Integrated Monitoring of Water Quality, Metal Ions, and Antibiotic Residues, with Isolation and Optimization of Enrofloxacin-Degrading Bacteria in American Shad (Alosa sapidissima) Aquaculture Systems
Abstract
1. Introduction
2. Materials and Methods
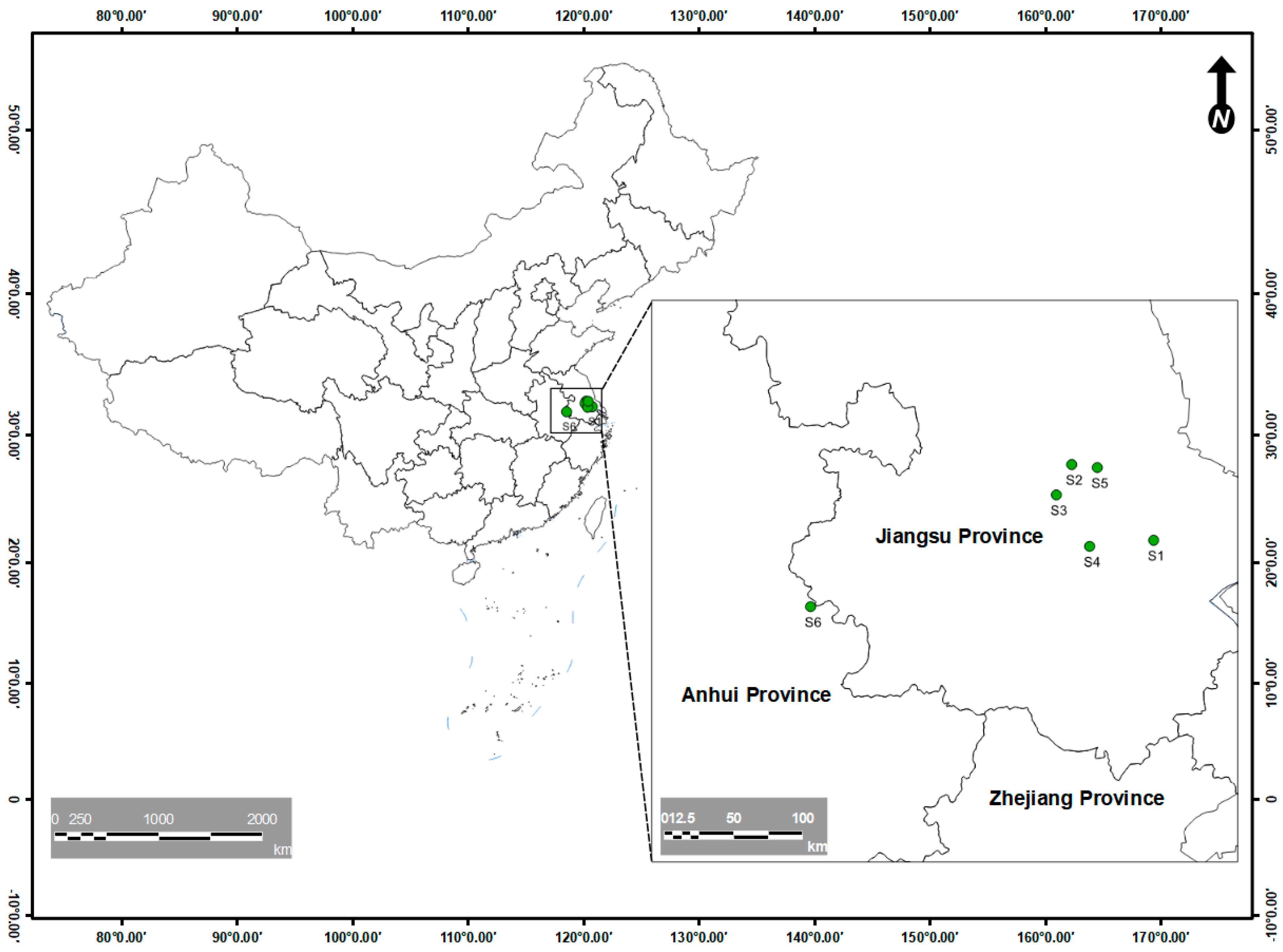
2.1. Sample Collection and Experimental Setup
2.2. Water Quality and Metal Ion Determination for Each Sample Point
2.3. Enrofloxacin and Ciprofloxacin Determination
2.4. Screening of ENR Degradation Bacteria
2.5. Optimization Conditions for ENR Degradation
2.6. Data Statistical Analysis
3. Results
3.1. Water Quality, Heavy Metals and Metal Ions
3.2. Enrofloxacin and Ciprofloxacin
| Samples | TN, <6 mg·L−1 | TP, <0.8 mg·L−1 | NH3-N, <0.2 mg·L−1 | CODMn, <25 mg·L−1 | TSS, <100 mg·L−1 | pH, 6~9 |
|---|---|---|---|---|---|---|
| S1 (pond water from the river) | 3.58 ± 0.28 b | 0.21 ± 0.10 b | 0.92 ± 0.02 c | 9.84 ± 1.60 ab | 103.80 ± 23.80 b | 7.72 ± 0.30 a |
| S2 (adult fish pond) | 3.88 ± 0.20 b | 0.21 ± 0.01 b | 1.52 ± 0.02 b | 12.12 ± 2.30 a | 129.67 ± 29.10 ab | 7.70 ± 0.30 a |
| S2 (juvenile fish pond) | 2.88 ± 0.10 c | 0.22 ± 0.01 b | 1.71 ± 0.01 a | 9.60 ± 1.70 b | 196.67 ± 32.40 a | 7.82 ± 0.40 a |
| S3 (well water) | 1.22 ± 0.01 d | 0.05 ± 0.01 d | 0.22 ± 0.01 f | 6.47 ± 1.30 b | 118.00 ± 21.30 b | 7.51 ± 0.20 a |
| S3 (greenhouse pond) | 2.93 ± 0.13 c | 0.25 ± 0.04 b | 0.35 ± 0.01 e | 7.28 ± 1.40 b | 146.00 ± 32.00 ab | 8.14 ± 0.10 a |
| S4 (well water) | 3.71 ± 0.14 b | 0.30 ± 0.01 b | 0.50 ± 0.01 d | 9.81 ± 1.90 ab | 134.00 ± 31.20 ab | 7.34 ± 0.40 a |
| S4 (pond water) | 13.30 ± 2.00 a | 0.80 ± 0.02 a | 0.60 ± 0.02 d | 10.00 ± 1.80 ab | 142.00 ± 26.10 ab | 8.10 ± 0.10 a |
| S5 (well water) | 2.51 ± 0.11 c | 0.11 ± 0.01 c | 0.35 ± 0.01 e | 10.76 ± 2.10 ab | 112.67 ± 22.00 b | 7.92 ± 0.30 a |
| S5 (pond water) | 4.41 ± 0.29 b | 0.19 ± 0.01 b | 1.79 ± 0.01 a | 10.13 ± 2.30 ab | 138.00 ± 19.80 ab | 8.09 ± 0.20 a |
| Medium | Sampling Points | Mn (μg·L−1) | Fe (μg·L−1) | Co (μg·L−1) | Ni (μg·L−1) <50 μg·L−1 | As (μg·L−1) <50 μg·L−1 | Se (μg·L−1) | Sr (μg·L−1) | Cd (μg·L−1), <5 μg·L−1 | Ba (μg·L−1) | Pb (μg·L−1) <50 μg·L−1 | Cu (μg·L−1) <10 μg·L−1 | Zn (mg·L−1) <0.1 mg·L−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| water | S1 | ND d | ND c | 0.13 ± 0.03 b | 1.84 ± 0.64 b | 5.05 ± 1.27 b | 0.98 ± 0.07 a | 333.46 ± 57.68 c | ND b | 10.41 ± 1.00 b | ND d | 4.61 ± 1.43 b | 0.06 ± 0.00 a |
| S2 | ND d | ND c | 0.06 ± 0.02 c | 2.86 ± 0.25 a | 1.07 ± 0.13 d | 1.00 ± 0.05 a | 318.95 ± 70.94 c | ND b | 8.16 ± 0.47 b | ND d | 1.25 ± 0.15 d | 0.07 ± 0.00 a | |
| S3 | 0.56 ± 0.08 b | 1.07 ± 0.44 a | 0.13 ± 0.02 b | 2.31 ± 0.26 a | 4.87 ± 1.41 b | 1.06 ± 0.15 a | 562.85 ± 28.63 a | ND b | 3.65 ± 0.91 c | 5.53 ± 0.35 b | 7.77 ± 0.79 a | 0.07 ± 0.00 a | |
| S4 | 5.00 ± 0.09 a | ND c | 0.19 ± 0.11 a | 2.44 ± 0.17 a | 3.77 ± 1.09 c | 1.06 ± 0.13 a | 410.08 ± 16.61 b | ND b | 17.47 ± 1.09 a | 0.27 ± 0.11 c | 3.83 ± 0.18 b | 0.06 ± 0.00 a | |
| S5 | 0.05 ± 0.01 c | 0.54 ± 0.12 b | 0.11 ± 0.05 b | 0.38 ± 0.12 c | 9.82 ± 0.09 a | 1.38 ± 0.11 a | 171.20 ± 7.03 d | ND b | 5.06 ± 0.16 c | 19.98 ± 1.74 a | 2.05 ± 0.28 c | 0.07 ± 0.00 a | |
