Effects of a Proteinase Inhibitor from Inga laurina Seeds (ILTI) on Aedes aegypti Larval Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Reagents
2.2. ILTI Purification
2.3. Protein Quantification
2.4. Ae. aegypti Larvae
2.5. Effect of ILTI on Larval Development
2.6. Midgut Larvae
2.7. Enzymatic Activity Assays
2.7.1. Tryptic Activity
2.7.2. Chymotryptic Activity
2.7.3. Acetylcholinesterase Activity
2.7.4. Acid and Alkaline Phosphatases
2.8. Ex Vivo Inhibition of Tryptic Activity in Aedes aegypti L4 Exposed to ILTI
2.9. Molecular Modelling
2.10. Molecular Docking
2.11. Statistical Analysis
3. Results
3.1. Effect of ILTI on Ae. aegypti Larval Development
3.2. Effects of ILTI on Ae. aegypti Larval Weight
3.3. Effects of ILTI on Ae. aegypti Larval Survival
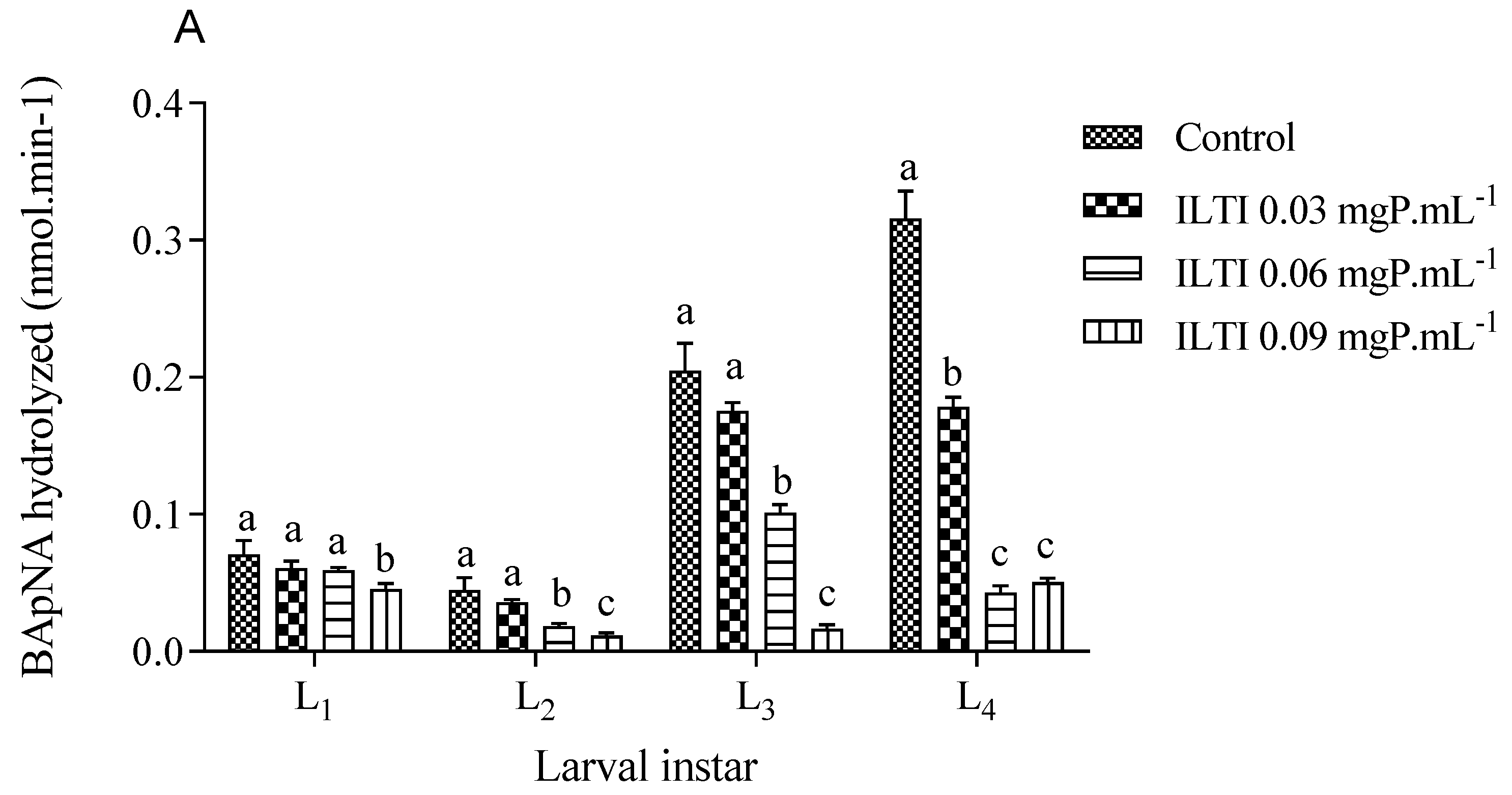
3.4. Effect of ILTI on the Tryptic and Chymotryptic Activities of Ae. aegypti
3.5. Effect of ILTI on the Acetylcholinesterase Activity of Ae. aegypti
3.6. Effect of ILTI on the Activity of Acid and Alkaline Phosphatases in Ae. aegypti
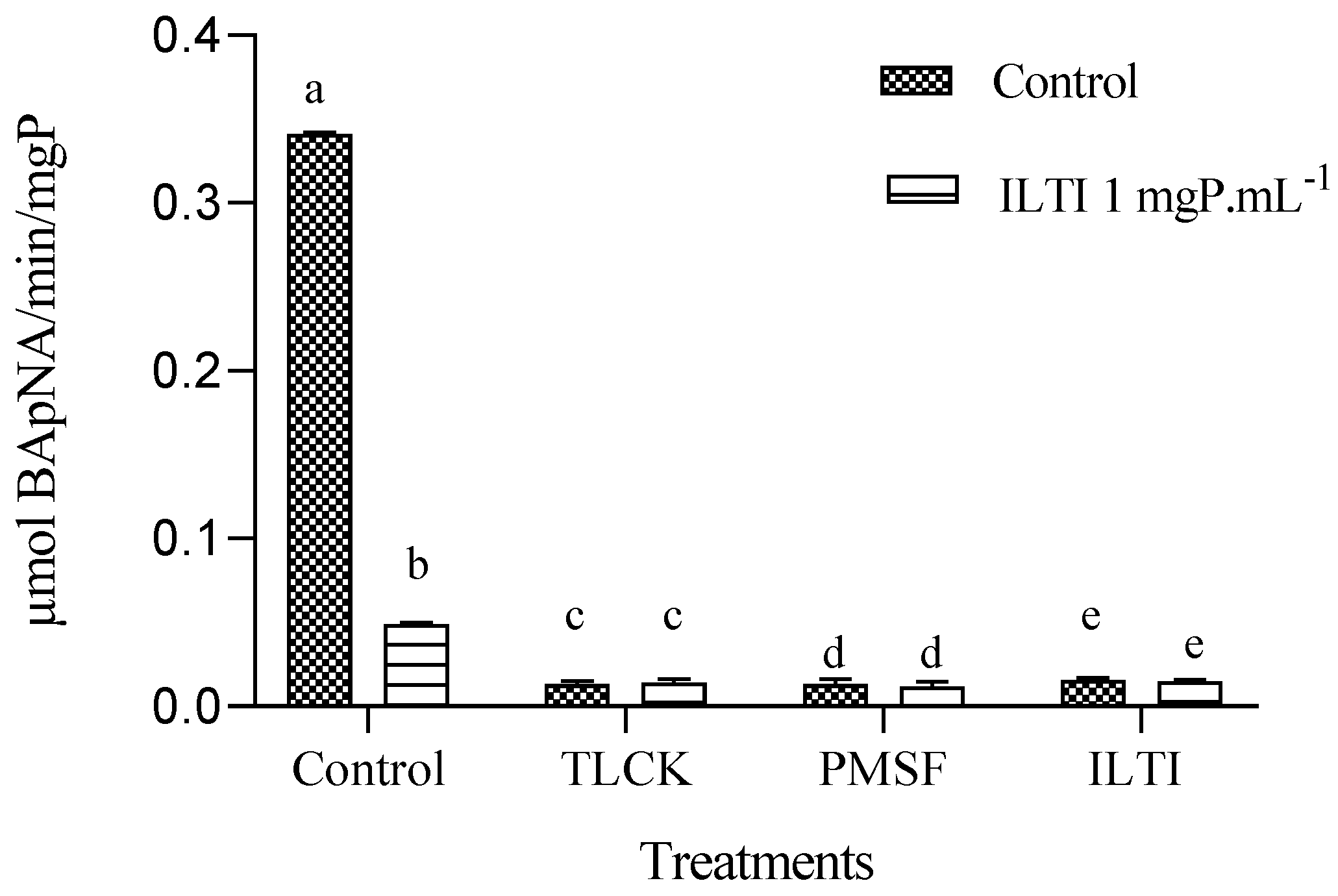
3.7. Ex Vivo Inhibition of Tryptic Activity in Aedes aegypti L4 Exposed to ILTI
3.8. Molecular Modelling and Docking Simulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO – World Health Organization. Updated WHO Guidance for Controlling Vector-Borne Diseases Through Indoor Residual Spraying. 2024. Available online: https://www.who.int/news/item/15-02-2024-updated-who-guidance-for-controlling-vector-borne-diseases-through-indoor-residual-spraying (accessed on 5 August 2024).
- Kudom, A.A. Entomological surveillance to assess potential outbreak of Aedes-borne arboviruses and insecticide resistance status of Aedes aegypti from Cape Coast, Ghana. Acta Tropica 2020, 202, 105257. [Google Scholar] [CrossRef] [PubMed]
