A Systematic Review on Contamination of Marine Species by Chromium and Zinc: Effects on Animal Health and Risk to Consumer Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Focus Question
2.2. Information Sources
2.3. Risk of Bias Assessment
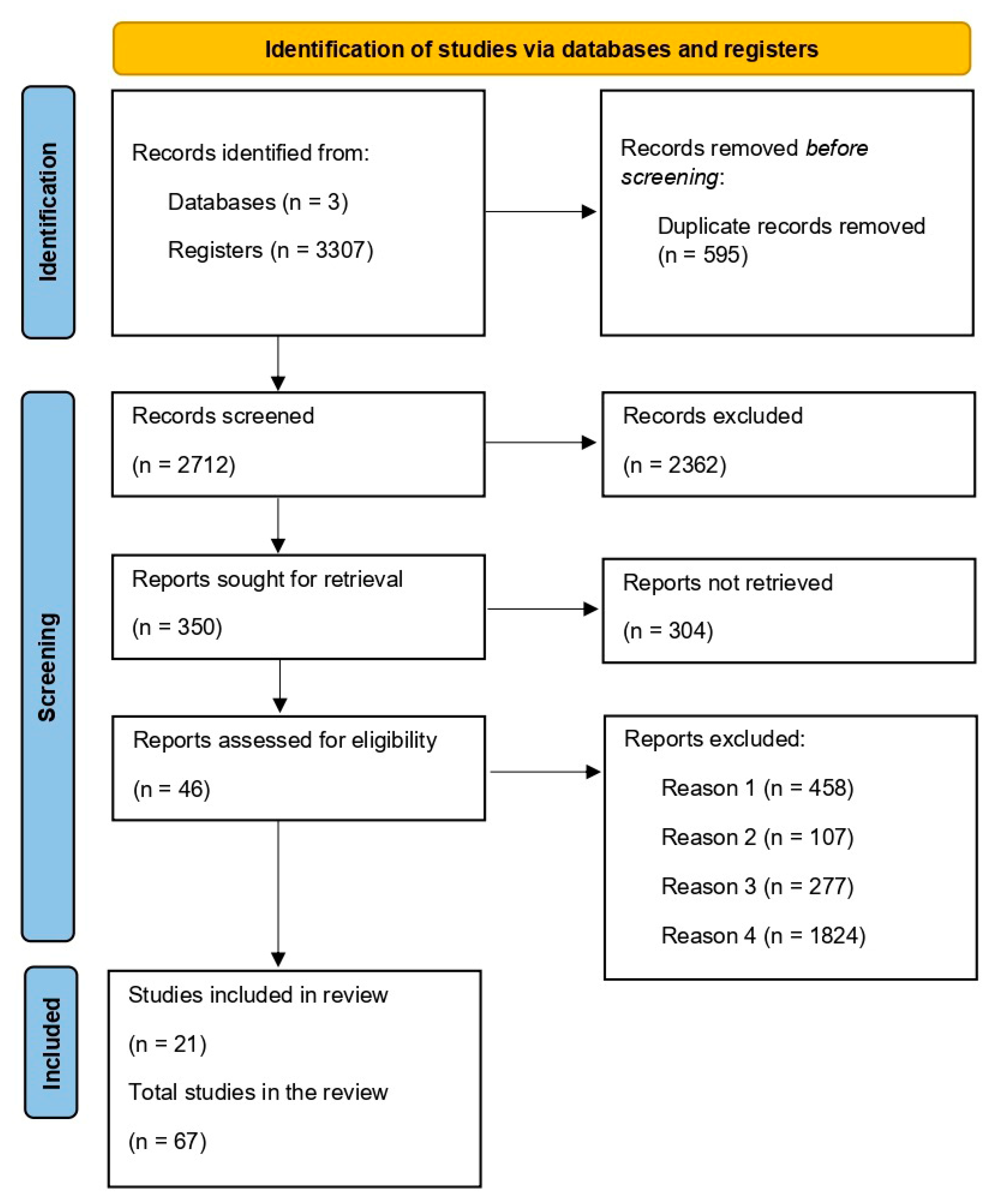
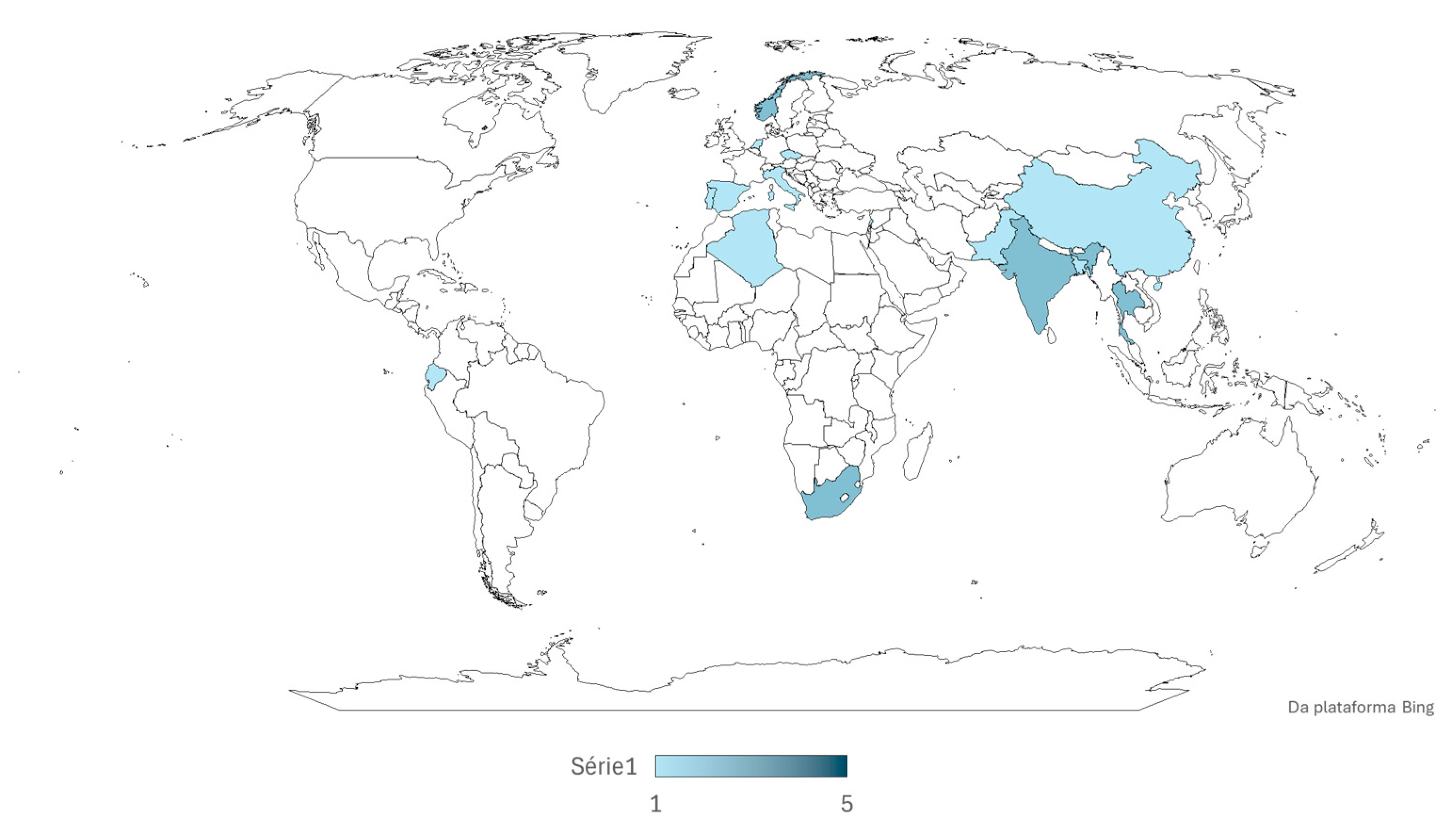
3. Results
4. Discussion
4.1. Origin and Circulation of Cr and Zn in the Marine Environment
4.2. Maximum Cr and Zn Levels and the Response in Aquatic Animals
4.3. Metal Levels in the Studied Seafood Species
4.4. Effects of Zn Overexposure to Humans
4.5. Chromium Intoxication in Humans
4.6. Consumer Health Risk Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Firth, D.C.; Salie, K.; O’Neill, B.; Hoffman, L.C. Monitoring of trace metal accumulation in two South African farmed mussel species, Mytilus galloprovincialis and Choromytilus meridionalis. Mar. Pollut. Bull. 2019, 141, 529–534. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Aoun, M.; Arnaudguilhem, C.; El Samad, O.; Khozam, R.B.; Lobinski, R. Impact of a phosphate fertilizer plant on the contamination of marine biota by heavy elements. Environ. Sci. Pollut. Res. 2015, 22, 14940–14949. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Pavesi, T.; Moreira, J.C. Mechanisms and individuality in chromium toxicity in humans. J. Appl. Toxicol. 2020, 40, 1183–1197. [Google Scholar] [CrossRef]
- Kamila, S.; Shaw, P.; Islam, S. Chattopadhyay Ecotoxicology of hexavalent chromium in fish: An updated review. Sci. Total Environ. 2023, 890, 164395. [Google Scholar] [CrossRef] [PubMed]
- Park, R.M.; Bena, J.F.; Stayner, L.T.; Smith, R.J.; Gibb, H.J.; Lees, P.S. Hexavalent chromium and lung cancer in the chromate industry: A quantitative risk assessment. Risk Anal. 2004, 24, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Murphy, A.; Sun, H.; Costa, M. Molecular and epigenetic mechanisms of Cr (VI)-induced carcinogenesis. Toxicol. Appl. Pharmacol. 2019, 377, 114636. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Franco-Fuentes, E.; Moity, N.; Ramírez-González, J.; Andrade-Vera, S.; Hardisson, A.; González-Weller, D.; Paz, S.; Rubio, C.; Gutiérrez, Á.J. Metals in commercial fish in the Galapagos Marine Reserve: Contribution to food security and toxic risk assessment. J. Environ. Manag. 2021, 286, 112188. [Google Scholar] [CrossRef]
- Hong, A.H.; Hargan, K.E.; Williams, B.; Nuangsaeng, B.; Siriwong, S.; Tassawad, P.; Chaiharn, C.; Huertos, M.L. Examining molluscs as bioindicators of shrimp aquaculture effluent contamination in a southeast Asian mangrove. Ecol. Indic. 2020, 115, 106365. [Google Scholar] [CrossRef]
- Sujitha, S.B.; Jonathan, M.P.; Aurioles-Gamboa, D.; Villegas, L.E.C.; Bohórquez-Herrera, J.; Hernández-Camacho, C.J. Trace elements in marine organisms of Magdalena Bay, Pacific Coast of Mexico: Bioaccumulation in a pristine environment. Environ. Geochem. Health 2018, 41, 1075–1089. [Google Scholar] [CrossRef]
