Occurrence of 97 Pharmaceuticals in Wastewater and Receiving Waters: Analytical Validation and Treatment Influence
Abstract
1. Introduction
2. Materials and Methods
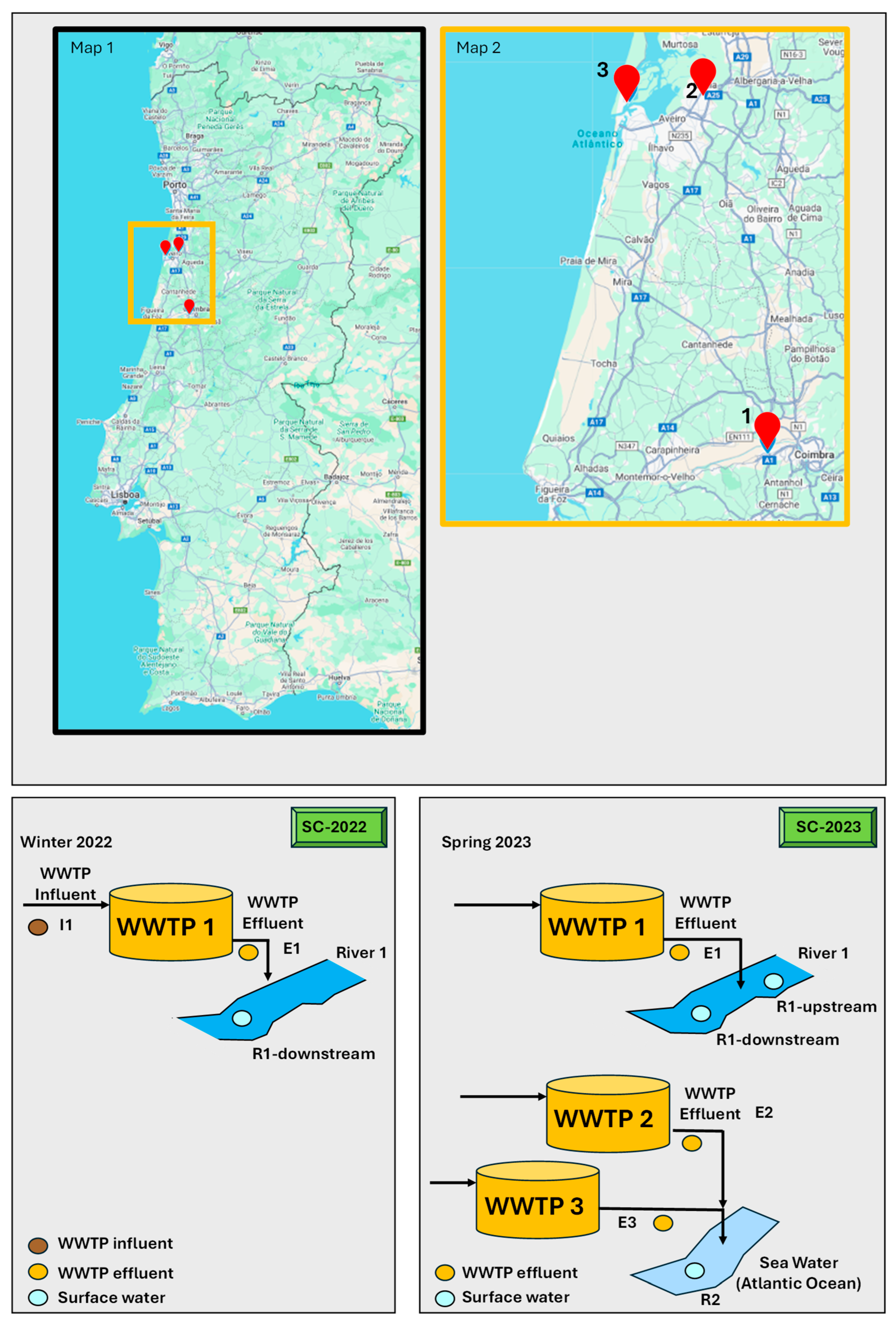
2.1. Sampling Campaign
2.2. Description of the Study WWTPs
2.3. Collection and Pre-Treatment of the Samples
2.4. Eluents, Solvents, and Reagents
2.5. Targeted Pharmaceutical Compounds
2.6. Analytical Method
2.6.1. Sample Pre-Treatment
2.6.2. Chromatographic Analysis
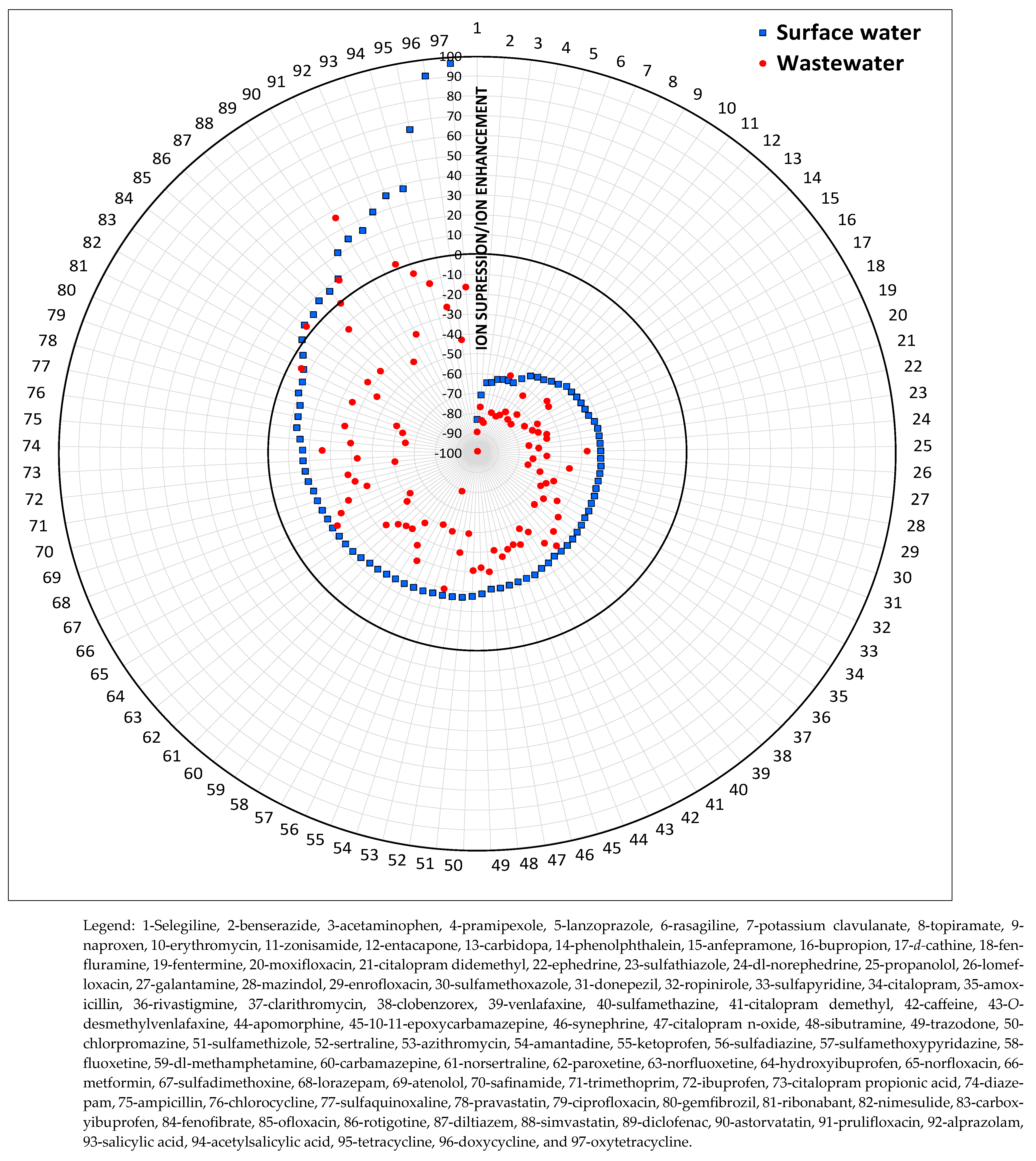
2.7. Analytical Method Validation
2.8. UHPLC-MS/MS Analytical Sequence
2.9. Environmental Risk Characterization
3. Results and Discussion
3.1. Validation Results
3.2. Determination of Pharmaceutical Compounds
3.2.1. Detected Pharmaceuticals: Quantity, Concentration, and Frequency
Detection Summary and Overview
Spatial Distribution of Pharmaceutical Detections
Influence of WWTP Characteristics on Detection Patterns
Trends in Pharmaceutical Concentrations
Exceptions to Expected Dilution Patterns
Potential Sources of Elevated Downstream Concentrations
Frequency of Pharmaceutical Detection and Relevant Environmental Implications
High-Concentration Compounds and Their Environmental Significance
3.2.2. Comparison of Sampling Campaigns Performed in 2022 and 2023
3.2.3. Highest Concentration Levels of the Detected Compounds
3.2.4. R1-Upstream vs. R1-Downstream in the 2023 Sampling Campaign
3.2.5. Influent I1 vs. Effluent E1 in the 2022 Sampling Campaign
3.2.6. Analysis of Pharmaceutical Detection Across Therapeutic Classes
3.2.7. Pharmaceuticals and Their Transformation Products
3.2.8. Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECOSAR | Ecological Structure–Activity Relationships |
| ILIS | Isotopically Labeled Internal Standards |
| MDL | Method Detection Limit |
| ME | Matrix Effect |
| MQL | Method Quantification Limit |
| MRM | Multiple Reaction Monitoring |
| MS/MS | Tandem Mass Spectrometry |
| n.d. | Not detected |
