Green Carbon Dots from Pinecones and Pine Bark for Amoxicillin and Tetracycline Detection: A Circular Economy Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Pretreatment
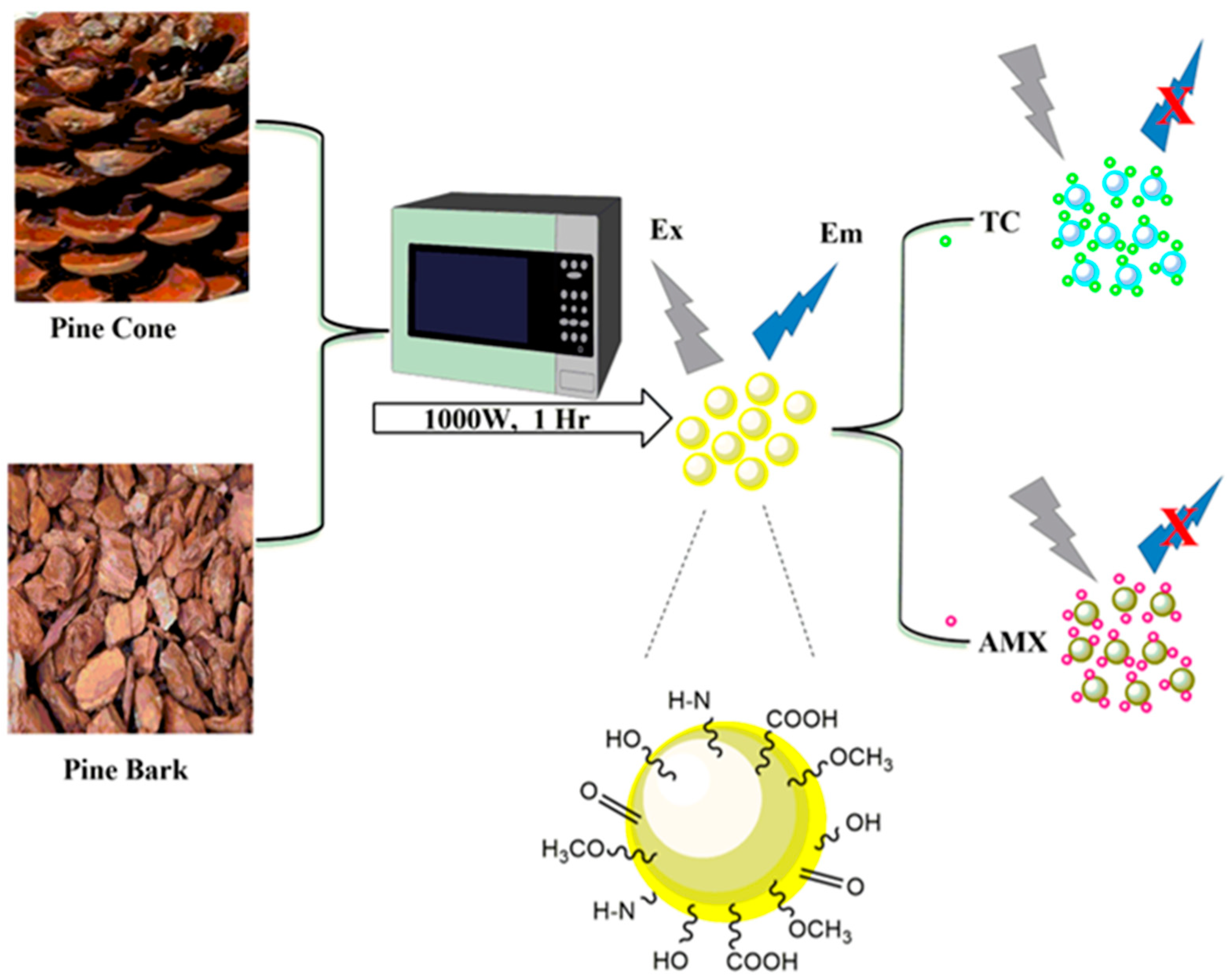
2.2. Synthesis of PCCDs and PBCDs
2.3. Instrumentation
2.4. Detection of TC and AMX
3. Results
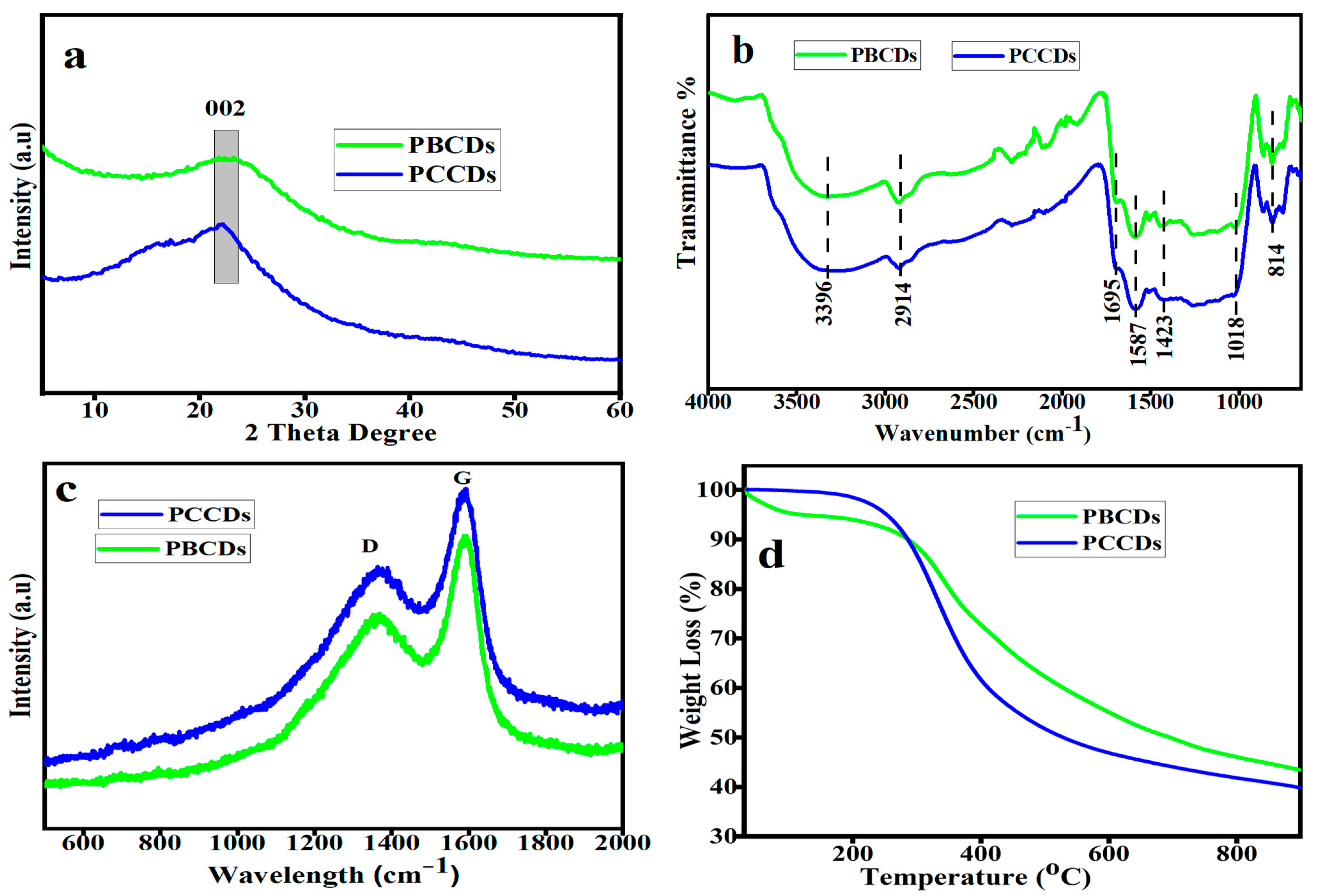
3.1. Characterization Analysis
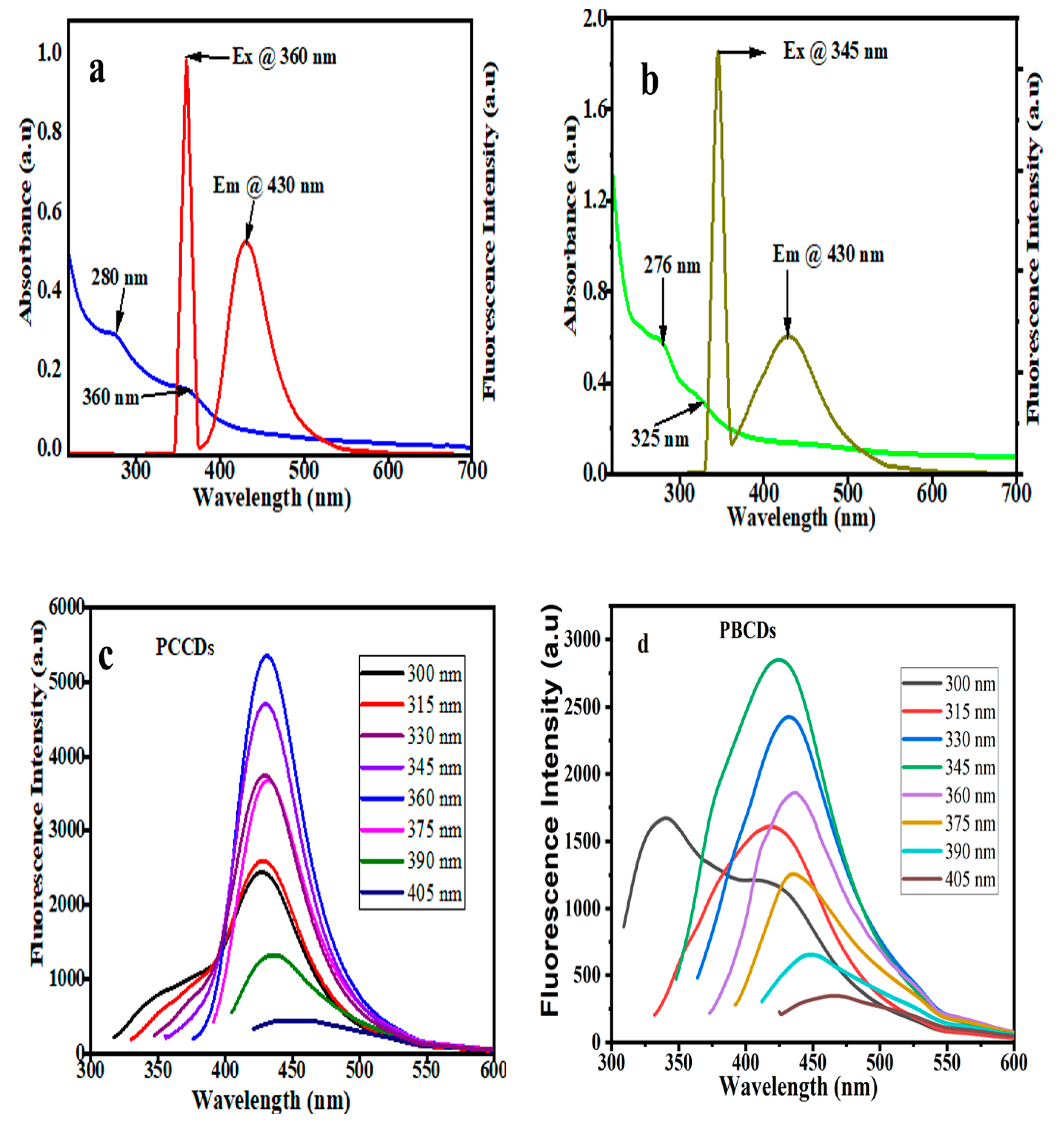
3.2. Optical Performance of the CDs
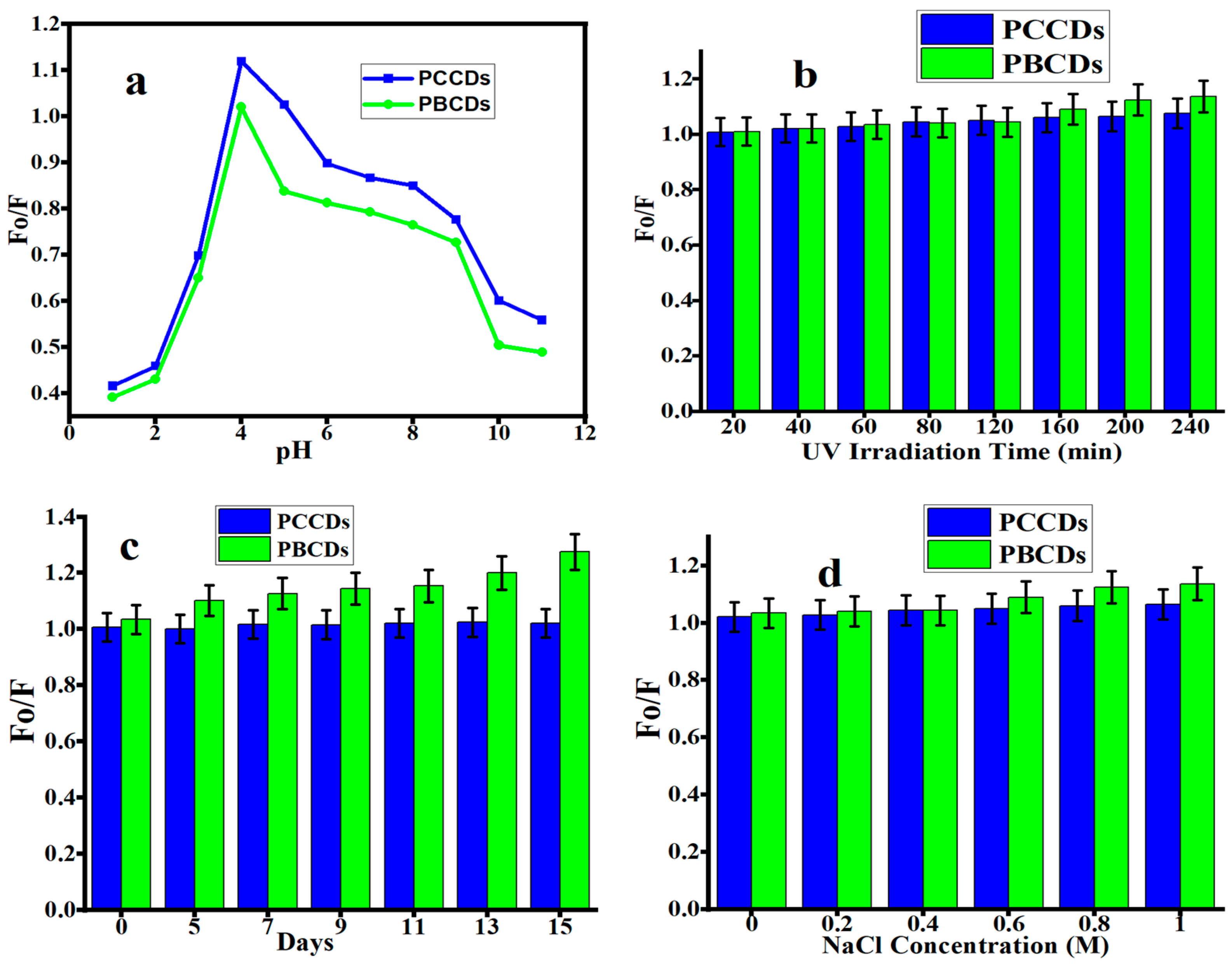
3.3. The Stability Studies
3.4. Application of PCCDs and PBCDs as Fluorescent Probes
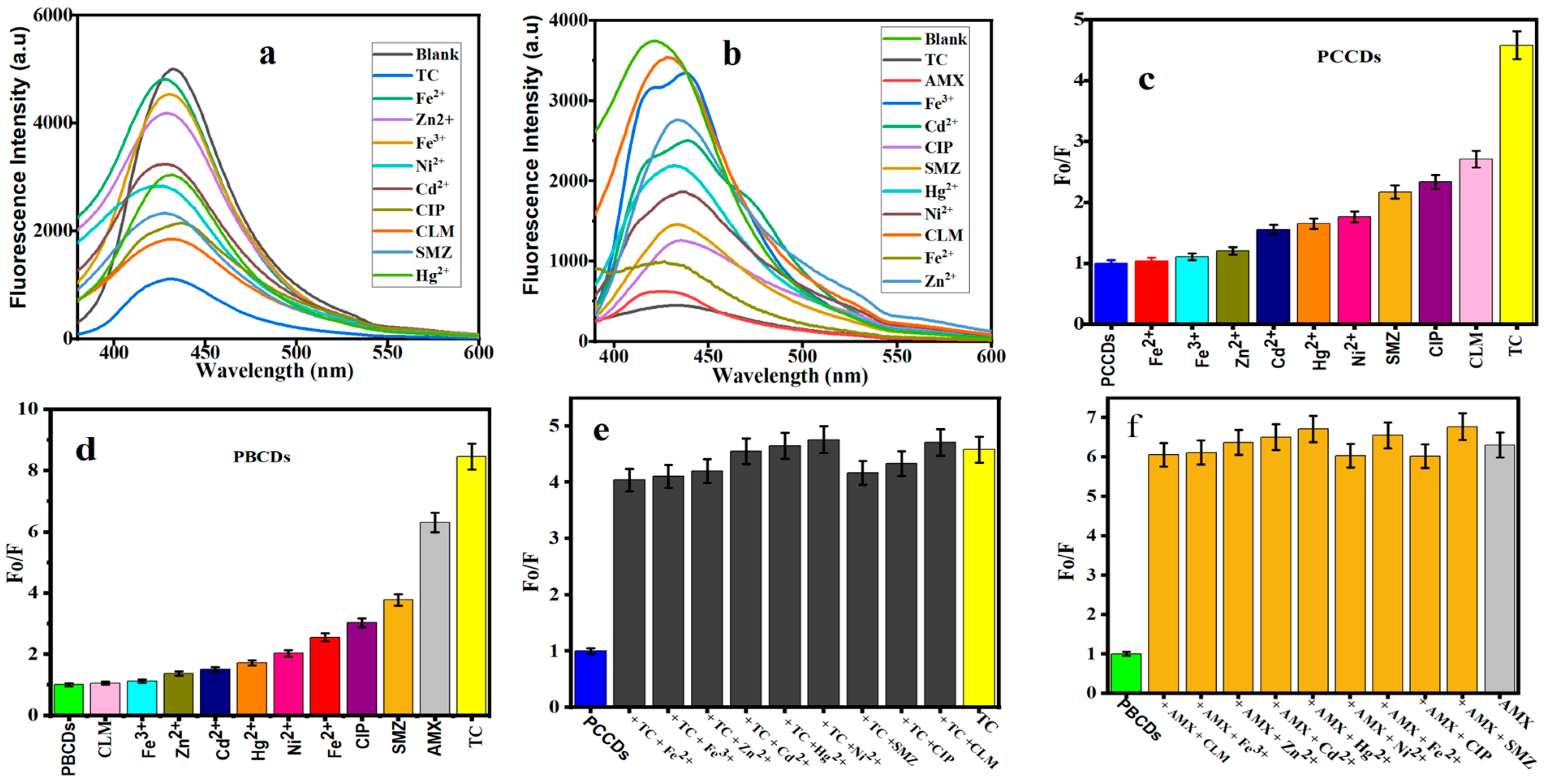
3.4.1. Fluorescence Detection of Tetracycline and Amoxicillin
3.4.2. Sensitive Detection of TC by PCCDs and PBCDs
3.4.3. Sensitive Detection of AMX by PBCDs
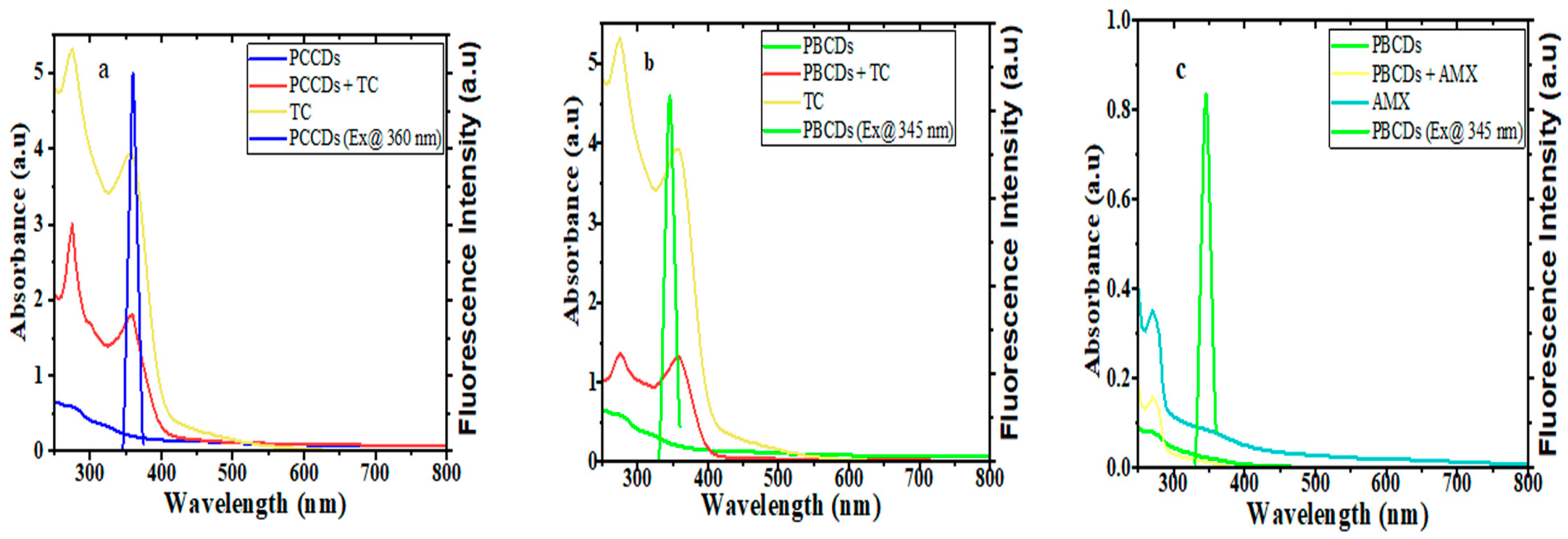
3.5. Detection Mechanism
3.6. Real Water Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priya, B.; Shandilya, P.; Raizada, P.; Thakur, P.; Singh, N.; Singh, P. Photocatalytic mineralization and degradation kinetics of ampicillin and oxytetracycline antibiotics using graphene sand composite and chitosan supported BiOCl. J. Mol. Catal. A Chem. 2016, 423, 400–413. [Google Scholar] [CrossRef]
- Raizada, P.; Kumari, J.; Shandilya, P.; Singh, P. Kinetics of photocatalytic mineralization of oxytetracycline and ampicillin using activated carbon supported ZnO/ZnWO4 nanocomposite in simulated wastewater. Desalination Water Treat. 2017, 79, 204–213. [Google Scholar] [CrossRef]
- Adil, M.; Iqbal, M.; Kanwal, S.; Abbas, G. Toxicity and Adverse Effects of Veterinary Pharmaceuticals in Animals. In Pharmaceuticals in Aquatic Environments; CRC Press: Boca Raton, FL, USA, 2024; pp. 141–159. [Google Scholar]
- Bayode, A.A.; Osti, A.; Glisenti, A. Sonophotocatalytic degradation of sulfamethoxazole using lanthanum ferrite perovskite oxide anchored on an ultrasonically exfoliated porous graphitic carbon nitride nanosheet. RSC Adv. 2024, 14, 22063–22075. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.K.; Kumar, M.; Singh, D.K. Insight into the amoxicillin resistance, ecotoxicity, and remediation strategies. J. Water Process Eng. 2021, 39, 101858. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Mmuoegbulam, A.O.; Okezie, O.; Durumin Iya, N.I.; Mohammed, S.a.E.; James, P.H.; Muhammad, A.B.; Unimke, A.A.; Alim, S.A.; Yahaya, S.M. Recent progress in carbon-based nanomaterials: Critical review. J. Nanoparticle Res. 2024, 26, 106. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal nanoparticles and carbon-based nanomaterials for improved performances of electrochemical (Bio) sensors with biomedical applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef]
- Ozyurt, D.; Al Kobaisi, M.; Hocking, R.K.; Fox, B. Properties, synthesis, and applications of carbon dots: A review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Khan, M.E.; Mohammad, A.; Yoon, T. State-of-the-art developments in carbon quantum dots (CQDs): Photo-catalysis, bio-imaging, and bio-sensing applications. Chemosphere 2022, 302, 134815. [Google Scholar] [CrossRef]
- Raeispour, S.; Rahmandoust, M.; Kouchakzadeh, H. A nanocarrier system based on CQDs for efficient mitoxantrone drug delivery. Heliyon 2024, 10, e31674. [Google Scholar] [CrossRef]
- Yadav, P.K.; Chandra, S.; Kumar, V.; Kumar, D.; Hasan, S.H. Carbon quantum dots: Synthesis, structure, properties, and catalytic applications for organic synthesis. Catalysts 2023, 13, 422. [Google Scholar] [CrossRef]
- Manikandan, V.; Lee, N.Y. Green synthesis of carbon quantum dots and their environmental applications. Environ. Res. 2022, 212, 113283. [Google Scholar] [CrossRef]
- Islam, R.; Ahammad, R.; Islam, M.M.; Shoeb, M.; Mamun, M.I.R. High performance liquid chromatography assessment of antibiotic residues in poultry and fish feeds in Bangladesh. Curr. Res. Biosci. Biotechnol. 2024, 6, 13–19. [Google Scholar]
- Montone, C.M.; Moneta, B.G.; Laganà, A.; Piovesana, S.; Taglioni, E.; Cavaliere, C. Transformation products of antibacterial drugs in environmental water: Identification approaches based on liquid chromatography-high resolution mass spectrometry. J. Pharm. Biomed. Anal. 2024, 238, 115818. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, H.; Feng, R.; Wang, M.; Hu, P.; Pan, J.; Niu, X. Facile molecular imprinting on magnetic nanozyme surface for highly selective colorimetric detection of tetracycline. Sens. Actuators B Chem. 2022, 370, 132451. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Lin, L. Recent development of microfluidic biosensors for the analysis of antibiotic residues. TrAC Trends Anal. Chem. 2022, 157, 116797. [Google Scholar] [CrossRef]