| S6 | ND d | ND c | 0.14 ± 0.06 b | 0.57 ± 0.04 c | 3.47 ± 0.13 c | 1.17 ± 0.14 a | 455.72 ± 12.04 b | 0.05 ± 0.01 a | 18.23 ± 2.01 a | 0.44 ± 0.02 c | 1.30 ± 0.12 d | 0.07 ± 0.00 a | |
| Mn (mg·kg−1) | Fe (mg·kg−1) | Ag (mg·kg−1) | Ni (mg·kg−1) | As (mg·kg−1) | Se (mg·kg−1) | Cr (mg·kg−1) | Cd (mg·kg−1) | Hg (mg·kg−1) | Pb (mg·kg−1) | Cu (mg·kg−1) | Zn (mg·kg−1) | ||
| sediment | S1 | 11.97 ± 1.31 c | 525.52 ± 18.21 b | 0.01 ± 0.00 a | 5.73 ± 0.42 b | 1.69 ± 0.13 b | 0.20 ± 0.04 a | 9.25 ± 1.18 b | 0.02 ± 0.01 a | 0.13 ± 0.03 a | 2.93 ± 0.53 b | 3.63 ± 0.79 c | 0.02 ± 0.00 b |
| S2 | 12.70 ± 1.83 c | 450.47 ± 29.36 b | 0.00 ± 0.00 a | 5.16 ± 1.06 b | 1.13 ± 0.26 b | 0.20 ± 0.06 a | 7.80 ± 1.75 b | 0.02 ± 0.01 a | ND b | 2.50 ± 0.73 b | 3.82 ± 1.08 c | 0.02 ± 0.00 b | |
| S3 | 18.49 ± 1.19 b | 539.85 ± 49.90 b | 0.01 ± 0.00 a | 6.36 ± 0.92 b | 1.83 ± 0.16 b | 0.22 ± 0.03 a | 9.61 ± 1.09 b | 0.09 ± 0.01 a | ND b | 3.05 ± 1.01 b | 4.90 ± 0.98 b | 0.04 ± 0.02 ab | |
| S4 | - | - | - | - | - | - | - | - | - | - | - | - | |
| S5 | 29.94 ± 4.43 a | 762.26 ± 52.53 a | 0.03 ± 0.00 a | 10.18 ± 0.75 a | 6.27 ± 0.57 a | 0.29 ± 0.02 a | 12.72 ± 0.87 a | 0.23 ± 0.01 a | ND b | 10.92 ± 0.73 a | 10.49 ± 0.60 a | 0.06 ± 0.00 a | |
| S6 | 14.47 ± 3.39 c | 500.96 ± 43.43 b | 0.00 ± 0.00 a | 5.70 ± 0.49 b | 1.39 ± 0.12 b | 0.17 ± 0.02 a | 8.42 ± 0.64 b | 0.02 ± 0.01 a | ND b | 2.51 ± 0.38 b | 3.85 ± 0.72 c | 0.02 ± 0.00 b |
| Medium | Sampling Points | Li (μg·L−1) | Na (μg·L−1) | Mg (μg·L−1) | Al (μg·L−1) | K (μg·L−1) | Ca (μg·L−1) |
|---|---|---|---|---|---|---|---|
| water | S1 | 17.07 ± 4.47 b | 14329.97 ± 398.18 a | 1550.47 ± 38.77 c | 0.45 ± 0.12 b | 684.50 ± 18.69 a | 5432.18 ± 105.42 b |
| S2 | 8.71 ± 2.10 c | 6068.23 ± 188.74 c | 2692.39 ± 76.86 b | 0.32 ± 0.05 c | 273.58 ± 89.73 e | 7150.73 ± 179.04 a | |
| S3 | 136.51 ± 10.55 a | 17753.26 ± 99.33 a | 2542.42 ± 67.51 b | 0.45 ± 0.11 b | 236.39 ± 12.22 e | 3994.18 ± 153.03 c | |
| S4 | 19.04 ± 4.33 b | 9443.96 ± 212.33 b | 3740.67 ± 70.59 a | 0.44 ± 0.14 b | 432.94 ± 34.99 c | 6754.77 ± 346.13 a | |
| S5 | 18.62 ± 2.52 b | 2556.60 ± 37.56 d | 1419.67 ± 67.81 c | 0.83 ± 0.14 a | 397.91 ± 15.73 d | 2261.61 ± 57.68 d | |
| S6 | 10.80 ± 4.63 c | 2989.24 ± 132.74 d | 1647.38 ± 72.40 c | 0.55 ± 0.14 b | 566.81 ± 27.38 b | 7016.82 ± 125.21 a | |
| Na (mg·kg−1) | Mg (mg·kg−1) | K (mg·kg−1) | Ca (mg·kg−1) | ||||
| sediment | S1 | 5.86 ± 0.67 b | 88.72 ± 8.22 bc | 39.56 ± 4.52 c | 170.29 ± 18.27 d | ||
| S2 | 2.77 ± 0.82 c | 65.49 ± 13.51 c | 39.62 ± 4.14 c | 168.85 ± 11.68 d | |||
| S3 | 8.31 ± 0.50 a | 97.80 ± 15.85 b | 54.41 ± 6.97 ab | 306.25 ± 20.26 b | |||
| S4 | - | - | - | - | |||
| S5 | 4.22 ± 0.31 b | 294.87 ± 15.23 a | 69.29 ± 5.84 a | 693.51 ± 27.29 a | |||
| S6 | 2.82 ± 0.26 c | 88.04 ± 9.76 bc | 47.05 ± 3.66 b | 236.55 ± 12.16 c |
| Water | ENR Avg. | ENR in Spring | ENR in Summer | ENR In Autumn | ENR in Winter | CIP Avg. | CIP in Spring | CIP in Summer | CIP in Autumn | CIP in Winter |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 32.37 ± 1.90 d | 29.63 ± 1.59 d | 19.57 ± 1.07 d | 11.2 ± 1.03 d | 54.97 ± 6.26 d | 5.98 ± 0.85 c | 5.47 ± 1.11 c | 4.30 ± 0.45 d | 2.00 ± 0.12 e | 9.50 ± 0.26 d |
