Brain Cytochrome P450: Navigating Neurological Health and Metabolic Regulation
Abstract
1. Introduction
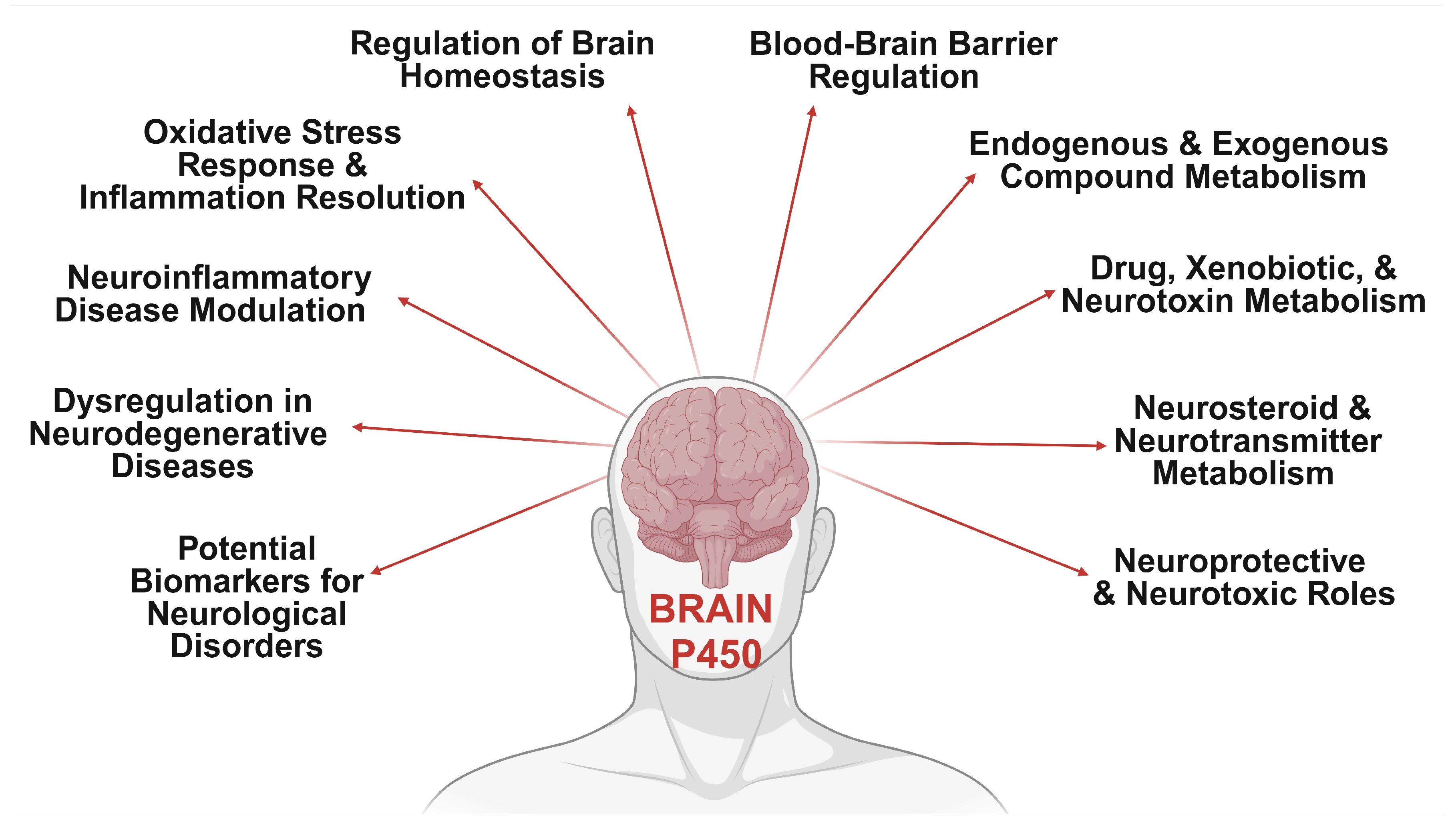
2. Neurological Impacts of Brain CYP Metabolism
3. Differential Expression Patterns of Brain CYPs
| CYP Family | Isoform | Brain Regions | Gene Expression | Protein Levels | Transcript Levels | Enzyme Activity | Experimental Models | Techniques Employed | References |
|---|---|---|---|---|---|---|---|---|---|
| CYP1 | CYP1A1 | Cortex, Hippocampus, Cerebellum | High in cerebral cortex and cerebellum | Predominantly in neurons | Elevated levels noted | Detectable activity in neuronal cultures | Animal and human studies | qPCR, Western blot, immunohistochemistry | [99,100,101] |
| CYP1B1 | Cortex, Hippocampus, Other Regions | Present in neural tissue; varies | Significant in blood–brain barrier, human neurons, and astrocytes | Variable across samples | Active in xenobiotic and steroid metabolism | Genetic models | IHC, in situ hybridization | [102,103,104,105,106] | |
| CYP2 | CYP2D6 | Cortex, Cerebellum, Hippocampus, Amygdala, Olfactory Bulbs | High in substantia nigra | Significant across brain regions (hippocampus, thalamus, hypothalamus, cortex) | Variability in expression observed | Critical for drug metabolism | Transgenic and knockout models | qPCR, LC-MS, Western blot | [97,107,108,109,110] |
| CYP2C19 | Cortex, Hippocampus | Expressed variably across regions | Detectable levels notably in pyramidal neurons | High variability in expression | Modulated by pharmacological agents | Experimental models | PCR, IHC, drug response assays | [107,111,112,113,114,115] | |
| CYP3 | CYP3A4 | Frontal Cortex, Hippocampus | Expressed in hippocampus and cortex | Neuronal expression high in certain areas | High variability reported | Important for drug metabolism | Animal models | Immunohistochemistry | [63,116,117] |
| CYP3A5 | Cortex, Hippocampus | Expressed variably across brain areas | Levels vary across brain regions | High in specific regions | Documented activity in drug metabolism | Human and animal studies | Western blot, ELISA | [31,116,117,118] | |
| CYP27 | CYP27A1 | Cortex, Hippocampus, Cerebellum, Microglia | Elevated in brain regions involved in cholesterol metabolism | Variable across studies, notably high | Confirmed RNA levels across regions | Active in cholesterol oxidation | Knockout and transgenic studies | RT-PCR, Western blot | [119,120,121,122,123] |