- Ramzan, M.; Raza, A.; un Nisa, Z.; Abdel-Massih, R.M.; Al Bakain, R.; Cabrerizo, F.M.; Cruz, T.E.D.; Aziz, R.K.; Musharraf, S.G. Detection of antimicrobial resistance (AMR) and antimicrobial susceptibility testing (AST) using advanced spectroscopic techniques: A review. TrAC Trends Anal. Chem. 2024, 172, 117562. [Google Scholar] [CrossRef]
- El-Ghobashy, M.R.; Abo-Talib, N.F. Spectrophotometric methods for the simultaneous determination of binary mixture of metronidazole and diloxanide furoate without prior separation. J. Adv. Res. 2010, 1, 323–329. [Google Scholar] [CrossRef]
- Imran, M.; Ahmed, S.; Abdullah, A.Z.; Hakami, J.; Chaudhary, A.A.; Rudayni, H.A.; Khan, S.U.D.; Khan, A.; Basher, N.S. Nanostructured material-based optical and electrochemical detection of amoxicillin antibiotic. Luminescence 2023, 38, 1064–1086. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, X.; Shi, X.; Guo, Y.; Sun, Y.; Qu, L.; Li, Z. A fluorescence-switchable carbon dot for the reversible turn-on sensing of molecular oxygen. J. Mater. Chem. C 2021, 9, 4300–4306. [Google Scholar] [CrossRef]
- Amjadi, M.; Jalili, R. A molecularly imprinted dual-emission carbon dot-quantum dot mesoporous hybrid for ratiometric determination of anti-inflammatory drug celecoxib. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2018, 191, 345–351. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Tu, H.; Zhang, H.; Silvester, D.S.; Banks, C.E.; Zou, G.; Hou, H.; Ji, X. The development of carbon dots: From the perspective of materials chemistry. Mater. Today 2021, 51, 188–207. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Hui, S. Carbon dots (CDs): Basics, recent potential biomedical applications, challenges, and future perspectives. J. Nanoparticle Res. 2023, 25, 68. [Google Scholar] [CrossRef]
