, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Tanideh, N.; Ghafari, V.; Ebrahimi, R.; Habibagahi, R.; Koohi-Hosseinabadi, O.; Iraji, A. Effects of Calendula officinalis and Hypericum perforatum on antioxidant, anti-inflammatory, and histopathology indices of induced periodontitis in male rats. J. Dent. 2020, 21, 314. [Google Scholar]
- Yeşilada, E.; Gürbüz, İ.; Shibata, H. Screening of Turkish anti-ulcerogenic folk remedies for anti-Helicobacter pylori activity. J. Ethnopharmacol. 1999, 66, 289–293. [Google Scholar] [CrossRef]
- Vollmer, A.; Al-Ahmad, A.; Argyropoulou, A.; Thurnheer, T.; Hellwig, E.; Attin, T.; Vach, K.; Wittmer, A.; Ferguson, K.; Skaltsounis, A.L.; et al. Antimicrobial photoinactivation using visible light plus water-filtered infrared-a (vis + wira) and Hypericum perforatum modifies in situ oral biofilms. Sci. Rep. 2019, 9, 20325. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Oyardı, O.; Akkol, E.K.; Ozçelik, B. Antimicrobial effect of the extracts from Hypericum perforatum against oral bacteria and biofilm formation. Pharm. Biol. 2016, 54, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Alahmad, A.; Alghoraibi, I.; Zein, R.; Kraft, S.; Dra, G.; Walter, J. Identification of Major Constituents of Hypericum perforatum L. Extracts in Syria by Development of a Rapid, Simple, and Reproducible HPLC-ESI-Q-TOF MS Analysis and Their Antioxidant Activities. ACS Omega 2022, 7, 13475–13493. [Google Scholar] [CrossRef] [PubMed]
- Lasik, M.; Nowak, J.; Stachowiak, B.; Czarnecki, Z. Evaluation of the antagonistic properties of natural antibacterial substances extracted from herbs: Poster presentation. Eurobiotech 2007, 54, 10. [Google Scholar]
- Delcanale, P.; Hally, C.; Nonell, S.; Bonardi, S.; Viappiani, C.; Abbruzzetti, S. Photodynamic action of Hypericum perforatum hydrophilic extract against Staphylococcus aureus. Photochem. Photobiol. Sci. 2020, 19, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Taherikalani, M.; Khaksarian, M.; Soroush, S.; Ashrafi, B.; Heydari, R. Phytochemical profiles and antibacterial activities of hydroalcoholic extracts of Origanum vulgare and Hypericum perforatum and Carvacrol and Hypericin as a Promising Anti-Staphylococcus aureus. Mini Rev. Med. Chem. 2019, 19, 923–932. [Google Scholar] [CrossRef]
- Arpag, O.F.; Duran, N.; Açikgül, F.C.; Türkmen, M. Comparison of Minimum Inhibitory Concentrations of Hypericum perforatum, L. Essential Oils, 0.2% Chlorhexidine and 10% Povidone-iodine Over Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis. J. Essent. Oil Bear. Plants 2020, 23, 1192–1205. [Google Scholar] [CrossRef]
- Nawchoo, I.A.; Yousuf, M.; Aslam, K.; Wani, B.; Dar, N. In vitro antibacterial activity and phytochemical studies of methanolic extract of leaves of Hypericum perforatum L. growing wild in Kashmir Himalaya. Asian J. Plant Sci. Res. 2012, 2, 414–420. [Google Scholar]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–490. [Google Scholar] [CrossRef]
| Organism * | Non-Growth Zone Diameters (mm) | MIC (µg/mL) | MBC (µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| Pure Oil | 50% Oil | Antibiotic | H. perforatum Oil | Antibiotic | H. perforatum Oil | Antibiotic | |
| E. faecalis | 15.67 ± 1.53 | 15 ± 1 | 16.33 ± 0.58 | 42.67 ± 18.48 | 21.33 ± 9.24 | 149.33 ± 97.76 | 64 ± 0 |
| S. aureus | 14.67 ± 1.15 | 13.67 ± 1.53 | 18.33 ± 0.58 | 85.33 ± 36.95 | 64 ± 0 | 213.33 ± 73.9 | 106.67 ± 36.95 |
| S. mutans | 15.67 ± 1.53 | 13.67 ± 0.58 | 24.33 ± 0.58 | 106.67 ± 36.95 | 37.33 ± 24.44 | 213.33 ± 73.9 | 53.33 ± 18.48 |
| E. coli | 19.67 ± 1.53 | 17.33 ± 0.58 | 19.67 ± 1.15 | 213.33 ± 73.9 | 170.67 ± 73.9 | 512 ± 0 | 213.33 ± 73.9 |
| P. gingivalis | 15 ± 1 | 11.33 ± 0.58 | 21.67 ± 0.58 | 53.33 ± 18.48 | 64 ± 0 | 256 ± 221.7 | 85.33 ± 36.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagheri, R.; Bohlouli, S.; Maleki Dizaj, S.; Shahi, S.; Memar, M.Y.; Salatin, S. The Antimicrobial and Anti-Biofilm Effects of Hypericum perforatum Oil on Common Pathogens of Periodontitis: An In Vitro Study. Clin. Pract. 2022, 12, 1009-1019. https://doi.org/10.3390/clinpract12060104
Bagheri R, Bohlouli S, Maleki Dizaj S, Shahi S, Memar MY, Salatin S. The Antimicrobial and Anti-Biofilm Effects of Hypericum perforatum Oil on Common Pathogens of Periodontitis: An In Vitro Study. Clinics and Practice. 2022; 12(6):1009-1019. https://doi.org/10.3390/clinpract12060104
Chicago/Turabian StyleBagheri, Reza, Sepideh Bohlouli, Solmaz Maleki Dizaj, Shahriar Shahi, Mohammad Yousef Memar, and Sara Salatin. 2022. "The Antimicrobial and Anti-Biofilm Effects of Hypericum perforatum Oil on Common Pathogens of Periodontitis: An In Vitro Study" Clinics and Practice 12, no. 6: 1009-1019. https://doi.org/10.3390/clinpract12060104
APA StyleBagheri, R., Bohlouli, S., Maleki Dizaj, S., Shahi, S., Memar, M. Y., & Salatin, S. (2022). The Antimicrobial and Anti-Biofilm Effects of Hypericum perforatum Oil on Common Pathogens of Periodontitis: An In Vitro Study. Clinics and Practice, 12(6), 1009-1019. https://doi.org/10.3390/clinpract12060104