| n.e. | Not eliminated |
| RQ | Risk Quotient |
| SPE | Solid Phase Extraction |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
References
- Bartolo, N.S.; Azzopardi, L.M.; Serracino-Inglott, A. Pharmaceuticals and the environment. Early Hum. Dev. 2021, 155, 105218. [Google Scholar] [CrossRef] [PubMed]
- Beek, T.a.d.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical Pollution in Aquatic Environments: A Concise Review of Environmental Impacts and Bioremediation Systems. Front. Microbiol. 2022, 13, 869332. [Google Scholar] [CrossRef] [PubMed]
- Kayode-Afolayan, S.D.; Ahuekwe, E.F.; Nwinyi, O.C. Impacts of pharmaceutical effluents on aquatic ecosystems. Sci. Afr. 2022, 17, e01288. [Google Scholar] [CrossRef]
- Paíga, P.; Correia, M.; Fernandes, M.J.; Silva, A.; Carvalho, M.; Vieira, J.; Jorge, S.; Silva, J.G.; Freire, C.; Delerue-Matos, C. Assessment of 83 pharmaceuticals in WWTP influent and effluent samples by UHPLC-MS/MS: Hourly variation. Sci. Total Environ. 2019, 648, 582–600. [Google Scholar] [CrossRef]
- Paíga, P.; Santos, L.H.M.L.M.; Ramos, S.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Presence of pharmaceuticals in the Lis river (Portugal): Sources, fate and seasonal variation. Sci. Total Environ. 2016, 573, 164–177. [Google Scholar] [CrossRef]
- Paíga, P.; Correia-Sá, L.; Correia, M.; Figueiredo, S.; Vieira, J.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Temporal Analysis of Pharmaceuticals as Emerging Contaminants in Surface Water and Wastewater Samples: A Case Study. J. Xenobiot. 2024, 14, 873–892. [Google Scholar] [CrossRef]
- Domínguez-García, P.; Almirall, X.O.; Gómez-Canela, C. A POCIS-based approach for the monitoring of pharmaceuticals in wastewater treatment plants: Calibration and deployment challenges. Environ. Pollut. 2025, 367, 125641. [Google Scholar] [CrossRef]
