Carbon-Nanotube-Based Nanocomposites in Environmental Remediation: An Overview of Typologies and Applications and an Analysis of Their Paradoxical Double-Sided Effects
Abstract
1. What Are Carbon Nanotubes (CNTs)?
1.1. Possible Structures of CNTs
1.2. Main Physicochemical Properties of CNTs
1.3. Methods for Synthesizing CNTs
Environmentally Friendly Synthesis of CNTs: Utilization of Biomass Raw Materials
2. CNTs for the Removal of Environmental Xenobiotics
2.1. CNTs for Water Detoxification
- Provisioning benefits, intended as the withdrawal of GW for human water use purposes, including agriculture, industry and human settlements.
- Regulatory assistance, intended as the buffer capacity of GW, capable of regulating GW systems’ quantity and quality.
- Supporting services, on which GW-dependent ecosystems (GWDEs) and other GW-related environmental features rely.
- Cultural assistance, linked to leisure activities, tradition, religion or spiritual values, which are associated with specific sites.
2.1.1. CNTs for Decontamination of Water with Inorganic Xenobiotics via Different Absorption Mechanisms
2.1.2. CNTs for Organic Xenobiotic Removal by Absorption
2.1.3. CNTs for Organic Xenobiotic Removal by Photocatalytic Degradation
CNT-Assisted Photocatalytic Degradation of Organic Pollutants: The Proposed Mechanism
2.1.4. Application of CNTs in Removing Pathogens and Cyanobacteria Toxins from Water
Adsorption of Microorganisms on CNTs
Adsorption of Cyanobacterial Toxins on CNTs
2.1.5. Water Treatments Using CNT-Based Filtering Membranes
CNT-Based Membranes for Water Desalination
2.2. CNTs for Detection and Remediation of Gaseous Pollutants
2.3. Regeneration of Exhausted CNTs
3. The Other Side of the Coin: The Paradoxical Toxicity of CNTs Towards the Environment and Living Beings
3.1. In Vitro Studies
3.2. In Vivo Studies: Pulmonary Toxicity
4. How Can the Toxicity of CNTs Be Moderated?
5. Conclusions, Preventive Behavior and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, G.C. Antimicrobial Nanotubes: From Synthesis and Promising Antimicrobial Upshots to Unanticipated Toxicities, Strategies to Limit Them, and Regulatory Issues. Nanomaterials 2025, 15, 633. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, G.C. Nanotubes: Carbon-Based Fibers and Bacterial Nano-Conduits Both Arousing a Global Interest and Conflicting Opinions. Fibers 2022, 10, 75. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A Review on Carbon Nanotube: An Overview of Synthesis, Properties, Functionalization, Characterization, and the Application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Mingo, N.; Stewart, D.A.; Broido, D.A.; Srivastava, D. Phonon Transmission through Defects in Carbon Nanotubes from First Principles. Phys. Rev. B 2008, 77, 033418. [Google Scholar] [CrossRef]
- Kumanek, B.; Janas, D. Thermal Conductivity of Carbon Nanotube Networks: A Review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef]
- Koziol, K.K.; Janas, D.; Brown, E.; Hao, L. Thermal Properties of Continuously Spun Carbon Nanotube Fibres. Physica E Low. Dimens. Syst. Nanostruct. 2017, 88, 104–108. [Google Scholar] [CrossRef]
- Hong, S.; Myung, S. A Flexible Approach to Mobility. Nat. Nanotechnol. 2007, 2, 207–208. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Jin, Z.; Wang, J.; Li, Y.; Jiang, K.; Fan, S. Fabrication of Ultralong and Electrically Uniform Single-Walled Carbon Nanotubes on Clean Substrates. Nano Lett. 2009, 9, 3137–3141. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z. Curved Pi-Conjugation, Aromaticity, and the Related Chemistry of Small Fullerenes (<C60) and Single-Walled Carbon Nanotubes. Chem. Rev. 2005, 105, 3643–3696. [Google Scholar] [CrossRef]
- Hussain, A.; Haider, Q.; Rehman, A.; Ahmad, H.; Baili, J.; Aljahdaly, N.H.; Hassan, A. A Thermal Conductivity Model for Hybrid Heat and Mass Transfer Investigation of Single and Multi-Wall Carbon Nano-Tubes Flow Induced by a Spinning Body. Case Stud. Therm. Eng. 2021, 28, 101449. [Google Scholar] [CrossRef]
- Sobamowo, M.G.; Akanmu, J.O.; Adeleye, O.A.; Akingbade, S.A.; Yinusa, A.A. Coupled Effects of Magnetic Field, Number of Walls, Geometric Imperfection, Temperature Change, and Boundary Conditions on Nonlocal Nonlinear Vibration of Carbon Nanotubes Resting on Elastic Foundations. Forces Mech. 2021, 3, 100010. [Google Scholar] [CrossRef]
- Monthioux, M.; Kuznetsov, V.L. Who Should Be given the Credit for the Discovery of Carbon Nanotubes? Carbon 2006, 44, 1621–1623. [Google Scholar] [CrossRef]
- Aslam, M.M.-A.; Kuo, H.-W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mota, J.P.B.; Rostam-Abadi, M.; Rood, M.J. Structural Characterization of Single-Walled Carbon Nanotube Bundles by Experiment and Molecular Simulation. Langmuir 2005, 21, 896–904. [Google Scholar] [CrossRef]
- Hemraj-Benny, T.; Bandosz, T.J.; Wong, S.S. Effect of Ozonolysis on the Pore Structure, Surface Chemistry, and Bundling of Single-Walled Carbon Nanotubes. J. Colloid. Interface Sci. 2008, 317, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, C.; Li, F.; Cheng, H.-M. Pore Structures of Multi-Walled Carbon Nanotubes Activated by Air, CO2 and KOH. J. Porous Mater. 2006, 13, 141–146. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.C.; Jung, J.H.; Kim, H.J. Pore Characterization of Multi-Walled Carbon Nanotubes Modified by KOH. Chem. Phys. Lett. 2005, 416, 251–255. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Z.; Yokogawa, K. Investigation of Preparation and Structures of Activated Carbon Nanotubes. Mater. Res. Bull. 2006, 41, 1503–1512. [Google Scholar] [CrossRef]
- Niu, J.J.; Wang, J.N.; Jiang, Y.; Su, L.F.; Ma, J. An Approach to Carbon Nanotubes with High Surface Area and Large Pore Volume. Microporous Mesoporous Mater. 2007, 100, 1–5. [Google Scholar] [CrossRef]
- Liao, Q.; Sun, J.; Gao, L. Adsorption of Chlorophenols by Multi-Walled Carbon Nanotubes Treated with HNO3 and NH3. Carbon 2008, 46, 553–555. [Google Scholar] [CrossRef]
- Lu, C.; Su, F. Adsorption of Natural Organic Matter by Carbon Nanotubes. Sep. Purif. Technol. 2007, 58, 113–121. [Google Scholar] [CrossRef]
- Murr, L.E.; Bang, J.J.; Esquivel, E.V.; Guerrero, P.A.; Lopez, D.A. Carbon Nanotubes, Nanocrystal Forms, and Complex Nanoparticle Aggregates in Common Fuel-Gas Combustion Sources and the Ambient Air. J. Nanopart. Res. 2004, 6, 241–251. [Google Scholar] [CrossRef]
- Vander Wal, R.L. Fe-Catalyzed Single-Walled Carbon Nanotube Synthesis within a Flame Environment. Combust. Flame 2002, 130, 37–47. [Google Scholar] [CrossRef]
- Saveliev, A.V.; Merchan-Merchan, W.; Kennedy, L.A. Metal Catalyzed Synthesis of Carbon Nanostructures in an Opposed Flow Methane Oxygen Flame. Combust. Flame 2003, 135, 27–33. [Google Scholar] [CrossRef]
- Height, M.J.; Howard, J.B.; Tester, J.W.; Vander Sande, J.B. Flame Synthesis of Single-Walled Carbon Nanotubes. Carbon 2004, 42, 2295–2307. [Google Scholar] [CrossRef]
- Sen, S.; Puri, I.K. Flame Synthesis of Carbon Nanofibres and Nanofibre Composites Containing Encapsulated Metal Particles. Nanotechnology 2004, 15, 264–268. [Google Scholar] [CrossRef]
- Naha, S.; Sen, S.; De, A.K.; Puri, I.K. A Detailed Model for the Flame Synthesis of Carbon Nanotubes and Nanofibers. Proc. Combust. Inst. 2007, 31, 1821–1829. [Google Scholar] [CrossRef]
- Keidar, M. Factors Affecting Synthesis of Single Wall Carbon Nanotubes in Arc Discharge. J. Phys. D Appl. Phys. 2007, 40, 2388–2393. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Carbon Nanotube–Metal Oxide Nanocomposites: Fabrication, Properties and Applications. Chem. Eng. J. 2016, 302, 344–367. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef]
- Griffiths, O.G.; O’Byrne, J.P.; Torrente-Murciano, L.; Jones, M.D.; Mattia, D.; McManus, M.C. Identifying the Largest Environmental Life Cycle Impacts during Carbon Nanotube Synthesis via Chemical Vapour Deposition. J. Clean. Prod. 2013, 42, 180–189. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. State-of-the-Art on the Production and Application of Carbon Nanomaterials from Biomass. Green. Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef]
- Ge, L.; Zhao, C.; Zuo, M.; Tang, J.; Ye, W.; Wang, X.; Zhang, Y.; Xu, C. Review on the Preparation of High Value-Added Carbon Materials from Biomass. J. Anal. Appl. Pyrolysis 2022, 168, 105747. [Google Scholar] [CrossRef]
- Omoriyekomwan, J.E.; Tahmasebi, A.; Dou, J.; Wang, R.; Yu, J. A Review on the Recent Advances in the Production of Carbon Nanotubes and Carbon Nanofibers via Microwave-Assisted Pyrolysis of Biomass. Fuel Process. Technol. 2021, 214, 106686. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Liu, W.J.; Li, W.W.; Jiang, H.; Yu, H.Q. Fates of Chemical Elements in Biomass during Its Pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef]
- Li, D.C.; Jiang, H. The Thermochemical Conversion of Non-Lignocellulosic Biomass to Form Biochar: A Review on Characterizations and Mechanism Elucidation. Bioresour. Technol. 2017, 246, 57–68. [Google Scholar] [CrossRef]
- Yu, J.; Maliutina, K.; Tahmasebi, A. A Review on the Production of Nitrogen-Containing Compounds from Microalgal Biomass via Pyrolysis. Bioresour. Technol. 2018, 270, 689–701. [Google Scholar] [CrossRef]
- Shafaghat, H.; Rezaei, P.S.; Ro, D.; Jae, J.; Kim, B.S.; Jung, S.C.; Sung, B.H.; Park, Y.K. In-Situ Catalytic Pyrolysis of Lignin in a Bench-Scale Fixed Bed Pyrolyzer. J. Ind. Eng. Chem. 2017, 54, 447–453. [Google Scholar] [CrossRef]
- Shahbaz, M.; AlNouss, A.; Parthasarathy, P.; Abdelaal, A.H.; Mackey, H.; McKay, G.; Al-Ansari, T. Investigation of Biomass Components on the Slow Pyrolysis Products Yield Using Aspen Plus for Techno-Economic Analysis. Biomass Convers. Biorefin 2022, 12, 669–681. [Google Scholar] [CrossRef]
- Lotfy, V.F.; Fathy, N.A.; Basta, A.H. Novel Approach for Synthesizing Different Shapes of Carbon Nanotubes from Rice Straw Residue. J. Environ. Chem. Eng. 2018, 6, 6263–6274. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, J.; Kwong, F.L.; Ng, D.H.L.; Tjong, S.C. Synthesis of Multiwalled Carbon Nanotubes from Bamboo Charcoal and the Roles of Minerals on Their Growth. Biomass Bioenergy 2012, 36, 12–19. [Google Scholar] [CrossRef]
- Fathy, N.A. Carbon Nanotubes Synthesis Using Carbonization of Pretreated Rice Straw through Chemical Vapor Deposition of Camphor. RSC Adv. 2017, 7, 28535–28541. [Google Scholar] [CrossRef]
- Araga, R.; Sharma, C.S. One Step Direct Synthesis of Multiwalled Carbon Nanotubes from Coconut Shell Derived Charcoal. Mater. Lett. 2017, 188, 205–207. [Google Scholar] [CrossRef]
- Ge, L.; Zuo, M.; Wang, Y.; Wang, R.; Rong, N.; Qi, Z.; Zhao, C.; Zhang, Y.; Xu, C. A Review of Comprehensive Utilization of Biomass to Synthesize Carbon Nanotubes: From Chemical Vapor Deposition to Microwave Pyrolysis. J. Anal. Appl. Pyrolysis 2024, 177, 106320. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A. Carbon Nanotube Connectors and Planar Jungle Gyms. Phys. Lett. A 1992, 172, 173–176. [Google Scholar] [CrossRef]
- Menon, M.; Srivastava, D. Carbon Nanotube “T Junctions”: Nanoscale Metal-Semiconductor-Metal Contact Devices. Phys. Rev. Lett. 1997, 79, 4453–4456. [Google Scholar] [CrossRef]
- Ma, K.L.; Yan, X.H.; Guo, Y.D.; Xiao, Y. Electronic Transport Properties of Junctions between Carbon Nanotubes and Graphene Nanoribbons. Eur. Phys. J. B 2011, 83, 487–492. [Google Scholar] [CrossRef]
- Harris, P.J.F.; Suarez-Martinez, I.; Marks, N.A. The Structure of Junctions between Carbon Nanotubes and Graphene Shells. Nanoscale 2016, 8, 18849–18854. [Google Scholar] [CrossRef]
- Lambin, P.; Vigneron, J.P.; Fonseca, A.; Nagy, J.B.; Lucas, A.A. Atomic Structure and Electronic Properties of a Bent Carbon Nanotube. Synth. Met. 1996, 77, 249–252. [Google Scholar] [CrossRef]
- Dimitrakakis, G.K.; Tylianakis, E.; Froudakis, G.E. Pillared Graphene: A New 3-D Network Nanostructure for Enhanced Hydrogen Storage. Nano Lett. 2008, 8, 3166–3170. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Kwaczala, A.T.; Kanakia, S.; Patel, S.C.; Judex, S.; Sitharaman, B. Fabrication and Characterization of Three-Dimensional Macroscopic All-Carbon Scaffolds. Carbon 2013, 53, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Gopalan, A.; D’Agati, M.; Srinivas Sankaran, J.; Judex, S.; Qin, Y.-X.; Sitharaman, B. Porous Three-Dimensional Carbon Nanotube Scaffolds for Tissue Engineering. J. Biomed. Mater. Res. A 2015, 103, 3212–3225. [Google Scholar] [CrossRef]
- Noyce, S.G.; Vanfleet, R.R.; Craighead, H.G.; Davis, R.C. High Surface-Area Carbon Microcantilevers. Nanoscale Adv. 2019, 1, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Tan, Y.H.; Abdullah, E.C.; Rahman, M.E.; Karri, R.R. A Comprehensive Review on Micropollutants Removal Using Carbon Nanotubes-Based Adsorbents and Membranes. J. Environ. Chem. Eng. 2021, 9, 106647. [Google Scholar] [CrossRef]
- Jain, N.; Gupta, E.; Kanu, N.J. Plethora of Carbon Nanotubes Applications in Various Fields–A State-of-the-Art-Review. Smart Sci. 2022, 10, 1–24. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, H.; Kong, F.; Zhang, Y.; Wang, S.; Liu, S.; Lucia, L.A.; Fatehi, P.; Pang, H. Fabrication, Characteristics and Applications of Carbon Materials with Different Morphologies and Porous Structures Produced from Wood Liquefaction: A Review. Chem. Eng. J. 2019, 364, 226–243. [Google Scholar] [CrossRef]
- Tan, C.W.; Tan, K.H.; Ong, Y.T.; Mohamed, A.R.; Zein, S.H.S.; Tan, S.H. Energy and Environmental Applications of Carbon Nanotubes. Environ. Chem. Lett. 2012, 10, 265–273. [Google Scholar] [CrossRef]
- Duarte, E.D.V.; Oliveira, M.G.; Spaolonzi, M.P.; Costa, H.P.S.; Silva, T.L.d.; Silva, M.G.C.d.; Vieira, M.G.A. Adsorption of Pharmaceutical Products from Aqueous Solutions on Functionalized Carbon Nanotubes by Conventional and Green Methods: A Critical Review. J. Clean Prod. 2022, 372, 133743. [Google Scholar] [CrossRef]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Tan, S.H. A Review on Carbon Nanotubes in an Environmental Protection and Green Engineering Perspective. Braz. J. Chem. Eng. 2010, 27, 227–242. [Google Scholar] [CrossRef]
- Kant Tripathi, G. Thriving Perspectives of Nanotechnology: A Review. Nanomed. Nanotechnol. Open Access 2020, 5, 1–10. [Google Scholar] [CrossRef]