| S2 | 16.68 ± 1.39 e | 13.95 ± 2.47 e | 19.20 ± 0.88 d | 27.7 ± 0.89 c | 8.63 ± 0.40 f | 3.81 ± 0.15 d | 3.95 ± 0.16 d | 4.57 ± 0.19 d | 5.10 ± 0.18 d | 2.10 ± 0.35 f |
| S3 | 1532.10 ± 149.61 b | 40.10 ± 1.59 c | 744.20 ± 21.54 b | 3486.60 ± 43.29 b | 1875.5 ± 26.47 b | 401.20 ± 31.76 a | 6.00 ± 0.51 c | 313.30 ± 30.56 b | 537.6 ± 60.20 b | 747.70 ± 65.32 b |
| S4 | 80.33 ± 4.96 c | 100.05 ± 10.04 b | 96.63 ± 4.78 c | 7.23 ± 0.41 e | 117.53 ± 17.26 c | 29.25 ± 9.14 b | 31.31 ± 1.34 b | 34.64 ± 1.28 c | 15.86 ± 2.67 c | 35.31 ± 3.11 c |
| S5 | 18.63 ± 1.26 e | 18.96 ± 2.34 e | 9.56 ± 1.27 e | 9.74 ± 1.58 d | 36.45 ± 2.41 e | 3.90 ± 0.17 d | 3.85 ± 0.21 d | 2.71 ± 0.13 e | 2.75 ± 0.34 e | 6.48 ± 0.39 e |
| S6 | 3215.95 ± 153.10 a | 1552.30 ± 68.92 a | 2473.71 ± 150.87 a | 6268.39 ± 142.35 a | 3239.98 ± 116.23 a | 459.60 ± 20.12 a | 185.42 ± 10.34 a | 386.55 ± 17.04 a | 894.83 ± 64.38 a | 455.88 ± 49.95 a |
| Sediment | ENR Avg. | ENR in Spring | ENR in Summer | ENR in Autumn | ENR in Winter | CIP Avg. | CIP in Spring | CIP in Summer | CIP in Autumn | CIP in Winter |
| S1 | 41.43 ± 1.23 c | 28.17 ± 2.47 e | 48.57 ± 3.67 b | 25.52 ± 3.10 d | 57.12 ± 4.06 c | 21.86 ± 1.74 a | 15.12 ± 1.36 b | 18.21 ± 2.08 b | 30.25 ± 1.39 b | 21.78 ± 1.45 a |
| S2 | 65.67 ± 3.69 b | 47.23 ± 2.56 d | 24.36 ± 1.09 c | 80.56 ± 4.11 c | 74.14 ± 5.21 b | 20.56 ± 1.72 a | 14.14 ± 2.00 b | 35.10 ± 3.14 a | 17.02 ± 1.77 cd | 21.36 ± 1.05 a |
| S3 | 70.14 ± 4.91 b | 72.14 ± 5.68 c | 124.15 ± 6.23 a | 20.15 ± 1.36 d | 65.53 ± 2.14 bc | 23.71 ± 1.04 a | 37.12 ± 2.18 a | 20.53 ± 2.74 b | 21.52 ± 2.36 c | 22.52 ± 1.36 a |
| S4 | - | - | - | - | - | - | - | - | - | - |
| S5 | 133.67 ± 6.14 a | 227.25 ± 9.22 a | 121.36 ± 7.23 a | 184.12 ± 10.14 a | 74.53 ± 4.99 b | 23.67 ± 1.29 a | 33.12 ± 1.42 a | 15.52 ± 2.18 c | 46.23 ± 2.45 a | 16.12 ± 1.38 b |
| S6 | 125.36 ± 7.44 a | 98.26 ± 4.21 b | 120.36 ± 4.77 a | 130.69 ± 7.78 b | 94.78 ± 8.41 a | 12.36 ± 1.34 b | 9.58 ± 0.75 c | 10.33 ± 1.23 d | 14.77 ± 1.06 d | 13.05 ± 1.06 c |
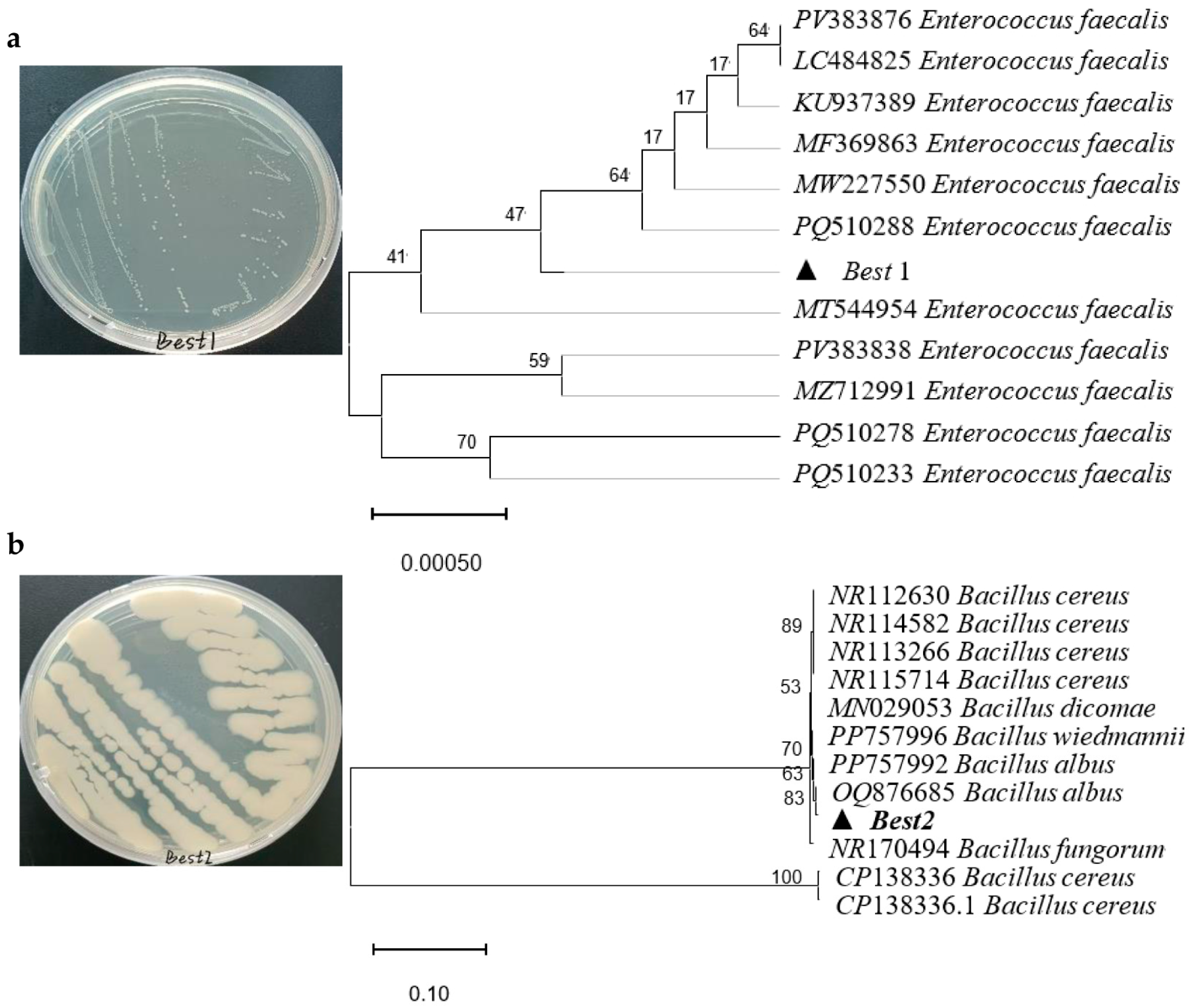
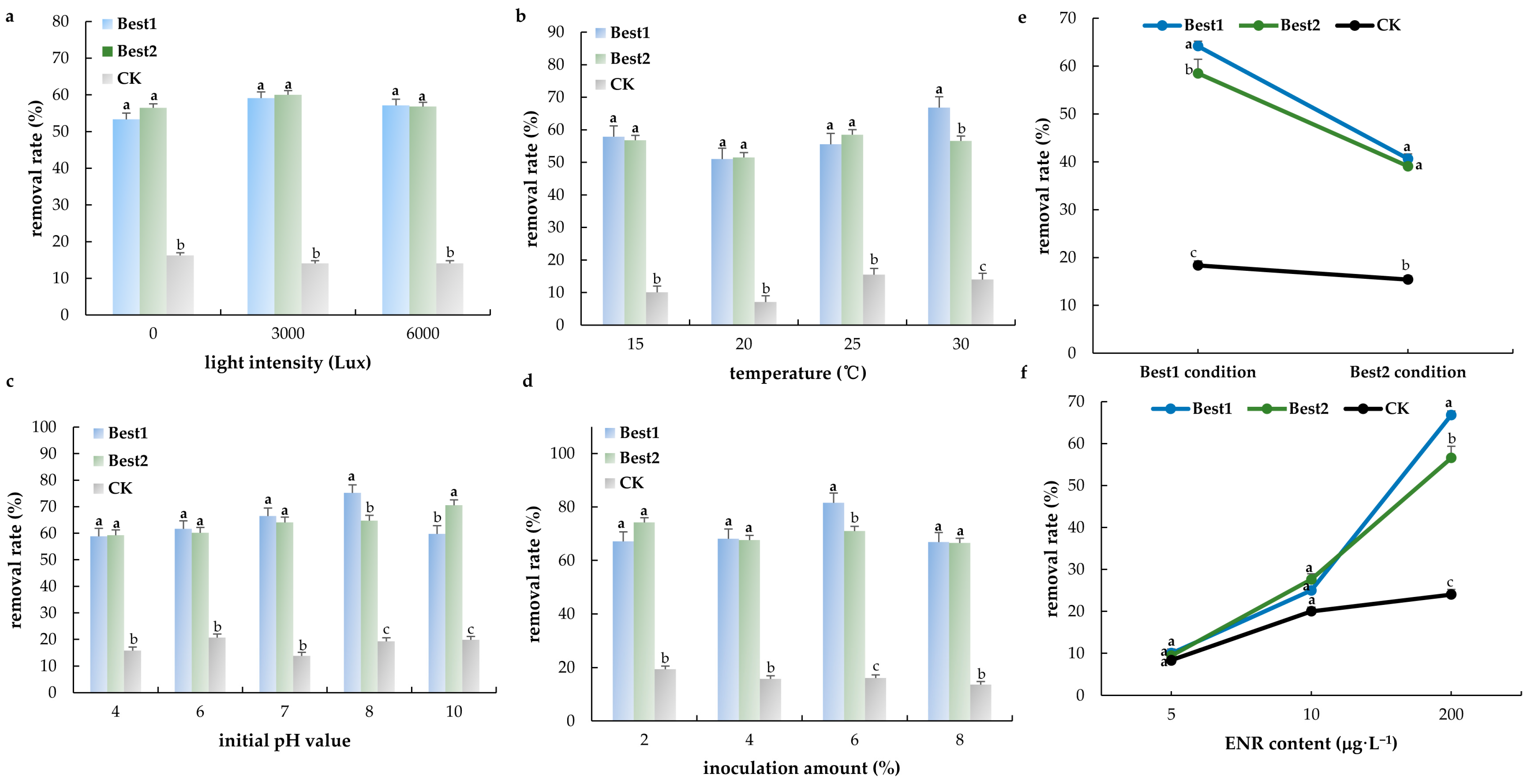
3.3. ENR Degradation Bacteria and Their Optimization Conditions
4. Discussion
4.1. Environmental Stressors and the Reliance on Antibiotics in American Shad Culture
4.2. Factors Shaping ENR Degradation and Optimization of Microbial Remediation
4.3. Toward Sustainable Aquaculture and One Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shardo, J.D. Comparative embryology of teleostean fishes. I. Development and staging of the american shad, Alosa sapidissima (Wilson, 1811). J. Morphol. 1995, 225, 125–167. [Google Scholar] [CrossRef]
- Wu, X.; Tang, Z.; Li, W.; Chu, Z.; Hong, X.; Zhu, X.; Xu, H. Identifying the germ cells during embryogenesis and gametogenesis by germ-line gene vasa in an anadromous fish, American shad Alosa sapidissima. J. Fish Biol. 2018, 92, 1422–1434. [Google Scholar] [CrossRef]
- Bayse, S.M.; Regish, A.M.; McCormick, S.D. Survival and spawning success of American shad (Alosa sapidissima) in varying temperatures and levels of glochidia infection. Fish Physiol. Biochem. 2021, 47, 1821–1836. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, Z.; Guan, C.; Huang, B.; Lei, J.; Li, J.; Guo, Z.; Wang, Y.; Hong, L. Developmental changes in digestive enzyme activity in American shad, Alosa sapidissima, during early ontogeny. Fish Physiol. Biochem. 2017, 43, 397–409. [Google Scholar] [CrossRef]