| CYP46 | CYP46A1 | Cortex, Hippocampus, Cerebellum | Primarily in neurons | Expression linked to neurodegeneration | High in specific regions | Essential for cholesterol metabolism | Gene therapy models | qPCR, immunofluorescence | [39,92,124,125,126] |
4. Metabolic and Regulatory Interactions of Brain CYPs
4.1. Endogenous Substance Dynamics of Cerebral CYPs
4.1.1. Cerebral CYPs in Cholesterol Metabolism
4.1.2. Cerebral CYPs in Dopamine Metabolism
4.1.3. Cerebral CYPs in Serotonin Metabolism
4.1.4. Cerebral CYPs in Polyunsaturated Fatty Acid Metabolism
4.2. Exogenous Substance Dynamics of Cerebral CYPs
4.2.1. Cerebral CYPs in Opioid Metabolism
Hydrocodone Metabolism by Cerebral CYPs
Oxycodone Metabolism by Cerebral CYPs
Morphine Metabolism by Cerebral CYPs
Cannabinoid Metabolism by Cerebral CYPs
4.2.2. Cerebral CYPs in Nicotine and Ethanol Metabolism
4.2.3. Cerebral CYPs in Psychotropic and Psychedelic Metabolism
5. Brain CYPs: Implications in Neurodegenerative Diseases
5.1. Interplay of Alzheimer’s Disease and Cerebral CYPs
5.2. Interplay of Parkinson’s Disease and Cerebral CYPs
5.2.1. Interrelation Between Smoking and Parkinson’s Disease
5.3. Interplay of Huntington’s Disease and Cerebral CYPs
5.4. Interplay of Neuropsychiatric Disorders and Cerebral CYPs
6. Emergence of CYP-Based Neuropathological Biomarkers
7. Future Perspectives in Brain CYPs: Emerging Insights and Therapeutic Opportunities
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CYP | Cytochrome P450 |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| BBB | Blood–brain barrier |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| HD | Huntington disease |
| SCZ | Schizophrenia |
| ROS | Reactive oxygen species |
| LXR | Liver X receptor |
| NMDAR | N-methyl-D-aspartate receptor |
| APP | Amyloid precursor protein |
| Aβ | Amyloid β |
| 24-HC | 24S-hydroxycholesterol |
| 5-HT | Serotonin |
| 5-HTP | 5-hydroxytryptophan |
| 5-MT | 5-methoxytryptamine |
| PUFA | Polyunsaturated fatty acid |
| AA | Arachidonic acid |
| EET | Epoxyeicosatrienoic acid |
| Ep-PUFA | Epoxy polyunsaturated fatty acid |
| EH | Epoxide hydrolases |
| LA | Linoleic acid |
| DHET | Dihydroxyeicosatrienoic acids |
| M3G | Morphine-3-glucuronide |
| M6G | Morphine-6-glucuronide |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MPP+ | 1-methyl-4-phenylpyridinum |
| HTT | Huntingtin |
| mHTT | Mutant HTT |
| 25(OH)D | 25-hydroxycholecalciferol |