- Ahmad, W.; Tareen, A.K.; Khan, K.; Khan, M.; Khan, Q.; Wang, Z.; Maqbool, M. A Review of the Synthesis, Fabrication, and Recent Advances in Mixed Dimensional Heterostructures for Optoelectronic Devices Applications. Appl. Mater. Today 2023, 30, 101717. [Google Scholar] [CrossRef]
- Ahmad, W.; Gong, Y.; Abbas, G.; Khan, K.; Khan, M.; Ali, G.; Shuja, A.; Tareen, A.K.; Khan, Q.; Li, D. Evolution of Low-Dimensional Material-Based Field-Effect Transistors. Nanoscale 2021, 13, 5162–5186. [Google Scholar] [CrossRef]
- Ghasemzadeh, G.; Momenpour, M.; Omidi, F.; Hosseini, M.R.; Ahani, M.; Barzegari, A. Applications of Nanomaterials in Water Treatment and Environmental Remediation. Front. Environ. Sci. Eng. 2014, 8, 471–482. [Google Scholar] [CrossRef]
- Arora, B.; Attri, P. Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification. J. Compos. Sci. 2020, 4, 135. [Google Scholar] [CrossRef]
- Das, R.; Vecitis, C.D.; Schulze, A.; Cao, B.; Ismail, A.F.; Lu, X.; Chen, J.; Ramakrishna, S. Recent Advances in Nanomaterials for Water Protection and Monitoring. Chem. Soc. Rev. 2017, 46, 6946–7020. [Google Scholar] [CrossRef]
- Singh, R.; Wang, K.; Qureshi, M.B.; Rangel, I.C.; Brown, N.J.; Shahrestani, S.; Gottfried, O.N.; Patel, N.P.; Bydon, M. Robotics in Neurosurgery: Current Prevalence and Future Directions. Surg. Neurol. Int. 2022, 13, 373. [Google Scholar] [CrossRef]
- Ogunsola, S.S.; Oladipo, M.E.; Oladoye, P.O.; Kadhom, M. Carbon Nanotubes for Sustainable Environmental Remediation: A Critical and Comprehensive Review. Nano-Struct. Nano-Objects 2024, 37, 101099. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Desorption of Polycyclic Aromatic Hydrocarbons from Carbon Nanomaterials in Water. Environ. Pollut. 2007, 145, 529–537. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, Q.; Mei, Y.; He, S.; Zhang, Z.; Wang, R.; Wang, W. Insights on Functionalized Carbon Nanotubes for Cancer Theranostics. J. Nanobiotechnol. 2021, 19, 423. [Google Scholar] [CrossRef] [PubMed]
- Belloni, F.; Kütahyali, C.; Rondinella, V.V.; Carbol, P.; Wiss, T.; Mangione, A. Can Carbon Nanotubes Play a Role in the Field of Nuclear Waste Management? Environ. Sci. Technol. 2009, 43, 1250–1255. [Google Scholar] [CrossRef]
- Cinke, M.; Li, J.; Bauschlicher, C.W.; Ricca, A.; Meyyappan, M. CO2 Adsorption in Single-Walled Carbon Nanotubes. Chem. Phys. Lett. 2003, 376, 761–766. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Cheng, C.K.; Luque, R.; Thomas, S.; Banh, T.L.; Pham, V.V.; Nguyen, X.P. Heavy Metal Removal by Biomass-Derived Carbon Nanotubes as a Greener Environmental Remediation: A Comprehensive Review. Chemosphere 2022, 287, 131959. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, R.; Kaushal, S.; Thakur, N.; Umar, A.; Akbar, S.; Ibrahim, A.A.; Baskoutas, S. Biomass Waste-Derived Carbon Materials for Sustainable Remediation of Polluted Environment: A Comprehensive Review. Chemosphere 2023, 345, 140419. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, O.N.; Talapatra, S.; Vajtai, R.; Ajayan, P.M. Carbon Nanotube Filters. Nat. Mater. 2004, 3, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Fallgren, P.H.; Morris, J.M.; Chen, Q. Removal of Bacteria and Viruses from Waters Using Layered Double Hydroxide Nanocomposites. Sci. Technol. Adv. Mater. 2007, 8, 67–70. [Google Scholar] [CrossRef]
- Tahaikt, M.; El Habbani, R.; Ait Haddou, A.; Achary, I.; Amor, Z.; Taky, M.; Alami, A.; Boughriba, A.; Hafsi, M.; Elmidaoui, A. Fluoride Removal from Groundwater by Nanofiltration. Desalination 2007, 212, 46–53. [Google Scholar] [CrossRef]
- Wei, C.; Dai, L.; Roy, A.; Tolle, T.B. Multifunctional Chemical Vapor Sensors of Aligned Carbon Nanotube and Polymer Composites. J. Am. Chem. Soc. 2006, 128, 1412–1413. [Google Scholar] [CrossRef]
- Van Hieu, N.; Thuy, L.T.B.; Chien, N.D. Highly Sensitive Thin Film NH3 Gas Sensor Operating at Room Temperature Based on SnO2/MWCNTs Composite. Sens. Actuators B Chem. 2008, 129, 888–895. [Google Scholar] [CrossRef]
- Bondavalli, P.; Legagneux, P.; Pribat, D. Carbon Nanotubes Based Transistors as Gas Sensors: State of the Art and Critical Review. Sens. Actuators B Chem. 2009, 140, 304–318. [Google Scholar] [CrossRef]
- Lu, J.; Kumar, B.; Castro, M.; Feller, J.F. Vapour Sensing with Conductive Polymer Nanocomposites (CPC): Polycarbonate-Carbon Nanotubes Transducers with Hierarchical Structure Processed by Spray Layer by Layer. Sens. Actuators B Chem. 2009, 140, 451–460. [Google Scholar] [CrossRef]
- Zhang, W.D.; Zhang, W.H. Carbon Nanotubes as Active Components for Gas Sensors. J. Sens. 2009, 2009, 107–143. [Google Scholar] [CrossRef]
- Albayati, N.; Waisi, B.; Al-Furaiji, M.; Kadhom, M.; Alalwan, H. Effect of COVID-19 on Air Quality and Pollution in Different Countries. J. Transp. Health 2021, 21, 101061. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Sundaram, B. Efficacy and Challenges of Carbon Nanotube in Wastewater and Water Treatment. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100764. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Vigneswaran, S.; Naidu, R. Permeable Reactive Barrier for Groundwater Remediation. J. Ind. Eng. Chem. 2008, 14, 145–156. [Google Scholar] [CrossRef]
- Desouky, A.M. Remove Heavy Metals from Groundwater Using Carbon Nanotubes Grafted with Amino Compound. Sep. Sci. Technol. 2018, 53, 1698–1702. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Yuan, X.; Dong, H.; Zeng, G.; Wu, H.; Wang, H.; Liu, J.; Hua, S.; Zhang, S.; et al. Facile Synthesis of Alumina-Decorated Multi-Walled Carbon Nanotubes for Simultaneous Adsorption of Cadmium Ion and Trichloroethylene. Chem. Eng. J. 2015, 273, 101–110. [Google Scholar] [CrossRef]
- Jha, K.C.; Liu, Z.; Vijwani, H.; Nadagouda, M.; Mukhopadhyay, S.M.; Tsige, M. Carbon Nanotube Based Groundwater Remediation: The Case of Trichloroethylene. Molecules 2016, 21, 953. [Google Scholar] [CrossRef]
- Lico, D.; Vuono, D.; Siciliano, C.; Nagy, J.B.; De Luca, P. Removal of Unleaded Gasoline from Water by Multi-Walled Carbon Nanotubes. J. Environ. Manag. 2019, 237, 636–643. [Google Scholar] [CrossRef]
- Mpouras, T.; Polydera, A.; Dermatas, D.; Verdone, N.; Vilardi, G. Multi Wall Carbon Nanotubes Application for Treatment of Cr(VI)-Contaminated Groundwater; Modeling of Batch & Column Experiments. Chemosphere 2021, 269, 128749. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, D.; Hu, Z.; Xiao, Y.; He, C.; Jiang, F.; Zhao, N.; Zhao, C.; Zhang, W.; Qiu, R. The Adsorption and Reduction of Anionic Cr(VI) in Groundwater by Novel Iron Carbide Loaded on N-Doped Carbon Nanotubes: Effects of Fe-Confinement. Chem. Eng. J. 2023, 452, 139357. [Google Scholar] [CrossRef]
- Ye, L.; Wang, L.; Wei, Z.; Zhou, S.; Yao, Z.; Fan, F.; Mei, Y. Thin Film Composite Nanofiltration Membrane with Tannic Acid-Fe(III) Complexes Functionalized CNTs Interlayer toward Energy Efficient Remediation of Groundwater. Desalination 2023, 552, 116438. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Z.; Yu, D.; Chen, X.; Cheng, R.; Min, S.; Wang, J.; Xiao, Q.; Wang, J. Overview of Membrane Technology Applications for Industrial Wastewater Treatment in China to Increase Water Supply. Resour. Conserv. Recycl. 2015, 105, 1–10. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Oil and Grease Removal from Wastewaters: Sorption Treatment as an Alternative to State-of-the-Art Technologies. A Critical Review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- Hayat, K.; Menhas, S.; Bundschuh, J.; Chaudhary, H.J. Microbial Biotechnology as an Emerging Industrial Wastewater Treatment Process for Arsenic MitigationA Critical Review. J. Clean. Prod. 2017, 151, 427–438. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of Microbial Cells for the Biotreatment of Wastewater: A Review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Bello, M.M.; Raman, A.A.A. Synergy of Adsorption and Advanced Oxidation Processes in Recalcitrant Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 1125–1142. [Google Scholar] [CrossRef]
- Ahn, C.H.; Baek, Y.; Lee, C.; Kim, S.O.; Kim, S.; Lee, S.; Kim, S.H.; Bae, S.S.; Park, J.; Yoon, J. Carbon Nanotube-Based Membranes: Fabrication and Application to Desalination. J. Ind. Eng. Chem. 2012, 18, 1551–1559. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A Review of Reverse Osmosis Membrane Materials for Desalination-Development to Date and Future Potential. J. Memb. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, F.; Chen, M.; Xu, Z.; Zhu, Z. Adsorption Behavior of Methylene Blue on Carbon Nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Khadem, E. Carbon Nanotubes for Heavy Metals Removal. In Composite Nanoadsorbents; Elsevier: Amsterdam, The Netherlands, 2019; pp. 181–210. [Google Scholar]
- Wang, X.; Chen, C.; Hu, W.; Ding, A.; Xu, D.; Zhou, X. Sorption of 243Am(III) to Multiwall Carbon Nanotubes. Environ. Sci. Technol. 2005, 39, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface Functional Groups of Carbon-Based Adsorbents and Their Roles in the Removal of Heavy Metals from Aqueous Solutions: A Critical Review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Salipira, K.L.; Mamba, B.B.; Krause, R.W.; Malefetse, T.J.; Durbach, S.H. Cyclodextrin Polyurethanes Polymerised with Carbon Nanotubes for the Removal of Organic Pollutants in Water. Water 2008, 34, 113. [Google Scholar] [CrossRef]
- Awad, A.M.; Jalab, R.; Benamor, A.; Nasser, M.S.; Ba-Abbad, M.M.; El-Naas, M.; Mohammad, A.W. Adsorption of Organic Pollutants by Nanomaterial-Based Adsorbents: An Overview. J. Mol. Liq. 2020, 301, 112335. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, L.; Xing, B. Adsorption of Polycyclic Aromatic Hydrocarbons by Carbon Nanomaterials. Environ. Sci. Technol. 2006, 40, 1855–1861. [Google Scholar] [CrossRef]
- Savage, N.; Diallo, M.S. Nanomaterials and Water Purification: Opportunities and Challenges. J. Nanoparticle Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Peng, J.; He, Y.; Zhou, C.; Su, S.; Lai, B. The Carbon Nanotubes-Based Materials and Their Applications for Organic Pollutant Removal: A Critical Review. Chin. Chem. Lett. 2021, 32, 1626–1636. [Google Scholar] [CrossRef]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Alfei, S.; Grasso, F.; Orlandi, V.; Russo, E.; Boggia, R.; Zuccari, G. Cationic Polystyrene-Based Hydrogels as Efficient Adsorbents to Remove Methyl Orange and Fluorescein Dye Pollutants from Industrial Wastewater. Int. J. Mol. Sci. 2023, 24, 2948. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Inamuddin Carbon Nanotube-Based Adsorbents for the Removal of Dyes from Waters: A Review. Environ. Chem. Lett. 2020, 18, 605–629. [Google Scholar] [CrossRef]
- Lazar, T. Color Chemistry: Synthesis, Properties, and Applications of Organic Dyes and Pigments, 3rd Revised Edition. Color. Res. Appl. 2005, 30, 313–314. [Google Scholar] [CrossRef]
- Shabaan, O.A.; Jahin, H.S.; Mohamed, G.G. Removal of Anionic and Cationic Dyes from Wastewater by Adsorption Using Multiwall Carbon Nanotubes. Arab. J. Chem. 2020, 13, 4797–4810. [Google Scholar] [CrossRef]
- Nazir, M.A.; Bashir, M.A.; Najam, T.; Javed, M.S.; Suleman, S.; Hussain, S.; Kumar, O.P.; Shah, S.S.A.; Rehman, A. ur Combining Structurally Ordered Intermetallic Nodes: Kinetic and Isothermal Studies for Removal of Malachite Green and Methyl Orange with Mechanistic Aspects. Microchem. J. 2021, 164, 105973. [Google Scholar] [CrossRef]
- Pirkarami, A.; Olya, M.E.; Yousefi Limaee, N. Decolorization of Azo Dyes by Photo Electro Adsorption Process Using Polyaniline Coated Electrode. Prog. Org. Coat. 2013, 76, 682–688. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, Y.; Ge, S.; Ding, X.; Cui, Z.; Shao, Q. One-Step Microwave Hydrothermal Preparation of Cd/Zr-Bimetallic Metal–Organic Frameworks for Enhanced Photochemical Properties. Adv. Compos. Hybrid. Mater. 2021, 4, 150–161. [Google Scholar] [CrossRef]
- Luo, C.; Wang, S.; Wu, D.; Cheng, X.; Ren, H. UV/Nitrate Photocatalysis for Degradation of Methylene Blue in Wastewater: Kinetics, Transformation Products, and Toxicity Assessment. Environ. Technol. Innov. 2022, 25, 102198. [Google Scholar] [CrossRef]
- Zghal, S.; Jedidi, I.; Cretin, M.; Cerneaux, S.; Abdelmouleh, M. Adsorptive Removal of Rhodamine B Dye Using Carbon Graphite/CNT Composites as Adsorbents: Kinetics, Isotherms and Thermodynamic Study. Materials 2023, 16, 1015. [Google Scholar] [CrossRef]
- Geyikçi, F. Adsorption of Acid Blue 161 (AB 161) Dye from Water by Multi-Walled Carbon Nanotubes. Fuller. Nanotub. Carbon. Nanostruct. 2013, 21, 579–593. [Google Scholar] [CrossRef]
- Zare, K.; Sadegh, H.; Shahryari-Ghoshekandi, R.; Maazinejad, B.; Ali, V.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Enhanced Removal of Toxic Congo Red Dye Using Multi Walled Carbon Nanotubes: Kinetic, Equilibrium Studies and Its Comparison with Other Adsorbents. J. Mol. Liq. 2015, 212, 266–271. [Google Scholar] [CrossRef]
- Vuono, D.; Catizzone, E.; Aloise, A.; Policicchio, A.; Agostino, R.G.; Migliori, M.; Giordano, G. Study of Adsorption Behavior of Multi-Walled Carbon Nanotubes towards Dyes Applied in Textile Applications. Adv. Sci. Lett. 2017, 23, 5851–5854. [Google Scholar] [CrossRef]
- Dong, M.; Guo, J.; Wang, Y.; Gai, X.; Xiong, X.; Zeng, J.; Wang, Y.; Wu, Y. Humic Acid Non-Covalent Functionalized Multi-Walled Carbon Nanotubes Composite Membrane and Its Application for the Removal of Organic Dyes. J. Environ. Chem. Eng. 2022, 10, 107320. [Google Scholar] [CrossRef]
- Lu, Q.; Moore, J.M.; Huang, G.; Mount, A.S.; Rao, A.M.; Larcom, L.L.; Ke, P.C. RNA Polymer Translocation with Single-Walled Carbon Nanotubes. Nano Lett. 2004, 4, 2473–2477. [Google Scholar] [CrossRef]
- Cui, D.; Tian, F.; Ozkan, C.S.; Wang, M.; Gao, H. Effect of Single Wall Carbon Nanotubes on Human HEK293 Cells. Toxicol. Lett. 2005, 155, 73–85. [Google Scholar] [CrossRef]
- Tan, S.N.; Yuen, M.L.; Ramli, R.A. Photocatalysis of Dyes: Operational Parameters, Mechanisms, and Degradation Pathway. Green. Anal. Chem. 2025, 12, 100230. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; ul Haq, A.; Akram, N. Photocatalysis: An Effective Tool for Photodegradation of Dyes—A Review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Sobhanardakani, S.; Zandipak, R.; Sahraei, R. Removal of Janus Green Dye from Aqueous Solutions Using Oxidized Multi-Walled Carbon Nanotubes. Toxicol. Environ. Chem. 2013, 95, 909–918. [Google Scholar] [CrossRef]