- Dorta, P.; Rubio, C.; Lozano, G.; González-Weller, D.; Gutiérrez, Á.; Hardisson, A.; Revert, C. Metals in Mullus surmuletus and Pseudupeneus prayensis from the Canary Islands (Atlantic Ocean). J. Food Prot. 2015, 78, 2257–2263. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.S.; Saher, N.U. Interferences of trace metals between sediment and Dotillid crab (Ilyoplax frater) from three tidal creeks, Karachi, Pakistan. SN Appl. Sci. 2021, 3, 109. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Anacleto, P.; Barbosa, V.; Sloth, J.J.; Rasmussen, R.R.; Tediosi, A.; Fernandez-Tejedor, M.; van den Heuvel, F.H.M.; Kotterman, L.; Marques, A. Toxic elements and speciation in seafood samples from different contaminated sites in Europe. Environ. Res. 2015, 143, 72–81. [Google Scholar] [CrossRef]
- Miller, R.J.; Adeleye, A.S.; Page, H.M.; Kui, L.; Lenihan, H.S.; Keller, A.A. Nano and traditional copper and zinc antifouling coatings: Metal release and impact on marine sessile invertebrate communities. J. Nanoparticle Res. 2020, 22, 129. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mohanty, B. Effect of sublethal hexavalent chromium exposure on the pituitary-ovarian axis of a teleost, Channa punctatus (Bloch). Environ. Toxicol. 2012, 27, 415–422. [Google Scholar] [CrossRef]
- Vutukuru, S.S. Chromium induced alterations in some biochemical profiles of the Indian major carp, Labeo rohita (Hamilton). Bull. Environ. Contam. Toxicol. 2003, 70, 118–123. [Google Scholar] [CrossRef]
- Krumschnabel, G.; Nawaz, M. Acute toxicity of hexavalent chromium in isolated teleost hepatocytes. Aquat. Toxicol. 2004, 70, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Wepener, V.; van Vuren, J.H.; Du Preez, H.H. The effect of hexavalent chromium at different pH values on the haematology of Tilapia sparrmanii (Cichlidae). Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1992, 101, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Billard, R.; Roubaud, P. The effect of metals and cyanide on fertilization in rainbow trout (Salmo gairdneri). Water Res. 1985, 19, 209–214. [Google Scholar] [CrossRef]
- Van Pittius, G.M.; van Vuren, J.H.; Du Preez, H.H. Effects of chromium during pH change on blood coagulation in Tilapia sparrmanii (Cichlidae). Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1992, 101, 371–374. [Google Scholar] [CrossRef]
- Arunkumar, R.I.; Rajasekaran, P.; Michael, R.D. Differential effect of chromium compounds on the immune response of the African mouth breeder Oreochromis mossambicus (Peters). Fish Shellfish. Immunol. 2000, 10, 667–676. [Google Scholar] [CrossRef]
- Farag, A.M.; May, T.; Marty, G.D.; Easton, M.; Harper, D.D.; Little, E.E.; Cleveland, L. The effect of chronic chromium exposure on the health of Chinook salmon (Oncorhynchus tshawytscha). Aquat. Toxicol. 2006, 76, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, L.; Zhang, P.; Xiao, J.; Guo, Z.; Fu, Q. Bioaccumulation of dietary CrPic, Cr(III) and Cr(VI) in juvenile coral trout (Plectropomus leopardus). Ecotoxicol. Environ. Saf. 2022, 240, 113692. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.R.; El-Houseiny, W.; El-Murr, A.E.; Ebraheim, L.L.M.; Ahmed, A.I.; Ek-Hakim, Y.M.A. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicol. Environ. Saf. 2020, 188, 109890. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.P.; de Moraes, F.R.; Fujimoto, R.Y.; da Cruz, C.; Belo, M.A.; de Moraes, J.R. Acute toxicity by water containing hexavalent or trivalent chromium in native Brazilian fish, Piaractus mesopotamicus: Anatomopathological alterations and mortality. Bull. Environ. Contam. Toxicol. 2014, 92, 213–219. [Google Scholar] [CrossRef]