- Othman, K.A.; Ali, L.I.A.; Qader, A.F.; Omer, R.A.; Amin, A.A. Synthesis, Characterization, and Applications of Carbon Dots for Determination of Pharmacological and Biological Samples: A Review. J. Fluoresc. 2024, 35, 561–582. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Ali, F.; Dawood, A.; Hussain, A.; Alnasir, M.H.; Khan, M.A.; Butt, T.M.; Janjua, N.K.; Hamid, A. Fueling the future: Biomass applications for green and sustainable energy. Discov. Sustain. 2024, 5, 156. [Google Scholar] [CrossRef]
- Gan, J.; Chen, L.; Chen, Z.; Zhang, J.; Yu, W.; Huang, C.; Wu, Y.; Zhang, K. Lignocellulosic biomass-based carbon dots: Synthesis processes, properties, and applications. Small 2023, 19, 2304066. [Google Scholar] [CrossRef]
- Wangmo, D.; Rana, R. Pine Needles as Green Material for Removal of Metal Ions and Dyes from Wastewater. In Green Chemistry in Environmental Sustainability and Chemical Education; Springer: Berlin/Heidelberg, Germany, 2024; pp. 61–72. [Google Scholar]
- Liu, Y.; Ge, G.; Liu, H.; Wang, Y.; Zhou, P.; Li, B.; Zhu, G. Fast and eco-friendly synthesis of carbon dots from pinecone for highly effective detection of 2, 4, 6-trinitrophenol in environmental samples. Environ. Technol. 2024, 46, 719–730. [Google Scholar] [CrossRef]
- Shahba, H.; Sabet, M. Two-step and green synthesis of highly fluorescent carbon quantum dots and carbon nanofibers from pine fruit. J. Fluoresc. 2020, 30, 927–938. [Google Scholar] [CrossRef]
- Sanni, S.O.; Moundzounga, T.H.G.; Oseghe, E.O.; Haneklaus, N.H.; Viljoen, E.L.; Brink, H.G. One-Step Green Synthesis of Water-Soluble Fluorescent Carbon Dots and Its Application in the Detection of Cu2+. Nanomaterials 2022, 12, 958. [Google Scholar] [CrossRef]
- Miller, J.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Pearson Education: London, UK, 2018. [Google Scholar]
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon Dots for Heavy-Metal Sensing, pH-Sensitive Cargo Delivery, and Antibacterial Applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhao, L.; Sun, L.; Zhao, X.; Xia, Y. κ-Carrageenan-derived carbon dots for highly selective and sensitive detection of Fe3+ and oxytetracycline. J. Mater. Sci. 2021, 56, 1272–1285. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumari, M.; Chauhan, P.; Ram Chaudhary, G. Upcycling of plastic waste into fluorescent carbon dots: An environmentally viable transformation to biocompatible C-dots with potential prospective in analytical applications. Waste Manag. 2021, 120, 675–686. [Google Scholar] [CrossRef]