| 1,25(OH)2D | 25-Dihydroxyvitamin D |
| MSA | Multiple-System Atrophy |
References
- Winkler, M.; Geier, M.; Hanlon, S.P.; Nidetzky, B.; Glieder, A. Human Enzymes for Organic Synthesis. Angew. Chem. Int. Ed. Engl. 2018, 57, 13406–13423. [Google Scholar] [CrossRef] [PubMed]
- Dubey, K.D.; Shaik, S. Cytochrome P450-The Wonderful Nanomachine Revealed through Dynamic Simulations of the Catalytic Cycle. Acc. Chem. Res. 2019, 52, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Rajakumara, E.; Saniya, D.; Bajaj, P.; Rajeshwari, R.; Giri, J.; Davari, M.D. Hijacking Chemical Reactions of P450 Enzymes for Altered Chemical Reactions and Asymmetric Synthesis. Int. J. Mol. Sci. 2022, 24, 214. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ang, E.L.; Zhao, H. Recent developments in the application of P450 based biocatalysts. Curr. Opin. Chem. Biol. 2018, 43, 1–7. [Google Scholar] [CrossRef]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 Monooxygenases in Biotechnology and Synthetic Biology. Trends Biotechnol. 2019, 37, 882–897. [Google Scholar] [CrossRef]
- Schröder, G.C.; Smit, M.S.; Opperman, D.J. Harnessing heme chemistry: Recent advances in the biocatalytic applications of cytochrome P450 monooxgenases. Curr. Opin. Green Sustain. Chem. 2023, 39, 100734. [Google Scholar] [CrossRef]
- Bernhardt, R.; Urlacher, V.B. Cytochromes P450 as promising catalysts for biotechnological application: Chances and limitations. Appl. Microbiol. Biotechnol. 2014, 98, 6185–6203. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef]
- Nelson, D.R. Cytochrome P450 diversity in the tree of life. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 141–154. [Google Scholar] [CrossRef]
- Durairaj, P.; Li, S. Functional expression and regulation of eukaryotic cytochrome P450 enzymes in surrogate microbial cell factories. Eng. Microbiol. 2022, 2, 100011. [Google Scholar] [CrossRef]
- Durairaj, P.; Fan, L.; Du, W.; Ahmad, S.; Mebrahtu, D.; Sharma, S.; Ashraf, R.A.; Liu, J.; Liu, Q.; Bureik, M. Functional expression and activity screening of all human cytochrome P450 enzymes in fission yeast. FEBS Lett. 2019, 593, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Ortiz De Montellano, P.R. (Ed.) Cytochrome P450: Structure, Mechanism, and Biochemistry; Springer International Publishing: Cham, Switzerland, 2015; Available online: https://link.springer.com/10.1007/978-3-319-12108-6 (accessed on 17 February 2025).