- Laporta, G.Z.; Potter, A.M.; Oliveira, J.F.A.; Bourke, B.P.; Pecor, D.B.; Linton, Y.-M. Global Distribution of Aedes aegypti and Aedes albopictus in a Climate Change Scenario of Regional Rivalry. Insetos 2023, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Asgarian, T.; Vatandoost, H.; Hanafi-Bojd, A.; Nikpoor, F. Worldwide Status of Insecticide Resistance of Aedes aegypti and Ae. albopictus, Vectors of Arboviruses of Chikungunya, Dengue, Zika and Yellow Fever. J. Arthropod-Borne Dis. 2023, 17, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.; Chrissian, C.; Rankin-Turner, S.; Desgaste, M.; Camacho, E.; Broderick, N.A.; McMeniman, C.J.; Stark, R.E.; Casadevall, A. Cuticular profiling of insecticide resistant Aedes aegypti. Sci. Rep. 2023, 13, 10154. [Google Scholar] [CrossRef]
- Silva, L.S.S.; Fernandes, K.M.; Miranda, F.R.; Silva, S.C.C.; Coelho, L.C.B.B.; Navarro, D.M.D.A.F.; Napoleão, T.H.; Martins, G.F.; Paiva, P.M.G. Exposure of mosquito (Aedes aegypti) larvae to the water extract and lectin-rich fraction of Moringa oleifera seeds impairs their development and future fecundity. Ecotoxicol. Environ. Saf. 2019, 183, 109583. [Google Scholar] [CrossRef]
- Carvalho, F.D.; Moreira, L.A. Why is Aedes aegypti Linnaeus so successful as a species? Neotrop. Entomol. 2017, 46, 243–255. [Google Scholar] [CrossRef]
- Cotabarren, J.; Lufrano, D.; Parisi, M.G.; Obregón, W.D. Biotechnological, biomedical, and agronomical applications of plant protease inhibitors with high stability: A systematic review. J. Plant Sci. 2020, 292, 110398. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Zeng, R. Insect Response to Plant Defensive Protease Inhibitors. Ann. Rev. Entomol. 2015, 60, 233–252. [Google Scholar] [CrossRef]
- Wilkins, R. Insecticide resistance and intracellular proteases. Pest. Manag. Sci. 2017, 73, 2403–2412. [Google Scholar] [CrossRef]
- Manzato, V.M.; Torquato, R.J.S.; Lemos, F.J.A.; Nishiduka, E.; Tashima, A.K.; Tanaka, A.S. A versatile inhibitor of digestive enzymes in Aedes aegypt larvae selected from a pacifastin (TiPi) phage display library. Biochem. Biophys. Res. Commun. 2022, 590, 139–144. [Google Scholar] [CrossRef]
- Borovsky, D.; Rougé, P. Cloning and characterization of Aedes aegypti blood downregulated chymotrypsin II. Arch. Insect Biochem. Physiol. 2023, 114, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Macedo, M.L.R.; Garcia, V.A.; Freire, M.G.M.; Richardson, M. Characterization of a Kunitz trypsin inhibitor with a single disulfide bridge from seeds of Inga laurina (SW.) Wild. Phytochemistry 2007, 68, 1104–1111. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 7, 248–254. [Google Scholar] [CrossRef]