- Castaño-Ortiz, J.M.; Gil-Solsona, R.; Ospina-Álvarez, N.; Alcaraz-Hernández, J.D.; Farré, M.; León, V.M.; Barceló, D.; Santos, L.H.M.L.M.; Rodríguez-Mozaz, S. Fate of pharmaceuticals in the Ebro River Delta region: The combined evaluation of water, sediment, plastic litter, and biomonitoring. Sci. Total Environ. 2024, 906, 167467. [Google Scholar] [CrossRef]
- Castaño-Ortiz, J.M.; Gago-Ferrero, P.; Barceló, D.; Rodríguez-Mozaz, S.; Gil-Solsona, R. HRMS-based suspect screening of pharmaceuticals and their transformation products in multiple environmental compartments: An alternative to target analysis? J. Hazard. Mater. 2024, 465, 132974. [Google Scholar] [CrossRef]
- González-Alonso, S.; Merino, L.M.; Esteban, S.; López de Alda, M.; Barceló, D.; Durán, J.J.; López-Martínez, J.; Aceña, J.; Pérez, S.; Mastroianni, N.; et al. Occurrence of pharmaceutical, recreational and psychotropic drug residues in surface water on the northern Antarctic Peninsula region. Environ. Pollut. 2017, 229, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A 2012, 1248, 104–121. [Google Scholar]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Hatami, A.M.; Sabour, M.R.; Hajbabaie, M.R.; Nematollahi, H. Global trends of VOSviewer research, emphasizing Environment and Energy areas: A bibliometric analysis during 2000–2020. Environ. Energy Econ. Res. 2022, 6, S030. [Google Scholar]
- Bukar, U.A.; Sayeed, M.S.; Razak, S.F.A.; Yogarayan, S.; Amodu, O.A.; Mahmood, R.A.R. A method for analyzing text using VOSviewer. MethodsX 2023, 11, 102339. [Google Scholar] [CrossRef]
- Sabour, M.R.; Derhamjani, G.; Akbari, M.; Hatami, A.M. Global trends and status in waste foundry sand management research during the years 1971-2020: A systematic analysis. Environ. Sci. Pollut. Res. 2021, 28, 37312–37321. [Google Scholar] [CrossRef]
- EU. Directive (EU) 2024/3019 of the European Parliament and of the Council of 27 November 2024 Concerning Urban Wastewater Treatment Off J. Eur. Inion, L Series. pp. 1–59. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/?uri=OJ:L_202403019 (accessed on 1 January 2025).