- Almoisheer, N.; Alseroury, F.A.; Kumar, R.; Almeelbi, T.; Barakat, M.A. Synthesis of Graphene Oxide/Silica/Carbon Nanotubes Composite for Removal of Dyes from Wastewater. Earth Syst. Environ. 2019, 3, 651–659. [Google Scholar] [CrossRef]
- Salem, M.A.S.; Khan, A.M.; Manea, Y.K.; Wani, A.A. Nano Chromium Embedded in F-CNT Supported CoBi-LDH Nanocomposites for Selective Adsorption of Pb2+ and Hazardous Organic Dyes. Chemosphere 2022, 289, 133073. [Google Scholar] [CrossRef]
- Gholami, S.; Llacuna, J.L.; Vatanpour, V.; Dehqan, A.; Paziresh, S.; Cortina, J.L. Impact of a New Functionalization of Multiwalled Carbon Nanotubes on Antifouling and Permeability of PVDF Nanocomposite Membranes for Dye Wastewater Treatment. Chemosphere 2022, 294, 133699. [Google Scholar] [CrossRef]
- Zong, P.; Wang, S.; Liang, G.; Shao, M.; Yan, N.; Xu, X.; Xu, M.; Li, W.; Yang, Y.; Chen, J.; et al. Eco-Friendly Approach for Effective Removal for Congo Red Dye from Wastewater Using Reusable Zn-Al Layered Double Hydroxide Anchored on Multiwalled Carbon Nanotubes Supported Sodium Dodecyl Sulfonate Composites. J. Mol. Liq. 2022, 349, 118468. [Google Scholar] [CrossRef]
- Gabal, M.A.; Al-Zahrani, N.G.; Al Angari, Y.M. Beading of Co1-XZnxFe2O4 Nanoparticles on MWCNTs. Synthesis Characterization, Effect of Zn-Substitution and Dye Removal Capability. Mater. Chem. Phys. 2018, 213, 220–230. [Google Scholar] [CrossRef]
- Eskandarian, L.; Arami, M.; Pajootan, E. Evaluation of Adsorption Characteristics of Multiwalled Carbon Nanotubes Modified by a Poly(Propylene Imine) Dendrimer in Single and Multiple Dye Solutions: Isotherms, Kinetics, and Thermodynamics. J. Chem. Eng. Data 2014, 59, 444–454. [Google Scholar] [CrossRef]
- Mohammadi, A.; Veisi, P. High Adsorption Performance of β-Cyclodextrin-Functionalized Multi-Walled Carbon Nanotubes for the Removal of Organic Dyes from Water and Industrial Wastewater. J. Environ. Chem. Eng. 2018, 6, 4634–4643. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Polat, T.G.; Bozoǧlan, B.K.I. Synthesis of Magnetic Oxidized Multiwalled Carbon Nanotube-κ-Carrageenan-Fe3O4 Nanocomposite Adsorbent and Its Application in Cationic Methylene Blue Dye Adsorption. Carbohydr. Polym. 2016, 147, 79–88. [Google Scholar] [CrossRef]
- Boukhalfa, N.; Boutahala, M.; Djebri, N.; Idris, A. Kinetics, Thermodynamics, Equilibrium Isotherms, and Reusability Studies of Cationic Dye Adsorption by Magnetic Alginate/Oxidized Multiwalled Carbon Nanotubes Composites. Int. J. Biol. Macromol. 2019, 123, 539–548. [Google Scholar] [CrossRef]
- Qureshi, S.S.; Shah, V.; Nizamuddin, S.; Mubarak, N.M.; Karri, R.R.; Dehghani, M.H.; Ramesh, S.; Khalid, M.; Rahman, M.E. Microwave-Assisted Synthesis of Carbon Nanotubes for the Removal of Toxic Cationic Dyes from Textile Wastewater. J. Mol. Liq. 2022, 356, 119045. [Google Scholar] [CrossRef]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy Metal Removal from Aqueous Solution by Advanced Carbon Nanotubes: Critical Review of Adsorption Applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Altowyan, A.S.; Toghan, A.; Ahmed, H.A.; Pashameah, R.A.; Mwafy, E.A.; Alrefaee, S.H.; Mostafa, A.M. Removal of Methylene Blue Dye from Aqueous Solution Using Carbon Nanotubes Decorated by Nickel Oxide Nanoparticles via Pulsed Laser Ablation Method. Radiat. Phys. Chem. 2022, 198, 110268. [Google Scholar] [CrossRef]
- Nath, A.; Shah, A.; Singh, L.R.; Mahato, M. Waste Plastic-Derived NiO-MWCNT Composite as Visible Light Photocatalyst for Degradation of Methylene Blue Dye. Nanotechnol. Environ. Eng. 2021, 6, 70. [Google Scholar] [CrossRef]
- Ali, H.; Tiama, T.M.; Ismail, A.M. New and Efficient NiO/Chitosan/Polyvinyl Alcohol Nanocomposites as Antibacterial and Dye Adsorptive Films. Int. J. Biol. Macromol. 2021, 186, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Ardebilchi Marand, S.; Almasi, H.; Ardebilchi Marand, N. Chitosan-Based Nanocomposite Films Incorporated with NiO Nanoparticles: Physicochemical, Photocatalytic and Antimicrobial Properties. Int. J. Biol. Macromol. 2021, 190, 667–678. [Google Scholar] [CrossRef]
- Shinde, S.G.; Patil, M.P.; Do Kim, G.; Shrivastava, V.S. Ni, C, N, S Multi-Doped ZrO2 Decorated on Multi-Walled Carbon Nanotubes for Effective Solar Induced Degradation of Anionic Dye. J. Environ. Chem. Eng. 2020, 8, 103769. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.K.; Arora, I.; Samim, M. Hierarchically Grown NiO-Decorated Polyaniline-Reduced Graphene Oxide Composite for Ultrafast Sunlight-Driven Photocatalysis. ACS Omega 2018, 3, 7846–7855. [Google Scholar] [CrossRef] [PubMed]
- Alwan, S.H.; Alshamsi, H.A. In Situ Synthesis NiO/F-MWCNTs Nanocomposite for Adsorption of Malachite Green Dye from Polluted Water. Carbon. Lett. 2022, 32, 1073–1084. [Google Scholar] [CrossRef]
- Yadav, A.; Bagotia, N.; Yadav, S.; Sharma, A.K.; Kumar, S. Adsorptive Studies on the Removal of Dyes from Single and Binary Systems Using Saccharum Munja Plant-Based Novel Functionalized CNT Composites. Environ. Technol. Innov. 2021, 24, 102015. [Google Scholar] [CrossRef]
- Jain, A.; Vaya, D. Photocatalytic Activity of TiO2 Nanomaterial. J. Chil. Chem. Soc. 2017, 62, 3683–3690. [Google Scholar] [CrossRef]
- Singh, R. Different Anticipated Criteria to Achieve Novel and Efficient Photocatalysis via Green ZnO: Scope and Challenges. Int. J. Environ. Sci. Technol. 2022, 19, 9209–9242. [Google Scholar] [CrossRef]
- Das, R.; Leo, B.F.; Murphy, F. The Toxic Truth About Carbon Nanotubes in Water Purification: A Perspective View. Nanoscale Res. Lett. 2018, 13, 183. [Google Scholar] [CrossRef]
- Jain, N.; Kanu, N.J. The Potential Application of Carbon Nanotubes in Water Treatment: A State-of-the-Art-Review. Mater. Today Proc. 2021, 43, 2998–3005. [Google Scholar] [CrossRef]
- Yousif, E. Potential of Carbon Nanotubes in Enhance of Photocatalyst Activity. Arch. Nanomed. Open Access J. 2018, 1, 1–6. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhao, J.; Zhu, H.; Orthman, J. Photodegradation of Dye Pollutants on TiO2 Nanoparticles Dispersed in Silicate under UV–VIS Irradiation. Appl. Catal. B 2002, 37, 331–338. [Google Scholar] [CrossRef]
- Alagumalai, K.; Musuvadhi Babulal, S.; Chen, S.M.; Shanmugam, R.; Yesuraj, J. Electrochemical Evaluation of Naproxen through Au@f-CNT/GO Nanocomposite in Environmental Water and Biological Samples. J. Ind. Eng. Chem. 2021, 104, 32–42. [Google Scholar] [CrossRef]
- Berry, D.; Xi, C.; Raskin, L. Microbial Ecology of Drinking Water Distribution Systems. Curr. Opin. Biotechnol. 2006, 17, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Escobar, I.C.; Randall, A.A.; Taylor, J.S. Bacterial Growth in Distribution Systems: Effect of Assimilable Organic Carbon and Biodegradable Dissolved Organic Carbon. Environ. Sci. Technol. 2001, 35, 3442–3447. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Harrington, G.W.; Xagoraraki, I.; Goel, R. Factors Affecting Bulk to Total Bacteria Ratio in Drinking Water Distribution Systems. Water Res. 2008, 42, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Upadhyayula, V.K.K.; Deng, S.; Mitchell, M.C.; Smith, G.B. Application of Carbon Nanotube Technology for Removal of Contaminants in Drinking Water: A Review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef]
- Dugan, N.R.; Williams, D.J. Cyanobacteria Passage through Drinking Water Filters during Perturbation Episodes as a Function of Cell Morphology, Coagulant and Initial Filter Loading Rate. Harmful Algae 2006, 5, 26–35. [Google Scholar] [CrossRef]
- Majsterek, I.; Sicinska, P.; Tarczynska, M.; Zalewski, M.; Walter, Z. Toxicity of Microcystin from Cyanobacteria Growing in a Source of Drinking Water. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 175–179. [Google Scholar] [CrossRef]
- Nuzzo, J.B. The Biological Threat to U.S. Water Supplies: Toward a National Water Security Policy. Biosecurity Bioterrorism 2006, 4, 147–159. [Google Scholar] [CrossRef]
- Camper, A.K.; LeChevallier, M.W.; Broadaway, S.C.; McFeters, G.A. Growth and Persistence of Pathogens on Granular Activated Carbon Filters. Appl. Environ. Microbiol. 1985, 50, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.W.; Mains, C.N.; Anderson, R.E.; Bissonnette, G.K. Effect of Point-of-Use, Activated Carbon Filters on the Bacteriological Quality of Rural Groundwater Supplies. Appl. Environ. Microbiol. 1995, 61, 4291–4295. [Google Scholar] [CrossRef]
- Nepal, D.; Balasubramanian, S.; Simonian, A.L.; Davis, V.A. Strong Antimicrobial Coatings: Single-Walled Carbon Nanotubes Armored with Biopolymers. Nano Lett. 2008, 8, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Watari, F. Capture of Bacteria by Flexible Carbon Nanotubes. Acta Biomater. 2009, 5, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Physicochemical Determinants of Multiwalled Carbon Nanotube Bacterial Cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef]
- Arias, L.R.; Yang, L. Inactivation of Bacterial Pathogens by Carbon Nanotubes in Suspensions. Langmuir 2009, 25, 3003–3012. [Google Scholar] [CrossRef]
- Krishna, V.; Pumprueg, S.; Lee, S.H.; Zhao, J.; Sigmund, W.; Koopman, B.; Moudgil, B.M. Photocatalytic Disinfection with Titanium Dioxide Coated Multi-Wall Carbon Nanotubes. Process Saf. Environ. Prot. 2005, 83, 393–397. [Google Scholar] [CrossRef]
- Zhu, Y.; Ran, T.; Li, Y.; Guo, J.; Li, W. Dependence of the Cytotoxicity of Multi-Walled Carbon Nanotubes on the Culture Medium. Nanotechnology 2006, 17, 4668–4674. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Kong, Y.L.; Yong, P.V.C.; Wong, E.H. An Overview of Antimicrobial Properties of Carbon Nanotubes-Based Nanocomposites. Adv Pharm Bull. 2022, 12, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Wick, P.; Manser, P.; Limbach, L.; Dettlaffweglikowska, U.; Krumeich, F.; Roth, S.; Stark, W.; Bruinink, A. The Degree and Kind of Agglomeration Affect Carbon Nanotube Cytotoxicity. Toxicol. Lett. 2007, 168, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial Nanomaterials for Water Disinfection and Microbial Control: Potential Applications and Implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Huang, T.S.; Tzeng, Y.; Liu, Y.K.; Chen, Y.C.; Walker, K.R.; Guntupalli, R.; Liu, C. Immobilization of Antibodies and Bacterial Binding on Nanodiamond and Carbon Nanotubes for Biosensor Applications. Diam. Relat. Mater. 2004, 13, 1098–1102. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K.; Deng, S.; Smith, G.B.; Mitchell, M.C. Adsorption of Bacillus Subtilis on Single-Walled Carbon Nanotube Aggregates, Activated Carbon and NanoCeramTM. Water Res. 2009, 43, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Ounaies, Z.; Park, C.; Wise, K.E.; Siochi, E.J.; Harrison, J.S. Electrical Properties of Single Wall Carbon Nanotube Reinforced Polyimide Composites. Compos. Sci. Technol. 2003, 63, 1637–1646. [Google Scholar] [CrossRef]
- Suzuki, M. Application of Fiber Adsorbents in Water Treatment. Water Sci. Technol. 1991, 23, 1649–1658. [Google Scholar] [CrossRef]
- Deng, S.; Upadhyayula, V.K.K.; Smith, G.B.; Mitchell, M.C. Adsorption Equilibrium and Kinetics of Microorganisms on Single-Wall Carbon Nanotubes. IEEE Sens. J. 2008, 8, 954–962. [Google Scholar] [CrossRef]
- Brady-Estévez, A.S.; Kang, S.; Elimelech, M. A Single-Walled-Carbon-Nanotube Filter for Removal of Viral and Bacterial Pathogens. Small 2008, 4, 481–484. [Google Scholar] [CrossRef]
- Brady-Estévez, A.S.; Nguyen, T.H.; Gutierrez, L.; Elimelech, M. Impact of Solution Chemistry on Viral Removal by a Single-Walled Carbon Nanotube Filter. Water Res. 2010, 44, 3773–3780. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Microbial Cytotoxicity of Carbon-Based Nanomaterials: Implications for River Water and Wastewater Effluent. Environ. Sci. Technol. 2009, 43, 2648–2653. [Google Scholar] [CrossRef]
- Lilly, M.; Dong, X.; McCoy, E.; Yang, L. Inactivation of Bacillus Anthracis Spores by Single-Walled Carbon Nanotubes Coupled with Oxidizing Antimicrobial Chemicals. Environ. Sci. Technol. 2012, 46, 13417–13424. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Tiwari, N.; Kanojia, S.; Mukhopadhyay, K.; Saxena, A.K. Insight into the Mechanism of Decontamination and Disinfection at the Functionalized Carbon Nanotube-Polymer Interfaces. J. Phys. Chem. C 2015, 119, 16678–16687. [Google Scholar] [CrossRef]
- Kim, J.P.; Kim, J.H.; Kim, J.; Lee, S.N.; Park, H.O. A Nanofilter Composed of Carbon Nanotube-Silver Composites for Virus Removal and Antibacterial Activity Improvement. J. Environ. Sci. 2016, 42, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ahmed, W.; Lal, S.; Sen, T. Novel Multifunctional Carbon Nanotube Containing Silver and Iron Oxide Nanoparticles for Antimicrobial Applications in Water Treatment. Mater. Today Proc. 2017, 4, 57–64. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Gong, J.-L.; Zeng, G.-M.; Ou, X.-M.; Song, B.; Guo, M.; Zhang, J.; Liu, H.-Y. Antimicrobial Behavior Comparison and Antimicrobial Mechanism of Silver Coated Carbon Nanocomposites. Process Saf. Environ. Prot. 2016, 102, 596–605. [Google Scholar] [CrossRef]
- Sui, M.; Zhang, L.; Sheng, L.; Huang, S.; She, L. Synthesis of ZnO Coated Multi-Walled Carbon Nanotubes and Their Antibacterial Activities. Sci. Total Environ. 2013, 452–453, 148–154. [Google Scholar] [CrossRef]
- Kim, J.; Van Der Bruggen, B. The Use of Nanoparticles in Polymeric and Ceramic Membrane Structures: Review of Manufacturing Procedures and Performance Improvement for Water Treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef]
- Pasquini, L.M.; Hashmi, S.M.; Sommer, T.J.; Elimelech, M.; Zimmerman, J.B. Impact of Surface Functionalization on Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. Environ. Sci. Technol. 2012, 46, 6297–6305. [Google Scholar] [CrossRef]
- Ahmed, F.; Santos, C.M.; Mangadlao, J.; Advincula, R.; Rodrigues, D.F. Antimicrobial PVK: SWNT Nanocomposite Coated Membrane for Water Purification: Performance and Toxicity Testing. Water Res. 2013, 47, 3966–3975. [Google Scholar] [CrossRef]
- Ahmed, F.; Santos, C.M.; Vergara, R.A.M.V.; Tria, M.C.R.; Advincula, R.; Rodrigues, D.F. Antimicrobial Applications of Electroactive PVK-SWNT Nanocomposites. Environ. Sci. Technol. 2012, 46, 1804–1810. [Google Scholar] [CrossRef]