- Sasaki, D.Y.; Jacobowski, A.C.; de Souza, A.P.; Cardoso, M.H.; Franco, O.L.; Macedo, M.L. Effects of proteinase inhibitor from Adenanthera pavonina seeds on short- and long term larval development of Aedes aegypti. Biochimie 2015, 112, 172–186. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides. 2005. Available online: https://www.who.int/publications/i/item/WHO-CDS-WHOPES-GCDPP-2005.13 (accessed on 5 August 2024).
- Coelho, J.S.; Santos, N.D.; Napoleao, T.H.; Gomes, F.S.; Ferreira, R.S.; Zingali, R.B.; Coelho, L.C.; Leite, S.P.; Navarro, D.M.; Paiva, P.M. Effect of Moringa oleifera lectin on development and mortality of Aedes aegypti larvae. Chemosphere 2009, 77, 934–938. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Nokowsky, N.; Cohen, W. The preparation and properties of two chromogenic substrates of trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef]
- Christeller, J.T.; Laing, W.A.; Markwick, N.P.; Burgess, E.P.J. Midgut protease activities in 12 phytophagous lepidopteran larvae: Dietary and protease inhibitor interactions. Insect Biochem. Mol. Biol. 1992, 22, 735–746. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Asakura, K. Phosphatase activity in the larva of the euryhaline mosquito, Aedes togoi (Theobald), with special reference to seawater adaptation. J. Exp. Mar. Biol. Ecol. 1978, 31, 325–337. [Google Scholar] [CrossRef]
- Kall, L.; Krogh, A.; Sonnhammer, E.L.L. Advantages of combined transmembrane topology and signal peptide prediction—the Phobius web server. Nucleic Acids Res. 2007, 35, 429–432. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Jabłońska, J.; Pravda, L.; Vařeková, R.S.; Thornton, J.M. PDBsum: Structural summaries of PDB entries. Protein Sci. 2018, 27, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendal III, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D Struc. Biol. 2010, 66, 12–21. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multi- threading. J. Comput. Chem. 2010, 31, 455e461. [Google Scholar] [CrossRef]