- EU. Commission Implementing Decision (EU) 2025/439 of 28 February 2025 Establishing a Watch List of Substances for Union-wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off J. Eur. Inion, L Series. pp. 1–6. Available online: https://eur-lex.europa.eu/eli/dec_impl/2025/439/oj (accessed on 1 March 2025).
- Paíga, P.; Delerue-Matos, C. Tracing Pharmaceuticals in Water Systems: Focus on Neurodegenerative and Psychiatric Treatments. J. Xenobiot. 2024, 14, 1807–1825. [Google Scholar] [CrossRef] [PubMed]
- EMEA. Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use. European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use. London, 01 June 2006. Doc. Ref. EMEA/CHMP/SWP/4447/00 Rev. 1. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-environmental-risk-assessment-medicinal-products-human-use-revision-1_en.pdf (accessed on 16 February 2024).
- US Environmental Protection Agency (USEPA). Ecological Structure Activity Relationships (ECOSAR) Predictive Model v1.11. 2012. Available online: https://goo.gl/xBM2VN (accessed on 2 December 2023).
- Wei, D.; Kisuno, A.; Kameya, T.; Urano, K. A new method for evaluating biological safety of environmental water with algae, daphnia and fish toxicity ranks. Sci. Total Environ. 2006, 371, 383–390. [Google Scholar] [CrossRef]
- Ginebreda, A.; Muñoz, I.; López de Alda, M.; Brix, R.; López-Doval, J.; Barceló, D. Environmental risk assessment of pharmaceuticals in rivers: Relationships between hazard indexes and aquatic macroinvertebrate diversity indexes in the Llobregat River (NE Spain). Environ. Int. 2010, 36, 153–162. [Google Scholar] [CrossRef]
- Furlong, E.T.; Noriega, M.C.; Kanagy, C.J.; Kanagy, L.K.; Coffey, L.J.; Burkhardt, M.R. Determination of Human-Use Pharmaceuticals in Filtered Water by Direct Aqueous Injection–High-Performance Liquid Chromatography/Tandem Mass Spectrometry. Chapter 10 of Section B, Methods of the National Water Quality Laboratory Book 5, Laboratory Analysis. Techniques and Methods 5–B10. U.S. Department of the Interior. U.S. Geological Survey. Available online: https://pubs.usgs.gov/tm/5b10/pdf/tm10-b5.pdf (accessed on 2 May 2025).
- EPA Method 1694: Pharmaceuticals and Personal Care Products in Water, Soil, Sediment, and Biosolids by HPLC/MS/MS, December 2007. Available online: https://www.epa.gov/sites/default/files/2015-10/documents/method_1694_2007.pdf (accessed on 2 May 2025).
- Almeida, C.M.M. Overview of Sample Preparation and Chromatographic Methods to Analysis Pharmaceutical Active Compounds in Waters Matrices. Separations 2021, 8, 16. [Google Scholar] [CrossRef]
- Renew, J.E.; Huang, C.H. Simultaneous determination of fluoroquinolone, sulfonamide, and trimethoprim antibiotics in wastewater using tandem solid phase extraction and liquid chromatography–electrospray mass spectrometry. J. Chromatogr. A 2004, 1042, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Lacey, C.; McMahon, G.; Bones, J.; Barron, L.; Morrissey, A.; Tobin, J.M. An LCMS method for the determination of pharmaceutical compounds in wastewater treatment plant influent and effluent samples. Talanta 2008, 75, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Borecka, M.; Białk-Bielinska, A.; Siedlewicz, G.; Stepnowski, P.; Pazdro, K. The Influence of Matrix Effects on Trace Analysis of Pharmaceutical Residues in Aqueous Environmental Samples. In Insights on Environmental Changes; GeoPlanet: Earth and Planetary Sciences; Zieliński, T., Pazdro, K., Dragan-Górska, A., Weydmann, A., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–16. [Google Scholar]
- Steen, R.J.C.A.; Leonards, P.E.G.; Brinkman, U.A.T. Ecological risk assessment of agrochemicals in European estuaries. Environ. Toxicol. Chem. 1999, 18, 1574–1581. [Google Scholar] [CrossRef]
- PremierTech Ltd. Why Do We Need to Treat Wastewater? Available online: https://www.premiertechaqua.com/en-gb/wastewater/why-do-we-need-treat-wastewater (accessed on 4 May 2025).