- Tiraferri, A.; Vecitis, C.D.; Elimelech, M. Covalent Binding of Single-Walled Carbon Nanotubes to Polyamide Membranes for Antimicrobial Surface Properties. ACS Appl. Mater. Interfaces 2011, 3, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.; Park, H.; Choi, H.; Choi, H. Carbon Nanotube Blended Polyethersulfone Membranes for Fouling Control in Water Treatment. Water Res. 2011, 45, 274–282. [Google Scholar] [CrossRef]
- Shawky, H.A.; Chae, S.R.; Lin, S.; Wiesner, M.R. Synthesis and Characterization of a Carbon Nanotube/Polymer Nanocomposite Membrane for Water Treatment. Desalination 2011, 272, 46–50. [Google Scholar] [CrossRef]
- Al Momani, F.; Smith, D.W.; Gamal El-Din, M. Degradation of Cyanobacteria Toxin by Advanced Oxidation Processes. J. Hazard. Mater. 2008, 150, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Cheng, B.L.; Cheng, Y.L. Adsorption of Microcystin-LR by Three Types of Activated Carbon. J. Hazard. Mater. 2007, 141, 115–122. [Google Scholar] [CrossRef]
- Lambert, T.W.; Holmes, C.F.B.; Hrudey, S.E. Adsorption of Microcystin-LR by Activated Carbon and Removal in Full Scale Water Treatment. Water Res. 1996, 30, 1411–1422. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, S.C.; Cho, I.C.; Kim, S.J.; Kim, S.W. Photocatalytic Oxidation of Microcystin-LR in a Fluidized Bed Reactor Having TiO2-Coated Activated Carbon. Sep. Purif. Technol. 2004, 34, 59–66. [Google Scholar] [CrossRef]
- Wang, H.; Ho, L.; Lewis, D.M.; Brookes, J.D.; Newcombe, G. Discriminating and Assessing Adsorption and Biodegradation Removal Mechanisms during Granular Activated Carbon Filtration of Microcystin Toxins. Water Res. 2007, 41, 4262–4270. [Google Scholar] [CrossRef]
- Warhurst, A.M.; Raggett, S.L.; McConnachie, G.L.; Pollard, S.J.T.; Chipofya, V.; Codd, G.A. Adsorption of the Cyanobacterial Hepatotoxin Microcystin-LR by a Low-Cost Activated Carbon from the Seed Husks of the Pan-Tropical Tree, Moringa Oleifera. Sci. Total Environ. 1997, 207, 207–211. [Google Scholar] [CrossRef]
- Yan, H.; Gong, A.; He, H.; Zhou, J.; Wei, Y.; Lv, L. Adsorption of Microcystins by Carbon Nanotubes. Chemosphere 2006, 62, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, P.; Schumann, R.; Wong, S.H. Microcystin-LR Adsorption by Activated Carbon. J. Colloid. Interface Sci. 2001, 240, 1–8. [Google Scholar] [CrossRef]
- Yan, H.; Pan, G.; Zou, H.; Li, X.; Chen, H. Effective Removal of Microcystins Using Carbon Nanotubes Embedded with Bacteria. Chin. Sci. Bull. 2004, 49, 1694–1698. [Google Scholar] [CrossRef]
- de Albuquerque, E.C.; Méndez, M.O.A.; dos Reis Coutinho, A.; Franco, T.T. Removal of Cyanobacteria Toxins from Drinking Water by Adsorption on Activated Carbon Fibers. Mater. Res. 2008, 11, 371–380. [Google Scholar] [CrossRef]
- Ye, C.; Gong, Q.; Lu, F.; Liang, J. Adsorption of Middle Molecular Weight Toxins on Carbon Nanotubes. Acta Phys. Chim. Sin. 2007, 23, 1321–1324. [Google Scholar] [CrossRef]
- Chufa, B.M.; Murthy, H.C.A.; Gonfa, B.A.; Anshebo, T.Y. Carbon Nanotubes: A Review on Green Synthesis, Growth Mechanism and Application as a Membrane Filter for Fluoride Remediation. Green Chem. Lett. Rev. 2021, 14, 647–664. [Google Scholar] [CrossRef]
- Ihsanullah. Carbon Nanotube Membranes for Water Purification: Developments, Challenges, and Prospects for the Future. Sep. Purif. Technol. 2019, 209, 307–337. [Google Scholar] [CrossRef]
- Barrejón, M.; Prato, M. Carbon Nanotube Membranes in Water Treatment Applications. Adv. Mater. Interfaces 2021, 9, 2101260. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Zhang, S.; Pan, B. Application Potential of Carbon Nanotubes in Water Treatment: A Review. J. Environ. Sci. 2013, 25, 1263–1280. [Google Scholar] [CrossRef]
- Selvaraj, M.; Hai, A.; Banat, F.; Haija, M.A. Application and Prospects of Carbon Nanostructured Materials in Water Treatment: A Review. J. Water Process Eng. 2020, 33, 100996. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.S.; Yun, E.T.; Ham, S.Y.; Park, J.H.; Ahn, C.H.; Lee, S.H.; Park, H.D. Vertically Aligned Carbon Nanotube Membranes: Water Purification and Beyond. Membranes 2020, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sreekumar, T.V.; Liu, T.; Kumar, S. Properties and Structure of Nitric Acid Oxidized Single Wall Carbon Nanotube Films. J. Phys. Chem. B 2004, 108, 16435–16440. [Google Scholar] [CrossRef]
- Lyons, P.E.; De, S.; Blighe, F.; Nicolosi, V.; Pereira, L.F.C.; Ferreira, M.S.; Coleman, J.N. The Relationship between Network Morphology and Conductivity in Nanotube Films. J. Appl. Phys. 2008, 104, 044302. [Google Scholar] [CrossRef]
- Rashed, A.O.; Merenda, A.; Kondo, T.; Lima, M.; Razal, J.; Kong, L.; Huynh, C.; Dumée, L.F. Carbon Nanotube Membranes—Strategies and Challenges towards Scalable Manufacturing and Practical Separation Applications. Sep. Purif. Technol. 2021, 257, 117929. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yang, S.J.; Park, J.Y.; Kim, T.; Lee, K.; Kim, Y.S.; Han, H.N.; Park, C.R. Easy Preparation of Self-Assembled High-Density Buckypaper with Enhanced Mechanical Properties. Nano Lett. 2015, 15, 3588. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Z.; Liu, Y.; Leng, J. Buckypaper and Its Composites for Aeronautic Applications. Compos. B Eng. 2020, 199, 108231. [Google Scholar] [CrossRef]
- Alosime, E.M.; Alshahrani, A.A.; Nghiem, L.D.; in het Panhuis, M. The Preparation and Characterization of Buckypaper Made from Carbon Nanotubes Impregnated with Chitosan. Polym. Compos. 2020, 41, 1393–1404. [Google Scholar] [CrossRef]
- Dumée, L.; Campbell, J.L.; Sears, K.; Schütz, J.; Finn, N.; Duke, M.; Gray, S. The Impact of Hydrophobic Coating on the Performance of Carbon Nanotube Bucky-Paper Membranes in Membrane Distillation. Desalination 2011, 283, 64–67. [Google Scholar] [CrossRef]
- Dumée, L.F.; Sears, K.; Schütz, J.; Finn, N.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Characterization and Evaluation of Carbon Nanotube Bucky-Paper Membranes for Direct Contact Membrane Distillation. J. Memb. Sci. 2010, 351, 36–43. [Google Scholar] [CrossRef]
- Yang, X.; Lee, J.; Yuan, L.; Chae, S.R.; Peterson, V.K.; Minett, A.I.; Yin, Y.; Harris, A.T. Removal of Natural Organic Matter in Water Using Functionalised Carbon Nanotube Buckypaper. Carbon 2013, 59, 160–166. [Google Scholar] [CrossRef]
- Elnabawy, E.; Elsherbiny, I.M.A.; Abdelsamad, A.M.A.; Anis, B.; Hassan, A.; Ulbricht, M.; Khalil, A.S.G. Tailored CNTs Buckypaper Membranes for the Removal of Humic Acid and Separation of Oil-in- Water Emulsions. Membranes 2020, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Jun, L.Y.; Karri, R.R.; Mubarak, N.M.; Yon, L.S.; Bing, C.H.; Khalid, M.; Jagadish, P.; Abdullah, E.C. Modelling of Methylene Blue Adsorption Using Peroxidase Immobilized Functionalized Buckypaper/Polyvinyl Alcohol Membrane via Ant Colony Optimization. Environ. Pollut. 2020, 259, 113940. [Google Scholar] [CrossRef] [PubMed]
- Tomai, P.; Martinelli, A.; Morosetti, S.; Curini, R.; Fanali, S.; Gentili, A. Oxidized Buckypaper for Stir-Disc Solid Phase Extraction: Evaluation of Several Classes of Environmental Pollutants Recovered from Surface Water Samples. Anal. Chem. 2018, 90, 6827–6834. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Mastropietro, T.F.; Bruno, R.; Baratta, M.; Ferrando-Soria, J.; Mashin, A.I.; Nicoletta, F.P.; Pardo, E.; De Filpo, G.; Armentano, D. Synthesis and Enhanced Capture Properties of a New BioMOF@SWCNT-BP: Recovery of the Endangered Rare-Earth Elements from Aqueous Systems. Adv. Mater. Interfaces 2021, 8, 2100730. [Google Scholar] [CrossRef]
- Baratta, M.; Mastropietro, T.F.; Bruno, R.; Tursi, A.; Negro, C.; Ferrando-Soria, J.; Mashin, A.I.; Nezhdanov, A.; Nicoletta, F.P.; De Filpo, G.; et al. Multivariate Metal-Organic Framework/Single-Walled Carbon Nanotube Buckypaper for Selective Lead Decontamination. ACS Appl. Nano Mater. 2022, 5, 5223–5233. [Google Scholar] [CrossRef]
- Baratta, M.; Tursi, A.; Curcio, M.; Cirillo, G.; Nicoletta, F.P.; De Filpo, G. GO-SWCNT Buckypapers as an Enhanced Technology for Water Decontamination from Lead. Molecules 2022, 27, 4044. [Google Scholar] [CrossRef]
- Baratta, M.; Tursi, A.; Curcio, M.; Cirillo, G.; Nezhdanov, A.V.; Mashin, A.I.; Nicoletta, F.P.; De Filpo, G. Removal of Non-Steroidal Anti-Inflammatory Drugs from Drinking Water Sources by GO-SWCNT Buckypapers. Molecules 2022, 27, 7674. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, T.; Jing, Z.; Xu, J.; Qiu, F.; Yang, D.; Yu, L. In Situ Fabrication of Dynamic Nano Zero-Valent Iron/Activated Carbon Nanotubes Membranes for Tellurium Separation. Chem. Eng. Sci. 2019, 205, 278–286. [Google Scholar] [CrossRef]
- Barrejón, M.; Syrgiannis, Z.; Burian, M.; Bosi, S.; Montini, T.; Fornasiero, P.; Amenitsch, H.; Prato, M. Cross-Linked Carbon Nanotube Adsorbents for Water Treatment: Tuning the Sorption Capacity through Chemical Functionalization. ACS Appl. Mater. Interfaces 2019, 11, 12920–12930. [Google Scholar] [CrossRef]
- Niu, H.Y.; Cai, Y.Q.; Shi, Y.L.; Wei, F.S.; Liu, J.M.; Jiang, G.B. A New Solid-Phase Extraction Disk Based on a Sheet of Single-Walled Carbon Nanotubes. Anal. Bioanal. Chem. 2008, 392, 927–935. [Google Scholar] [CrossRef]
- Berned-Samatán, V.; Rubio, C.; Galán-González, A.; Muñoz, E.; Benito, A.M.; Maser, W.K.; Coronas, J.; Téllez, C. Single-Walled Carbon Nanotube Buckypaper as Support for Highly Permeable Double Layer Polyamide/Zeolitic Imidazolate Framework in Nanofiltration Processes. J. Memb. Sci. 2022, 652, 120490. [Google Scholar] [CrossRef]
- Dovjuu, O.; Kim, S.; Lee, A.; Baek, S.; Kim, J.; Noh, J.; Huh, S.; Choi, B.; Sung, Y.; Jeong, H. Structural Characterization of the Crystalline Nanocellulose and Nanocellulose-Reinforced Carbon Buckypaper. Diam. Relat. Mater. 2020, 106, 107821. [Google Scholar] [CrossRef]
- Alshahrani, A.; Alharbi, A.; Alnasser, S.; Almihdar, M.; Alsuhybani, M.; AlOtaibi, B. Enhanced Heavy Metals Removal by a Novel Carbon Nanotubes Buckypaper Membrane Containing a Mixture of Two Biopolymers: Chitosan and i-Carrageenan. Sep. Purif. Technol. 2021, 276, 119300. [Google Scholar] [CrossRef]
- Sweetman, L.J.; Alcock, L.J.; McArthur, J.D.; Stewart, E.M.; Triani, G.; In Het Panhuis, M.; Ralph, S.F. Bacterial Filtration Using Carbon Nanotube/Antibiotic Buckypaper Membranes. J. Nanomater. 2013, 2013, 781212. [Google Scholar] [CrossRef]
- Wang, R.; Chen, D.; Wang, Q.; Ying, Y.; Gao, W.; Xie, L. Recent Advances in Applications of Carbon Nanotubes for Desalination: A Review. Nanomaterials 2020, 10, 1203. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Pal, K.; Sagadevan, S.; Yehye, W.A.; Johan, R.B.; Shah, S.T.; Adebesi, A.; Ali, M.E.; Islam, M.S.; Rafique, R.F. Electrochemically Active Carbon Nanotube (CNT) Membrane Filter for Desalination and Water Purification, cap.10. In Emerging Technologies for Sustainable Desalination Handbook; Gude, V.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 333–363. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal Oxide-Based Gas Sensor Research: How To? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Penza, M.; Rossi, R.; Alvisi, M.; Signore, M.A.; Cassano, G.; Dimaio, D.; Pentassuglia, R.; Piscopiello, E.; Serra, E.; Falconieri, M. Characterization of Metal-Modified and Vertically-Aligned Carbon Nanotube Films for Functionally Enhanced Gas Sensor Applications. Thin Solid. Films 2009, 517, 6211–6216. [Google Scholar] [CrossRef]
- Wei, L.; Shizhen, H.; Wenzhe, C. An MWCNT-Doped SNO2 Thin Film NO2 Gas Sensor by RF Reactive Magnetron Sputtering. J. Semicond. 2010, 31, 024006. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon Nanotubes as Adsorbents in Environmental Pollution Management: A Review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of Pollutants by Porous Carbon, Carbon Nanotubes and Fullerene- An Overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. [Google Scholar] [CrossRef]
- Yeow, J.T.W.; Wang, Y. A Review of Carbon Nanotubes-Based Gas Sensors. J. Sens. 2009, 2009, 493904. [Google Scholar]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, F.; Tognolini, S.; Borghetti, P.; Drera, G.; Pagliara, S.; Goldoni, A.; Sangaletti, L. Enhancing the Sensitivity of Chemiresistor Gas Sensors Based on Pristine Carbon Nanotubes to Detect Low-Ppb Ammonia Concentrations in the Environment. Analyst 2013, 138, 7392–7399. [Google Scholar] [CrossRef] [PubMed]
- Sayago, I.; Terrado, E.; Aleixandre, M.; Horrillo, M.C.; Fernández, M.J.; Lozano, J.; Lafuente, E.; Maser, W.K.; Benito, A.M.; Martinez, M.T.; et al. Novel Selective Sensors Based on Carbon Nanotube Films for Hydrogen Detection. Sens. Actuators B Chem. 2007, 122, 75–80. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, I.; Chaturvedi, P.; Chouksey, A.; Tandon, R.P.; Chaudhury, P.K. Study of Simultaneous Reversible and Irreversible Adsorption on Single-Walled Carbon Nanotube Gas Sensor. Mater. Chem. Phys. 2016, 177, 276–282. [Google Scholar] [CrossRef]
- Kathirvelan, J.; Vijayaraghavan, R. Detection of Methane Using Multi-Walled Carbon Nanotubes. Bull. Mater. Sci. 2015, 38, 909–913. [Google Scholar] [CrossRef]
- Star, A.; Joshi, V.; Skarupo, S.; Thomas, D.; Gabriel, J.C.P. Gas Sensor Array Based on Metal-Decorated Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 21014–21020. [Google Scholar] [CrossRef]
- Jung, D.; Han, M.; Lee, G.S. Gas Sensor Using a Multi-Walled Carbon Nanotube Sheet to Detect Hydrogen Molecules. Sens. Actuators A Phys. 2014, 211, 51–54. [Google Scholar] [CrossRef]
- Bittencourt, C.; Felten, A.; Espinosa, E.H.; Ionescu, R.; Llobet, E.; Correig, X.; Pireaux, J.J. WO3 Films Modified with Functionalised Multi-Wall Carbon Nanotubes: Morphological, Compositional and Gas Response Studies. Sens. Actuators B Chem. 2006, 115, 33–41. [Google Scholar] [CrossRef]
- Hannon, A.; Lu, Y.; Li, J.; Meyyappan, M. Room Temperature Carbon Nanotube Based Sensor for Carbon Monoxide Detection. J. Sens. Sens. Syst. 2014, 3, 349–354. [Google Scholar] [CrossRef]
- Dilonardo, E.; Penza, M.; Alvisi, M.; Di Franco, C.; Rossi, R.; Palmisano, F.; Torsi, L.; Cioffi, N. Electrophoretic Deposition of Au NPs on MWCNT-Based Gas Sensor for Tailored Gas Detection with Enhanced Sensing Properties. Sens. Actuators B Chem. 2016, 223, 417–428. [Google Scholar] [CrossRef]