- Krishnaiah, P.; Atchudan, R.; Perumal, S.; Salama, E.-S.; Lee, Y.R.; Jeon, B.-H. Utilization of waste biomass of Poa pratensis for green synthesis of n-doped carbon dots and its application in detection of Mn2+ and Fe3+. Chemosphere 2022, 286, 131764. [Google Scholar] [CrossRef]
- Wang, J.; Fu, J.; Chen, M.; Zhang, J. Red-emitting carbon dots for fluorescent and smartphone-assisted dual-mode detection of Cu(II), Hg(II), and Fe(III) and investigation of the sensing mechanism. Mater. Today Chem. 2024, 41, 102331. [Google Scholar] [CrossRef]
- Sanni, S.O.; Viljoen, E.L.; Ofomaja, A.E. Three-dimensional hierarchical porous carbon structure derived from pinecone as a potential catalyst support in catalytic remediation of antibiotics. RSC Adv. 2020, 10, 8717–8728. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Bai, H.; Jia, P.; Zhao, Y.; Liu, Y.; Wang, L.; Zhuang, Y.; Yue, T. Fluorescent detection of tetracycline in foods based on carbon dots derived from natural red beet pigment. LWT 2022, 157, 113100. [Google Scholar] [CrossRef]
- Jessy Mercy, D.; Kiran, V.; Thirumalai, A.; Harini, K.; Girigoswami, K.; Girigoswami, A. Rice husk assisted carbon quantum dots synthesis for amoxicillin sensing. Results Chem. 2023, 6, 101219. [Google Scholar] [CrossRef]
- Sun, P.; Song, W.; Zou, Y.; Tian, M.; Zhang, F.; Chai, F. The fabrication of N-doped carbon dots by methionine and their utility in sensing Cu2+ in real water. Anal. Methods 2023, 15, 1631–1638. [Google Scholar] [CrossRef]
- Qi, H.; Liu, C.; Jing, J.; Jing, T.; Zhang, X.; Li, J.; Luo, C.; Qiu, L.; Li, Q. Two kinds of biomass-derived carbon dots with one-step synthesis for Fe3+ and tetracyclines detection. Dye. Pigment. 2022, 206, 110555. [Google Scholar] [CrossRef]
- Li, C.; Liu, L.; Zhang, D. Aggregation enhanced emissive orange carbon dots for information encryption and detection of Fe3+ and tetracycline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 305, 123504. [Google Scholar] [CrossRef]