- Marinaro, C.; Marino, A.; Bianchu, A.R.; Berman, B.; Trifuoggi, M.; Marano, A.; Palumbo, G.; Chianese, T.; Scudiero, R.; Rosati, L.; et al. Molecular and toxicological mechanisms behind the effects of chromium (VI) on the male reproductive system of Mytilus galloprovincialis: First evidence for poly-ADP-ribosylation of protamine-like II. Chem. Interact. 2024, 401, 111186. [Google Scholar] [CrossRef]
- McRae, N.K.; Gaw, S.; Glover, C.N. Mechanisms of zinc toxicity in the galaxiid fish, Galaxias maculatus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 179, 184–190. [Google Scholar] [CrossRef]
- Parolini, M.; Panseri, S.; Håland, G.F.; Rossi, L.; Dell’Anno, M.; Ceriani, F.; De Felice, B.; Rafoss, T.; Arioli, F.; Pilu, S.; et al. Trends and potential human health risk of trace elements accumulated in transplanted blue mussels during restoration activities of Flekkefjord fjord (Southern Norway). Environ. Monit. Assess. 2022, 194, 208. [Google Scholar] [CrossRef]
- Selvam, S.; Manisha, A.; Roy, P.D.; Venkatramanan, S.; Chung, S.Y.; Muthukumar, P.; Jesuraja, K.; Elgorban, A.M.; Ahmed, B.; Elzain, H.E. Microplastics and trace metals in fish species of the Gulf of Mannar (Indian Ocean) and evaluation of human health. Environ. Pollut. 2021, 291, 118089. [Google Scholar] [CrossRef]
- Lozano-Bilbao, E.; Lozano, G.; Jiménez, S.; Jurado-Ruzafa, A.; Hardisson, A.; Rubio, C.; Weller, D.G.; Paz, S.; Gutiérrez, Á.J. Influence of Biometric and Seasonal Parameters on the Metal Content of Scomber colias in Northwestern African Waters. Biol. Trace Elem. Res. 2020, 199, 3886–3897. [Google Scholar] [CrossRef]
- Debipersadh, S.; Sibanda, T.; Selvarajan, R.; Naidoo, R. Investigating toxic metal levels in popular edible fishes from the South Durban basin: Implications for public health and food security. Environ. Monit. Assess. 2018, 190, 476. [Google Scholar] [CrossRef]
- Afonso, A.; Gutiérrez, Á.J.; Lozano, G.; González-Weller, D.; Lozano-Bilbao, E.; Rubio, C.; Caballero, J.M.; Revert, C.; Hardisson, A. Metals in Diplodus sargus cadenati and Sparisoma cretense—A risk assessment for consumers. Environ. Sci. Pollut. Res. 2017, 25, 2630–2642. [Google Scholar] [CrossRef]
- Hanis, F.; Messaoudi, M.; Bouamra, M.; Abdelhadi, S.A.; Ouanezar, A.; Malki, A.; Arbaoui, F.; Lamouri, R.; Brahimi, A.; Rebiai, A.; et al. Analysis and Risk Assessment of Essential and Toxic Elements in Algerian Canned Tuna Fish. Biol. Trace Elem. Res. 2024, 202, 1212–1223. [Google Scholar] [CrossRef]
- Reyes-Marquez, A.; Sedeno-Diaz, J.E.; Anguiniga-Garcia, S.; Austria-Ortiz, G.M.; Lopez-Lopez, E. Health risk assessment by consumption of commercial biota contaminated with heavy metals in Tampamachoco coastal lagoon, Gulf of Mexico. Mar. Pollut. Bull. 2024, 206, 116757. [Google Scholar] [CrossRef]