- Sosa, E.R.; Landsberg, J.H.; Kiryu, Y.; Stephenson, C.M.; Cody, T.T.; Dukeman, A.K.; Wolfe, H.P.; Vandersea, M.W.; Litaker, R.W. Pathogenicity studies with the fungi Aphanomyces invadans, Achlya bisexualis, and Phialemonium dimorphosporum: Induction of skin ulcers in striped mullet. J. Aquat. Anim. Health. 2007, 19, 41–48. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Zhou, Z.; Tang, M.; Cui, J.; Han, W.; Li, J.; Dai, J.; Ren, X.; Jiang, H.; et al. Self-cross-linked collagen sponge from the Alosa sapidissima scale for hemostasis and wound healing applications. Biomacromolecules 2025, 26, 405–414. [Google Scholar] [CrossRef]
- Li, H.; Bao, L.; Pan, Y.; Zhu, X.; Cheng, J.; Zhang, J.; Chu, W. The role of miR-216a-mediated Nrf2 pathway in muscle oxidative stress of Siniperca chuatsi induced by cadmium. Ecotoxicol. Environ. Saf. 2024, 283, 116863. [Google Scholar] [CrossRef]
- Burada, A.; Maria-Catalina, T.; Georgescu, L.; Teodorof, L.; Despina (Nastase), C.; Seceleanu-Odor, D.; Iticescu, C. Heavy metals environment accumulation in Somova-parches aquatic complex from the Danube Delta area. Rev. Chim.-Buchar.-Orig. Ed. 2015, 66, 48–54. [Google Scholar]
- Yu, Y.; Dong, L.; Zhang, L.; Gan, J.; Peng, J.; Liu, T.; Chen, J.; Lu, X.; He, L.; Cheng, B. Effect of flowing water on the pharmacokinetic properties of norfloxacin in channel catfish (Ictalurus punctatus) after single-dose oral administration. Vet. Med. Sci. 2023, 9, 1201–1210. [Google Scholar] [CrossRef]
- Du, J.; Liu, Q.; Fu, L. Metabolic and transcriptional disruption of American shad (Alosa sapidissima) by enrofloxacin in commercial aquaculture. Environ. Sci. Pollut. Res. Int. 2022, 29, 2052–2062. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, Q.; Zhang, K. Exposure to enrofloxacin affects the early development and metabolic system of juvenile American shad, as indicated by host metabolism and the environmental microbiome. Lett. Appl. Microbiol. 2023, 76, ovac037. [Google Scholar] [CrossRef]
- Mai, Z.; Xiong, X.; Hu, H.; Jia, J.; Wu, C.; Wang, G. Occurrence, distribution, and ecological risks of antibiotics in Honghu Lake and surrounding aquaculture ponds, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 50732–50742. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, L.; Wang, Q.; Zeng, H.; Xu, J.; Chen, Z. Antibiotics in aquaculture ponds from Guilin, South of China: Occurrence, distribution, and health risk assessment. Environ. Res. 2022, 204 Pt B, 112084. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Han, Y.; Liu, J.; Li, B.; Li, J.; Li, W.; Shi, P.; Pan, Y.; Li, A. Occurrence, distribution and potential environmental risks of pollutants in aquaculture ponds during pond cleaning in Taihu Lake Basin, China. Sci. Total Environ. 2024, 939, 173610. [Google Scholar] [CrossRef]