- Wang, K.; Ji, Q.; Xu, J.; Li, H.; Zhang, D.; Liu, X.; Wu, Y.; Fan, H. Highly sensitive and selective detection of amoxicillin using carbon quantum dots derived from beet. J. Fluoresc. 2018, 28, 759–765. [Google Scholar] [CrossRef]
- Kaur, N.; Tiwari, P.; Abbas, Z.; Mobin, S.M. Doxycycline detection and degradation in aqueous media via simultaneous synthesis of Fe-N@ carbon dots and Fe3O4-carbon dot hybrid nanoparticles: A one arrow two hawk approach. J. Mater. Chem. B 2022, 10, 5251–5262. [Google Scholar] [CrossRef]
- Mathew, S.; John, B.K.; Mathew, J.; Korah, B.K.; Mathew, D.B. Green synthesized carbon dots as antibiotics sensor and fluorescent ink. J. Mol. Struct. 2023, 1294, 136422. [Google Scholar] [CrossRef]
- Borman, P.; Elder, D. Q2 (R1) validation of analytical procedures: Text and methodology. In ICH Quality Guidelines: An Implementation Guide; Wiley: Hoboken, NJ, USA, 2017; pp. 127–166. [Google Scholar] [CrossRef]
- Somatek. Validation of analytical procedures: Text and methodology. Q2 (R1). In ICH Harmonised Tripartite Guideline; Somatek: San Diego, CA, USA, 2005. [Google Scholar]
- Mu, Y.; Zhuang, Q.; Huang, S.; Hu, M.; Wang, Y.; Ni, Y. Adenine-stabilized carbon dots for highly sensitive and selective sensing of copper (II) ions and cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118531. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, J.H.; Li, R.S.; Li, Y.F.; Huang, C.Z.; Zhen, S.J. Carbon dots synthesized at room temperature for detection of tetracycline hydrochloride. Anal. Chim. Acta 2019, 1063, 144–151. [Google Scholar] [CrossRef]
- Wei, X.; Lv, L.; Zhang, Z.; Guan, W. Preparation of molecularly imprinted fluorescence sensor based on carbon quantum dots via precipitation polymerization for fluorescence detection of tetracycline. J. Appl. Polym. Sci. 2020, 137, 49126. [Google Scholar] [CrossRef]
- Long, D.; Peng, J.; Peng, H.; Xian, H.; Li, S.; Wang, X.; Chen, J.; Zhang, Z.; Ni, R. A quadruple-channel fluorescent sensor array based on label-free carbon dots for sensitive detection of tetracyclines. Analyst 2019, 144, 3307–3313. [Google Scholar] [CrossRef]