- Lin, H.; Luo, X.; Yu, D.; He, C.; Cao, W.; He, L.; Liang, Z.; Zhou, J.; Fang, G. Risk assessment of As, Cd, Cr, and Pb via the consumption of seafood in Haikou. Sci. Rep. 2024, 14, 19549. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Vashishth, R. Assessing the risk of consuming fish from Kanyakumari (Tamil Nadu), India: An evaluative study on bioaccumulated heavy metals in different fish species using inductively coupled plasma mass spectrometry. Toxicol. Rep. 2024, 13, 101727. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Ravelo, A.; Gutiérrez, Á.J.; Paz, S.; Carrascosa-Iruzubieta, C.; González-Weller, D.; Caballero, J.M.; Revert, C.; Rubio, C.; Hardisson, A. Toxic Metals (Al, Cd, Pb) and Trace Element (B, Ba, Co, Cu, Cr, Fe, Li, Mn, Mo, Ni, Sr, V, Zn) Levels in Sarpa Salpa from the North-Eastern Atlantic Ocean Region. Int. J. Environ. Res. Public Health 2020, 17, 7212. [Google Scholar] [CrossRef]
- Nath, K.; Kumar, N. Hexavalent chromium: Toxicity and its impact on certain aspects of carbohydrate metabolism of the freshwater teleost, Colisa fasciatus. Sci. Total. Environ. 1988, 72, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, W.X. Significance of zinc re-absorption in Zn dynamic regulation in marine fish revealed by pharmacokinetic model. Environ. Pollut. 2024, 363, 125106. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.; Sultana, M.; Hasan, T.; Khan, I.N.; Irfan, N.M.; Ahmed, K. Heavy Metals in Common Fishes Consumed in Dhaka, a Megacity of Asia: A Probabilistic Carcinogenic and Non-Carcinogenic Health Hazard. Biol. Trace Elem. Res. 2025, 203, 384–399. [Google Scholar] [CrossRef]
- Afonso, A.; Gutiérrez, A.J.; Lozano, G.; González-Weller, D.; Rubio, C.; Caballero, J.M.; Hardisson, A.; Revert, C. Determination of toxic metals, trace and essentials, and macronutrients in Sarpa salpa and Chelon labrosus: Risk assessment for the consumers. Environ. Sci. Pollut. Res. Int. 2017, 24, 10557–10569. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, J.; Li, W.; Liu, Y.; Xin, C.; Yang, L. Trace elements concentrations in squids consumed in Shandong Province China and their associated risks to the human health. Mar. Pollut. Bull. 2018, 128, 267–274. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, P.; Baitha, R.; Singh, D.K.; Reddy, K.S. Integrative biomonitoring in Litopenaeus vannamei: Metal analysis and biochemical markers. Mar. Pollut. Bull. 2025, 212, 117544. [Google Scholar] [CrossRef]
- GB 2762-2014; China’s Maximium Levels for Contamination in Foods(CMLCF). National Food Safety Standard of Maximum Levels of Contaminants in Foods. Ministry of Health of the People’s Republic of China: Beijing, China, 2014. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Maximum%20Levels%20of%20Contaminants%20in%20Foods%20_Beijing_China%20-%20Peoples%20Republic%20of_12-11-2014.pdf (accessed on 22 May 2025).
- Food and Drug Administration. Guidance Document for Chromium in Shellfish; U.S. Department of Health and Human Services, Public Health Service, Office of Seafood (HFS416): Washington, DC, USA, 1993; 40p. [Google Scholar]
- Center of Food Safety (CSF) Hong Kong. Food Adulteration (Metallic Contamination) Regulations; Center of Food Safety (CSF): Hong Kong, China, 2018; Chapter 132. Available online: https://www.elegislation.gov.hk/hk/cap132V (accessed on 24 July 2025).