- Xiong, W.; Sun, Y.; Zhang, T.; Ding, X.; Li, Y.; Wang, M.; Zeng, Z. Antibiotics, antibiotic resistance genes, and bacterial community composition in fresh water aquaculture environment in China. Microb. Ecol. 2015, 70, 425–432. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, X.; Xu, X.; Wang, X.; Yuan, X.; Ding, Z.; Zhao, S.; Wang, S. Antibiotics in marine aquaculture farms surrounding Laizhou Bay, Bohai Sea: Distribution characteristics considering various culture modes and organism species. Sci. Total Environ. 2021, 760, 143863. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, D.; Fabrizi, L.; Guandalini, E.; Podestà, E.; Marvasi, L.; Zaghini, A.; Coni, E. Long depletion time of enrofloxacin in rainbow trout (Oncorhynchus mykiss). Antimicrob. Agents Chemother. 2004, 48, 3912–3917. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Xu, X.; Diao, Z.; Sun, K.; Hao, Q.; Liu, S.; Ying, G. Tissue distribution, bioaccumulation characteristics and health risk of antibiotics in cultured fish from a typical aquaculture area. J. Hazard. Mater. 2018, 343, 140–148. [Google Scholar] [CrossRef]
- He, L.; He, L.; Tang, Y.; Qiao, L.; Xu, M.; Zhou, Z.; Bai, H.; Zhang, M.; Ying, G. Deciphering spread of quinolone resistance in mariculture ponds: Cross-species and cross-environment transmission of resistome. J. Hazard. Mater. 2025, 487, 137198. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.; Liu, X.; Lee, S.; Kho, Y.; Kim, W.K.; Kim, P.; Choi, K. Ecological risk assessment of amoxicillin, enrofloxacin, and neomycin: Are their current levels in the freshwater environment safe? Toxics 2021, 9, 196. [Google Scholar] [CrossRef]
- Corum, O.; Terzi, E.; Durna Corum, D.; Tastan, Y.; Gonzales, R.C.; Kenanoglu, O.N.; Arriesgado, D.M.; Navarro, V.R.; Bilen, S.; Sonmez, A.Y.; et al. Plasma and muscle tissue disposition of enrofloxacin in Nile tilapia (Oreochromis niloticus) after intravascular, intraperitoneal, and oral administrations. Food. Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2022, 39, 1806–1817. [Google Scholar] [CrossRef]
- Huang, C.; Chen, L.; Li, H.; Mu, Y.; Yang, Z. Synthesis and application of Bi2WO6 for the photocatalytic degradation of two typical fluoroquinolones under visible light irradiation. RSC Adv. 2019, 9, 27768–27779. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Chen, T.; Sun, J.; Ma, X.; Wang, Y.; Wang, J.; Xie, Z. Preparation of TiO2-modified biochar and its characteristics of photo-catalysis degradation for enrofloxacin. Sci. Rep. 2020, 10, 6588. [Google Scholar] [CrossRef] [PubMed]
- Annabi, C.; Fourcade, F.; Soutrel, I.; Geneste, F.; Floner, D.; Bellakhal, N.; Amrane, A. Degradation of enoxacin antibiotic by the electro-Fenton process: Optimization, biodegradability improvement and degradation mechanism. J. Environ. Manag. 2016, 165, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hao, H.; Cheng, G.; Liu, C.; Ahmed, S.; Shabbir, M.A.B.; Hussain, H.I.; Dai, M.; Yuan, Z. Microbial shifts in the intestinal microbiota of Salmonella infected chickens in response to enrofloxacin. Fron.t Microbiol. 2017, 8, 1711. [Google Scholar] [CrossRef]
- Grabowski, Ł.; Gaffke, L.; Pierzynowska, K.; Cyske, Z.; Choszcz, M.; Węgrzyn, G.; Węgrzyn, A. Enrofloxacin-the ruthless killer of eukaryotic cells or the last hope in the fight against bacterial infections? Int. J. Mol. Sci. 2022, 23, 3648. [Google Scholar] [CrossRef]
- Method 200.7; Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry. EPA: Cincinnati, OH, USA, 1994.