- Rendic, S.; Guengerich, F.P. Survey of Human Oxidoreductases and Cytochrome P450 Enzymes Involved in the Metabolism of Xenobiotic and Natural Chemicals. Chem. Res. Toxicol. 2015, 28, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Intersection of the Roles of Cytochrome P450 Enzymes with Xenobiotic and Endogenous Substrates: Relevance to Toxicity and Drug Interactions. Chem. Res. Toxicol. 2017, 30, 2–12. [Google Scholar] [CrossRef]
- Rendic, S.P.; Peter Guengerich, F. Human cytochrome P450 enzymes 5-51 as targets of drugs and natural and environmental compounds: Mechanisms, induction, and inhibition—Toxic effects and benefits. Drug Metab. Rev. 2018, 50, 256–342. [Google Scholar] [CrossRef]
- Rendic, S.P.; Guengerich, F.P. Human Family 1-4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: An update. Arch. Toxicol. 2021, 95, 395–472. [Google Scholar] [CrossRef]
- Šrejber, M.; Navrátilová, V.; Paloncýová, M.; Bazgier, V.; Berka, K.; Anzenbacher, P.; Otyepka, M. Membrane-attached mammalian cytochromes P450: An overview of the membrane’s effects on structure, drug binding, and interactions with redox partners. J. Inorg. Biochem. 2018, 183, 117–136. [Google Scholar] [CrossRef]
- Esteves, F.; Campelo, D.; Gomes, B.C.; Urban, P.; Bozonnet, S.; Lautier, T.; Rueff, J.; Truan, G.; Kranendonk, M. The Role of the FMN-Domain of Human Cytochrome P450 Oxidoreductase in Its Promiscuous Interactions with Structurally Diverse Redox Partners. Front. Pharmacol. 2020, 11, 299. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Y.; Guengerich, F.P.; Ma, L.; Li, S.; Zhang, W. Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications. J. Biol. Chem. 2020, 295, 833–849. [Google Scholar] [CrossRef]
- Xu, L.-H.; Du, Y.-L. Rational and semi-rational engineering of cytochrome P450s for biotechnological applications. Synth. Syst. Biotechnol. 2018, 3, 283–290. [Google Scholar] [CrossRef]
- Park, H.; Park, G.; Jeon, W.; Ahn, J.-O.; Yang, Y.-H.; Choi, K.-Y. Whole-cell biocatalysis using cytochrome P450 monooxygenases for biotransformation of sustainable bioresources (fatty acids, fatty alkanes, and aromatic amino acids). Biotechnol. Adv. 2020, 40, 107504. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Waterman, M.R.; Egli, M. Recent Structural Insights into Cytochrome P450 Function. Trends Pharmacol. Sci. 2016, 37, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guan, X.; Dai, Z.; He, R.; Ding, X.; Yang, L.; Ge, G. Molecular probes for human cytochrome P450 enzymes: Recent progress and future perspectives. Coord. Chem. Rev. 2021, 427, 213600. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P450 Enzymes as Drug Targets in Human Disease. Drug Metab. Dispos. Biol. Fate Chem. 2024, 52, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Roles of Individual Human Cytochrome P450 Enzymes in Drug Metabolism. Pharmacol. Rev. 2024, 76, 1104–1132. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Pircalabioru, G.G.; Bezirtzoglou, E. The Role of Cytochromes P450 in Infection. Front. Immunol. 2018, 9, 89. [Google Scholar] [CrossRef]
- Nebert, D.W.; Wikvall, K.; Miller, W.L. Human cytochromes P450 in health and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120431. [Google Scholar] [CrossRef]
- Rettie, A.E.; Liggett, S.B. Preface to Special Issue on “Cytochrome P450 Variation in Pharmacogenomics”. J. Pers. Med. 2018, 8, 23. [Google Scholar] [CrossRef]
- Carrera-Pacheco, S.E.; Mueller, A.; Puente-Pineda, J.A.; Zúñiga-Miranda, J.; Guamán, L.P. Designing cytochrome P450 enzymes for use in cancer gene therapy. Front. Bioeng. Biotechnol. 2024, 12, 1405466. [Google Scholar] [CrossRef]