- United States Environmental Protection Agency, US-EPA. Risk assessment guidance for superfund. In Human Health Evaluation Manual (Part A), Interim Final; EPA 540/1–89/002; United States Environmental Protection Agency: Washington, DC, USA, 1989; Volume I, p. 291. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/10001FQY.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1986+Thru+1990&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C86thru90%5CTxt%5C00000003%5C10001FQY.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 2 June 2023).
- Hogstrand, C. Homeostasis and Toxicology of Essential Metals. Fish Physiol. 2011, 31A, 135–200. [Google Scholar]
- Elderfield, H. Chromium speciation in sea water. Earth Planet. Sci. Lett. 1970, 9, 10–16. [Google Scholar] [CrossRef]
- United State Environmental Protection Agency (USEPA). Chromium in Drinking Water. Available online: https://www.epa.gov/sdwa/chromium-drinking-water#:~:text=Agency%20will%20follow%3F-What%20are%20EPA′s%20drinking%20water%20regulations%20for%20chromium%3F,chromium%2C%20including%20chromium%2D6 (accessed on 4 May 2025).
- Zhao, X.; Xie, X.; Xie, Z.; Peng, W.; Zhao, Z. Algal blooms exacerbate cadmium/zinc bioaccumulation in filter-feeding fish in contaminated water: Mechanisms and environmental implications. Aquaculture 2024, 590, 741053. [Google Scholar] [CrossRef]
- Fernandez, P.M.; Vinarta, S.C.; Bernal, A.R.; Cruz, E.L.; Figueroa, L.I.C. Bioremediation strategies for chromium removal: Current research, scale-up approach and future perspectives. Chemosphere 2018, 208, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Velma, V.; Vutukuru, S.S.; Tchounwou, P.B. Ecotoxicology of hexavalent chromium in freshwater fish: A critical review. Rev. Environ. Health 2009, 24, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Panigrahi, A.K. A comprehensive review on chromium induced alterations in fresh water fishes. Toxicol. Rep. 2018, 5, 440–447. [Google Scholar] [CrossRef]
- Mantovani, A.; Frazzoli, C.; Cubadda, F. Organic forms of trace elements as feed additives: Assessment of risks and benefits for farm animals and consumers. Pure Appl. Chem. 2010, 82, 393–407. [Google Scholar] [CrossRef]
- Huang, W.; Cao, L.; Shan, X.; Xiao, Z.; Wang, Q.; Dou, S. Toxic effects of zinc on the development, growth, and survival of red sea bream Pagrus major embryos and larvae. Arch. Environ. Contam. Toxicol. 2010, 58, 140–150. [Google Scholar] [CrossRef]
- Wu, F.; Deng, Y.; Sokolov, E.P.; Falfushynska, H.; Glanzer, A.; Xie, L.; Sokolova, I.M. Nanopollutants (nZnO) amplify hypoxia-induced cellular stress in a keystone marine bivalve, Mytilus edulis. Environ. Res. 2025, 274, 121346. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Zhu, Q.L.; Hu, X.C.; Parsons, D.; Lawson, R.; Hogstrand, C. Transgenerational effects of zinc in zebrafish following early life stage exposure. Sci. Total Environ. 2022, 828, 154443. [Google Scholar] [CrossRef]
- Rodrigues, P.d.A.; Ferrari, R.G.; Kato, L.S.; Hauser-Davis, R.A.; Conte-Junior, C.A. A Systematic Review on Metal Dynamics and Marine Toxicity Risk Assessment Using Crustaceans as Bioindicators. Biol. Trace Elem. Res. 2022, 200, 881–903. [Google Scholar] [CrossRef]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Brown, M.A.; Thom, J.V.; Orth, G.L.; Cova, P.; Juarez, J. Food poisoning involving zinc contamination. Arch. Environ. Health 1964, 8, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.S.; Monaghan, S.A.; Miller, M.L.; McKenna, R.W.; Perkins, W.D.; Levinson, B.S.; Bhushan, V.; Kroft, S.H. Zinc-induced copper deficiency: A report of three cases initially recognized on bone marrow examination. Am. J. Clin. Pathol. 2005, 123, 125–131. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Ambiental (USEPA). Tolerable Daily Intake by Metals. 2007. Available online: https://www.epa.gov/risk/framework-metals-risk-assessment (accessed on 24 July 2025).
