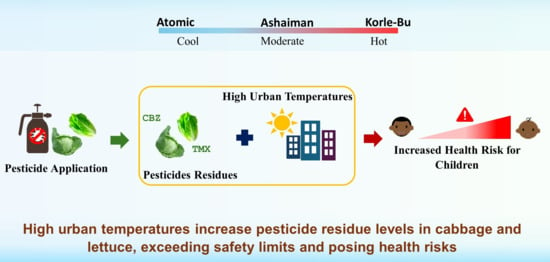
The Impact of High Urban Temperatures on Pesticide Residues Accumulation in Vegetables Grown in the Greater Accra Metropolitan Area of Ghana
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design, Sample Size, and Sampling Procedure
2.3. Sample Collection
2.4. Sample Preparation of Pesticide Residue
2.5. Pesticide Detection Using Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
2.6. Quality Control and Assurance
2.7. Human Health Index Computation
2.8. Statistical Analysis
3. Results
3.1. Concentrations of Pesticide Residue in Vegetables Based on Land Surface Temperatures
3.2. Assessment of Public Health Risks of Pesticide Residues
4. Discussion
5. Conclusions
Limitations and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Boahen, E.; Fosu-mensah, B.Y.; Koranteng, S.S.; Darko, D.A.; Obuobi, G.; Mensah, M. Potentially Toxic Elements’ Accumulation and Health Risk of Consuming Vegetables Cultivated along the Accra-Tema Motorway. J. Chem. 2024, 2024, 6438563. [Google Scholar] [CrossRef]
- Cowan, J.S. Vegetables—Definition Webster’s Definition of “Vegetable” Vegetables—Definition Vegetables—Other Features Macronutrients: Micronutrients; Elsevier: London, UK, 2018; pp. 1–37. [Google Scholar]
- Ramya, V.; Patel, P. Health benefits of vegetables. Int. J. Chem. Stud. 2019, 7, 82–87. [Google Scholar]
- Beyuo, J.; Sackey, L.N.A.; Yeboah, C.; Kayoung, P.Y.; Koudadje, D. The implications of pesticide residue in food crops on human health: A critical review. Discov. Agric. 2024, 2, 123. [Google Scholar] [CrossRef]
- Machekano, H.; Masamba, W.; Mvumi, B.M.; Nyamukondiwa, C. Cabbage or ‘pesticide’ on the platter? Chemical analysis reveals multiple and excessive residues in African vegetable markets. Int. J. Food Contam. 2019, 6, 2. [Google Scholar] [CrossRef]
- Bouchard, L.; Leis, A.; Lorenzo, P. The Impact of Weather Variability and Climate Change on pesticides applications in the US—An empirical investigation. Glob. Health Promot. 2014, 21, 64–81. [Google Scholar]
- Gbedemah, S.F.; Attasse, G.A.; Hosu-Porbley, G.S.; Kusi Frimpong, L.; Amfo-Otu, R.; Adanu, S.K.; Doe, E.K. Analysis of heavy metals and pathogen levels in vegetables cultivated using selected water bodies in urban areas of the Greater Accra Metropolis of Ghana. Heliyon 2024, 10, e27924. [Google Scholar] [CrossRef]
- Xi, N.; Xia, X.; Li, Y. Climate warming inhibits neonicotinoid photodegradation on vegetable leaves: Important role of the olefin group in leaf wax. Sci. Total Environ. 2023, 882, 163399. [Google Scholar] [CrossRef]
- Gyimah, R.R.; Kwang, C.; Antwi, R.A.; Morgan Attua, E.; Owusu, A.B.; Doe, E.K. Trading greens for heated surfaces: Land surface temperature and perceived health risk in Greater Accra Metropolitan Area, Ghana. Egypt. J. Remote Sens. Space Sci. 2023, 26, 861–880. [Google Scholar] [CrossRef]
- UN-DESA. World Urbanization Prospects: The 2018 Revision, Demographic Research; United Nations Secretariat: New York, NY, USA, 2018. [Google Scholar]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Atitsogbey, P.; Kyereh, E.; Ofori, H.; Johnson, P.T.; Steiner-asiedu, M. Heliyon Heavy metal, microbial and pesticides residue contaminations are limiting the potential consumption of green leafy vegetables in Ghana: An overview. Heliyon 2023, 9, e15466. [Google Scholar] [CrossRef]
- Quansah, J.K.; Saalia, F.K.; Chen, J. Pesticides residues in leafy green vegetables and irrigation waters in Accra, Ghana. Food Addit. Contam. Part B 2024, 17, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Zhou, E.; Xiao, P.; Sun, X.; Yang, J. Pesticide overuse in vegetable production: A case study of urban agriculture in city x, China. Environ. Res. Commun. 2023, 5, 085012. [Google Scholar] [CrossRef]