- Willinger, M.G.; Neri, G.; Bonavita, A.; Micali, G.; Rauwel, E.; Herntrich, T.; Pinna, N. The Controlled Deposition of Metal Oxides onto Carbon Nanotubes by Atomic Layer Deposition: Examples and a Case Study on the Application of V 2O4 Coated Nanotubes in Gas Sensing. Phys. Chem. Chem. Phys. 2009, 11, 3615–3622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Yang, B.; Xiao, H. Enhancement of Gas Sensing Characteristics of Multiwalled Carbon Nanotubes by CF4 Plasma Treatment for SF6 Decomposition Component Detection. J. Nanomater. 2015, 2015, 171545. [Google Scholar] [CrossRef]
- Saito, T.; Matsushige, K.; Tanaka, K. Chemical Treatment and Modification of Multi-Walled Carbon Nanotubes. Phys. B Condens. Matter 2002, 323, 280–283. [Google Scholar] [CrossRef]
- Matveev, A.T.; Golberg, D.; Novikov, V.P.; Klimkovich, L.L.; Bando, Y. Synthesis of Carbon Nanotubes below Room Temperature. Carbon 2001, 39, 155–158. [Google Scholar] [CrossRef]
- Ahn, K.S.; Kim, J.S.; Kim, C.O.; Hong, J.P. Non-Reactive Rf Treatment of Multiwall Carbon Nanotube with Inert Argon Plasma for Enhanced Field Emission. Carbon 2003, 41, 2481–2485. [Google Scholar] [CrossRef]
- Kónya, Z.; Vesselenyi, I.; Niesz, K.; Kukovecz, A.; Demortier, A.; Fonseca, A.; Delhalle, J.; Mekhalif, Z.; Nagy, J.B.; Koós, A.A.; et al. Large Scale Production of Short Functionalized Carbon Nanotubes. Chem. Phys. Lett. 2002, 360, 429–435. [Google Scholar] [CrossRef]
- Zhao, Q.; Nardelli, M.B.; Lu, W.; Bernholc, J. Carbon Nanotube-Metal Cluster Composites: A New Road to Chemical Sensors? Nano Lett. 2005, 5, 847–851. [Google Scholar] [CrossRef]
- Leghrib, R.; Llobet, E. Quantitative Trace Analysis of Benzene Using an Array of Plasma-Treated Metal-Decorated Carbon Nanotubes and Fuzzy Adaptive Resonant Theory Techniques. Anal. Chim. Acta 2011, 708, 19–27. [Google Scholar] [CrossRef]
- Espinosa, E.H.; Ionescu, R.; Bittencourt, C.; Felten, A.; Erni, R.; Van Tendeloo, G.; Pireaux, J.J.; Llobet, E. Metal-Decorated Multi-Wall Carbon Nanotubes for Low Temperature Gas Sensing. Thin Solid. Films 2007, 515, 8322–8327. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G.; Rossi, R.; Alvisi, M.; Rizzo, A.; Signore, M.A.; Dikonimos, T.; Serra, E.; Giorgi, R. Enhancement of Sensitivity in Gas Chemiresistors Based on Carbon Nanotube Surface Functionalized with Noble Metal (Au, Pt) Nanoclusters. Appl. Phys. Lett. 2007, 90, 173123. [Google Scholar] [CrossRef]
- Zhao, W.; Fam, D.W.H.; Yin, Z.; Sun, T.; Tan, H.T.; Liu, W.; Tok, A.I.Y.; Boey, Y.C.F.; Zhang, H.; Hng, H.H.; et al. A Carbon Monoxide Gas Sensor Using Oxygen Plasma Modified Carbon Nanotubes. Nanotechnology 2012, 23, 425502. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, L.; Zhang, Z.; Li, Y.; Dong, Y.; Sun, Y. Synthesis of Multi-Walled Carbon Nanotube-Hydroxyapatite Composites and Its Application in the Sorption of Co(II) from Aqueous Solutions. J. Mol. Liq. 2013, 179, 46–53. [Google Scholar] [CrossRef]
- Ewels, C.P.; Glerup, M. Nitrogen Doping in Carbon Nanotubes. J. Nanosci. Nanotechnol. 2005, 5, 1345–1363. [Google Scholar] [CrossRef]
- Koós, A.A.; Dillon, F.; Obraztsova, E.A.; Crossley, A.; Grobert, N. Comparison of Structural Changes in Nitrogen and Boron-Doped Multi-Walled Carbon Nanotubes. Carbon 2010, 48, 3033–3041. [Google Scholar] [CrossRef]
- Battie, Y.; Ducloux, O.; Thobois, P.; Susi, T.; Kauppinen, E.I.; Loiseau, A. Selective Differential Ammonia Gas Sensor Based on N-Doped SWCNT Films. Phys. Status Solidi B Basic. Res. 2011, 248, 2462–2466. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K. Ab Initio Study of Doped Carbon Nanotube Sensors. Nano Lett. 2003, 3, 513–517. [Google Scholar] [CrossRef]
- Nicholls, R.J.; Aslam, Z.; Sarahan, M.C.; Koós, A.; Yates, J.R.; Nellist, P.D.; Grobert, N. Boron-Mediated Nanotube Morphologies. ACS Nano 2012, 6, 7800–7805. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, J.Y.; Yen, W.T. Low Temperature ITO Thin Film Deposition on PES Substrate Using Pulse Magnetron Sputtering. Appl. Surf. Sci. 2008, 254, 3262–3268. [Google Scholar] [CrossRef]
- Shah, T.H.; Rawal, A. Textiles in Filtration. In Handbook of Technical Textiles: Technical Textile Applications; Horrocks, A.R., Anand, S.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–110. [Google Scholar] [CrossRef]
- Vishwakarma, P.K.; Pandey, S.K.; Yadav, S.K.; Shukla, P.; Srivastava, A.; Giri, R. Multiwalled Carbon Nanotube-Based Freestanding Filters for Efficient Removal of Fine Particulate Matters (PM0.3), Microplastics (MP0.3), and Bioaerosols. ACS Appl. Nano Mater. 2022, 5, 9306–9318. [Google Scholar] [CrossRef]
- Osler, K.; Dheda, D.; Ngoy, J.; Wagner, N.; Daramola, M.O. Synthesis and Evaluation of Carbon Nanotubes Composite Adsorbent for CO2 Capture: A Comparative Study of CO2 Adsorption Capacity of Single-Walled and Multi-Walled Carbon Nanotubes. Int. J. Coal Sci. Technol. 2017, 4, 41–49. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Chung, A.J.; Liao, C.H. CO2 Capture with Amine-Loaded Carbon Nanotubes via a Dual-Column Temperature/Vacuum Swing Adsorption. Appl. Energy 2014, 113, 706–712. [Google Scholar] [CrossRef]
- Aghababaei-Talkhonche, R.; Ghorbani-Shahna, F.; Mohammadrezaei, A.; Farhadian, M. Catalytic Removal of NO2 by Nickel–Platinum Catalyst Supported on Multi-Wall Carbon Nanotubes. Glob. Nest J. 2020, 22, 231–239. [Google Scholar] [CrossRef]
- Santucci, S.; Picozzi, S.; Di Gregorio, F.; Lozzi, L.; Cantalini, C.; Valentini, L.; Kenny, J.M.; Delley, B. NO2 and CO Gas Adsorption on Carbon Nanotubes: Experiment and Theory. J. Chem. Phys. 2003, 119, 10904–10910. [Google Scholar] [CrossRef]
- Azam, M.A.; Alias, F.M.; Tack, L.W.; Seman, R.N.A.R.; Taib, M.F.M. Electronic Properties and Gas Adsorption Behaviour of Pristine, Silicon-, and Boron-Doped (8, 0) Single-Walled Carbon Nanotube: A First Principles Study. J. Mol. Graph. Model. 2017, 75, 85–93. [Google Scholar] [CrossRef]
- Amade, R.; Hussain, S.; Ocaña, I.R.; Bertran, E. Growth and Functionalization of Carbon Nanotubes on Quartz Filter for Environmental Applications. J. Environ. Eng. Ecol. Sci. 2014, 3, 2. [Google Scholar] [CrossRef]
- Hsu, S.; Lu, C. Modification of Single-Walled Carbon Nanotubes for Enhancing Isopropyl Alcohol Vapor Adsorption from Air Streams. Sep. Sci. Technol. 2007, 42, 2751–2766. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Yang, Y.; Jian, W.; Ma, D.; Jia, F. Kinetics and Thermodynamics of SO2 adsorption on Metal-Loaded Multiwalled Carbon Nanotubes. Open Phys. 2020, 18, 1201–1214. [Google Scholar] [CrossRef]
- Yang, S.; Xu, D.; Yan, W.; Xiong, Y. Effective NO and SO2 removal from Fuel Gas with H2O2 catalyzed by Fe3O4/Fe0/Fe3C Encapsulated in Multi-Walled Carbon Nanotubes. J. Environ. Chem. Eng. 2021, 9, 105413. [Google Scholar] [CrossRef]
- Wang, J.; Cheon, W.S.; Lee, J.Y.; Yan, W.; Jung, S.; Jang, H.W.; Shokouhimehr, M. Magnetic Boron Nitride Adorned with Pd Nanoparticles: An Efficient Catalyst for the Reduction of Nitroarenes in Aqueous Media. Dalton Trans. 2023, 52, 3567–3574. [Google Scholar] [CrossRef]
- Godlewska, K.; Paszkiewicz, M. Reusable Passive Sampler with Carbon Nanotubes for Monitoring Contaminants in Wastewater: Application, Regeneration and Reuse. Chemosphere 2023, 332, 138855. [Google Scholar] [CrossRef] [PubMed]
- Salvador, F.; Martin-Sanchez, N.; Sanchez-Hernandez, R.; Sanchez-Montero, M.J.; Izquierdo, C. Regeneration of Carbonaceous Adsorbents. Part I: Thermal Regeneration. Microporous Mesoporous Mater. 2015, 202, 259–276. [Google Scholar] [CrossRef]
- Xu, G.; Huo, D.; Hou, C.; Zhao, Y.; Bao, J.; Yang, M.; Fa, H. A Regenerative and Selective Electrochemical Aptasensor Based on Copper Oxide Nanoflowers-Single Walled Carbon Nanotubes Nanocomposite for Chlorpyrifos Detection. Talanta 2018, 178, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, X.; Zhang, R.; Wu, Y.; Farid, M.U.; Huang, H. Comparison of Chemical, Ultrasonic and Thermal Regeneration of Carbon Nanotubes for Acetaminophen, Ibuprofen, and Triclosan Adsorption. RSC Adv. 2017, 7, 52719–52728. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Anagnostopoulos, V.A.; Bhatnagar, A.; Mitropoulos, A.C.; Kyzas, G.Z. A Review for Chromium Removal by Carbon Nanotubes. Chem. Ecol. 2017, 33, 572–588. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorptive Removal of Dyes from Aqueous Solution onto Carbon Nanotubes: A Review. Adv. Colloid Interface Sci. 2013, 193–194, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Toński, M.; Paszkiewicz, M.; Dołżonek, J.; Flejszar, M.; Bielicka-Giełdoń, A.; Stepnowski, P.; Białk-Bielińska, A. Regeneration and Reuse of the Carbon Nanotubes for the Adsorption of Selected Anticancer Drugs from Water Matrices. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126355. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, T.; Vellingiri, K.; Tsang, D.C.W.; Shon, J.R.; Kim, K.H.; Kumar, S. Recycling and Regeneration of Carbonaceous and Porous Materials through Thermal or Solvent Treatment. Chem. Eng. J. 2019, 364, 514–529. [Google Scholar] [CrossRef]
- Chiang, P.C.; Chang, E.E.; Wu, J.S. Comparison of Chemical and Thermal Regeneration of Aromatic Compounds on Exhausted Activated Carbon. Water Sci. Technol. 1997, 35, 279–285. [Google Scholar] [CrossRef]
- Gui, X.; Zeng, Z.; Lin, Z.; Gan, Q.; Xiang, R.; Zhu, Y.; Cao, A.; Tang, Z. Magnetic and Highly Recyclable Macroporous Carbon Nanotubes for Spilled Oil Sorption and Separation. ACS Appl. Mater. Interfaces 2013, 5, 5845–5850. [Google Scholar] [CrossRef]
- Nakano, Y.; Hua, L.Q.; Nishijima, W.; Shoto, E.; Okada, M. Biodegradation of Trichloroethylene (TCE) Adsorbed on Granular Activated Carbon (GAC). Water Res. 2000, 34, 4139–4142. [Google Scholar] [CrossRef]
- Naghizadeh, A. Regeneration of Carbon Nanotubes Exhausted with Humic Acid Using Electro-Fenton Technology. Arab. J. Sci. Eng. 2016, 41, 155–161. [Google Scholar] [CrossRef]
- Methatham, T.; Lu, M.C.; Ratanatamskul, C. Kinetics of Electro-Fenton Ferrous Regeneration (EFFR) on Chlorinated Organic Compound Degradation. Desalination Water Treat. 2015, 54, 1044–1053. [Google Scholar] [CrossRef]
- Sirés, I.; Garrido, J.A.; Rodríguez, R.M.; Brillas, E.; Oturan, N.; Oturan, M.A. Catalytic Behavior of the Fe3+/Fe2+ System in the Electro-Fenton Degradation of the Antimicrobial Chlorophene. Appl. Catal. B 2007, 72, 382–394. [Google Scholar] [CrossRef]
- Oturan, M.A.; Brillas, E. Electrochemical Advanced Oxidation Processes (EAOPs) for Environmental Applications. Port. Electrochim. Acta 2007, 25, 1–18. [Google Scholar] [CrossRef]
- Derakhshani, E.; Naghizadeh, A. Ultrasound Regeneration of Multi Wall Carbon Nanotubes Saturated by Humic Acid. Desalination Water Treat. 2014, 52, 7468–7472. [Google Scholar] [CrossRef]
- Peyravi, A.; Hashisho, Z.; Crompton, D.; Anderson, J.E. Enhanced Microwave Regeneration of a Polymeric Adsorbent through Carbon Nanotubes Deposition. Sep. Purif. Technol. 2022, 278, 119616. [Google Scholar] [CrossRef]
- Wang, J.; Peng, X.; Luan, Z.; Zhao, C. Regeneration of Carbon Nanotubes Exhausted with Dye Reactive Red 3BS Using Microwave Irradiation. J. Hazard. Mater. 2010, 178, 1125–1127. [Google Scholar] [CrossRef]
- Prola, L.D.T.; Machado, F.M.; Bergmann, C.P.; de Souza, F.E.; Gally, C.R.; Lima, E.C.; Adebayo, M.A.; Dias, S.L.P.; Calvete, T. Adsorption of Direct Blue 53 Dye from Aqueous Solutions by Multi-Walled Carbon Nanotubes and Activated Carbon. J. Environ. Manag. 2013, 130, 166–175. [Google Scholar] [CrossRef]
- Liang, P.; Liu, Y.; Guo, L.; Zeng, J.; Lu, H. Multiwalled Carbon Nanotubes as Solid-Phase Extraction Adsorbent for the Preconcentration of Trace Metal Ions and Their Determination by Inductively Coupled Plasma Atomic Emission Spectrometry. J. Anal. At. Spectrom. 2004, 19, 1489–1492. [Google Scholar] [CrossRef]
- Jain, A.; Kumari, S.; Agarwal, S.; Khan, S. Water Purification via Novel Nano-Adsorbents and Their Regeneration Strategies. Process Saf. Environ. Prot. 2021, 152, 441–454. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lu, C. Kinetics, Thermodynamics and Regeneration of Molybdenum Adsorption in Aqueous Solutions with NaOCl-Oxidized Multiwalled Carbon Nanotubes. J. Ind. Eng. Chem. 2014, 20, 2521–2527. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H.; Liu, C. Removal of Zinc(II) from Aqueous Solution by Purified Carbon Nanotubes: Kinetics and Equilibrium Studies. Ind. Eng. Chem. Res. 2006, 45, 2850–2855. [Google Scholar] [CrossRef]
- Sui, K.; Li, Y.; Liu, R.; Zhang, Y.; Zhao, X.; Liang, H.; Xia, Y. Biocomposite Fiber of Calcium Alginate/Multi-Walled Carbon Nanotubes with Enhanced Adsorption Properties for Ionic Dyes. Carbohydr. Polym. 2012, 90, 399–406. [Google Scholar] [CrossRef]
- Gong, J.L.; Wang, B.; Zeng, G.M.; Yang, C.P.; Niu, C.G.; Niu, Q.Y.; Zhou, W.J.; Liang, Y. Removal of Cationic Dyes from Aqueous Solution Using Magnetic Multi-Wall Carbon Nanotube Nanocomposite as Adsorbent. J. Hazard. Mater. 2009, 164, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Ma-Hock, L.; Treumann, S.; Strauss, V.; Brill, S.; Luizi, F.; Mertler, M.; Wiench, K.; Gamer, A.O.; van Ravenzwaay, B.; Landsiedel, R. Inhalation Toxicity of Multiwall Carbon Nanotubes in Rats Exposed for 3 Months. Toxicol. Sci. 2009, 112, 468–481. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Nayak, A.K. Regulatory Considerations of Carbon Nanotubes. In Carbon Nanotubes for Targeted Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 103–106. [Google Scholar]
- Wikipedia. Toxicology of Carbon Nanotubes. Available online: https://en.wikipedia.org/wiki/Toxicology_of_carbon_nanomaterials#:~:text=The%20toxicity%20of%20carbon%20nanotubes%20has%20been%20an,in%20evaluating%20the%20toxicity%20of%20this%20heterogeneous%20material (accessed on 7 March 2025).
- Li, Y.; Wang, S.; Lv, Z.; Wang, Z.; Zhao, Y.; Xie, Y.; Xu, Y.; Qian, L.; Yang, Y.; Zhao, Z.; et al. Transforming the Synthesis of Carbon Nanotubes with Machine Learning Models and Automation. Matter 2025, 8, 101913. [Google Scholar] [CrossRef]
- Scoville, C.; Cole, R.; Hogg, J.; Farooque, O.; Russel, A. Carbon Nanotubes. Available online: https://courses.cs.washington.edu/courses/csep590a/08sp/projects/CarbonNanotubes.pdf#:~:text=The%20structure%20of%20a%20carbon%20nanotube%20is%20formed,and%20bonded%20together%20to%20form%20a%20carbon%20nanotube (accessed on 7 March 2025).