- Levina, A.; Pham, T.H.; Lay, P.A. Binding of Chromium(III) to Transferrin Could Be Involved in Detoxification of Dietary Chromium(III) Rather than Transport of an Essential Trace Element. Angew. Chem. Int. Ed. Engl. 2016, 55, 8104–8107. [Google Scholar] [CrossRef]
- USEPA. Guideline for Assessing Chemical Contaminant Data for Use in Fish Advisories, in Fish Sampling and Analysis, 3rd ed.; Document No. EPA 823-B-00-007; Office of Water, U.S. Environmental Protection Agency: Washington, DC, USA, 2000; Volume I. [Google Scholar]
- Amerizadeh, A.; Gholizadeh, M.; Karimi, R. Meta-analysis and health risk assessment of toxic heavy metals in muscles of commercial fishes in Caspian Sea. Environ. Monit. Assess. 2023, 195, 45. [Google Scholar] [CrossRef]
| References | Risk of Bias |
|---|---|
| Firth et al. [1], Auon et al. [3], Franco-Fuentes [10], Hong et al. [11], Sujitha et al. [12], Dorta et al. [13], Siddiqui and Saher [14], Maulvault et al. [15] | Study location bias—e.g., selection of areas, such as cropland, but without comparative control |
| Firth et al. [1], Auon et al. [3], Miller et al. [16], Mishra and Mohanty [17], Vutukuru [18], Krumschnabel and Nawaz [19], Wepener et al. [20], Billard and Roubaud [21], Van Pittius et al. [22], Arunkumar et al. [23], Farag et al. [24], Li et al. [25], Mohamed et al. [26], Castro et al. [27], Marinaro et al. [28], McRae et al. [29], Parolini et al. [30], Selvam et al. [31], Lozano-Bilbao et al. [32], Debipersadh et al. [33], Afonso et al. [34], Hanis et al. [35], Reyes-Marquez et al. [36], Lin et al. [37], Ray and Vashishth [38] | Analytical bias—e.g., poor analytical quality control or experimental conditions that cannot be extrapolated to natural conditions, insufficient sampling |
| Auon et al. [3], Gutiérez-Ravelo et al. [39] | Temporal bias—e.g., lack of sampling that considers the effects of seasonality |
| Auon et al. [3], Nath and Kumar [40], Ma and Wang [41], Shaheen et al. [42] | Statistical evaluation bias—e.g., lack of statistical details that hinder the full understanding of the evaluation carried out |
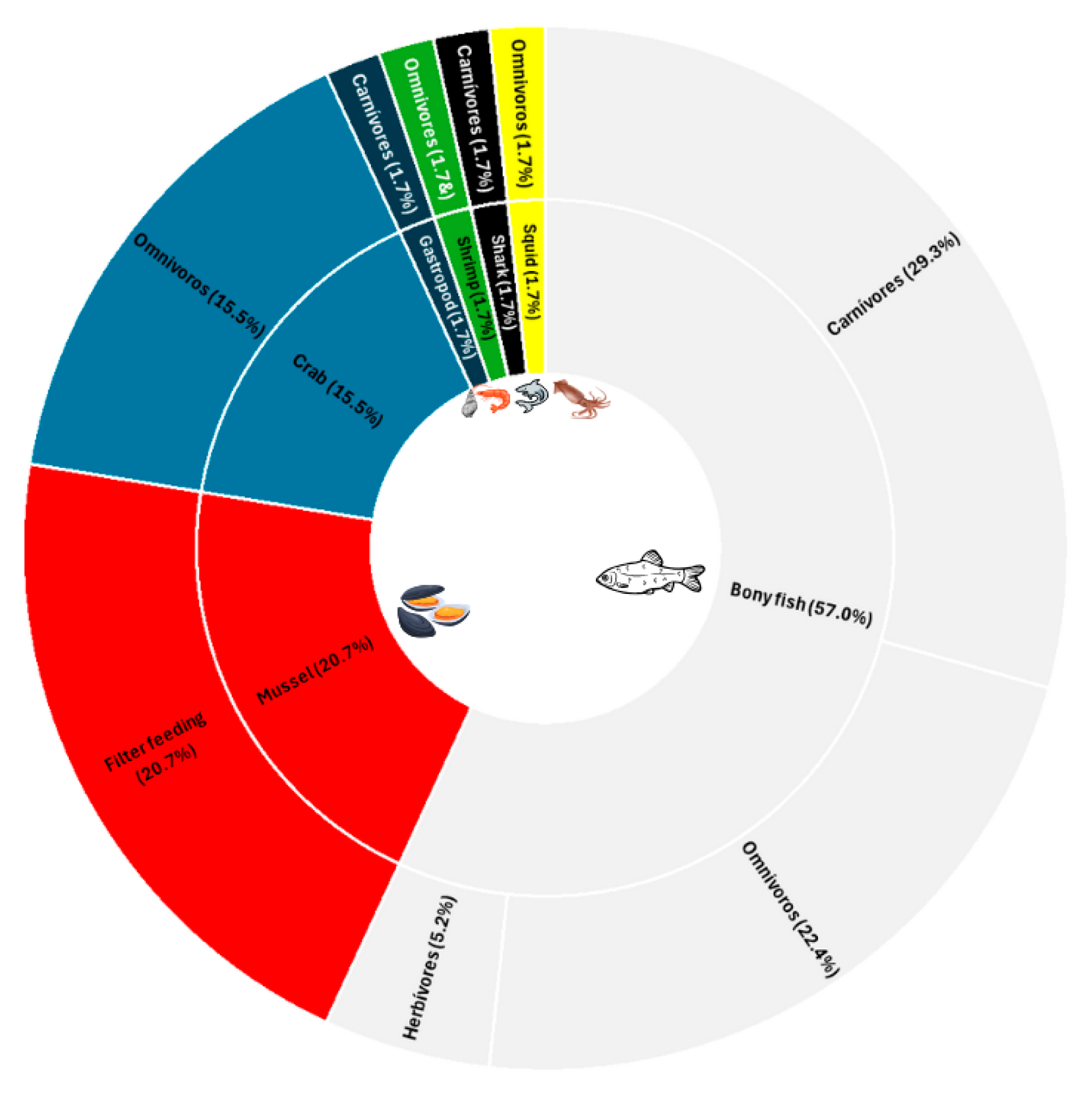
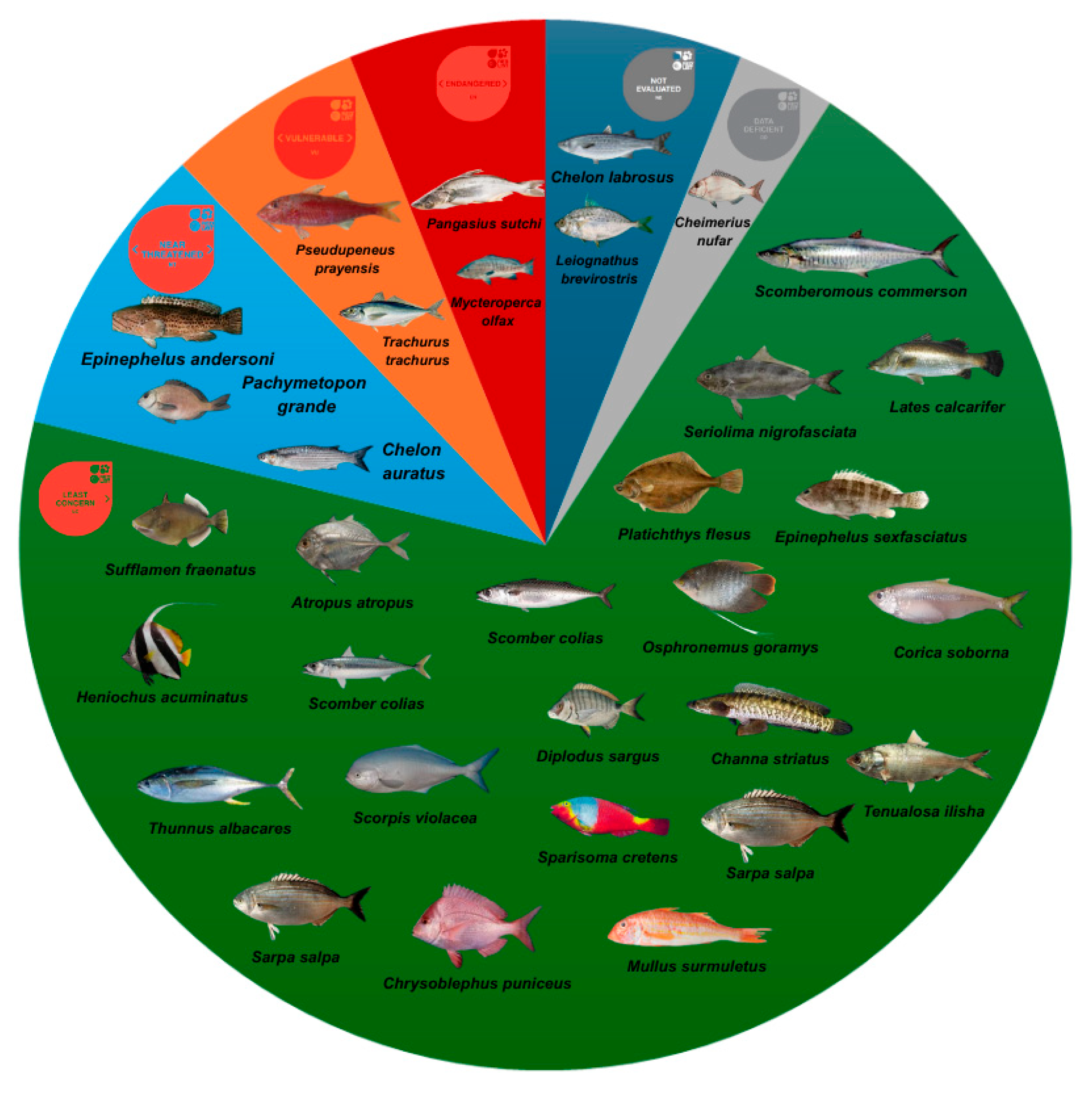
| Organism | Species | Zinc | Chromium | Location | References |
|---|---|---|---|---|---|
| Fish | Sufflamen fraenatus | 0.90 | 0.56 | Thoothukudi Coast, India | Selvam et al. [31] |
| Heniochus acuminatus | 1.00 | 0.46 | |||
| Pseudotriacanthus sp. | 0.44 | 0.13 | |||
| Leiognathus brevirostris | 0.32 | 0.41 | |||
| Atropus atropus | 0.28 | 0.12 | |||