- Valle, D.; Pimenta, D.N.; Cunha, R.V. Dengue: Teorias e Práticas (Online); Editora Fiocruz: Rio de Janeiro, Brazil, 2015; 450p, ISBN 978-85-7541-552-8. [Google Scholar] [CrossRef]
- Crow, J.F. Genetics of insect resistance to chemicals. Annu. Ver. Entomol. 1957, 2, 227–246. [Google Scholar] [CrossRef]
- Dittmer, J.; Gabrieli, P. Transstadial metabolic priming mediated by larval nutrition in female Aedes albopictus mosquitoes. J. Insect Physiol. 2020, 123, 104053. [Google Scholar] [CrossRef]
- Sá, G.C.S.; Bezerra, P.V.V.; da Silva, M.F.A.; da Silva, L.B.; Barra, P.B.; Ximenes, M.F.F.M.; Uchôa, A.F. Arbovirus vectors insects: Are botanical insecticides an alternative for its management? J. Pest. Sci. 2022, 96, 1–20. [Google Scholar] [CrossRef]
- Silva, L.L.M.; Garrido, R.G. Organophosphorus and organochlorines: Medical toxicology and environmental reflexes. Res. Soc. Dev. 2021, 10, e313101018853. [Google Scholar] [CrossRef]
- Almeida, W.A.; Novab, I.C.V.; Nascimento, J.S.; Mourab, M.C.; Agra-Neto, A.C.; da Costa, H.N.; Cruz, G.d.S.; Teixeira, Á.A.C.; Wanderley-Teixeira, V.; Ferreira, M.R.A.; et al. Effects of Plectranthus barbatus leaf extract on survival, digestive proteases, midgut morphophysiology and gut microbiota homeostasis of Aedes aegypti larvae. S. Afr. J. Bot. 2021, 141, 116–125. [Google Scholar] [CrossRef]
- Pontual, E.V.; Napoleão, T.H.; Dias de Assis, C.R.; de Souza Bezerra, R.; Xavier, H.S.; Navarro, D.M.; Coelho, L.C.B.B.; Paiva, P.M.G. Effect of Moringa oleifera flower extract on larval trypsin and acetylcholinesterase activities in Aedes aegypti. Arch. Insect Biochem. Physiol. 2012, 79, 135–152. [Google Scholar] [CrossRef]
- Viana, J.L.; Soares-da-Silva, J.; Vieira-Neta, M.R.A.; Tadei, W.P.; Oliveira, C.D.; Abdalla, F.C.; Peixoto, C.A.; Pinheiro, V.C.S. Isolates of Bacillus thuringiensis from Maranhão biomes with potential insecticidal action against Aedes aegypti larvae (Diptera, Culicidae). Braz. J. Biol. 2020, 81, 114–124. [Google Scholar] [CrossRef]