- Dankyi, E.; Gordon, C.; Carboo, D.; Fomsgaard, I.S. Quantification of neonicotinoid insecticide residues in soils from cocoa plantations using a QuEChERS extraction procedure and LC-MS/MS. Sci. Total Environ. 2014, 499, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Nishant, N.; Upadhyay, R. Presence of pesticide residue in vegetable crops: A review. Agric. Rev. 2016, 37, 173–185. [Google Scholar] [CrossRef]
- Santarelli, G.A.; Migliorati, G.; Pomilio, F.; Marfoglia, C.; Centorame, P.; D’Agostino, A.; D’Aurelio, R.; Scarpone, R.; Battistelli, N.; Di Simone, F.; et al. Assessment of pesticide residues and microbial contamination in raw leafy green vegetables marketed in Italy. Food Control 2018, 85, 350–358. [Google Scholar] [CrossRef]
- Tudi, M.; Li Hairong Li Hongying Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; Phung, D.T.; Sadler, R.; et al. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Grazia, C.; Malorgio, G. Food safety standards and international supply chain organization: A case study of the moroccan fruit and vegetable exports. Food Control 2015, 55, 190–199. [Google Scholar] [CrossRef]
- Omari, R.; Frempong, G. Food safety concerns of fast food consumers in urban Ghana. Appetite 2016, 98, 49–54. [Google Scholar] [CrossRef]
- Sharma, N.; Kumar, A.; Singh, S.; Kumar, S.; Joshi, R. Multi-residue determination of pesticides in vegetables and assessment of human health risks in Western Himalayan region of India. Environ. Monit. Assess. 2022, 194, 332. [Google Scholar] [CrossRef]
- Ma, C.-S.; Zhang, W.; Peng, Y.; Zhao, F.; Chang, X.-Q.; Xing, K.; Zhu, L.; Ma, G.; Yang, H.-P.; Rudolf, V.H.W. Climate warming promotes pesticide resistance through expanding overwintering range of a global pest. Nat. Commun. 2021, 12, 5351. [Google Scholar] [CrossRef]
- Bloomfield, J.P.; Williams, R.J.; Gooddy, D.C.; Cape, J.N.; Guha, P. Impacts of climate change on the fate and behaviour of pesticides in surface and groundwater—A UK perspective. Sci. Total Environ. 2006, 369, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Ghana Statistical Service. Ghana 2021 Population and Housing Census, Poulation of Regions and Districts; Ghana Statistical Service: Accra, Ghana, 2021. [Google Scholar]
- Wemegah, C.S.; Yamba, E.I.; Aryee, J.N.A.; Sam, F.; Amekudzi, L.K. Assessment of urban heat island warming in the greater accra region. Sci. Afr. 2020, 8, e00426. [Google Scholar] [CrossRef]
- Bonsu, K.; Bonin, O. Assessing landscape fragmentation and its implications for biodiversity conservation in the Greater Accra Metropolitan Area (GAMA) of Ghana. Discov. Environ. 2023, 1, 22. [Google Scholar] [CrossRef]
- Lente, I.; Ofosu-Anim, J.; Brimah, A.K.; Atiemo, S. Heavy metal pollution of vegetable crops irrigated with wastewater in Accra, Ghana. West Afr. J. Appl. Ecol. 2014, 22. [Google Scholar]
- Cunha, S.C.; Lehotay, S.J.; Mastovska, K.; Fernandes, J.O.; Beatriz, M.; Oliveira, P.P. Evaluation of the QuEChERS sample preparation approach for the analysis of pesticide residues in olives. J. Sep. Sci. 2007, 30, 620–632. [Google Scholar] [CrossRef]
- Kelle, H.I.; Oluade, O.A.; Achem, D. Human Health Risk Assessment of Pesticide Residues in Solanum lycopersicum Fruit Sold in Lagos Metropolis, South-West Nigeria. Commun. Phys. Sci. 2020, 2020, 533–543. [Google Scholar]
- Lehotay, S.J.; O’Neil, M.; Tully, J.; García, A.V.; Contreras, M.; Mol, H.; Heinke, V.; Anspach, T.; Lach, G.; Fussell, R.; et al. Determination of Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate: Collaborative Study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [CrossRef]