| Scomber colias | 8.07 ± 3.18 | 0.20 ± 0.26 | Canary Islands | Lozano-Bilbao et al. [32] | |
| Scorpis violacea | 10.82 ± 1 1.45 | 0.13 ± 0.09 | Galapagos Islands, Ecuador | Franco-Fuentes et al. [10] | |
| Mycteroperca olfax | 15.75 ± 11.20 | 0.18 ± 0.09 | |||
| Thunnus albacares | 22.55 ± 7.94 | 0.16 ± 0.06 | |||
| Sarpa salpa | 16.3 ± 6.34 | 0.35 ± 0.68 | Canary Islands | Gutiérrez-Ravelo et al. [39] | |
| Epinephelus andersoni | 21.0 ± 4.18 | 0.34 ± 0.15 | Durban, South Africa | Debipersadh et al. [33] | |
| Chrysoblephus puniceus | 31.63 ± 10.5 | - | |||
| Cheimerius nufar | 16.37 ± 1.84 | 1.09 ± 0.32 | |||
| Pachymetopon grande | 31.07 ± 5.57 | 1.30 ± 0.11 | |||
| Trachurus trachurus | 56.71 ± 1.55 | 1.81 ± 0.47 | |||
| Scomber colias | 22.06 ± 6.87 | 0.24 ± 0.43 | |||
| Diplodus sargus cadenati | 4.51 ± 2.42 | 0.18 ± 0.15 | Canary Islands | Afonso et al. [34] | |
| Sparisoma cretens | 2.43 ± 0.37 | 0.13 ± 0.11 | |||
| Mullus surmuletus | 5.99–268.36 | 0.00–2.31 | Canary Islands | Dorta et al. [13] | |
| Pseudupeneus prayensis | 1.86–14.00 | 0.00–0.16 | |||
| Platichthys flesus | 21 ± 4 | 0.00 | Tagus estuary (Portugal), Ebro Delta estuary (Spain), Sacca di Goro estuary (Italy), Western Scheldt estuary (Netherlands) and Solund (Norway). | Maulvault et al. [15] | |
| Chelon auratus | 19 ± 1 | 0.00 | |||
| Seriolina nigrofasciata | 21.23 | 6.40 | |||
| Scomberomous commerson | 24.29 | 8.09 | |||
| Lates calcarifer | 22.86 | 7.41 | |||
| Epinephelus sexfasciatus | 20.61 | 7.86 | |||
| Sarpa salpa | 10.18 ± 2.96 | 0.12 ± 0.08 | Canary Islands | Afonso et al. [43] | |
| Chelon labrosus | 3.25 ± 1.80 | 0.11 ± 0.07 | |||
| Thunnus sp. | 2.86 to 35.90 | 0.12 to 1.21 | Algeria | Hanis et al. [35] | |
| Corica soborna | 392.06 ± 19.22 | 1.75 ± 0.12 | Bangladesh | Shaheen et al. [42] | |
| Tenualosa ilisha | 16.42 ± 1.32 | 6.78 ± 0.40 | |||
| Crab | Ilyoplax frater | 75.44–128 | 9.35–12.3 | Karachi, Pakistan | Siddiqui and Saher [14] |
| Macrophthalmus depressus | 42.60–155 | 5.65–24.30 | |||
| Macrophthalmus japonicas | 38.02–85.11 | 0.37–6.03 | |||
| Macrophthalmus depressus | 68.8–268.8 | - | |||
| Opusia indica | 73.94–82.56 | 7.40–10.94 | |||
| Austruca sindensis | 2.0–76.9 | 0.54–0.60 | |||
| Uca pugilator | 7.00–7.08 | <LOD | |||
| Uca tangeri | 14.6–81.9 | <LOD | |||
| Uca annulipes | 10.2–23.8 | <LOD | |||
| Portunus trituberculatus | 2700 | 2.3 | Lebanon, Mediterranean Sea | Aoun et al. [3] | |
| Mussel | Mytilus galloprovincialis | 153 ± 23 | 4.5 ± 0.5 | Tagus estuary, Ebro Delta estuary, Sacca di Goro estuary, Western Scheldt estuary, and Solund. | Maulvault et al. [15] |