- Aukidy, M.A.; Verlicchi, P.; Jelic, A.; Petrovic, M.; Barcelò, D. Monitoring release of pharmaceutical compounds: Occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley, Italy. Sci. Total Environ. 2012, 438, 15–25. [Google Scholar] [CrossRef]
- Rodrigues, F.; Durães, L.; Simões, N.E.C.; Pereira, A.M.P.T.; Silva, L.J.G.; Feio, M.J. Pharmaceuticals in urban streams: A review of their detection and effects in the ecosystem. Water Res. 2025, 268 Pt B, 122657. [Google Scholar] [CrossRef]
- Hughes, J.; Cowper-Heays, K.; Olesson, E.; Bell, R.; Stroombergen, A. Impacts and implications of climate change on wastewater systems: A New Zealand perspective. Clim. Risk Manag. 2021, 31, 100262. [Google Scholar] [CrossRef]
- ECCC. Environment and Climate Change Canada. Water Pollution: Erosion and Sedimentation. Available online: https://www.canada.ca/en/environment-climate-change/services/water-overview/pollution-causes-effects/erosion-sedimentation.html (accessed on 7 March 2025).
- Paíga, P.; Delerue-Matos, C. Anthropogenic contamination of Portuguese coastal waters during the bathing season: Assessment using caffeine as a chemical marker. Mar. Pollut. Bull. 2017, 120, 355–363. [Google Scholar] [CrossRef]
- Paíga, P.; Ramos, S.; Jorge, S.; Gabriel Silva, J.; Delerue-Matos, C. Monitoring survey of caffeine in surface waters (Lis River) and wastewaters located at Leiria Town in Portugal. Environ. Sci. Pollut. Res. 2019, 26, 33440–33450. [Google Scholar] [CrossRef]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Seasonal Variation in the Occurrence of Pharmaceuticals in Effluents From a Sewage Treatment Plant in the Recipient Water. Environ. Sci. Technol. 2005, 39, 8220–8226. [Google Scholar] [CrossRef]
- Vidal-Dorsch, D.; SM, B.; Maruya, K.; Snyder, S.; Trenholm, R.; BJ, V. Contaminants of emerging concern in municipal wastewater effluents and marine receiving water. Environ. Toxicol. Chem. 2012, 31, 2674–2682. [Google Scholar] [CrossRef]
- Hedgespeth, M.L.; Sapozhnikova, Y.; Pennington, P.; Clum, A.; Fairey, A.; Wirth, E. Pharmaceuticals and personal care products (PPCPs) in treated wastewater discharges into Charleston Harbor, South Carolina. Sci. Total Environ. 2012, 437, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ferech, M.; Coenen, S.; Dvorakova, K.; Hendrickx, E.; Suetens, C.; Goossens, H. European Surveillance of Antimicrobial Consumption (ESAC): Outpatient penicillin use in Europe. J. Antimicrob. Chemother. 2006, 58, 408–412. [Google Scholar] [CrossRef]
- Santos, V.; Anjos, J.; de Medeiros, J.; Montagner, C. Impact of agricultural runoff and domestic sewage discharge on the spatial–temporal occurrence of emerging contaminants in an urban stream in São Paulo, Brazil. Environ. Monit. Assess. 2022, 194, 637. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Paíga, P.; Lolić, A.; Hellebuyck, F.; Santos, L.H.M.L.M.; Correia, M.; Delerue-Matos, C. Development of a SPE–UHPLC–MS/MS methodology for the determination of non-steroidal anti-inflammatory and analgesic pharmaceuticals in seawater. J. Pharm. Biomed. Anal. 2015, 106, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Buerge, I.J.; Poiger, T.; Muller, M.D.; Buser, H.-R. Caffeine, an Anthropogenic Marker for Wastewater Contamination of Surface Waters. Environ. Sci. Technol. 2003, 37, 691–700. [Google Scholar] [CrossRef]
- Seiler, R.L.; Zaugg, S.D.; Thomas, J.M.; Howcroft, D.L. Caffeine and pharmaceuticals as indicators of wastewater contamination in wells. Ground Water 1999, 37, 405–410. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Thai, P.K.; Kaserzon, S.L.; O’Brien, J.W.; Eaglesham, G.; Mueller, J.F. Assessment of drugs and personal care products biomarkers in the influent and effluent of two wastewater treatment plants in Ho Chi Minh City, Vietnam. Sci. Total Environ. 2018, 631–632, 469–475. [Google Scholar] [CrossRef]
- Li, S.; Wen, J.; He, B.; Wang, J.; Hu, X.; Juan, L. Occurrence of caffeine in the freshwater environment: Implications for ecopharmacovigilance. Environ. Pollut. 2020, 263 Pt B, 114371. [Google Scholar] [CrossRef]
- Ferrando-Climent, L.; Collado, N.; Buttiglieri, G.; Gros, M.; Rodriguez-Roda, I.; Rodriguez-Mozaz, S.; Barceló, D. Comprehensive study of ibuprofen and itsmetabolites in activated sludge batch experiments and aquatic environment. Sci. Total Environ. 2012, 438, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Farrell, S. Aspirin Stability. Available online: https://pharmahub.org/resources/535/download/ASA_Freshman_Lab_Handout.pdf (accessed on 7 March 2025).