- Pikuleva, I.A.; Waterman, M.R. Cytochromes p450: Roles in diseases. J. Biol. Chem. 2013, 288, 17091–17098. [Google Scholar] [CrossRef]
- Ghosh, C.; Hossain, M.; Solanki, J.; Dadas, A.; Marchi, N.; Janigro, D. Pathophysiological implications of neurovascular P450 in brain disorders. Drug Discov. Today 2016, 21, 1609–1619. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, H.; Wu, T.; Zhu, L.; Liu, L.; Liu, X. Alterations of Cytochrome P450s and UDP-Glucuronosyltransferases in Brain Under Diseases and Their Clinical Significances. Front. Pharmacol. 2021, 12, 650027. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.M.; Tyndale, R.F. CYP-mediated drug metabolism in the brain impacts drug response. Pharmacol. Ther. 2018, 184, 189–200. [Google Scholar] [CrossRef]
- Stocco, M.R.; Tyndale, R.F. Cytochrome P450 enzymes and metabolism of drugs and neurotoxins within the mammalian brain. In Advances in Pharmacology; Academic Press Inc.: Cambridge, MA, USA, 2022; Volume 95, pp. 73–106. [Google Scholar]
- Zhang, M.; Rottschäfer, V.; de Lange, E.C.M. The potential impact of CYP and UGT drug-metabolizing enzymes on brain target site drug exposure. Drug Metab. Rev. 2024, 56, 1–30. [Google Scholar] [CrossRef]
- van Lier, J.E. New Therapeutic Targets for Brain Function and Disease. J. Med. Chem. 2020, 63, 6474–6476. [Google Scholar] [CrossRef]
- Toselli, F.; Dodd, P.R.; Gillam, E.M.J. Emerging roles for brain drug-metabolizing cytochrome P450 enzymes in neuropsychiatric conditions and responses to drugs. Drug Metab. Rev. 2016, 48, 379–404. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef]
- Ferguson, C.S.; Tyndale, R.F. Cytochrome P450 enzymes in the brain: Emerging evidence of biological significance. Trends Pharmacol. Sci. 2011, 32, 708–714. [Google Scholar] [CrossRef]
- Vance, J.E. Dysregulation of cholesterol balance in the brain: Contribution to neurodegenerative diseases. Dis. Models Mech. 2012, 5, 746–755. [Google Scholar] [CrossRef]
- Haduch, A.; Daniel, W.A. The engagement of brain cytochrome P450 in the metabolism of endogenous neuroactive substrates: A possible role in mental disorders. Drug Metab. Rev. 2018, 50, 415–429. [Google Scholar] [CrossRef]
- Djelti, F.; Braudeau, J.; Hudry, E.; Dhenain, M.; Varin, J.; Bièche, I.; Marquer, C.; Chali, F.; Ayciriex, S.; Auzeil, N.; et al. CYP46A1 inhibition, brain cholesterol accumulation and neurodegeneration pave the way for Alzheimer’s disease. Brain 2015, 138, 2383–2398. [Google Scholar] [CrossRef]
- Burlot, M.-A.; Braudeau, J.; Michaelsen-Preusse, K.; Potier, B.; Ayciriex, S.; Varin, J.; Gautier, B.; Djelti, F.; Audrain, M.; Dauphinot, L.; et al. Cholesterol 24-hydroxylase defect is implicated in memory impairments associated with Alzheimer-like Tau pathology. Hum. Mol. Genet. 2015, 24, 5965–5976. [Google Scholar] [CrossRef] [PubMed]
- Mast, N.; Saadane, A.; Valencia-Olvera, A.; Constans, J.; Maxfield, E.; Arakawa, H.; Li, Y.; Landreth, G.; Pikuleva, I.A. Cholesterol-metabolizing enzyme cytochrome P450 46A1 as a pharmacologic target for Alzheimer’s disease. Neuropharmacology 2017, 123, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Ong, C.E.; Pan, Y. Unveiling the Role of Cytochrome P450 (2E1) in Human Brain Specifically in Parkinson’s Disease—Literature Review. Curr. Drug Metab. 2021, 22, 698–708. [Google Scholar] [CrossRef] [PubMed]