- Simon-Deckers, A.; Gouget, B.; Mayne-L’Hermite, M.; Herlin-Boime, N.; Reynaud, C.; Carrière, M. In Vitro Investigation of Oxide Nanoparticle and Carbon Nanotube Toxicity and Intracellular Accumulation in A549 Human Pneumocytes. Toxicology 2008, 253, 137–146. [Google Scholar] [CrossRef]
- Pulskamp, K.; Diabate, S.; Krug, H. Carbon Nanotubes Show No Sign of Acute Toxicity but Induce Intracellular Reactive Oxygen Species in Dependence on Contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Inman, A.O.; Wang, Y.Y.; Nemanich, R.J. Surfactant Effects on Carbon Nanotube Interactions with Human Keratinocytes. Nanomedicine 2005, 1, 293–299. [Google Scholar] [CrossRef]
- Pantarotto, D.; Briand, J.-P.; Prato, M.; Bianco, A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem. Commun. 2004, 1, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.K.; Sarkar, S.; Barr, J.; Wise, K.; Barrera, E.V.; Jejelowo, O.; Rice-Ficht, A.C.; Ramesh, G.T. Single-Walled Carbon Nanotube Induces Oxidative Stress and Activates Nuclear Transcription Factor-ΚB in Human Keratinocytes. Nano Lett. 2005, 5, 1676–1684. [Google Scholar] [CrossRef]
- Shvedova, A.; Castranova, V.; Kisin, E.; Schwegler-Berry, D.; Murray, A.; Gandelsman, V.; Maynard, A.; Baron, P. Exposure to Carbon Nanotube Material: Assessment of Nanotube Cytotoxicity Using Human Keratinocyte Cells. J. Toxicol. Environ. Health A 2003, 66, 1909–1926. [Google Scholar] [CrossRef]
- Francis, A.P.; Devasena, T. Toxicity of Carbon Nanotubes: A Review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, H.; Lacotte, S.; Pastorin, G.; Marega, R.; Wu, W.; Bonifazi, D.; Briand, J.-P.; Prato, M.; Muller, S.; Bianco, A. Functionalized Carbon Nanotubes Are Non-Cytotoxic and Preserve the Functionality of Primary Immune Cells. Nano Lett. 2006, 6, 1522–1528. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Inman, A.O. Challenges for Assessing Carbon Nanomaterial Toxicity to the Skin. Carbon 2006, 44, 1070–1078. [Google Scholar] [CrossRef]
- Sayes, C.M.; Liang, F.; Hudson, J.L.; Mendez, J.; Guo, W.; Beach, J.M.; Moore, V.C.; Doyle, C.D.; West, J.L.; Billups, W.E.; et al. Functionalization Density Dependence of Single-Walled Carbon Nanotubes Cytotoxicity in Vitro. Toxicol. Lett. 2006, 161, 135–142. [Google Scholar] [CrossRef]
- Shi Kam, N.W.; Jessop, T.C.; Wender, P.A.; Dai, H. Nanotube Molecular Transporters: Internalization of Carbon Nanotube−Protein Conjugates into Mammalian Cells. J. Am. Chem. Soc. 2004, 126, 6850–6851. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Z.; Meng, J.; Meng, J.; Duan, J.; Xie, S.; Lu, X.; Zhu, Z.; Wang, C.; Chen, S.; et al. Carbon Nanotubes Enhance Cytotoxicity Mediated by Human Lymphocytes In Vitro. PLoS ONE 2011, 6, e21073. [Google Scholar] [CrossRef]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of Carbon Nanomaterials: Single-Wall Nanotube, Multi-Wall Nanotube, and Fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef]
- Kisin, E.R.; Murray, A.R.; Keane, M.J.; Shi, X.-C.; Schwegler-Berry, D.; Gorelik, O.; Arepalli, S.; Castranova, V.; Wallace, W.E.; Kagan, V.E.; et al. Single-Walled Carbon Nanotubes: Geno- and Cytotoxic Effects in Lung Fibroblast V79 Cells. J. Toxicol. Environ. Health A 2007, 70, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Kisin, E.R.; Mercer, R.; Murray, A.R.; Johnson, V.J.; Potapovich, A.I.; Tyurina, Y.Y.; Gorelik, O.; Arepalli, S.; Schwegler-Berry, D.; et al. Unusual Inflammatory and Fibrogenic Pulmonary Responses to Single-Walled Carbon Nanotubes in Mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L698–L708. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.-F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory Toxicity of Multi-Wall Carbon Nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar] [CrossRef]
- Hirano, S.; Kanno, S.; Furuyama, A. Multi-Walled Carbon Nanotubes Injure the Plasma Membrane of Macrophages. Toxicol. Appl. Pharmacol. 2008, 232, 244–251. [Google Scholar] [CrossRef]
- Murr, L.E.; Garza, K.M.; Soto, K.F.; Carrasco, A.; Powell, T.G.; Ramirez, D.A.; Guerrero, P.A.; Lopez, D.A.; Venzor, J. Cytotoxicity Assessment of Some Carbon Nanotubes and Related Carbon Nanoparticle Aggregates and the Implications for Anthropogenic Carbon Nanotube Aggregates in the Environment. Int. J. Environ. Res. Public Health 2005, 2, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Bottini, M.; Bruckner, S.; Nika, K.; Bottini, N.; Bellucci, S.; Magrini, A.; Bergamaschi, A.; Mustelin, T. Multi-Walled Carbon Nanotubes Induce T Lymphocyte Apoptosis. Toxicol. Lett. 2006, 160, 121–126. [Google Scholar] [CrossRef]
- Chou, C.C.; Hsiao, H.Y.; Hong, Q.S.; Chen, C.H.; Peng, Y.W.; Chen, H.W.; Yang, P.C. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008, 8, 437–445. [Google Scholar] [CrossRef]
- Warheit, D.B. Comparative Pulmonary Toxicity Assessment of Single-Wall Carbon Nanotubes in Rats. Toxicol. Sci. 2003, 77, 117–125. [Google Scholar] [CrossRef]
- Li, Z.; Hulderman, T.; Salmen, R.; Chapman, R.; Leonard, S.S.; Young, S.-H.; Shvedova, A.; Luster, M.I.; Simeonova, P.P. Cardiovascular Effects of Pulmonary Exposure to Single-Wall Carbon Nanotubes. Environ. Health Perspect. 2007, 115, 377–382. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon Nanotubes Introduced into the Abdominal Cavity of Mice Show Asbestos-like Pathogenicity in a Pilot Study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hirohashi, M.; Ogami, A.; Oyabu, T.; Myojo, T.; Todoroki, M.; Yamamoto, M.; Hashiba, M.; Mizuguchi, Y.; Lee, B.W.; et al. Pulmonary Toxicity of Well-Dispersed Multi-Wall Carbon Nanotubes Following Inhalation and Intratracheal Instillation. Nanotoxicology 2012, 6, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Grubek-Jaworska, H.; Nejman, P.; Czumińska, K.; Przybyłowski, T.; Huczko, A.; Lange, H.; Bystrzejewski, M.; Baranowski, P.; Chazan, R. Preliminary Results on the Pathogenic Effects of Intratracheal Exposure to One-Dimensional Nanocarbons. Carbon 2006, 44, 1057–1063. [Google Scholar] [CrossRef]
- Francis, A.P.; Ganapathy, S.; Palla, V.R.; Murthy, P.B.; Ramaprabhu, S.; Devasena, T. One Time Nose-Only Inhalation of MWCNTs: Exploring the Mechanism of Toxicity by Intermittent Sacrifice in Wistar Rats. Toxicol. Rep. 2015, 2, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, P.P.; Erdely, A. Engineered Nanoparticle Respiratory Exposure and Potential Risks for Cardiovascular Toxicity: Predictive Tests and Biomarkers. Inhal. Toxicol. 2009, 21, 68–73. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Massimiani, M.; Fenoglio, I.; Colonna, M.; Valentini, F.; Palleschi, G.; Camaioni, A.; Magrini, A.; Siracusa, G.; Bergamaschi, A.; et al. Low Doses of Pristine and Oxidized Single-Wall Carbon Nanotubes Affect Mammalian Embryonic Development. ACS Nano 2011, 5, 4624–4633. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Y.; Zhang, J.; Mu, Q.; Zhang, W.; Butch, E.R.; Snyder, S.E.; Yan, B. Repeated Administrations of Carbon Nanotubes in Male Mice Cause Reversible Testis Damage without Affecting Fertility. Nat. Nanotechnol. 2010, 5, 683–689. [Google Scholar] [CrossRef]
- Philbrook, N.A.; Walker, V.K.; Afrooz, A.R.M.N.; Saleh, N.B.; Winn, L.M. Investigating the Effects of Functionalized Carbon Nanotubes on Reproduction and Development in Drosophila Melanogaster and CD-1 Mice. Reprod. Toxicol. 2011, 32, 442–448. [Google Scholar] [CrossRef]
- Møller, P.; Wils, R.S.; Di Ianni, E.; Gutierrez, C.A.T.; Roursgaard, M.; Jacobsen, N.R. Genotoxicity of Multi-Walled Carbon Nanotube Reference Materials in Mammalian Cells and Animals. Mutat. Res./Rev. Mutat. Res. 2021, 788, 108393. [Google Scholar] [CrossRef] [PubMed]
- Sargent, L.M.; Hubbs, A.F.; Young, S.-H.; Kashon, M.L.; Dinu, C.Z.; Salisbury, J.L.; Benkovic, S.A.; Lowry, D.T.; Murray, A.R.; Kisin, E.R.; et al. Single-Walled Carbon Nanotube-Induced Mitotic Disruption. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2012, 745, 28–37. [Google Scholar] [CrossRef]
- Lash, L.H. Special Issue: Membrane Transporters in Toxicology. Toxicol. Appl. Pharmacol. 2005, 204, 197. [Google Scholar] [CrossRef]
- Lam, C.-W. Pulmonary Toxicity of Single-Wall Carbon Nanotubes in Mice 7 and 90 Days After Intratracheal Instillation. Toxicol. Sci. 2003, 77, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.R.; Kisin, E.; Leonard, S.S.; Young, S.H.; Kommineni, C.; Kagan, V.E.; Castranova, V.; Shvedova, A.A. Oxidative Stress and Inflammatory Response in Dermal Toxicity of Single-Walled Carbon Nanotubes. Toxicology 2009, 257, 161–171. [Google Scholar] [CrossRef]
- Orecchioni, M.; Bedognetti, D.; Sgarrella, F.; Marincola, F.M.; Bianco, A.; Delogu, L.G. Impact of Carbon Nanotubes and Graphene on Immune Cells. J. Transl. Med. 2014, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Svadlakova, T.; Holmannova, D.; Kolackova, M.; Malkova, A.; Krejsek, J.; Fiala, Z. Immunotoxicity of Carbon-Based Nanomaterials, Starring Phagocytes. Int. J. Mol. Sci. 2022, 23, 8889. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Pan, D.; Castranova, V. Engineered Nanoparticle Exposure and Cardiovascular Effects: The Role of a Neuronal-Regulated Pathway. Inhal. Toxicol. 2018, 30, 335–342. [Google Scholar] [CrossRef]
- Alidori, S.; Bowman, R.L.; Yarilin, D.; Romin, Y.; Barlas, A.; Mulvey, J.J.; Fujisawa, S.; Xu, K.; Ruggiero, A.; Riabov, V.; et al. Deconvoluting Hepatic Processing of Carbon Nanotubes. Nat. Commun. 2016, 7, 12343. [Google Scholar] [CrossRef]
- Reddy, A.R.N.; Reddy, Y.N.; Krishna, D.R.; Himabindu, V. Multi Wall Carbon Nanotubes Induce Oxidative Stress and Cytotoxicity in Human Embryonic Kidney (HEK293) Cells. Toxicology 2010, 272, 11–16. [Google Scholar] [CrossRef]
- Ghanbari, F.; Nasarzadeh, P.; Seydi, E.; Ghasemi, A.; Taghi, J.M.; Ashtari, K.; Akbari, M. Mitochondrial oxidative stress and dysfunction induced by single- and multiwall carbon nanotubes: A comparative study. J. Biomed. Mater. Res. Part A 2017, 105A, 2047–2055. [Google Scholar] [CrossRef]
- Facciolà, A.; Visalli, G.; La Maestra, S.; Ceccarelli, M.; D’Aleo, F.; Nunnari, G.; Pellicanò, G.F.; Di Pietro, A. Carbon Nanotubes and Central Nervous System: Environmental Risks, Toxicological Aspects and Future Perspectives. Environ. Toxicol. Pharmacol. 2019, 65, 23–30. [Google Scholar] [CrossRef]
- Zheng, W.; McKinney, W.; Kashon, M.; Salmen, R.; Castranova, V.; Kan, H. The Influence of Inhaled Multi-Walled Carbon Nanotubes on the Autonomic Nervous System. Part. Fibre Toxicol. 2015, 13, 8. [Google Scholar] [CrossRef]