- Lajeunesse, A.; Gagnon, C.; Sauvé, S. Determination of basic antidepressants and their N-desmethyl metabolites in raw sewage and wastewater using solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal. Chem. 2008, 80, 5325–5333. [Google Scholar] [CrossRef]
- Colzani, L.; Forni, C.; Clerici, L.; Barreca, S.; Dellavedova, P. Determination of pollutants, antibiotics, and drugs in surface water in Italy as required by the third EU Water Framework Directive Watch List: Method development, validation, and assessment. Environ. Sci. Pollut. Res. 2024, 31, 14791–14803. [Google Scholar] [CrossRef]
- Domínguez-García, P.; Aljabasini, O.; Barata, C.; Gómez-Canela, C. Environmental risk assessment of pharmaceuticals in wastewaters and reclaimed water from catalan main river basins. Sci. Total Environ. 2024, 949, 175020. [Google Scholar] [CrossRef]
- Gulkowska, A.; Leung, H.W.; So, M.K.; Taniyasu, S.; Yamashita, N.; Yeung, L.W.Y.; Richardson, B.J.; Lei, A.P.; Giesy, J.P.; Lam, P.K.S. Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and Shenzhen, China. Water Res. 2008, 42, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Kermia, A.E.B.; Fouial-Djebbar, D.; Trari, M. Occurrence, fate and removal efficiencies of pharmaceuticals in wastewater treatment plants (WWTPs) discharging in the coastal environment of Algiers. Comptes Rendus. Chim. 2016, 19, 963–970. [Google Scholar] [CrossRef]
- Maurer, M.; Escher, B.I.; Richle, P.; Schaffner, C.; Alder, A.C. Elimination of β-blockers in sewage treatment plants. Water Res. X 2007, 41, 1614–1622. [Google Scholar] [CrossRef]
- Sun, Q.; Lv, M.; Hu, A.; Yang, X.; Yu, C.-P. Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China. J. Hazard. Mater. 2014, 277, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Bagnati, R.; Fanelli, R.; Pomati, F.; Calamari, D.; Zuccato, E. Removal of pharmaceuticals in sewage treatment plants in Italy. Environ. Sci. Technol. 2006, 40, 357–363. [Google Scholar] [CrossRef]
- Joss, A.; Keller, E.; Alder, A.C.; Göbel, A.; McArdell, C.S.; Ternes, T.; Siegrist, H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005, 39, 3139–3152. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543 Pt A, 547–569. [Google Scholar] [CrossRef]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Radjenovic, J.; Petrovic, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Mlunguza, N.Y.; Ncube, S.; Mahlambi, P.N.; Chimuka, L.; Madikizela, L.M. Adsorbents and removal strategies of non-steroidal anti-inflammatory drugs from contaminated water bodies. J. Environ. Chem. Eng. 2019, 7, 103142. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions—A review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Ben, W.; Zhu, B.; Yuan, X.; Zhang, Y.; Yang, M.; Qiang, Z. Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: Comparison of wastewater treatment processes. Water Res. 2018, 130, 38–46. [Google Scholar] [CrossRef]
- Zorita, S.; Mårtensson, L.; Mathiasson, L. Occurrence and removal of pharmaceuticals in a municipal sewage treatment system in the south of Sweden. Sci. Total Environ. 2009, 407, 2760–2770. [Google Scholar] [CrossRef]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Gómez, M.J.; Bueno, M.M.; Lacorte, S.; Fernández-Alba, A.R.; Agüera, A. Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere 2007, 66, 993–1002. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E.; Kompare, B. Removal of pharmaceutical residues in a pilot wastewater treatment plant. Anal. Bioanal. 2007, 387, 1379–1387. [Google Scholar] [CrossRef]
- Lindqvist, N.; Tuhkanen, T.; Kronberg, L. Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters. Water Res. 2005, 39, 2219–2228. [Google Scholar] [CrossRef]
- Golbaz, S.; Zamanzadeh, M.; Yaghmaeian, K.; Nabizadeh, R.; Rastkari, N.; Esfahani, H. Occurrence and removal of psychiatric pharmaceuticals in the Tehran South Municipal Wastewater Treatment Plant. Environ. Sci. Pollut. Res. 2023, 30, 27041–27055. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Heath Organization. Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 11 May 2025).