- ur Rasheed, M.S.; Mishra, A.K.; Singh, M.P. Cytochrome P450 2D6 and Parkinson’s Disease: Polymorphism, Metabolic Role, Risk and Protection. Neurochem. Res. 2017, 42, 3353–3361. [Google Scholar] [CrossRef]
- Boussicault, L.; Alves, S.; Lamazière, A.; Planques, A.; Heck, N.; Moumné, L.; Despres, G.; Bolte, S.; Hu, A.; Pagès, C.; et al. CYP46A1, the rate-limiting enzyme for cholesterol degradation, is neuroprotective in Huntington’s disease. Brain 2016, 139, 953–970. [Google Scholar] [CrossRef]
- Nóbrega, C.; Conceição, A.; Costa, R.G.; Koppenol, R.; Sequeira, R.L.; Nunes, R.; Carmo-Silva, S.; Marcelo, A.; Matos, C.A.; Betuing, S.; et al. The cholesterol 24-hydroxylase activates autophagy and decreases mutant huntingtin build-up in a neuroblastoma culture model of Huntington’s disease. BMC Res. Notes 2020, 13, 210. [Google Scholar] [CrossRef]
- Kacher, R.; Mounier, C.; Caboche, J.; Betuing, S. Altered Cholesterol Homeostasis in Huntington’s Disease. Front. Aging Neurosci. 2022, 14, 797220. [Google Scholar] [CrossRef]
- Ma, L.; Shcherbina, A.; Chetty, S. Variations and expression features of CYP2D6 contribute to schizophrenia risk. Mol. Psychiatry 2021, 26, 2605–2615. [Google Scholar] [CrossRef]
- Shin, W.; Bang, M.; Kim, A.; Cho, D.Y.; Lee, S.H. Influence of cytochrome P450 2D6 polymorphism on hippocampal white matter and treatment response in schizophrenia. NPJ Schizophr. 2021, 7, 5. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Naderi, E.; Wijninga, M.J.; Liemburg, E.J.; Group Investigators; Cath, D.; Bruggeman, R.; Alizadeh, B.Z. Pharmacogenetics of Long-Term Outcomes of Schizophrenia Spectrum Disorders: The Functional Role of CYP2D6 and CYP2C19. J. Pers. Med. 2023, 13, 1354. [Google Scholar] [CrossRef]
- Serna-Rodríguez, M.F.; Cienfuegos-Jiménez, O.; Cerda-Flores, R.M.; Marino-Martínez, I.A.; Hernández-Ordoñez, M.A.; Ontiveros-Sánchez de la Barquera, J.A.; Pérez-Maya, A.A. The Relationship Between CYP46A1 Polymorphism and Suicide Risk: A Preliminary Investigation. Neuromol. Med. 2024, 26, 11. [Google Scholar] [CrossRef] [PubMed]
- Messedi, M.; Makni-Ayadi, F. 24S-Hydroxycholesterol in Neuropsychiatric Diseases: Schizophrenia, Autism Spectrum Disorder, and Bipolar Disorder. Adv. Exp. Med. Biol. 2024, 1440, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Sodero, A.O. 24S-hydroxycholesterol: Cellular effects and variations in brain diseases. J. Neurochem. 2021, 157, 899–918. [Google Scholar] [CrossRef]
- Tripodi, D.; Vitarelli, F.; Spiti, S.; Leoni, V. The Diagnostic Use of the Plasma Quantification of 24S-Hydroxycholesterol and Other Oxysterols in Neurodegenerative Disease. Adv. Exp. Med. Biol. 2024, 1440, 337–351. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, C.; Liu, Y. Navigating the metabolic maze: Anomalies in fatty acid and cholesterol processes in Alzheimer’s astrocytes. Alzheimers Res. Ther. 2024, 16, 63. [Google Scholar] [CrossRef]
- Petrov, A.M.; Mast, N.; Li, Y.; Denker, J.; Pikuleva, I.A. Brain sterol flux mediated by cytochrome P450 46A1 affects membrane properties and membrane-dependent processes. Brain Commun. 2020, 2, fcaa043. [Google Scholar] [CrossRef]
- Stingl, J.C.; Brockmöller, J.; Viviani, R. Genetic variability of drug-metabolizing enzymes: The dual impact on psychiatric therapy and regulation of brain function. Mol. Psychiatry 2013, 18, 273–287. [Google Scholar] [CrossRef]
- Song, Y.; Li, C.; Liu, G.; Liu, R.; Chen, Y.; Li, W.; Cao, Z.; Zhao, B.; Lu, C.; Liu, Y. Drug-Metabolizing Cytochrome P450 Enzymes Have Multifarious Influences on Treatment Outcomes. Clin. Pharmacokinet. 2021, 60, 585–601. [Google Scholar] [CrossRef]