- GB11607-1989; Fisheries Water Quality Standards. State Environmental Protection Administration of China: Beijing, China, 1989.
- Chemtai, C.; Kengara, F.O.; Ngigi, A.N. Levels and ecological risk of pharmaceuticals in River Sosiani, Kenya. Environ. Monit. Assess. 2023, 195, 431. [Google Scholar] [CrossRef]
- Popa, P.; Murariu, G.; Mihaela, T.; Georgescu, L.; Iticescu, C. Multivariate statistical analyses of water quality of Danube river at Galati, Romania. Environ. Eng. Manag. J. 2015. [Google Scholar] [CrossRef]
- Brown, C.R.; Sergio, A.J.A.; Bate, C.S.; Koopman, N.; Roland, J.B.; Notman-Grobler, O.D.P.; Mastrodimitropoulos, P.M.B.; Piczak, M.L.; Lennox, R.J. A review of migratory Alosidae marine ecology in the northwest Atlantic. J. Fish Biol. 2025, 106, 677–695. [Google Scholar] [CrossRef]
- Sarker, K.K.; Lu, L.; Huang, J.; Zhou, T.; Wang, L.; Hu, Y.; Jiang, L.; Naher, H.; Baki, M.A.; Sarker, A.; et al. First report of de novo assembly and annotation from brain and blood transcriptome of an anadromous shad, Alosa sapidissima. BMC Genom. Data 2022, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Velotta, J.P.; Iqbal, A.R.; Glenn, E.S.; Franckowiak, R.P.; Formenti, G.; Mountcastle, J.; Balacco, J.; Tracey, A.; Sims, Y.; Howe, K.; et al. A Complete assembly and annotation of the American shad genome yields insights into the origins of diadromy. Genome Biol. Evol. 2025, 17, evae276. [Google Scholar] [CrossRef]
- Wu, T.; Hu, J.; Giap, V.; Wang, Q.; Lu, L.; Li, C. A chromosome-level genome assembly of the Chinese herring (Ilisha elongata) uncovered its population dynamics and genes related to lipid metabolism. J. Hered. 2025, esaf028. [Google Scholar] [CrossRef]
- Qiu, C.; Shi, Y.; Huang, X.; Chen, Z. Impact of probiotics on enzyme activities, intestinal microbial remodeling, and metabolic pathways in American shad (Alosa sapidissima) at high temperatures. Mar. Biotechnol. 2025, 27, 58. [Google Scholar] [CrossRef]
- Luo, M.; Feng, B.; Zhu, W.; Liang, Z.; Xu, W.; Fu, J.; Miao, L.; Dong, Z. Proteomics and metabolomics analysis of American shad (Alosa sapidissima) liver responses to heat stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2024, 296, 111686. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhu, W.; Liang, Z.; Feng, B.; Xie, X.; Li, Y.; Liu, Y.; Shi, X.; Fu, J.; Miao, L.; et al. High-temperature stress response: Insights into the molecular regulation of American shad (Alosa sapidissima) using a multi-omics approach. Sci. Total Environ. 2024, 916, 170329. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Z.; Li, Y.; Zhu, W.; Feng, B.; Xu, W.; Fu, J.; Wei, P.; Luo, M.; Dong, Z. Integrated transcriptome and microRNA analysis reveals molecular responses to high-temperature stress in the liver of American shad (Alosa sapidissima). BMC Genom. 2024, 25, 656. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Hu, S.; Dong, Y.; Miao, L.; Zhu, W.; Feng, B.; Fu, J.; Luo, M.; Wang, L.; Dong, Z. Molecular characterization and function of hif1a and fih1 in response to acute thermal stress in American shad (Alosa sapidissima). Fish Physiol. Biochem. 2024, 50, 1563–1581. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, Q.; Liu, J.; Zhang, K.; Huang, W. Structural and functional comparisons of the environmental microbiota of pond and tank environments at different locations for the commercial aquaculture of American shad. Lett. Appl. Microbiol. 2022, 75, 51–60. [Google Scholar] [CrossRef]
- Qiu, C.; Gao, C.; Deng, P.; Sun, Z.; Liu, Y.; Xu, J.; Shi, Y. Transcriptome analysis reveals the mechanism by which probiotic alleviate the response of juvenile American shad (Alosa sapidissima) to high temperatures. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef]
- Nakamoto, B.J.; Jeffres, C.A.; Corline, N.J.; Ogaz, M.; Bradley, C.J.; Viers, J.H.; Fogel, M.L. Multiple trophic pathways support fish on floodplains of California’s Central Valley. J. Fish Biol. 2023, 102, 155–171. [Google Scholar] [CrossRef]
- Harrabi, M.; Alexandrino, D.A.M.; Aloulou, F.; Elleuch, B.; Liu, B.; Jia, Z.; Almeida, C.M.R.; Mucha, A.P.; Carvalho, M.F. Biodegradation of oxytetracycline and enrofloxacin by autochthonous microbial communities from estuarine sediments. Sci. Total Environ. 2019, 648, 962–972. [Google Scholar] [CrossRef]
- Melnyk, L.J.; Lazorchak, J.M.; Kusnierz, D.H.; Perlman, G.D.; Lin, J.; Venkatapathy, R.; Sundaravadivelu, D.; Thorn, J.; Durant, J.; Pugh, K.; et al. One Health assessment of persistent organic chemicals and PFAS for consumption of restored anadromous fish. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 1035–1044. [Google Scholar] [CrossRef]
- Alexandrino, D.A.M.; Mucha, A.P.; Almeida, C.M.R.; Gao, W.; Jia, Z.; Carvalho, M.F. Biodegradation of the veterinary antibiotics enrofloxacin and ceftiofur and associated microbial community dynamics. Sci. Total Environ. 2017, 581–582, 359–368. [Google Scholar] [CrossRef]
- Xu, C.; Feng, Y.; Li, H.; Liu, M.; Yao, Y.; Li, Y. Enhanced degradation of enrofloxacin in mariculture wastewater based on marine bacteria and microbial carrier. J. Hazard. Mater. 2024, 472, 134555. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Liu, J. A novel enrofloxacin-degrading fungus, Humicola sp. KC0924g, isolated from the rhizosphere sediment of the submerged macrophyte Vallisneria spiralis L. Int. Microbiol. 2024, 27, 1693–1705. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, H.; Yuan, L.; Li, Y.; Yang, W.; Huang, Y. Biotransformation of enrofloxacin-copper combined pollutant in aqueous environments by fungus Cladosporium cladosporioides (CGMCC 40504). World J. Microbiol. Biotechnol. 2024, 40, 397. [Google Scholar] [CrossRef]