| Chamelea gallina | 75 ± 3 | 1.7 ± 0.1 | |||
| Mytilus edulis | 2384 | 3.4 | Lebanon, Mediterranean Sea | Aoun et al. [3] | |
| Patella vulgata | 841 | 1.22 | |||
| Choromytilus meridionalis | 15.5 ± 0.32 | 0.1 ± 0.01 | Saldanha Bay, South Africa | Firth et al. [1] | |
| Mytilus galloprovincialis | 25.9 ± 0.52 | 0.2 ± 0.01 | |||
| Mytilus edulis | 14.87 ± 0.79 | 0.29 ± 0.01 | Norway | Parolini et al. [30] | |
| Cerithidea obtusa | 66.0 ± 32 | 9.3 ± 4.9 | Thailand Gulf | Hong et al. [11] | |
| Littoraria sp. | 118.2 ± 66 | 7.1 ± 3.5 | |||
| Nerita balteata | 69.5 ± 35 | 13.5 ± 6.7 | |||
| Ellobium aurisjudae | 150.2 ± 65 | 9.6 ± 9.9 | |||
| Isognomon ephippium | 1938.5 ± 835 | 4.9 ± 2.9 | |||
| Thais gradata | 657.8 ± 536 | 20.5 ± 24.2 | |||
| Squid | Loligo chinesi | 15.05 | 0.542 | Shandong Province, China | Jiao et al. [44] |
| Shrimp | Litopenaeus vannamei | 12.15–29.08 | 0.295–1.683 | East Midnapore West Bengal, India. | Kumar et al. [45] |
| Gastropod | Conus princeps | 9.01 ± 5.28 | 9.01 ± 5.28 | Galapagos Islands | Franco-Fuentes et al. [10] |
| Limits | Seafood | - | 2.0 | China | CMLCF [46] |
| - | 12.0 | United States | FDA [47] | ||
| - | 2.0 | Hong Kong | CFS [48] | ||
| 40.0 | - | FAO | USEPA [49] | ||
| Water | 40.0 | 0.1 | FAO | USEPA [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Filho, A.M.; Rodrigues, P.d.A.; de Oliveira, A.T.; Conte-Junior, C.A. A Systematic Review on Contamination of Marine Species by Chromium and Zinc: Effects on Animal Health and Risk to Consumer Health. J. Xenobiot. 2025, 15, 121. https://doi.org/10.3390/jox15040121
Ramos-Filho AM, Rodrigues PdA, de Oliveira AT, Conte-Junior CA. A Systematic Review on Contamination of Marine Species by Chromium and Zinc: Effects on Animal Health and Risk to Consumer Health. Journal of Xenobiotics. 2025; 15(4):121. https://doi.org/10.3390/jox15040121
Chicago/Turabian StyleRamos-Filho, Alexandre Mendes, Paloma de Almeida Rodrigues, Adriano Teixeira de Oliveira, and Carlos Adam Conte-Junior. 2025. "A Systematic Review on Contamination of Marine Species by Chromium and Zinc: Effects on Animal Health and Risk to Consumer Health" Journal of Xenobiotics 15, no. 4: 121. https://doi.org/10.3390/jox15040121
APA StyleRamos-Filho, A. M., Rodrigues, P. d. A., de Oliveira, A. T., & Conte-Junior, C. A. (2025). A Systematic Review on Contamination of Marine Species by Chromium and Zinc: Effects on Animal Health and Risk to Consumer Health. Journal of Xenobiotics, 15(4), 121. https://doi.org/10.3390/jox15040121