- Gregori, S.D.; Gregori, M.D.; Ranzani, G.N.; Allegri, M.; Minella, C.; Regazzi, M. Morphine metabolism, transport and brain disposition. Metab. Brain Dis. 2012, 27, 1–5. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, Z. Impact of transporters and enzymes from blood–cerebrospinal fluid barrier and brain parenchyma on CNS drug uptake. Expert Opin. Drug Metab. Toxicol. 2018, 14, 961–972. [Google Scholar] [CrossRef]
- Agarwal, V.; Kommaddi, R.P.; Valli, K.; Ryder, D.; Hyde, T.M.; Kleinman, J.E.; Strobel, H.W.; Ravindranath, V. Drug Metabolism in Human Brain: High Levels of Cytochrome P4503A43 in Brain and Metabolism of Anti-Anxiety Drug Alprazolam to Its Active Metabolite. PLoS ONE 2008, 3, e2337. [Google Scholar] [CrossRef] [PubMed]
- Fanni, D.; Pinna, F.; Gerosa, C.; Paribello, P.; Carpiniello, B.; Faa, G.; Manchia, M. Anatomical distribution and expression of CYP in humans: Neuropharmacological implications. Drug Dev. Res. 2021, 82, 628–667. [Google Scholar] [CrossRef] [PubMed]
- Miksys, S.; Tyndale, R. Cytochrome P450–mediated drug metabolism in the brain. J. Psychiatry Neurosci. 2013, 38, 152–163. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef]
- Go, R.-E.; Hwang, K.-A.; Choi, K.-C. Cytochrome P450 1 family and cancers. J. Steroid Biochem. Mol. Biol. 2015, 147, 24–30. [Google Scholar] [CrossRef]
- Wahid, M.; Mahjabeen, I.; Baig, R.M.; Kayani, M.A. Expression of CYP1A1 and GSTP1 in Human Brain Tumor Tissues in Pakistan. Asian Pac. J. Cancer Prev. 2013, 14, 7187–7191. [Google Scholar] [CrossRef]
- García-Suástegui, W.A.; Ramos-Chávez, L.A.; Rubio-Osornio, M.; Calvillo-Velasco, M.; Atzin-Méndez, J.A.; Guevara, J.; Silva-Adaya, D. The Role of CYP2E1 in the Drug Metabolism or Bioactivation in the Brain. Oxidative Med. Cell. Longev. 2017, 2017, 4680732. [Google Scholar] [CrossRef]
- Zhong, Y.; Dong, G.; Luo, H.; Cao, J.; Wang, C.; Wu, J.; Feng, Y.-Q.; Yue, J. Induction of brain CYP2E1 by chronic ethanol treatment and related oxidative stress in hippocampus, cerebellum, and brainstem. Toxicology 2012, 302, 275–284. [Google Scholar] [CrossRef]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 2020, 367, eaay5947. [Google Scholar] [CrossRef]
- Heit, C.; Dong, H.; Chen, Y.; Thompson, D.C.; Deitrich, R.A.; Vasiliou, V.K. The role of CYP2E1 in alcohol metabolism and sensitivity in the central nervous system. Subcell. Biochem. 2013, 67, 235–247. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed]
- Tseng, E.; Walsky, R.L.; Luzietti, R.A.; Harris, J.J.; Kosa, R.E.; Goosen, T.C.; Zientek, M.A.; Obach, R.S. Relative Contributions of Cytochrome CYP3A4 Versus CYP3A5 for CYP3A-Cleared Drugs Assessed In Vitro Using a CYP3A4-Selective Inactivator (CYP3cide). Drug Metab. Dispos. 2014, 42, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Woodland, C.; Huang, T.T.; Gryz, E.; Bendayan, R.; Fawcett, J.P. Expression, Activity and Regulation of CYP3A in Human and Rodent Brain. Drug Metab. Rev. 2008, 40, 149–168. [Google Scholar] [CrossRef]
- Mast, N.; Anderson, K.W.; Johnson, K.M.; Phan, T.T.N.; Guengerich, F.P.; Pikuleva, I.A. In vitro cytochrome P450 46A1 (CYP46A1) activation by neuroactive compounds. J. Biol. Chem. 2017, 292, 12934–12946. [Google Scholar] [CrossRef] [PubMed]
- Pikuleva, I.A.; Cartier, N. Cholesterol Hydroxylating Cytochrome P450 46A1: From Mechanisms of Action to Clinical Applications. Front. Aging Neurosci. 2021, 13, 696778. [Google Scholar] [CrossRef]