- Deveci, B.; Golge, O.; Kabak, B. Quantification of 363 Pesticides in Leafy Vegetables (Dill, Rocket and Parsley) in the Turkey Market by Using QuEChERS with LC-MS/MS and GC-MS/MS. Foods 2023, 12, 1034. [Google Scholar] [CrossRef]
- Jallow, M.F.A.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Ahmad, N. Monitoring of pesticide residues in commonly used fruits and vegetables in Kuwait. Int. J. Environ. Res. Public Health 2017, 14, 833. [Google Scholar] [CrossRef]
- Verdini, E.; Pacini, T.; Orsini, S.; Sdogati, S.; Pecorelli, I. Chromatographic Comparison of Commercially Available Columns for Liquid Chromatography in Polar Pesticide Detection and Quantification Using a Score-Based Methodology. Foods 2024, 13, 3131. [Google Scholar] [CrossRef]
- Yuan, X.; Kim, C.J.; Lee, R.; Kim, M.; Shin, H.J.; Kim, L.; Jeong, W.T.; Shin, Y.; Kyung, K.S.; Noh, H.H. Validation of a Multi-Residue Analysis Method for 287 Pesticides in Citrus Fruits Mandarin Orange and Grapefruit Using Liquid Chromatography–Tandem Mass Spectrometry. Foods 2022, 11, 3522. [Google Scholar] [CrossRef] [PubMed]
- Okoffo, E.D.; Fosu-Mensah, B.Y.; Gordon, C. Persistent organochlorine pesticide residues in cocoa beans from Ghana, a concern for public health. Int. J. Food Contam. 2016, 3, 5. [Google Scholar] [CrossRef]
- USEPA. Human Health Risk Assessment Protocol. In Chapter 6: Quantifying Exposure, Multimedia Planning and Permitting Division, Office of Solid Waste, Center for Combustion Science and Engineering; 2005. Available online: https://archive.epa.gov/epawaste/hazard/tsd/td/web/pdf/05hhrap6.pdf (accessed on 27 April 2020).
- United States Environmental Protection Agency. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures; Risk Assessment Forum Technical Panel [EPA/630/R-00/002]; United Environmental Protection Agency States: Washington, DC, USA, 2000. [Google Scholar]
- Callahan, M.A.; Sexton, K. If cumulative risk assessment is the answer, what is the question? Environ. Health Perspect. 2007, 115, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.M.; Price, P.S. An analysis of cumulative risks based on biomonitoring data for six phthalates using the Maximum Cumulative Ratio. Environ. Int. 2018, 112, 77–84. [Google Scholar] [CrossRef]
- EPA U.S. Environmental Protection Agency (EPA). Pesticide Tolerances and Risk Assessments; U.S. EPA, Office of Pesticide Programs: Washington, DC, USA, 2021. Available online: https://www.epa.gov/pesticides (accessed on 18 March 2025).
- Minnesota Department of Health (MDH). MDH Health Risk Assessment Methods to Incorporate Human Equivalent Dose Calculations into Derivation of Oral Reference Doses; Originally published May 2011, revised 2017. Available online: https://www.health.state.mn.us/communities/environment/risk/docs/guidance/hedrefguide.pdf (accessed on 24 March 2024).
- Fuortes, L.J.; Ayebo, A.D.; Kross, B.C. Cholinesterase-inhibiting insecticide toxicity. Am. Fam. Physician 1993, 47, 1613–1620. [Google Scholar]
- Jennings, A.A.; Li, Z. Worldwide Regulatory Guidance Values Applied to Direct Contact Surface Soil Pesticide Contamination: Part I—Carcinogenic Pesticides. Air Soil Water Res. 2017, 10, 1178622117711930. [Google Scholar] [CrossRef]
- European Union (EU) Pesticides Database. Regulatory Framework for Pesticide Residues in Food: Maximum Residue Limits (MRLs); European Commission (EU): Brussels, Belgium, 2023; Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database_en (accessed on 18 March 2025).
- FAO/WHO (Codex Alimentarius). Codex Pesticides Residues in Food and Feed; Food and Agriculture Organization & World Health Organization: Rome, Italy, 2021; Available online: https://www.fao.org/fao-who-codexalimentarius (accessed on 18 March 2025).