- Uriarte, D.; Domini, C.; Garrido, M. New carbon dots based on glycerol and urea and its application in the determination of tetracycline in urine samples. Talanta 2019, 201, 143–148. [Google Scholar] [CrossRef]
- Yuan, M.; An, J.; Zhang, G.; Hu, Y.; Luo, M.; Shi, Y.; Liu, Y. In-situ nitrogen-doped carbon dots for fluorescence sensing of tetracycline antibiotic. Ceram. Int. 2022, 48, 4047–4054. [Google Scholar] [CrossRef]
- Guo, X.-R.; Dong, Y.-M.; Chen, X.-Y.; Chen, J. Sophorajaponica L. flower mediated carbon dots with nitrogen and sulfur co-doped as a sensitive fluorescent probe for amoxicillin detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 282, 121703. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Fan, C.; He, C.; Ma, S. Highly sensitive and selective detection of amoxicillin using molecularly imprinted ratiometric fluorescent nanosensor based on quantum dots. Microchim. Acta 2024, 191, 525. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; El-Wekil, M.M.; Ali, R.; Batakoushy, H.A.; Shahin, R.Y. Double-signal quantification of amoxicillin based on interaction with 4-aminoantipyrine at copper and nitrogen co-doped carbon quantum dots as an artificial nanozyme. Microchim. Acta 2022, 189, 183. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, Y.; Ji, Z.; Fan, J. Sensitive detection of amoxicillin in aqueous solution with novel fluorescent probes containing boron-doped carbon quantum dots. J. Mol. Liq. 2020, 311, 113278. [Google Scholar] [CrossRef]
- Jia, P.; Bu, T.; Sun, X.; Liu, Y.; Liu, J.; Wang, Q.; Shui, Y.; Guo, S.; Wang, L. A sensitive and selective approach for detection of tetracyclines using fluorescent molybdenum disulfide nanoplates. Food Chem. 2019, 297, 124969. [Google Scholar] [CrossRef]


| Probes | Precursors | Linear Range (μM) | Detection Limit (μM) | Reference |
|---|---|---|---|---|
| CDs | o-phenylenediamine | 10.0–400.0 | 6.0 | [51] |
| MIPs-AA/CQDs | Citric acid Polyethyleneimine | 1–60 | 0.17 | [52] |
| CDs | Citric acid Ethylenediamine | 1–300 | 0.30 | [53] |
| GUCDs | Glycerol Urea | 0.5–25 | 0.165 | [54] |
| RBP-CDs | Red beet pigment | 0.5–90 | 0.36 | [40] |