- Befenzi, H.; Ezzariai, A.; Baghor, J.; Arrach, H.; Armengaud, J.; Kielbasa, M.; Doan, A.; Lambert, J.; Lomascolo, A.; Albert, Q.; et al. Bjerkandera adusta TM11 for the bioremediation of fluoroquinolone antibiotics spiked in wastewater: A sustainable approach to pharmaceutical contaminant biotransformation. Ecotoxicol. Environ. Saf. 2025, 291, 117898. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, H.; Yuan, L.; Li, Y.; Yang, W.; Huang, Y. Characterization and removal mechanism of fluoroquinolone-bioremediation by fungus Cladosporium cladosporioides 11 isolated from aquacultural sediments. Environ. Sci. Pollut. Res. Int. 2024, 31, 29525–29535. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Jiao, Y.; Ji, X.; Li, Y.; Chen, Q.; Zhang, X.; Zhang, G. Efficient removal of enrofloxacin in swine wastewater using eukaryotic-bacterial symbiotic membraneless bioelectrochemical system. J. Hazard. Mater. 2025, 489, 137513. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sun, S.; Gu, X.; Li, Z.; Zhang, J.; Xing, Y.; Wang, L.; Zhang, W. Linking the removal of enrofloxacin to the extracellular polymers of microalgae in water bodies: A case study focusing on the shifts in microbial communities. Environ. Sci. Pollut. Res. Int. 2024, 31, 48062–48072. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xu, C.; Xu, K.; Chen, C.; Li, A.; Ji, B. Metabolic responses of microalgal-bacterial granular sludge to enrofloxacin and sulfamethoxazole exposure. Bioresour. Technol. 2025, 429, 132516. [Google Scholar] [CrossRef]
- Piddock, L.J.; Jin, Y.F.; Ricci, V.; Asuquo, A.E. Quinolone accumulation by Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli. J. Antimicrob. Chemother. 1999, 43, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Li, C.; Li, X.; Hu, B.; Lu, L.; Tomberlin, J.K.; Hu, W. Characteristics of tylosin and enrofloxacin degradation in swine manure digested by black soldier fly (Hermetia illucens L.) larvae. Environ. Pollut. 2022, 293, 118495. [Google Scholar] [CrossRef]
- Tong, Z.; Liu, F.; Tian, Y.; Zhang, J.; Liu, H.; Duan, J.; Bi, W.; Qin, J.; Xu, S. Effect of biochar on antibiotics and antibiotic resistance genes variations during co-composting of pig manure and corn straw. Front. Bioeng. Biotechnol. 2022, 10, 960476. [Google Scholar] [CrossRef]
- Abudureheman, M.; Ailijiang, N.; Mamat, A.; Feng, Y.; He, C.; Pu, M. Enhanced biodegradation of fluoroquinolones and the changes of bacterial communities and antibiotic-resistant genes under intermittent electrical stimulation. Environ. Res. 2023, 219, 115127. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, D.; Pan, L.; Su, C.; Li, Z.; Liu, C.; He, Q. Characterization and removal mechanism of a novel enrofloxacin-degrading microorganism, Microbacterium proteolyticum GJEE142 capable of simultaneous removal of enrofloxacin, nitrogen and phosphorus. J. Hazard. Mater. 2023, 454, 131452. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Cheng, M.; Hu, X.; Xue, M.; Jiang, N.; Liu, W.; Fan, Y.; Meng, Y.; Xu, C.; Zhou, Y. Pathological changes of highly pathogenic Bacillus cereus on Pelodiscus sinensis. Vet. Q. 2023, 43, 1–10. [Google Scholar] [CrossRef]
- Guo, C.; Ma, Y.; Li, Y.; Wang, Z.; Lin, S.; Dong, R.; Liu, S. Effects of Hydrothermal Pretreatment and Anaerobic Digestion of Pig Manure on the Antibiotic Removal and Methane Production. Appl. Biochem. Biotechnol. 2024, 196, 7104–7127. [Google Scholar] [CrossRef]
- Balasundaram, G.; Gahlot, P.; Ahmed, B.; Biswas, P.; Tyagi, V.K.; Svensson, K.; Kumar, V.; Kazmi, A.A. Advanced steam-explosion pretreatment mediated anaerobic digestion of municipal sludge: Effects on methane yield, emerging contaminants removal, and microbial community. Environ. Res. 2023, 238 Pt 2, 117195. [Google Scholar] [CrossRef]
- Zheng, Y.; Lv, P.; Yang, J.; Xu, G. Characterization and adsorption capacity of modified biochar for sulfamethylimidine and methylene blue in water. ACS Omega 2023, 8, 29966–29978. [Google Scholar] [CrossRef]
- Song, W.; Liao, Z.; Wang, L.; Li, Y.; Zhang, W.; Ji, Y.; Chen, J. The distribution and ecological risks of antibiotics in the sediments from a diverging area of the bifurcated river: Effects of hydrological properties. J. Environ. Manag. 2022, 320, 115787. [Google Scholar] [CrossRef]
- Kong, W.; Li, C.; Zhang, A.; Li, X.; Gong, Q.; Jin, B.; Jia, X.; Liu, X.; Kang, Y. A colorimetric-aptamer-based assay for the determination of enrofloxacin through triggering the aggregation of gold nanoparticles. Anal. Methods 2024, 16, 7121–7129. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Li, L.; Xu, G. Multi-omics platforms reveal the synergistic effect in tilapia intestine under microplastics and sulfamethoxazole, BDE153 acute co-exposure. Int. J. Mol. Sci. 2025, 26, 8441. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Zhu, H.; Hu, J.; Sun, Y.; Xu, G. Endocytosis, endoplasmic reticulum, actin cytoskeleton affected in tilapia liver under microplastics and BDE153 co-exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2025, 289, 110117. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Gao, J.; Jin, W.; Hu, J.; Sun, Y.; Zhu, H.; Xu, G. Apoptosis, MAPK signaling pathway affected in tilapia liver following microplastics and sulfamethoxazole co-exposure. Comp. Biochem. Physiol. Part D Genom. Proteom. 2025, 53, 101370. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Zheng, M.; Wang, G.; Zhao, H. Exposed to sulfamethoxazole induced hepatic lipid metabolism disorder and intestinal microbiota changes on zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 253, 109245. [Google Scholar] [CrossRef] [PubMed]