- Aragon, M.J.; Topper, L.; Tyler, C.R.; Sanchez, B.; Zychowski, K.; Young, T.; Herbert, G.; Hall, P.; Erdely, A.; Eye, T.; et al. Serum-Borne Bioactivity Caused by Pulmonary Multiwalled Carbon Nanotubes Induces Neuroinflammation via Blood–Brain Barrier Impairment. Proc. Natl. Acad. Sci. USA 2017, 114, E1968–E1976. [Google Scholar] [CrossRef]
- Gholamine, B.; Karimi, I.; Salimi, A.; Mazdarani, P.; Becker, L.A. Neurobehavioral Toxicity of Carbon Nanotubes in Mice. Toxicol. Ind. Health 2017, 33, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Larner, S.F.; Wang, J.; Goodman, J.; O’Donoghue Altman, M.B.; Xin, M.; Wang, K.K.W. In Vitro Neurotoxicity Resulting from Exposure of Cultured Neural Cells to Several Types of Nanoparticles. J. Cell Death 2017, 10, 117967071769452. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, X.; Nicholas, J.; Humes, S.T.; Loeb, J.C.; Robinson, S.E.; Bisesi, J.H.; Das, D.; Saleh, N.B.; Castleman, W.L.; et al. Single-Walled Carbon Nanotubes Modulate Pulmonary Immune Responses and Increase Pandemic Influenza a Virus Titers in Mice. Virol. J. 2017, 14, 242. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Choi, J.; Kim, J.-H.; Lee, B.-S.; Yoon, C.; Jeong, U.; Kim, Y. Subchronic Immunotoxicity and Screening of Reproductive Toxicity and Developmental Immunotoxicity Following Single Instillation of HIPCO-Single-Walled Carbon Nanotubes: Purity-Based Comparison. Nanotoxicology 2016, 10, 1188–1202. [Google Scholar] [CrossRef]
- Khang, D.; Lee, S.; Kim, S.-H. High Dispersity of Carbon Nanotubes Diminishes Immunotoxicity in Spleen. Int. J. Nanomed. 2015, 10, 2697–2710. [Google Scholar] [CrossRef]
- Qi, W.; Bi, J.; Zhang, X.; Wang, J.; Wang, J.; Liu, P.; Li, Z.; Wu, W. Damaging Effects of Multi-Walled Carbon Nanotubes on Pregnant Mice with Different Pregnancy Times. Sci. Rep. 2014, 4, 4352. [Google Scholar] [CrossRef]
- Cheng, J.; Cheng, S.H. Influence of Carbon Nanotube Length on Toxicity to Zebrafish Embryos. Int. J. Nanomed. 2012, 7, 3731–3739. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Sun, X.; Choi, K.-Y.; Niu, G.; Zhang, G.; Guo, J.; Lee, S.; Chen, X. The Genotype-Dependent Influence of Functionalized Multiwalled Carbon Nanotubes on Fetal Development. Biomaterials 2014, 35, 856–865. [Google Scholar] [CrossRef]
- Zhu, B.; Zhu, S.; Li, J.; Hui, X.; Wang, G.-X. The Developmental Toxicity, Bioaccumulation and Distribution of Oxidized Single Walled Carbon Nanotubes in Artemia salina. Toxicol. Res. 2018, 7, 897–906. [Google Scholar] [CrossRef]
- Arianti, S.F. Reproductive and developmental effects of carbon nanotube exposure: A systematic review. Sci. Midwifery 2025, 13, 345–354. [Google Scholar] [CrossRef]
- Lindberg, H.K.; Falck, G.C.-M.; Singh, R.; Suhonen, S.; Järventaus, H.; Vanhala, E.; Catalán, J.; Farmer, P.B.; Savolainen, K.M.; Norppa, H. Genotoxicity of Short Single-Wall and Multi-Wall Carbon Nanotubes in Human Bronchial Epithelial and Mesothelial Cells in Vitro. Toxicology 2013, 313, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Lettiero, B.; Andersen, A.J.; Hunter, A.C.; Moghimi, S.M. Complement System and the Brain: Selected Pathologies and Avenues toward Engineering of Neurological Nanomedicines. J. Control. Release 2012, 161, 283–289. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Peer, D.; Langer, R. Reshaping the Future of Nanopharmaceuticals: Ad. Iudicium. ACS Nano 2011, 5, 8454–8458. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Davis, C.; Cai, W.; He, L.; Chen, X.; Dai, H. Circulation and Long-Term Fate of Functionalized, Biocompatible Single-Walled Carbon Nanotubes in Mice Probed by Raman Spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fernando, K.A.S.; Liu, J.; Wang, J.; Sun, H.; Liu, Y.; Chen, M.; Huang, Y.; Wang, X.; Wang, H.; et al. Covalently PEGylated Carbon Nanotubes with Stealth Character In Vivo. Small 2008, 4, 940–944. [Google Scholar] [CrossRef]
- Pondman, K.M.; Pednekar, L.; Paudyal, B.; Tsolaki, A.G.; Kouser, L.; Khan, H.A.; Shamji, M.H.; ten Haken, B.; Stenbeck, G.; Sim, R.B.; et al. Innate Immune Humoral Factors, C1q and Factor H, with Differential Pattern Recognition Properties, Alter Macrophage Response to Carbon Nanotubes. Nanomedicine 2015, 11, 2109–2118. [Google Scholar] [CrossRef]
- Singh, R.P.; Das, M.; Thakare, V.; Jain, S. Functionalization density dependent toxicity of oxidized multiwalled carbon nanotubes in a murine macrophage cell line. Chem Res Toxicol. 2012, 25, 2127–2137. [Google Scholar] [CrossRef]
- Sallam, A.A.; El-Magd, M.A.; Ahmed, M.M.; Ghamry, H.I.; Alshahrani, M.Y.; Hegazy, R.A.; Magdy, A.; El-Fotoh, M.F.A. Quercetin alleviated multi-walled carbon nanotubes-induced neurotoxicity in mice through inhibition of oxidation, inflammation, and pyroptosis. Biomed. Pharmacother. 2022, 151, 113160. [Google Scholar] [CrossRef]
- Yang, H.; Yang, T.; Heng, C.; Zhou, Y.; Jiang, Z.; Qian, X.; Du, L.; Mao, S.; Yin, X.; Lu, Q. Quercetin Improves Nonalcoholic Fatty Liver by Ameliorating Inflammation, Oxidative Stress, and Lipid Metabolism in Db/Db Mice. Phytother. Res. 2019, 33, 3140–3152. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.A.; Ahmed, M.M.; El-Magd, M.A.; Magdy, A.; Ghamry, H.I.; Alshahrani, M.Y.; Abou El-Fotoh, M.F. Quercetin-Ameliorated, Multi-Walled Carbon Nanotubes-Induced Immunotoxic, Inflammatory, and Oxidative Effects in Mice. Molecules 2022, 27, 2117. [Google Scholar] [CrossRef]
- Zhang, D.; Deng, X.; Ji, Z.; Shen, X.; Dong, L.; Wu, M.; Gu, T.; Liu, Y. Long-Term Hepatotoxicity of Polyethylene-Glycol Functionalized Multi-Walled Carbon Nanotubes in Mice. Nanotechnology 2010, 21, 175101. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Luna, V.; Moreno-Aguilar, C.; Arauz-Lara, J.L.; Aranda-Espinoza, S.; Quintana, M. Interactions of Functionalized Multi-Wall Carbon Nanotubes with Giant Phospholipid Vesicles as Model Cellular Membrane System. Sci. Rep. 2018, 8, 17998. [Google Scholar] [CrossRef]
- Awasthi, S.; De, S.; Pandey, S.K. Surface Grafting of Carbon Nanostructures. In Handbook of Functionalized Carbon Nanostructures; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–45. [Google Scholar]
- Yang, M.; Zhang, M. Biodegradation of Carbon Nanotubes by Macrophages. Front. Mater. 2019, 6, 225. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the Toxicity of Carbon Nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Li, Q.; Li, Y.; Li, Y.; Zhang, X.; Huang, Q. Effects of Serum Proteins on Intracellular Uptake and Cytotoxicity of Carbon Nanoparticles. Carbon 2009, 47, 1351–1358. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Z.; Wang, X.; Leng, X. An Innovative MWCNTs/DOX/TC Nanosystem for Chemo-Photothermal Combination Therapy of Cancer. Nanomedicine 2017, 13, 2271–2280. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, R.; Hu, Y.; Sun, R.; Song, T.; Shi, X.; Yin, S. Stacking of Doxorubicin on Folic Acid-Targeted Multiwalled Carbon Nanotubes for in Vivo Chemotherapy of Tumors. Drug Deliv. 2018, 25, 1607–1616. [Google Scholar] [CrossRef]
- Rele, S.; Thakur, C.K.; Khan, F.; Baral, B.; Saini, V.; Karthikeyan, C.; Moorthy, N.S.H.N.; Jha, H.C. Curcumin Coating: A Novel Solution to Mitigate Inherent Carbon Nanotube Toxicity. J. Mater. Sci. Mater. Med. 2024, 35, 24. [Google Scholar] [CrossRef]
- Wu, L.; Man, C.; Wang, H.; Lu, X.; Ma, Q.; Cai, Y.; Ma, W. PEGylated Multi-Walled Carbon Nanotubes for Encapsulation and Sustained Release of Oxaliplatin. Pharm. Res. 2013, 30, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M.; Bourgognon, M.; Wang, J.T.-W.; Al-Jamal, K.T. Functionalised Carbon Nanotubes: From Intracellular Uptake and Cell-Related Toxicity to Systemic Brain Delivery. J. Control. Release 2016, 241, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial Cytotoxicity Is Composition, Size, and Cell Type Dependent. Part. Fibre Toxicol. 2010, 7, 22. [Google Scholar] [CrossRef]
- Heras, A.; Colina, A.; López-Palacios, J.; Ayala, P.; Sainio, J.; Ruiz, V.; Kauppinen, E.I. Electrochemical Purification of Carbon Nanotube Electrodes. Electrochem. Commun. 2009, 11, 1535–1538. [Google Scholar] [CrossRef]
- Mercier, G.; Hérold, C.; Marêché, J.-F.; Cahen, S.; Gleize, J.; Ghanbaja, J.; Lamura, G.; Bellouard, C.; Vigolo, B. Selective Removal of Metal Impurities from Single Walled Carbon Nanotube Samples. New J. Chem. 2013, 37, 790. [Google Scholar] [CrossRef]
- Pełech, I.; Narkiewicz, U.; Kaczmarek, A.; Jędrzejewska, A.; Pełech, R. Removal of Metal Particles from Carbon Nanotubes Using Conventional and Microwave Methods. Sep. Purif. Technol. 2014, 136, 105–110. [Google Scholar] [CrossRef]
- Madani, S.Y.; Mandel, A.; Seifalian, A.M. A Concise Review of Carbon Nanotube’s Toxicology. Nano Rev. 2013, 4, 21521. [Google Scholar] [CrossRef]
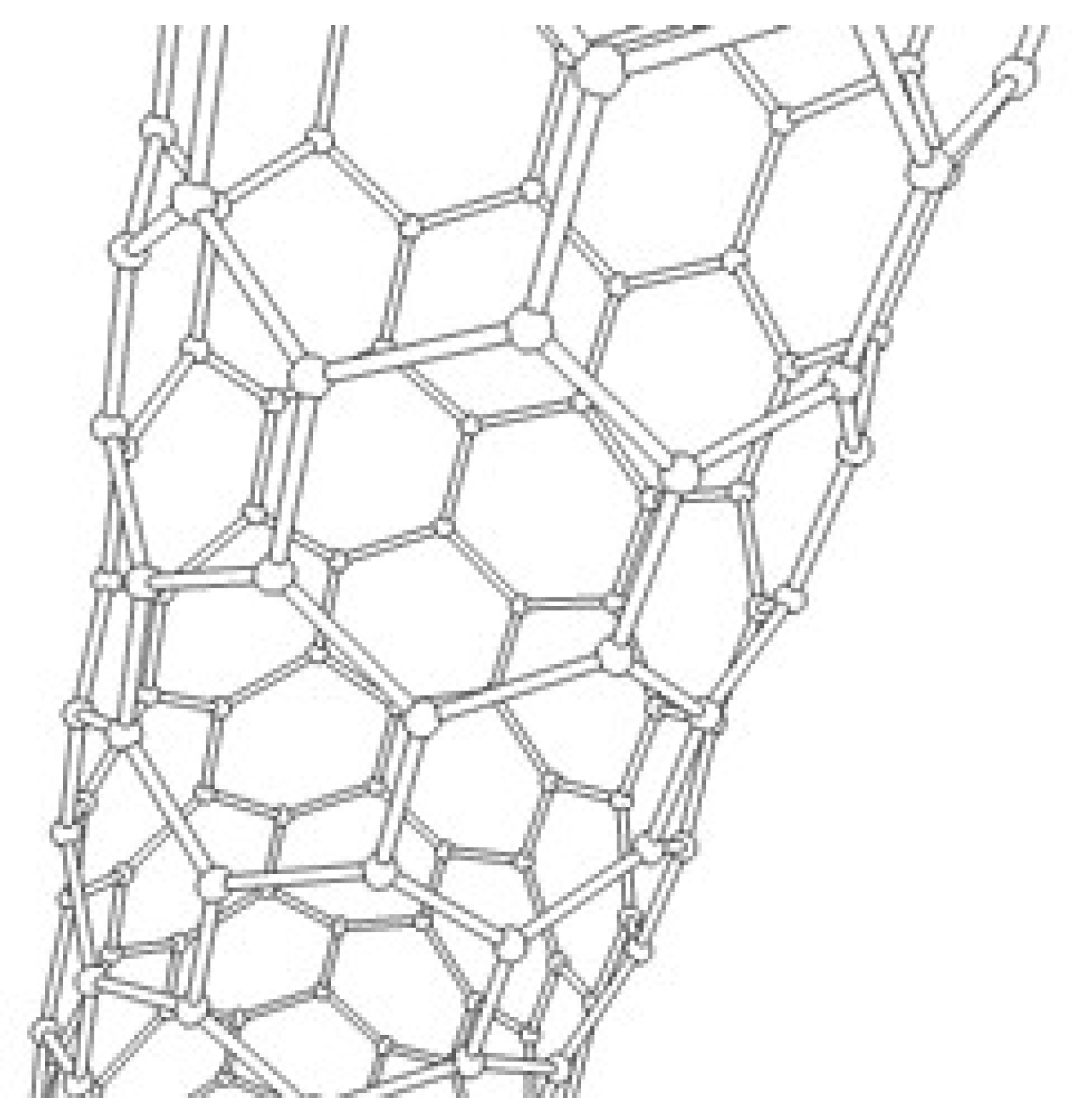
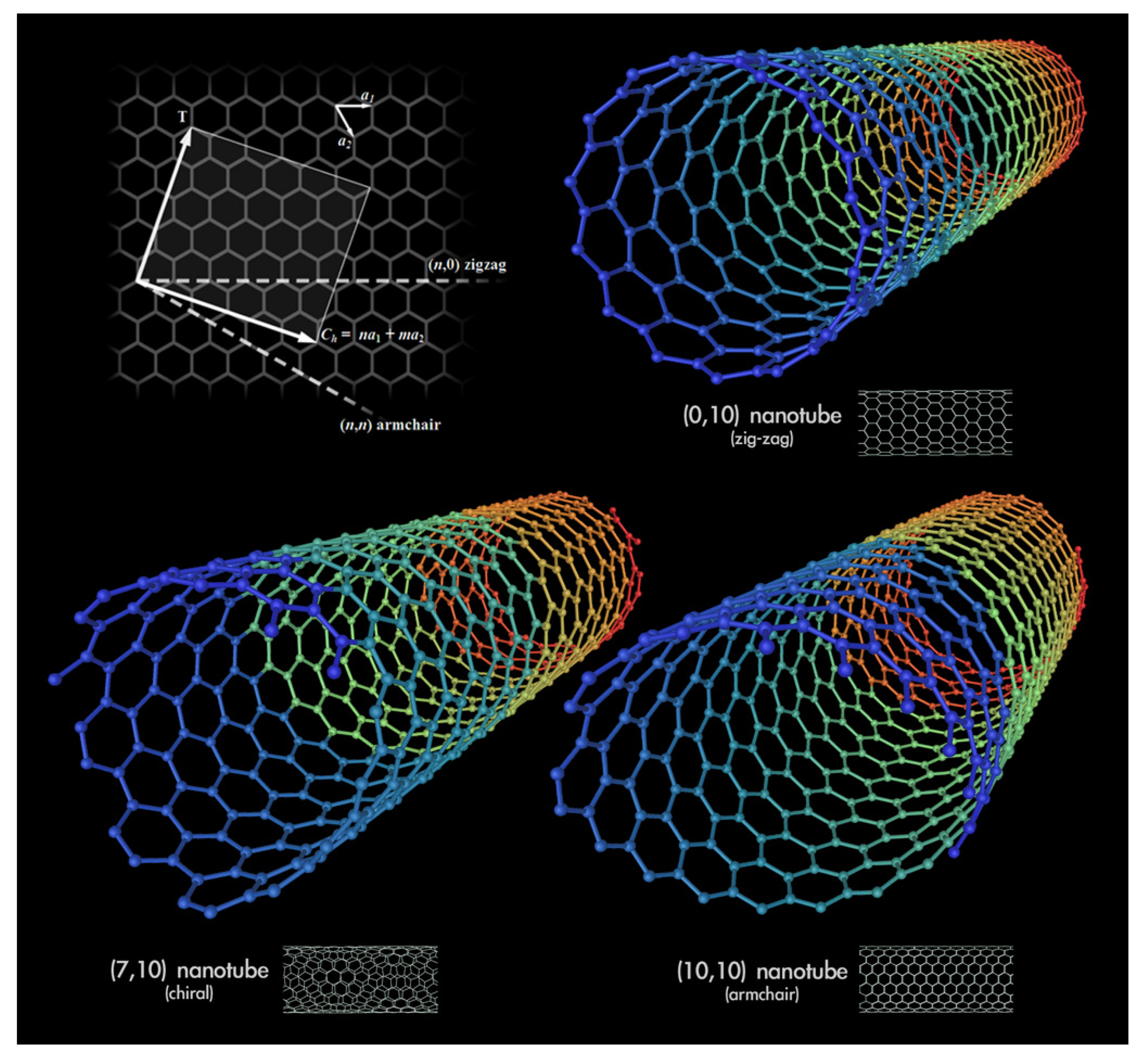

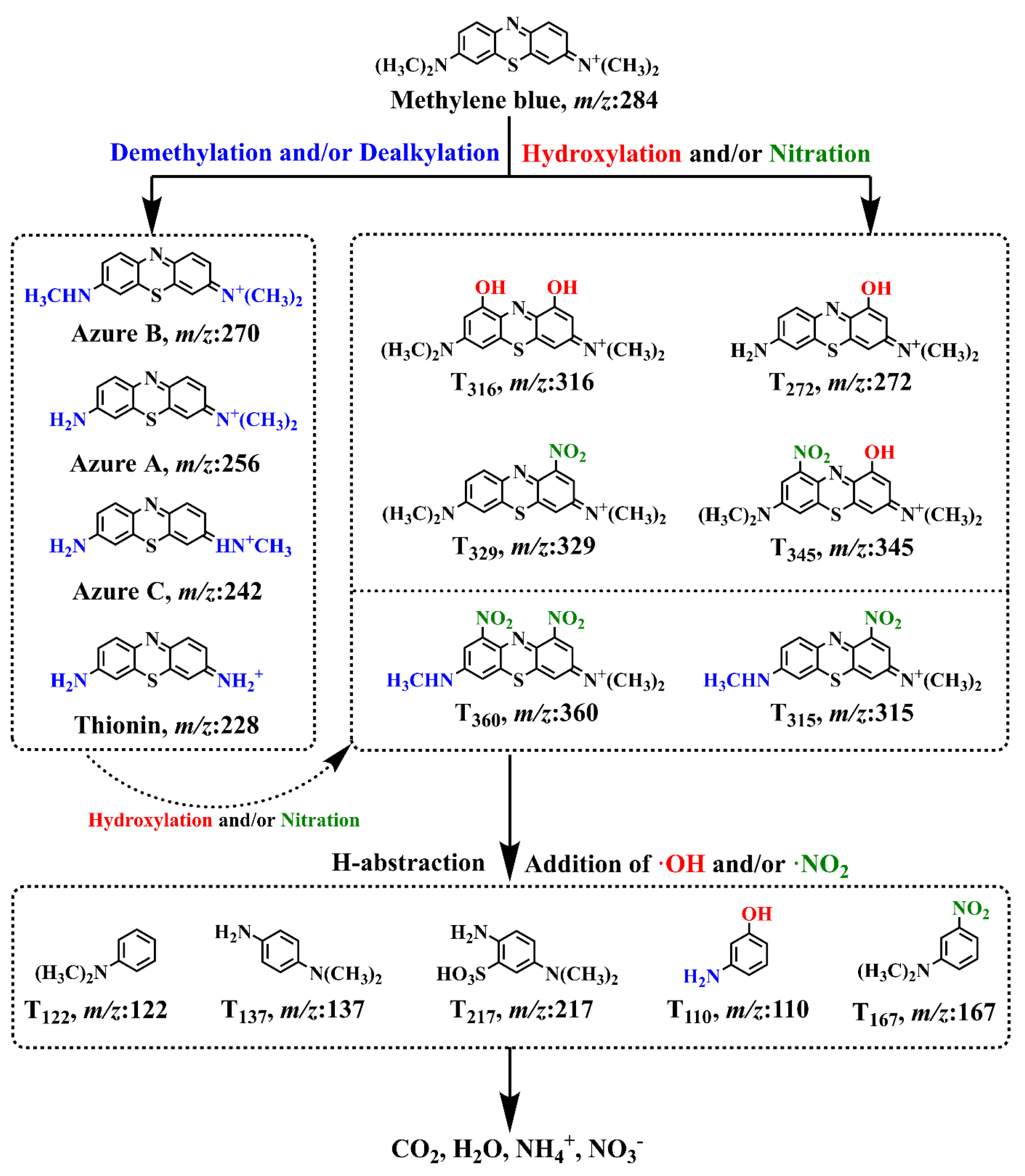

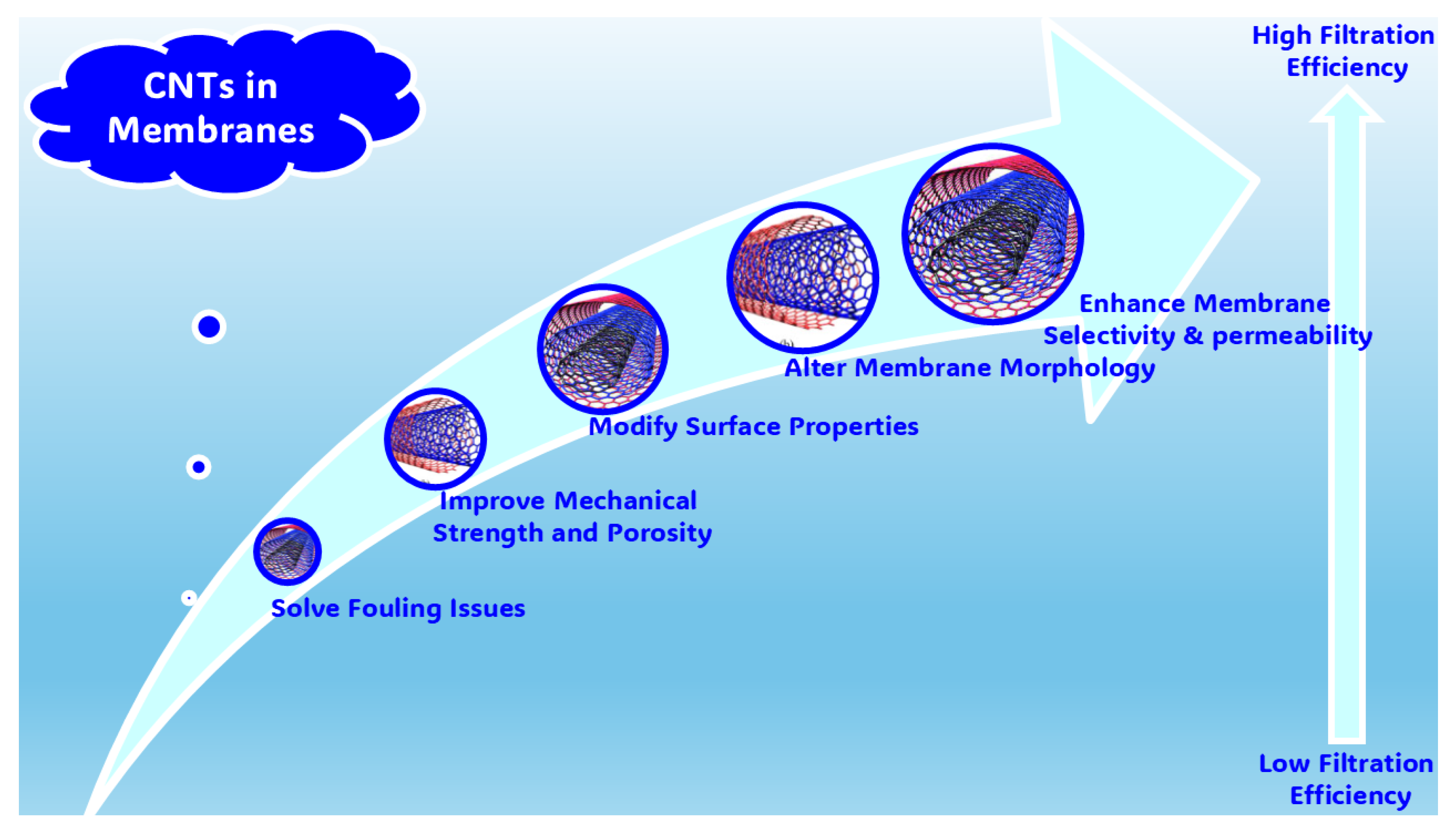
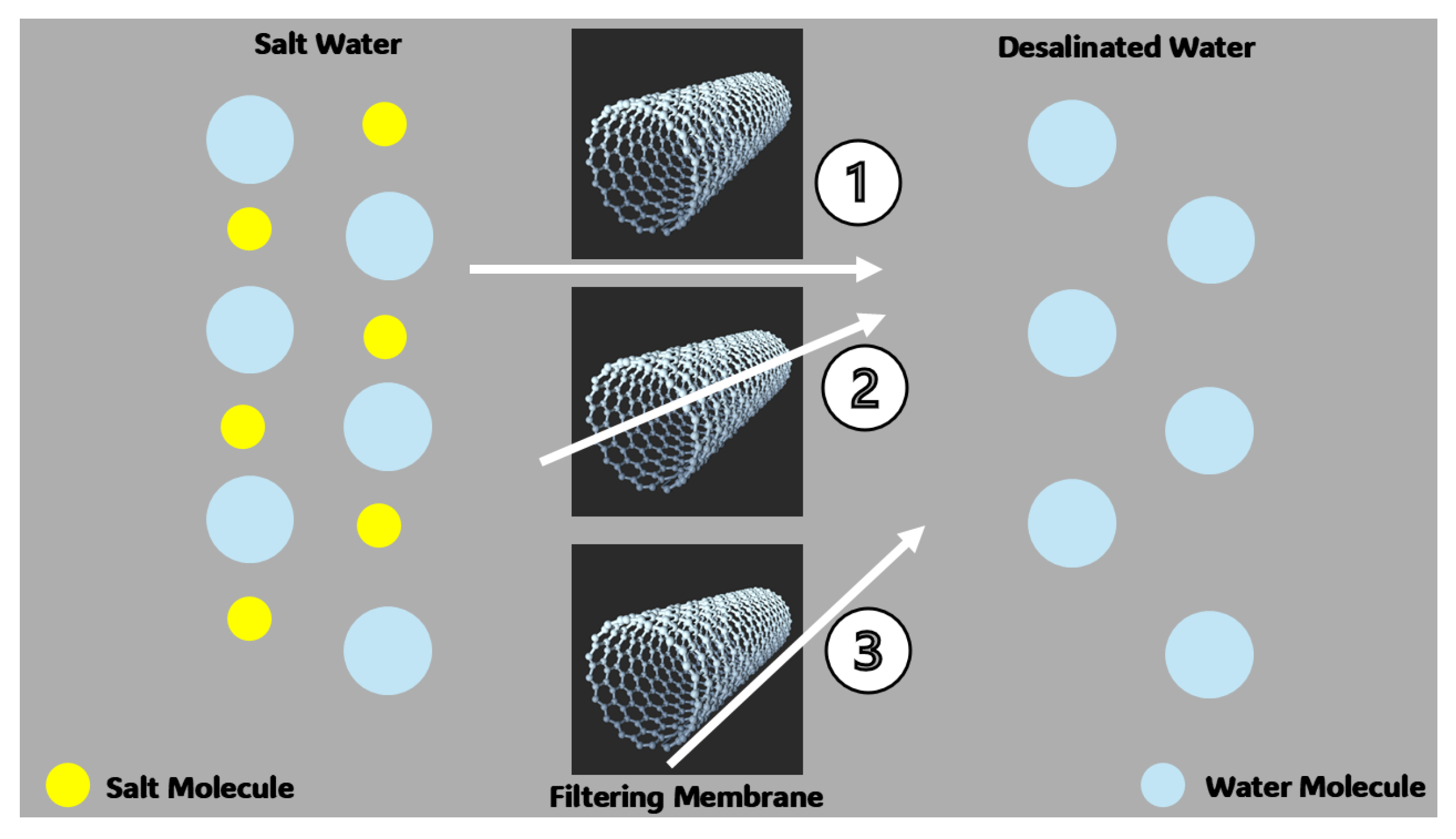
| Parameter | SWCNTs | MWCNTs |
|---|---|---|
| Graphene layer | Single | Multiple |
| Ø | 1.2–2 nm | 5–100 nm |
| ρ | 1.33–1.40 g cm−3 | 2.1 g cm−3 |
| Catalysis | Yes | No |
| SG | ~0.8 g cm−3 | <1.8 g cm−3 |
| TS | 150 GPa | 150 GPa |
| EC | 102–106 S cm−1 | 103–105 S cm−1 |
| EM | ∼105 cm2 V−1 s−1 | ∼105 cm2 V−1 s−1 |
| Purity | Poor | High |
| YM | 1054 GPa | 1200 GPa |
| TC | 6000 Wm−1 K−1 | 3000 Wm−1 K−1 |
| Twisting | Easy | Difficult |
| DL | High | Low |
| CNTs | BET (m2/g) | MIV (cm3/g) | MEV (cm3/g) | Characteristics | Refs. |
|---|---|---|---|---|---|
| Pristine | 150–1587 | 0.06–0.15 | 0.85 | N.A.P. | [15,16,17,18,19,20] |
| M-HNO3 | 157 | N.A. | 0.37 | AT provides O2 containing -COOH, -OH | [21] |
| M-NH3 | 195 | N.A. | 0.42 | NH3 removes -COOH, -OH | [21] |
| M-KOH | 785 | 0.17 | 1.04 | KOH ⬆ MV and MEV, ⬆ KOH/CNT ratio, ⬆ PV | [17,18,19,20] |
| A-Air | 270 | 0.06 | 0.56 | ⬇ MV, air removes catalyst metals/AC | [17] |
| A-CO2 | 420 | 0.10 | 0.67 | Large micropore volume | [17] |
| O3-T | 320 | 0.12 | 0.69 | Opens end caps, holes in sidewall OOCA O2 containing functional groups | [16] |
| HT | 550 | 0.18 | 0.97 | ⬆ External surface area | [22] |
| Biomass | Biomass Roles | Characteristics | Refs. |
|---|---|---|---|
| Rice straw | CS | MWCNTS, Ø = 15–40 nm, L = 14.6–47.9 nm Needle-shaped CNTs, Ø = 2.5–6.8 nm, L = 4–8 nm | [42] |
| Black fungus, seaweed Mushrooms Black sesame seeds | Catalyst | CNT array neatly arranged, Ø = 40–100 nm | [34] |
| Bamboo | Catalyst support | MWCNTs, L = several nm to µm, Ø < 20 nm | [43] |