- Charlson, F.; van Ommeren, M.; Flaxman, A.; Cornett, J.; Whiteford, H.; Saxena, S. New WHO prevalence estimates of mental disorders in conflict settings: A systematic review and meta-analysis. Lancet 2019, 394, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Placova, K.; Halfar, J.; Brozova, K.; Heviankova, S. Issues of Non-Steroidal Anti-Inflammatory Drugs in Aquatic Environments: A Review Study. Eng. Proc. 2023, 57, 13. [Google Scholar]
- Madikizela, L.M.; Ncube, S. Occurrence and ecotoxicological risk assessment of non-steroidal anti-inflammatory drugs in South African aquatic environment: What is known and the missing information? Chemosphere 2021, 280, 130688. [Google Scholar] [CrossRef]
- Myers, J.; Hennessey, M.; Arnold, J.-C.; McCubbin, K.D.; Lembo, T.; Mateus, A.; Kitutu, F.E.; Samanta, I.; Hutchinson, E.; Davis, A.; et al. Crossover-Use of Human Antibiotics in Livestock in Agricultural Communities: A Qualitative Cross-Country Comparison between Uganda, Tanzania and India. Antibiotics 2022, 11, 1342. [Google Scholar] [CrossRef]
- Lees, P.; Pelligand, L.; Giraud, E.; Toutain, P.-L. A history of antimicrobial drugs in animals: Evolution and revolution. J. Vet. Pharmacol. Ther. 2021, 44, 137–171. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Burgos, D.; Wilhelm, P.; Vögele, C.; Munsch, S. Food Restriction in Anorexia Nervosa in the Light of Modern Learning Theory: A Narrative Review. Behav. Sci. 2023, 13, 96. [Google Scholar] [CrossRef]
- Feng, B.; Harms, J.; Chen, E.; Gao, P.; Xu, P.; He, Y. Current Discoveries and Future Implications of Eating Disorders. Int. J. Environ. Res. Public Health 2023, 20, 6325. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Berry, D.J.; Bourgeois, B.F.D.; Cloyd, J.C.; Glauser, T.A.; Johannessen, S.I.; Leppik, I.E.; Tomson, T.; Perucca, E. Antiepileptic drugs—Best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008, 49, 1239–1276. [Google Scholar] [CrossRef]
- Celiz, M.D.; Tso, J.; Aga, D.S. Pharmaceutical metabolites in the environment: Analytical challenges and ecological risks. Critical Review. Environ. Toxicol. Chem. 2009, 28, 2473–2484. [Google Scholar] [CrossRef]
- Kosma, C.I.; Kapsi, M.G.; Konstas, P.-S.G.; Trantopoulos, E.P.; Boti, V.I.; Konstantinou, I.K.; Albanis, T.A. Assessment of multiclass pharmaceutical active compounds (PhACs) in hospital WWTP influent and effluent samples by UHPLC-Orbitrap MS: Temporal variation, removals and environmental risk assessment. Environ. Res. 2020, 191, 110152. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.; Alves, G.; Llerena, A.; Falcão, A. Venlafaxine pharmacokinetics focused on drug metabolism and potential biomarkers. Drug Metabol. Drug Interact. 2014, 29, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Schlüsener, M.P.; Hardenbicker, P.; Nilson, E.; Schulz, M.; Viergutz, C.; Ternes, T.A. Occurrence of venlafaxine, other antidepressants and selected metabolites in the Rhine catchment in the face of climate change. Environ. Pollut. 2015, 196, 247–256. [Google Scholar] [CrossRef] [PubMed]


| Pharmaceuticals | Concentration (ng/L) | Removal Efficiency (%) | |
|---|---|---|---|
| Wastewater 2022 | |||
| WWTP 1 | WWTP 1 | ||
| Influent | Effluent | ||
| Acetaminophen | 3638 | <MDL | 98.4 |
| Alprazolam | 105 | 114 | n.e. |
| Ampicillin | <MDL | <MDL | |
| Atorvastatin | 236 | 848 | n.e. |
| Azithromycin | 121 | 634 | n.e. |
| Bupropion | <MDL | 62.4 | n.e. |
| Caffeine | 12,664 | 7805 | 38.4 |
| Carbamazepine | 177 | 1359 | n.e. |
| Carboxyibuprofen | 7068 | 998 | 85.9 |
| Citalopram | 34.3 | 218 | n.e. |
| Citalopram propionic acid | 196 | 157 | 19.9 |
| Ciprofloxacin | 59.2 | n.d. | 100 |
| Clarithromycin | <MDL | 15.2 | n.e. |
| Clobenzorex | <MDL | n.d. | |
| O-desmethylvenlafaxine | 639 | 2011 | n.e. |
| Diclofenac | 1164 | 4882 | n.e. |
| Diltiazem | <MDL | 34.0 | n.e. |