- Macedo, M.L.R.; Durigan, R.A.; da Silva, D.S.; Marangoni, S.; Machado Freire, M.G.; Parra, J.R. Adenanthera pavonina trypsin inhibitor retard growth of Anagasta kuehniella (Lepidoptera: Pyralidae). Arch. Insect Biochem. Physiol. 2010, 73, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.W.; Oliveira, C.F.R.; Bezerra, C.S.; Machado Freire, M.G.; Kill, M.R.; Machado, O.L.T.; Marangoni, S.; Macedo, M.L.R. Purification of a Kunitz-type inhibitor from Acacia polyphylla DC Seeds: Characterization and insecticidal properties against Anagasta kuehniella Zeller (Lepidoptera: Pyralidae). J. Agric. Food Chem. 2013, 61, 2469–2478. [Google Scholar] [CrossRef]
- Dias, L.P.; Oliveira, J.T.; Rocha-Bezerra, L.C.; Sousa, D.O.; da Costa, H.P.S.; Araujo, N.M.; Carvalho, A.F.; Tabosa, P.M.; Monteiro-Moreira, A.C.; Lobo, M.D.; et al. A trypsin inhibitor purified from Cassia leiandra seeds has insecticidal activity against Aedes aegypti. Process. Biochem. 2017, 57, 228–238. [Google Scholar] [CrossRef]
- Ramos, V.S.; Cabrera, O.G.; Camargo, E.L.O.; Ambrósio, A.B.; Vidal, R.O.; Silva, D.S.; Guimarães, L.C.; Marangoni, S.; Parra, J.R.; Pereira, G.A.; et al. Molecular cloning and insecticidal effect of Inga laurina trypsin inhibitor on Diatraea saccharalis and Heliothis virescens. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2012, 156, 148–158. [Google Scholar] [CrossRef]
- Machado, S.W.; Oliveira, C.F.R.; Zério, N.G.; Parra, J.R.P.; Macedo, M.L.R. Inga laurina (ILTI) obstructs Spodoptera frugiperda trypsins expressed during adaptative mechanisms against plant protease inhibitors. Insect Biochem. Physiol. 2017, 95, e21393. [Google Scholar] [CrossRef]
- Borovsky, D.; Nauewelaers, S.; Powell, C.A.; Shatters Jr, R.G. Cloning, genetic engineering and characterization of TMOF expressed in Saccharomyces cerevisiae to control larval mosquitoes. J. Insect Physiol. 2018, 106 Pt. 2, 134–146. [Google Scholar] [CrossRef]
- Oliveira, C.F.R.; Marangoni, S.; Macedo, M.L.R. The trypsin inhibitor from Entada acaciifolia seeds affects negatively the development of Mediterranean flour moth, Anagasta kuehniella. Pestic. Biochem. Physiol. 2014, 108, 74–79. [Google Scholar] [CrossRef]