| Camphor, rice straw | Camphor is the CS Straw is a catalyst support | Bamboo-shaped CNTs, Ø = 22–66 nm | [44] |
| Coconut shell charcoal | Catalyst/catalyst support | MWCNTs, L = tens of µm, Ø = 25–30 nm | [45] |
| CNTs | MT | WP | OCs | AC/RE | Refs. |
|---|---|---|---|---|---|
| O-MWCNTs | BP * | HU | AF, T = 25 °C, AD = 50 mg, PD = 5 mg/L | −/93% | [221] |
| O-MWCNTs, MWCNTs | BP * | SO, Tol, CHCl3, HU | AF, T = 25 °C, AD = 150 mg, PD = 5–10 mg/L | 325, 300, 375 wt%−/>97% | [222] |
| JI-MWCNT-COOH | BP * | MB | BA, pH = 6, T = 20–50 °C, AD = 2.92 mg/mL (JP) PD = 10–80 mg/L, AS = 180 rpm, CT = 0–240 min | 85 mg/g | [223] |
| O-MWCNTs | BP * | PCL, CBZ, EST, PMC, met-THSF, met-MSF, CSF, CHSF, CBY, ATRA, i-PRO, DIU, KET, NAP, met-BSF, MBRO, TEST, NIME, AFLU, met-TESTO, LIN, MAL, FEN, PRO, pro-TEST | BA, T = 25 °C, AD = CBP PD = 1 ng/μL, 10 ng/μL CT = 16 h | −/79, −/82, −/100, −/100, −/73%, −/74, −/87, −/73, −/77, −/74, −/81, −/100, −/87, −/85, −/100, −/88, −/99, −/95, −/89, −/100, −/93, −/85, −/91, −/95, −/94% | [224] |
| BioMOF@SWCNT-COOH | BP * | Ce (III) | BA, pH = 6, T = 25 °C, AD = 40 mg PD = 1.35–50 mg/L, CT = 4200 min | 263 mg/g | [225] |
| MTV-MOF/SWCNT-COOH | BP * | Pb (II) | BA, pH = 6.5, T = 25 °C, AD = 40 mg PD = 0.2–100 mg/L, CT = 16 h | 310 mg/g | [226] |
| GO/SWCNT-COOH | BP * | Pb (II) | BA, pH = 4–8, T = 25 °C, AD = 50 mg PD = 1–50 mg/L, CT = 4000 min | 479 mg/g (pH = 6) | [227] |
| GO/SWCNT-COOH | BP * | DICLO, KET, NAP | BA, pH = 4–8, T = 25 °C, AD = 50 mg PD = 1–50 mg/L, AT = 4200 min | 118, 116, 126 mg/g (pH = 4) | [228] |
| Fe/MWCNT-OH | BP * | Te | BA, pH = 2–12, T = 30–60 °C, AD = 5 mg, PD = 100–500 mg/L, CT = 0–350 min | 800 mg/g (pH = 4.7) | [229] |
| SWCNTs | BP * | MB, DDB, 1-PA | AF, T = 25 °C, PD = 0.015 mg/mL, CT = 15–120 min | 1.4, 0.4, 0.6 μg/m2 | [230] |
| SWCNTs | BP * | 4-NPh, 4-t-OPh, bis-Ph A | BA, AD = 30 mg, PD = 10 ng/mL | −/≈100%, −/≈95%, −/≈90% | [231] |
| f-PA/ZIF-93 SWCNTs | BP * | RB, SY, AO | AF, T = 25 °C, PD = 20 mg/L, CT = 1 h | −/84, −/88, −/94% | [232] |
| f-CNC MWCNTs | BP * | Mn (VII) | AF, T = 25 °C, CT = 20 h | No data | [233] |
| MWCNTs/CH-CAR | BP * | Cu (II); Pb (II) | AF, pH = 7, PD = 2 mg/L, CT = 30 min | −/94%, −/91% | [234] |
| MWCNTs/CIP/MWCNTs/TX100 | BP * | Escherichia coli | AF, T = 21 °C, AD = 1 wt% (CIP, TX100) PD = 104 cells/mL | −/100%, −/58% | [235] |
| Filter Membrane | CNTs | VACNTs Membranes | Buckypaper |
|---|---|---|---|
| Set-up | Powder | Membrane | Membrane |
| Consistency | N.R. | Compact | Compact |
| CNT arrangement | N.R. | Perpendicular to surface | Random |
| CNT function | N.R. | Structural | Structural |
| Mechanical strength | High | Moderate | Moderate |
| Chemical stability | High | Limited | High |
| Water flux | N.R. | Extremely high | Moderate/high |
| Surface area | Extremely high | High | Extremely high |
| Fabrication process | N.R. | Complicated | Simple |
| Operating system | Simple | Complicated | Simple |
| Recovery | Difficult | Simple | Simple |
| Regeneration | Difficult | Slightly difficult | Simple |
| Nanomaterials | Cell Lines | Observation | Refs. |
|---|---|---|---|
| Metal oxide nanoparticles and SWCNTs | A549 | Penetrate the cell | [314] |
| CNTs | RM (NR8383), A549 | ⬆ Intracellular ROS | [315] |
| CNTs | MSTO-211H | Agglomerated CNTs had ⬆ cytotoxicity | [173] |
| MWCNTs | HEK | ⬇ Cell viability, ⬆ IL-8 | [316] |
| Functionalized SWCNTs | HF 3T6, MF 3T3 | Penetrated cells concentrating in the cytoplasm | [317] |
| SWCNTs | HaCaT, HeLa, H1299, A549 | ⬆ OS and inhibition of cell proliferation | [318] |
| SWCNTs | HaCaT | Cell death, OS, ⬆ lipid peroxides | [319] |
| SWCNTs | HaCaT and BEAS-2B | Loss of cellular integrity and cellular apoptosis | [320] |
| SWCNTs | Lym, macrophages | Uptake of SWCNTs | [321] |
| MWCNTs | HEK | Cell-cycle inhibition | [322] |
| Functionalized SWCNTs | HDFs | Less toxicity | [323] |
| SWCNT–streptavidin complex | HL60 | Low toxicity | [324] |
| CNTs | Lymphocytes | ⬆ Secretion of cytokines | [325] |
| SWCNTs | HEK293T | ⬇ Cell proliferation, ⬇ in cell adhesive ability | [125] |
| SWCNTs and MWCNTs | AMs | SWCNTs had ⬆ toxicity than MWCNTs | [326] |
| Purified SWCNTs | LF V79 | DNA damage | [327] |
| SWCNTs | RAW 264.7 | Production of TGF-β1 | [328] |
| Iron-rich SWCNTs | Macrophages | Phagocytosis of the SWCNTs, conversion of EXC O2– into OH radicals | [320] |
| Ground MWCNTs | RPMs | Cytotoxicity and ⬆⬆⬆ proinflammatory cytokines | [329] |
| MWCNTs | J774.1 | Cytotoxic effects by rupturing CM | [330] |
| CNTs | HAECs | ⬆ MCP-1, VCAM-1, and IL-8 | [320] |
| MWCNTs | AMs | Cell death | [331] |
| P-MWCNTs, O-MWCNTs | T lymphocytes | Apoptosis | [332] |
| Nanomaterials | Animals | Observation | Reference |
|---|---|---|---|
| SWCNTs | Mice | IR, granulomas | [333] |
| Pristine SWCNTs | Rat | IR, MFGs | [334] |
| SWCNTs/SiO2 | Mice | Granulomas, LF | [328] |
| MWCNTs | Rat | PF | [329] |
| SWCNTs | Mice | IR, OS, CD | [335] |
| MWCNTs | Mice | IR, granulomas | [336] |
| MWCNTs | Rat | Granuloma, CD | [337] |
| MWCNTs/SWCNTs | Guinea pigs | Pneumonitis | [338] |
| MWCNTs | Rat | Inflammation, granuloma, LF | [339] |
| Theme of the Study | Tested Cells | Toxic Effects | Findings | Refs. |
|---|---|---|---|---|
| Genotoxicity of MWCNTs in HCs | HeLa, MCF7, HREC | Genotoxicity | Damage to DNA and chromosomes, OS | [344,345] |
| Inhalation of CNTs induces PT | Mice | Pulmonary toxicity | Inflammation, granulomas, LF, ⬆ cytokine concentration | [346,347] |
| Skin exposure to CNTs to assess DT | JB6 P+, SKH-1 | Dermal toxicity | Irritation, inflammation, unclear penetration into deeper skin | [348] |
| Impact of CNTs on immune system | Lyms, T cells, Mons, DCs | Immunotoxicity | Immune responses, cytokine production, immune cell function alteration | [349,350] |
| Cardiovascular effects of CNTs in AMs | Mice | CT | Inflammation, ⬆ blood pressure and CD induced by OS | [351] |
| Liver toxicity/biodistribution of CNTs | Mice | Hepatoxicity | Liver damage, liver enzyme level alterations | [352] |
| Renal toxicity of MWCNTs in rats | HEK293 | Renal toxicity | Kidney CNT accumulation, renal inflammation, OS Kidney functionality impairment | [353] |
| OS induced by SWCNTs/MWCNTs in AMs | Mouse | OS | ⬆ ROS, ⬆ OS, MDs, ⬇ ATP | [354] |
| In vitro neurotoxicity of CNTs | Mammalian cell lines | Neurotoxicity | Neuroinflammation, neuron damage | [355] |
| CNTs | Models | Affected Organ | Results | Refs. |
|---|---|---|---|---|
| MWCNTs | Male Sprague Dawley rats | NS | Alterations of sympathetic and parasympathetic NSE | [356] |
| MWCNTs | Mice | NS and BBB | Damaged BBB integrity, NIR | [357] |
| CNTs | Male NMRI mice | NS | Behavioral toxicity, manifestation of sadness or anxiety | [358] |
| SWCNTs | PC-12 cells | NS | Toxicity to PC-12 cells, ⬆ harm to differentiated PC-12 cells | [359] |
| SWCNTs | Male C57BL/6 mice | PIS | ⬆ Vulnerability to respiratory virus infections | [360] |
| SWCNTs | IRC | IS, RS | Affected development, reproduction, IT | [361] |
| SWCNTs | MCs and J774A BALB/c mice | IS | IT * | [362] |
| MWCNTs | T lymphocytes | Concentration-dependent damage to human T cells | [332] | |
| SWCNTs | CD1, D3, NIH3T3 cells | Embryos | Harm to Mammalian embryos | [341] |
| CNTs | Kunming mice | Damage to embryos and fetuses, miscarriage | [363] | |
| MWCNTs | Zebrafish embryos | Significant length-dependent development risk | [364] | |
| CNTs | MEFs, C57BL/6J | Induction of hereditary embryotoxicity | [365] | |
| O-SWCNTs | Artemia salina | ROS overproduction, deformed Artemia salina | [366] | |
| CNTs | Mice/Humans | RS, pregnant, foetus | OS, HD, EA, TE, inflammation | [367] |
| SWCNTs/MWCNTs | MeT-5A and BEAS 2B cells | Genome | DNA damage in MeT-5A cells | [368] |
| Strategy | Goal | Modifying Agents/Methods | Results | Refs. |
|---|---|---|---|---|
| CNT surface modification with biocompatible materials or other molecules | ⬆ Dispersion in biological fluids Influenced CU, ⬆ solubility, ⬇ toxicity | Proteins, surfactants | ⬆ TT, ⬆ TBs, ⬇ toxicity | [383,384,385] |
| FA | ⬆ In vivo tumor targeting, ⬆ therapeutic benefits ⬇ Toxicity | [386] | ||
| PA hydrogels *, biomaterial, TiO2 | 100% survival of L929 mouse fibroblasts | [381] | ||
| Coatings of CNTs | ⬆ CNT biocompatibility ⬇ Potential toxicity Prevent direct contact with BSs ⬆ CNT solubility | Curcumin lysine ** | ⬇ IL-6, IL-8, IL-1β, TNFα, N-FκB ⬆ Antioxidant enzyme catalase, ⬇ ROS generation Recovery of MM, ⬇ cell death | [387] |
| CNTs encapsulation CNTs to entrap BAMs | ⬇ Direct cell exposure to CNTs Control of CNT release ⬇ CNTs’ impact on tissues | PEG (entrapping agent) Oxaliplatin (entrapped agent) | PEGylation lowered oxaliplatin release rate ⬆ Drug’s anticancer effects on HT-29 cells | [388] |
| Tailor Ø size and L | ⬇ Toxicity | N.A. | ⬆ SSA, ⬆ TM, ⬇ toxicity, ⬇ harm to lysosomes *** | [389,390] |
| Optimized PPs | Remove MIs Remove RCs | Chemical/electrochemical oxidation [391] High chlorine partial pressure [392] MA digestion [393] Incandescent annealing [394] | ⬇ Harmful effects | [394] |
| Engineering controls Suitable PPE | ⬇ Inhalation | Proper ventilation/respiratory protection | ⬇ Respiratory toxicity | N.R. |
| CA with AOs | ⬇ OS ⬇ Damage to cells | Quercetin | Prevention of oxidative damage ⬇ Inflammatory effects, ⬇ immunotoxic effects | [378] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S.; Zuccari, G. Carbon-Nanotube-Based Nanocomposites in Environmental Remediation: An Overview of Typologies and Applications and an Analysis of Their Paradoxical Double-Sided Effects. J. Xenobiot. 2025, 15, 76. https://doi.org/10.3390/jox15030076
Alfei S, Zuccari G. Carbon-Nanotube-Based Nanocomposites in Environmental Remediation: An Overview of Typologies and Applications and an Analysis of Their Paradoxical Double-Sided Effects. Journal of Xenobiotics. 2025; 15(3):76. https://doi.org/10.3390/jox15030076
Chicago/Turabian StyleAlfei, Silvana, and Guendalina Zuccari. 2025. "Carbon-Nanotube-Based Nanocomposites in Environmental Remediation: An Overview of Typologies and Applications and an Analysis of Their Paradoxical Double-Sided Effects" Journal of Xenobiotics 15, no. 3: 76. https://doi.org/10.3390/jox15030076
APA StyleAlfei, S., & Zuccari, G. (2025). Carbon-Nanotube-Based Nanocomposites in Environmental Remediation: An Overview of Typologies and Applications and an Analysis of Their Paradoxical Double-Sided Effects. Journal of Xenobiotics, 15(3), 76. https://doi.org/10.3390/jox15030076