| 10,11-epoxy carbamazepine | <MDL | <MDL | |
| Fluoxetine | <MDL | 34.4 | n.e. |
| Gemfibrozil | 13.3 | 15.2 | n.e. |
| 2-Hydroxyibuprofen | 4215 | 7767 | n.e. |
| Ibuprofen | 3733 | 4442 | n.e. |
| Ketoprofen | 891 | 1247 | n.e. |
| Mazindol | <MDL | 12.4 | n.e. |
| Naproxen | 963 | 828 | 14.0 |
| Ofloxacin | <MDL | 167 | n.e. |
| Salicylic acid | 8353 | 468 | 94.4 |
| Sertraline | <MDL | 166 | n.e. |
| Venlafaxine | 64.2 | 1029 | n.e. |
| Trazodone | <MDL | 503 | n.e. |
| Trimethoprim | <MDL | n.d. | |
| Topiramate | n.d. | 238 | n.e. |
| Pharmaceutical, Metabolites, Degradation Products, and Associated Ratios | Concentration (ng/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (ng/L) | Concentration (ng/L) | ||||||||
| 2022 | 2022 | 2023 | 2023 | 2023 | 2022 | 2023 | 2023 | 2023 | |
| I1 | E1 | E1 | E2 | E3 | R1 Downstream | R1 Upstream | R1 Downstream | R2 | |
| Carbamazepine (PC) | 177 | 1359 | 900 | 563 | 250 | 981 | 70.1 | 340 | n.d. |
| 10,11-epoxy carbamazepine (M1) | <MDL | <MDL | <MDL | <MDL | n.d. | <MDL | n.d. | <MDL | n.d. |
| Fluoxetine (PC) | <MDL | 34.4 | 6.80 | <MDL | <MDL | 7.55 | <MDL | <MDL | <MDL |
| Norfluoxetine (M1) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Sertraline (PC) | <MDL | 166 | 57.0 | <MDL | <MDL | 13.9 | <MDL | 20.7 | n.d. |
| Norsertraline (M1) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Acetylsalicylic acid (PC) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Salicylic acid (DP) | 8353 | 468 | 171 | 229 | 225 | 175 | 101 | 102 | 107 |
| Ibuprofen (PC) | 3733 | 4442 | 801 | 129 | 246 | 2267 | 176 | 242 | 28.6 |
| Carboxyibuprofen (M1) | 7068 | 998 | n.d. | n.d. | n.d. | n.d. | 38.9 | n.d. | n.d. |
| 2-Hydroxyibuprofen (M2) | 4215 | 7767 | 1545 | 430 | <MDL | 5623 | 515 | 515 | <MDL |
| Ratio M1/PC | 1.89 | 0.22 | 0.22 | ||||||
| Ratio M2/PC | 1.13 | 1.75 | 1.93 | 3.34 | 2.48 | 2.93 | 2.13 | ||
| Citalopram (PC) | 34.3 | 218 | 140 | 142 | 31.2 | 162 | 18.9 | 54.4 | n.d. |
| Citalopram demethyl (M1) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Citalopram didemethyl (M2) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Citalopram N-oxide (M3) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Citalopram propionic acid (M4) | 196 | 157 | n.d. | n.d. | n.d. | 311 | n.d. | n.d. | n.d. |
| Ratio M4/PC | 5.72 | 0.72 | 1.92 | ||||||
| Venlafaxine (PC) | 64.2 | 1029 | 663 | 955 | 173 | 796 | 51.1 | 234 | n.d. |
| O-Desmethylvenlafaxine (M1) | 639 | 2011 | 1828 | 2124 | 2364 | 2304 | 225 | 745 | n.d. |
| Ratio M1/PC | 9.95 | 1.96 | 2.76 | 2.22 | 13.7 | 2.90 | 4.41 | 3.19 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paíga, P.; Figueiredo, S.; Correia, M.; André, M.; Barbosa, R.; Jorge, S.; Delerue-Matos, C. Occurrence of 97 Pharmaceuticals in Wastewater and Receiving Waters: Analytical Validation and Treatment Influence. J. Xenobiot. 2025, 15, 78. https://doi.org/10.3390/jox15030078
Paíga P, Figueiredo S, Correia M, André M, Barbosa R, Jorge S, Delerue-Matos C. Occurrence of 97 Pharmaceuticals in Wastewater and Receiving Waters: Analytical Validation and Treatment Influence. Journal of Xenobiotics. 2025; 15(3):78. https://doi.org/10.3390/jox15030078
Chicago/Turabian StylePaíga, Paula, Sónia Figueiredo, Manuela Correia, Magda André, Roberto Barbosa, Sandra Jorge, and Cristina Delerue-Matos. 2025. "Occurrence of 97 Pharmaceuticals in Wastewater and Receiving Waters: Analytical Validation and Treatment Influence" Journal of Xenobiotics 15, no. 3: 78. https://doi.org/10.3390/jox15030078
APA StylePaíga, P., Figueiredo, S., Correia, M., André, M., Barbosa, R., Jorge, S., & Delerue-Matos, C. (2025). Occurrence of 97 Pharmaceuticals in Wastewater and Receiving Waters: Analytical Validation and Treatment Influence. Journal of Xenobiotics, 15(3), 78. https://doi.org/10.3390/jox15030078