- Lai, L.; Villanueva, M.; Muruzabal-Galarza, A.; Fernández, A.B.; Unzue, A.; Toledo-Arana, A.; Caballero, P.; Caballero, C.J. Bacillus thuringiensis Cyt Proteins as Enablers of Activity of Cry and Tpp Toxins against Aedes albopictus. Toxins 2023, 15, 211. [Google Scholar] [CrossRef]
- Pacheco, S.; Gallegos, A.S.; Peláez-Aguilar, E.; Sánchez, J.; Gómez, I.; Soberón, M.; Bravo, A. CRISPR-Cas9 knockout of membrane-bound alkaline phosphatase or cadherin does not confer resistance to Cry toxins in Aedes aegypti. PLoS Neglected Trop. Dis. 2024, 18, e0012256. [Google Scholar] [CrossRef]







| Predicted Structures | Sequence Length | Stereochemistry (G-Factors) | Most Favored (%) | Outliers (%) | Bad Bonds (%) | Bad Angles (%) | Poor Rotamers (%) | Favored Rotamers (%) |
|---|---|---|---|---|---|---|---|---|
| ILTI | 178 | −0.31 | 90.4 | 2.27 | 1.91 | 0.84 | 0.00 | 98.66 |
| Chymotrypsin | 238 | −0.36 | 93.64 | 3.81 | 2.22 | 0.83 | 0.52 | 98.96 |
| Trypsin | 264 | −0.31 | 93.51 | 3.44 | 2.76 | 0.82 | 0.00 | 98.21 |
| Residues | Positions | Atom Names | Distances (Å) | Residues | Positions | Atom Names | Interactions |
|---|---|---|---|---|---|---|---|
| Trypsin | ILTI | ||||||
| Lys | 64 | NZ | 3.3 | Arg | 144 | O | HB |
| Lys | 64 | CE | 3.7 | Arg | 144 | CA | H |
| Pro | 62 | O | 3.3 | Thr | 142 | O | HB |
| Tyr | 63 | O | 3.9 | Thr | 142 | O | HB |
| Ser | 61 | O | 3.4 | Thr | 142 | O | HB |
| Pro | 62 | CB | 4.0 | Phe | 136 | CZ | H |
| Lys | 64 | CG | 4.0 | Val | 143 | CB | H |
| Lys | 65 | CG | 3.8 | Val | 143 | CG1 | H |
| Gln | 110 | OE1 | 3.8 | Thr | 211 | OG1 | HB |
| Gln | 110 | CD | 3.7 | Thr | 211 | CB | H |
| Gln | 110 | CD | 3.8 | Thr | 211 | CG2 | H |
| Gln | 110 | OE1 | 4.0 | Gln | 212 | N | HB |
| Gln | 110 | OE1 | 3.3 | Gln | 212 | NE2 | HB |
| Gly | 141 | N | 3.6 | Arg | 27 | NH1 | HB |
| Asp | 143 | OD1 | 3.4 | Arg | 27 | NH2 | SB |
| Gln | 142 | O | 3.0 | Arg | 27 | NH2 | HB |
| Gly | 141 | O | 3.8 | Arg | 27 | NH2 | HB |
| Gln | 142 | O | 3.5 | Arg | 27 | NH1 | HB |