- Robledo Ardila, P.A.; Álvarez-Alonso, R.; Árcega-Cabrera, F.; Durán Valsero, J.J.; Morales García, R.; Lamas-Cosío, E.; Oceguera-Vargas, I.; DelValls, A. Assessment and review of heavy metals pollution in sediments of the Mediterranean Sea. Appl. Sci. 2024, 14, 1435. [Google Scholar] [CrossRef]
- Ardila, P.A.R.; Alonso, R.Á.; Valsero, J.J.D.; García, R.M.; Cabrera, F.Á.; Cosío, E.L.; Laforet, S.D. Assessment of heavy metal pollution in marine sediments from southwest of Mallorca island, Spain. Environ. Sci. Pollut. Res. 2023, 30, 16852–16866. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lu, X.; Hu, J.; Sun, Y.; Zhu, H.; Xu, G. Chlorella alleviates the intestinal damage of tilapia caused by microplastics. Chemosphere 2024, 353, 141644. [Google Scholar] [CrossRef]
- Zheng, Y.; Tang, H.; Hu, J.; Sun, Y.; Zhu, H.; Xu, G. Integrated transcriptomics and proteomics analyses reveal the ameliorative effect of hepatic damage in tilapia caused by microplastics with chlorella addition. Ecotoxicol. Environ. Saf. 2024, 285, 117076. [Google Scholar] [CrossRef]
- Wang, W.; Shi, Y.; Qiu, T.; Meng, J.; Ding, J.; Wang, W.; Wu, D.; Li, K.; Liu, J.; Wu, Y. Modified rougan decoction alleviates lipopolysaccharide-enrofloxacin-induced hepatotoxicity via activating the Nrf2/ARE pathway in chicken. Poult. Sci. 2023, 102, 102404. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, Y.; Sun, Y.; Xu, G. Resveratrol attenuated fatty acid synthesis through MAPK-PPAR pathway in red tilapia. Comp. Biochem. Physiol. C 2023, 268, 109598. [Google Scholar] [CrossRef]
- Ye, W.; Zheng, Y.; Sun, Y.; Li, Q.; Zhu, H.; Xu, G. Transcriptome analysis of the response of four immune related organs of tilapia (Oreochromis niloticus) to the addition of resveratrol in feed. Fish Shellfish Immunol. 2023, 133, 108510. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shi, Y.; Yang, X.; Gao, J.; Nie, Z.; Xu, G. Effects of resveratrol on lipid metabolism in liver of red tilapia Oreochromis niloticus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 261, 109408. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shao, N.; Yang, X.; Shi, Y.; Xu, G. Resveratrol ameliorates intestinal lipid metabolism through the PPAR signaling pathway in high-fat diet-fed red tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2024, 145, 109302. [Google Scholar] [CrossRef]
- Do, T.M.; Choi, D.; Oh, S.; Stuckey, D.C. Anaerobic membrane bioreactor performance with varying feed concentrations of ciprofloxacin. Sci. Total Environ. 2022, 803, 150108. [Google Scholar] [CrossRef]
- Dorival-García, N.; Zafra-Gómez, A.; Navalón, A.; González, J.; Vílchez, J.L. Removal of quinolone antibiotics from wastewaters by sorption and biological degradation in laboratory-scale membrane bioreactors. Sci. Total Environ. 2013, 442, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Buleté, A.; Vulliet, E.; Deshayes, S.; Zedek, S.; Mirande-Bret, C.; Eudes, V.; Bressy, A.; et al. Removal of a wide range of emerging pollutants from wastewater treatment plant discharges by micro-grain activated carbon in fluidized bed as tertiary treatment at large pilot scale. Sci. Total Environ. 2016, 542 Pt A, 983–996. [Google Scholar] [CrossRef]
- Yi, K.; Wang, D.; Qi, Y.; Li, X.; Chen, H.; Sun, J.; An, H.; Wang, L.; Deng, Y.; Liu, J.; et al. Effect of ciprofloxacin on biological nitrogen and phosphorus removal from wastewater. Sci. Total Environ. 2017, 605–606, 368–375. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Li, J.; Yona, A.; Wang, X.; Li, X.; Yuan, J.; Xu, G. Integrated Monitoring of Water Quality, Metal Ions, and Antibiotic Residues, with Isolation and Optimization of Enrofloxacin-Degrading Bacteria in American Shad (Alosa sapidissima) Aquaculture Systems. J. Xenobiot. 2025, 15, 174. https://doi.org/10.3390/jox15060174
Zheng Y, Li J, Yona A, Wang X, Li X, Yuan J, Xu G. Integrated Monitoring of Water Quality, Metal Ions, and Antibiotic Residues, with Isolation and Optimization of Enrofloxacin-Degrading Bacteria in American Shad (Alosa sapidissima) Aquaculture Systems. Journal of Xenobiotics. 2025; 15(6):174. https://doi.org/10.3390/jox15060174
Chicago/Turabian StyleZheng, Yao, Jiajia Li, Ampeire Yona, Xiaofei Wang, Xue Li, Julin Yuan, and Gangchun Xu. 2025. "Integrated Monitoring of Water Quality, Metal Ions, and Antibiotic Residues, with Isolation and Optimization of Enrofloxacin-Degrading Bacteria in American Shad (Alosa sapidissima) Aquaculture Systems" Journal of Xenobiotics 15, no. 6: 174. https://doi.org/10.3390/jox15060174
APA StyleZheng, Y., Li, J., Yona, A., Wang, X., Li, X., Yuan, J., & Xu, G. (2025). Integrated Monitoring of Water Quality, Metal Ions, and Antibiotic Residues, with Isolation and Optimization of Enrofloxacin-Degrading Bacteria in American Shad (Alosa sapidissima) Aquaculture Systems. Journal of Xenobiotics, 15(6), 174. https://doi.org/10.3390/jox15060174