- European Food Safety Authority (EFSA). The 2022 European Union report on pesticide residues in food. EFSA J. 2022, 20, e07271. [Google Scholar] [CrossRef]
- European Food Safety Authority(EFSA). Statement on the assessment of quality of data available to EFSA to derive the health-based guidance values for carbendazim. EFSA J. 2024, 22, e8756. [Google Scholar] [CrossRef]
- Costa, L.G.; Giordano, G.; Guizzetti, M.; Vitalone, A. Neurotoxicity of pesticides: A brief review. Front. Biosci. J. Virtual Libr. 2008, 13, 1240–1249. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019; WHO Press: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications-detail/classification-of-pesticides-by-hazard (accessed on 18 March 2025).
- Stockholm Convention on Persistent Organic Pollutants (POPs). Chlordane and Other Persistent Organic Pollutants: Global Elimination Strategy; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2017; Available online: https://chm.pops.int (accessed on 18 March 2025).
- Jang, S.; Shao, K.; Chiu, W.A. Beyond the cancer slope factor: Broad application of Bayesian and probabilistic approaches for cancer dose-response assessment. Environ. Int. 2023, 175, 107959. [Google Scholar] [CrossRef]
- Arbizu, M.P. pairwiseAdonis: Pairwise Multilevel Comparison Using Adonis, R Packag. version 0.4; GitHub, Inc.: San Francisco, CA, USA, 2020. [Google Scholar]
- Gad Alla, S.A.; Loutfy, N.M.; Shendy, A.H.; Ahmed, M.T. Hazard index, a tool for a long term risk assessment of pesticide residues in some commodities, a pilot study. Regul. Toxicol. Pharmacol. 2015, 73, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Lozowicka, B.; Rutkowska, E.; Jankowska, M.; Hrynko, I.; Kaczynski, P. Toxicological evaluation of multi-class pesticide residues in vegetables and associated human health risk study for adults and children. Hum. Ecol. Risk Assess. 2016, 22, 1480–1505. [Google Scholar] [CrossRef]
- Struciński, P.; Ludwicki, J.K.; Góralczyk, K.; Czaja, K.; Hernik, A.; Liszewska, M. Risk assessment for pesticides’ MRL non-compliances in Poland in the years 2011–2015. Rocz. Państw. Zakł. Hig. 2015, 66, 309–317. [Google Scholar]
- Rhodes, L.A.; McCarl, B.A. An Analysis of Climate Impacts on Herbicide, Insecticide, and Fungicide Expenditures. Agronomy 2020, 10, 745. [Google Scholar] [CrossRef]
- Shalaby, S.E.M.; Abdou, G.Y.; El-Metwally, I.M.; Abou-Elella, G.M.A. Health risk assessment of pesticide residues in vegetables collected from Dakahlia, Egypt. J. Plant Prot. Res. 2021, 61, 254–264. [Google Scholar] [CrossRef]
- Yu, R.; Liu, J.S.; Wang, Q.C.; Liu, Q.; Wang, Y. Huan jing ke xue. Huanjing Kexue 2015, 36, 3486–3492. [Google Scholar]
- Leal Filho, W.; Echevarria Icaza, L.; Emanche, V.O.; Quasem Al-Amin, A. An Evidence-Based Review of Impacts, Strategies and Tools to Mitigate Urban Heat Islands. Int. J. Environ. Res. Public Health 2017, 14, 1600. [Google Scholar] [CrossRef]
- Ulpiani, G. On the linkage between urban heat island and urban pollution island: Three-decade literature review towards a conceptual framework. Sci. Total Environ. 2021, 751, 141727. [Google Scholar] [CrossRef]
- Abolaji, A.; Awogbindin, I.; Adedara, I.; Farombi, E. Insecticide chlorpyrifos and fungicide carbendazim, common food contaminants mixture, induce hepatic, renal, and splenic oxidative damage in female rats. Hum. Exp. Toxicol. 2017, 36, 483–493. [Google Scholar] [CrossRef]