| Pro | 73 | CG | 3.7 | Tyr | 31 | CE2 | H |
| Pro | 73 | CB | 3.7 | Tyr | 31 | CE2 | H |
| Residues | Positions | Atom Names | Distances (Å) | Residues | Positions | Atom Names | Interactions |
|---|---|---|---|---|---|---|---|
| Chymotrypsin | ILTI (gi: AFG28551) | ||||||
| Tyr | 51 | CE1 | 3.9 | Ala | 25 | CB | H |
| Tyr | 51 | O | 3.4 | Thr | 236 | OG1 | HB |
| Tyr | 18 | OH | 3.7 | Glu | 170 | O | HB |
| Lys | 176 | NZ | 3.5 | Ser | 40 | OG | HB |
| Gly | 26 | O | 3.9 | His | 32 | NE2 | HB |
| Tyr | 80 | OH | 3.8 | Ile | 173 | N | HB |
| Tyr | 80 | OH | 3.2 | Ile | 173 | O | HB |
| Val | 85 | CG2 | 3.8 | Thr | 5 | CG2 | H |
| Val | 85 | CG1 | 3.9 | Ile | 6 | CG2 | H |
| Pro | 83 | O | 3.1 | Gln | 66 | OE1 | HB |
| Asn | 178 | ND2 | 4.0 | Ser | 169 | OG | HB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobowski, A.; Leite, W.; Júnior, A.M.; Reis, E.; Pires, L.; Silva, V.; Rocha, L.; Arruda, E.; Franco, O.; Cardoso, M.; et al. Effects of a Proteinase Inhibitor from Inga laurina Seeds (ILTI) on Aedes aegypti Larval Development. J. Xenobiot. 2025, 15, 77. https://doi.org/10.3390/jox15030077
Jacobowski A, Leite W, Júnior AM, Reis E, Pires L, Silva V, Rocha L, Arruda E, Franco O, Cardoso M, et al. Effects of a Proteinase Inhibitor from Inga laurina Seeds (ILTI) on Aedes aegypti Larval Development. Journal of Xenobiotics. 2025; 15(3):77. https://doi.org/10.3390/jox15030077
Chicago/Turabian StyleJacobowski, Ana, Welington Leite, Antolim Martinez Júnior, Eduarda Reis, Lorena Pires, Vitória Silva, Layza Rocha, Eduardo Arruda, Octávio Franco, Marlon Cardoso, and et al. 2025. "Effects of a Proteinase Inhibitor from Inga laurina Seeds (ILTI) on Aedes aegypti Larval Development" Journal of Xenobiotics 15, no. 3: 77. https://doi.org/10.3390/jox15030077
APA StyleJacobowski, A., Leite, W., Júnior, A. M., Reis, E., Pires, L., Silva, V., Rocha, L., Arruda, E., Franco, O., Cardoso, M., Hiane, P., & Macedo, M. (2025). Effects of a Proteinase Inhibitor from Inga laurina Seeds (ILTI) on Aedes aegypti Larval Development. Journal of Xenobiotics, 15(3), 77. https://doi.org/10.3390/jox15030077