| CRSSs-NH2@N-CDs | Aminated central radial silica spheres | 0.5–60 | 0.39 | [55] |
| PCCDs, PBCDs | Pinecones and Pine Bark | 5–100 | 0.062 and 0.2237 | This work |
| Probes | Precursors | Linear Range (μM) | Detection Limit (μM) | Reference |
|---|---|---|---|---|
| N, S-CDs-Sop | S. japonica flower | 5–80 | 3.40 | [56] |
| CDs@CdTe@MIP | 0.15 | [57] | ||
| Cu, N@CQDs | 0.2–120.0 | 0.06 | [58] | |
| B-CQDs | Citric acid monohydrate and boric acid | 1.43–429.12 | 0.825 | [59] |
| PBCDs | Pine Bark | 5–100 | 0.49 | This work |
| Water Sample | Added (µM) | Found (µM) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| 0 | ND | |||
| 15 | 15.4106 | 102.7374 | 1.1059 | |
| Tap Water (TC) | 20 | 19.2239 | 96.1199 | 1.1211 |
| 25 | 25.4194 | 101.6776 | 1.1362 | |
| 0 | ND | |||
| 15 | 14.8078 | 98.7192 | 0.3549 | |
| Tap Water (AMX) | 20 | 20.1679 | 100.8396 | 0.3617 |
| 25 | 24.7673 | 99.0691 | 0.3652 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanni, S.O.; Bayode, A.A.; Brink, H.G.; Haneklaus, N.H.; Fu, L.; Shang, J.; Fan, H.-J.S. Green Carbon Dots from Pinecones and Pine Bark for Amoxicillin and Tetracycline Detection: A Circular Economy Approach. J. Xenobiot. 2025, 15, 43. https://doi.org/10.3390/jox15020043
Sanni SO, Bayode AA, Brink HG, Haneklaus NH, Fu L, Shang J, Fan H-JS. Green Carbon Dots from Pinecones and Pine Bark for Amoxicillin and Tetracycline Detection: A Circular Economy Approach. Journal of Xenobiotics. 2025; 15(2):43. https://doi.org/10.3390/jox15020043
Chicago/Turabian StyleSanni, Saheed O., Ajibola A. Bayode, Hendrik G. Brink, Nils H. Haneklaus, Lin Fu, Jianping Shang, and Hua-Jun Shawn Fan. 2025. "Green Carbon Dots from Pinecones and Pine Bark for Amoxicillin and Tetracycline Detection: A Circular Economy Approach" Journal of Xenobiotics 15, no. 2: 43. https://doi.org/10.3390/jox15020043
APA StyleSanni, S. O., Bayode, A. A., Brink, H. G., Haneklaus, N. H., Fu, L., Shang, J., & Fan, H.-J. S. (2025). Green Carbon Dots from Pinecones and Pine Bark for Amoxicillin and Tetracycline Detection: A Circular Economy Approach. Journal of Xenobiotics, 15(2), 43. https://doi.org/10.3390/jox15020043