- Cantor, K.P.; Weisenburger, D.D.; Pahwa, P.; Dosman, J.A.; McLaughlin, J.R.; Demers, P.A.; Harris, S.A. Insecticide use and risk of non-Hodgkin lymphoma subtypes: A subset meta-analysis of the North American Pooled Project. Int. J. Cancer 2020, 147, 3370–3383. [Google Scholar] [CrossRef]
- Palanikumar, L.; Kumaraguru, A.K.; Ramakritinan, C.M.; Anand, M. Toxicity, biochemical and clastogenic response of chlorpyrifos and its impact on aquatic organisms: A review. Int. J. Aquat. Biol. 2014, 1, 281–288. [Google Scholar]
- Küblbeck, J.; Niskanen, J.; Honkakoski, P. Metabolism-Disrupting Chemicals and the Constitutive Androstane Receptor CAR. Cells 2020, 9, 2306. [Google Scholar] [CrossRef] [PubMed]











| Study Site | Reference LST * | Classified | Verified Air Temperature | Cabbage Sample/Farmer | Lettuce Sample/Farmer | Total |
|---|---|---|---|---|---|---|
| Korle Bu | High | 29.40 °C | 11 | 11 | 22 | |
| Ashaiman | Moderate | 28.17 °C | 11 | 11 | 22 | |
| Atomic (Haatso) | Low | 27.00 °C | 11 | 11 | 22 | |
| Total | 33 | 33 | 66 |
| Pesticide Active Ingredient | Description, Human Health Risk | Health Hazard | MRL (mg/kg) | RfD (mg/kg/day) | Agency |
|---|---|---|---|---|---|
| Acetochlor (ACT) | A chloroacetanilide herbicide for controlling grasses and broadleaf weeds, classified as a possible human carcinogen. | C | 0.4 | 0.0039 | [41,42] |
| Chlorothalonil (CTD) | A broad-spectrum fungicide used to protect vegetables from fungal diseases. Metabolite toxicity leads to strict residue limits in food. | NC | 0.02 | 0.015 | [41,43,44] |
| Thiamethoxam (TMX) | Neonicotinoid insecticide affects the nervous systems of insects. Outdoor use is banned by the European Union (EU) due to risks to pollinators. | C | 0.02 | 0.007 | [41,43,45] |
| Imidacloprid (IMI) | A neonicotinoid linked to pollinator decline and neurotoxic effects and reproductive issues. | NC | 0.01 | 0.06 | [41,43,46] |
| Carbendazim (CBZ) | A benzimidazole fungicide controlling fungal pathogens in vegetables. It causes endocrine disruption and reproductive toxicity. | NC | 0.1 | 0.012 | [47,48] |
| Acephate (ACP) | An organophosphate insecticide inhibiting acetylcholinesterase, affecting the nervous system and causing neurotoxicity concerns. | C | 10 | 0.02 | [41,43] |
| Azoxystrobin (AZL) | A strobilurin fungicide widely used in vegetable farming. It is safe within MRLs, and long-term exposure studies are ongoing. | NC | 0.02 | [41,47] | |
| Chlorfenvinphos (CHF) | An organophosphate insecticide with neurotoxic effects. It is banned by the EU due to health risks but still used under Codex MRLs. | NC | 0.01 | 0.0025 | [41,43,45] |
| Dimethoate (DMT) | A systemic organophosphate insecticide with high acute toxicity. The EFSA banned its use in food crops, citing developmental neurotoxicity risks. | C | 0.01 | 0.006 | [49] |
| Monocrotophos (MON) | A highly toxic organophosphate insecticide. It is classified as extremely hazardous due to neurotoxic effects. | NC | 0.01 | [43,50] | |
| Prochloraz (PRC) | An imidazole fungicide used to control fungal diseases in vegetables. It was assessed for its endocrine-disrupting potential. | C | 0.03 | 0.009 | [41,43,47] |
| Chlordane (CHL) | A chlorinated hydrocarbon insecticide with high persistence. It is banned globally under the Stockholm Convention on Persistent Organic Pollutants (SCPOP) due to carcinogenic and bioaccumulation. | C | 0.01 | 0.0005 | [43,51] |
| Factor | Df | Sum of Squares | R² | F-Value | p-Value |
|---|---|---|---|---|---|
| 1. Pairwise comparisons of mean pesticide residue concentration by site | |||||
| Atomic vs. Korle Bu | 1 | 0.064 | 0.0011 | 0.5362 | 0.608 |
| Atomic vs. Ashaiman | 1 | 0.063 | 0.0012 | 0.5541 | 0.569 |
| Korle Bu vs. Ashaiman | 1 | 0.039 | 0.0007 | 0.3293 | 0.776 |
| 2. Pairwise comparisons of mean pesticide residue concentration by composition | |||||
| Comparison | Df | Sum of Squares | R² | F-Value | p-Value |
| ACT vs. CTD | 1 | 0.1833 | 0.0097 | 1.1537 | 0.285 |
| ACT vs. TMX | 1 | 0.3781 | 0.0285 | 3.4668 | 0.03 * |
| ACT vs. ACP | 1 | 0.5134 | 0.0342 | 4.1764 | 0.024 * |
| ACT vs. CHF | 1 | 0.4571 | 0.0382 | 4.682 | 0.013 * |
| ACT vs. DMT | 1 | 0.6501 | 0.0555 | 6.9285 | 0.001 *** |
| ACT vs. PRC | 1 | 0.8016 | 0.0775 | 9.9119 | 0.001 *** |
| ACT vs. CHL | 1 | 0.4223 | 0.0323 | 3.9373 | 0.028 * |
| Factor | Df | Sum of Squares | R² | F-Value | p-Value |
|---|---|---|---|---|---|
| 1. Permanova results: mean pesticide residue concentration by vegetable and composition | |||||
| Model | 23 | 6.857 | 0.08122 | 2.6751 | 0.001 *** |
| Residual | 696 | 77.563 | 0.91878 | ||
| Total | 719 | 84.419 | 1 | ||
| 2. Pairwise comparisons of mean pesticide residue concentration by vegetables | |||||
| Cabbage vs. Lettuce | 1 | 0.077 | 0.00092 | 0.6576 | 0.505 |
| 3. Pairwise comparisons of mean pesticide residue concentration by composition | |||||
| ACT vs. CTD | 1 | 0.1833 | 0.0097 | 1.1537 | 0.297 |
| ACT vs. TMX | 1 | 0.3781 | 0.0285 | 3.4668 | 0.035 * |
| ACT vs. ACP | 1 | 0.5134 | 0.0342 | 4.1764 | 0.018 * |
| ACT vs. CHF | 1 | 0.4571 | 0.0382 | 4.682 | 0.01 ** |
| ACT vs. DMT | 1 | 0.6501 | 0.0555 | 6.9285 | 0.004 ** |
| ACT vs. PRC | 1 | 0.8016 | 0.0775 | 9.9119 | 0.002 ** |
| ACT vs. CHL | 1 | 0.4223 | 0.0323 | 3.9373 | 0.026 * |
| CTD vs. TMX | 1 | 0.6956 | 0.0411 | 5.0672 | 0.008 ** |
| CTD vs. CHL | 1 | 0.7235 | 0.0430 | 5.340 | 0.004 ** |
| IMI vs. PRC | 1 | 2.1752 | 0.1423 | 19.578 | 0.001 *** |
| IMI vs. CHL | 1 | 1.4291 | 0.0810 | 10.395 | 0.001 *** |
| CBZ vs. PRC | 1 | 0.5363 | 0.0469 | 5.8016 | 0.007 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumah, J.; Doe, E.K.; Fosu-Mensah, B.Y.; Ofori, B.D.; Kwawu, M.A.S.; Boahen, E.; Lartey, D.L.; Dordaa, S.D.D.P.; Gordon, C. The Impact of High Urban Temperatures on Pesticide Residues Accumulation in Vegetables Grown in the Greater Accra Metropolitan Area of Ghana. J. Xenobiot. 2025, 15, 103. https://doi.org/10.3390/jox15040103
Kumah J, Doe EK, Fosu-Mensah BY, Ofori BD, Kwawu MAS, Boahen E, Lartey DL, Dordaa SDDP, Gordon C. The Impact of High Urban Temperatures on Pesticide Residues Accumulation in Vegetables Grown in the Greater Accra Metropolitan Area of Ghana. Journal of Xenobiotics. 2025; 15(4):103. https://doi.org/10.3390/jox15040103
Chicago/Turabian StyleKumah, Joyce, Eric Kofi Doe, Benedicta Yayra Fosu-Mensah, Benjamin Denkyira Ofori, Millicent A. S. Kwawu, Ebenezer Boahen, Doreen Larkailey Lartey, Sampson D. D. P. Dordaa, and Christopher Gordon. 2025. "The Impact of High Urban Temperatures on Pesticide Residues Accumulation in Vegetables Grown in the Greater Accra Metropolitan Area of Ghana" Journal of Xenobiotics 15, no. 4: 103. https://doi.org/10.3390/jox15040103
APA StyleKumah, J., Doe, E. K., Fosu-Mensah, B. Y., Ofori, B. D., Kwawu, M. A. S., Boahen, E., Lartey, D. L., Dordaa, S. D. D. P., & Gordon, C. (2025). The Impact of High Urban Temperatures on Pesticide Residues Accumulation in Vegetables Grown in the Greater Accra Metropolitan Area of Ghana. Journal of Xenobiotics, 15(4), 103. https://doi.org/10.3390/jox15040103