- The GTEx Consortium; Aguet, F.; Anand, S.; Ardlie, K.G.; Gabriel, S.; Getz, G.A.; Graubert, A.; Hadley, K.; Handsaker, R.E.; Huang, K.H.; et al. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Miller, J.A.; Guillozet-Bongaarts, A.; Gibbons, L.E.; Postupna, N.; Renz, A.; Beller, A.E.; Sunkin, S.M.; Ng, L.; Rose, S.E.; Smith, K.A.; et al. Neuropathological and transcriptomic characteristics of the aged brain. eLife 2017, 6, e31126. [Google Scholar] [CrossRef]
- Clough, E.; Barrett, T. The Gene Expression Omnibus Database. Methods Mol. Biol. Clifton NJ 2016, 1418, 93–110. [Google Scholar] [CrossRef]
- Moreno, P.; Fexova, S.; George, N.; Manning, J.R.; Miao, Z.; Mohammed, S.; Muñoz-Pomer, A.; Fullgrabe, A.; Bi, Y.; Bush, N.; et al. Expression Atlas update: Gene and protein expression in multiple species. Nucleic Acids Res. 2022, 50, D129–D140. [Google Scholar] [CrossRef]
- Dutheil, F.; Beaune, P.; Loriot, M.-A. Xenobiotic metabolizing enzymes in the central nervous system: Contribution of cytochrome P450 enzymes in normal and pathological human brain. Biochimie 2008, 90, 426–436. [Google Scholar] [CrossRef]
- Dutheil, F.; Dauchy, S.; Diry, M.; Sazdovitch, V.; Cloarec, O.; Mellottée, L.; Bièche, I.; Ingelman-Sundberg, M.; Flinois, J.-P.; de Waziers, I.; et al. Xenobiotic-Metabolizing Enzymes and Transporters in the Normal Human Brain: Regional and Cellular Mapping as a Basis for Putative Roles in Cerebral Function. Drug Metab. Dispos. 2009, 37, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Sasame, H.A.; Ames, M.M.; Nelson, S.D. Cytochrome P-450 and NADPH cytochrome c reductase in rat brain: Formation of catechols and reactive catechol metabolites. Biochem. Biophys. Res. Commun. 1977, 78, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Pitanga, B.P.S.; Nascimento, R.P.; Silva, V.D.A.; Costa, S.L. The Role of Astrocytes in Metabolism and Neurotoxicity of the Pyrrolizidine Alkaloid Monocrotaline, the Main Toxin of Crotalaria retusa. Front. Pharmacol. 2012, 3, 144. [Google Scholar] [CrossRef] [PubMed]
- Bhamre, S.; Anandatheerthavarada, H.K.; Shankar, S.K.; Ravindranath, V. Microsomal cytochrome P450 in human brain regions. Biochem. Pharmacol. 1992, 44, 1223–1225. [Google Scholar] [CrossRef]
- Tirumalai, P.S.; Bhamre, S.; Upadhya, S.C.; Boyd, M.R.; Ravindranath, V. Expression of Multiple Forms of Cytochrome P450 and Associated Mono-oxygenase Activities in Rat Brain Regions. Biochem. Pharmacol. 1998, 56, 371–375. [Google Scholar] [CrossRef]
- Dauchy, S.; Dutheil, F.; Weaver, R.J.; Chassoux, F.; Daumas-Duport, C.; Couraud, P.-O.; Scherrmann, J.-M.; Waziers, I.D.; Declèves, X. ABC transporters, cytochromes P450 and their main transcription factors: Expression at the human blood–brain barrier. J. Neurochem. 2008, 107, 1518–1528. [Google Scholar] [CrossRef]
- Depaz, I.M.B.; Toselli, F.; Wilce, P.A.; Gillam, E.M.J. Differential Expression of Cytochrome P450 Enzymes from the CYP2C Subfamily in the Human Brain. Drug Metab. Dispos. 2015, 43, 353–357. [Google Scholar] [CrossRef]
- Tolledo, C.; Stocco, M.R.; Miksys, S.; Gonzalez, F.J.; Tyndale, R.F. Human CYP2D6 Is Functional in Brain In Vivo: Evidence from Humanized CYP2D6 Transgenic Mice. Mol. Neurobiol. 2020, 57, 2509–2520. [Google Scholar] [CrossRef]
- Mann, A.; Miksys, S.L.; Gaedigk, A.; Kish, S.J.; Mash, D.C.; Tyndale, R.F. The neuroprotective enzyme CYP2D6 increases in the brain with age and is lower in Parkinson’s disease patients. Neurobiol. Aging 2012, 33, 2160–2171. [Google Scholar] [CrossRef]
- Pikuleva, I. Targeting cytochrome P450 46A1 and brain cholesterol 24-hydroxylation to treat neurodegenerative diseases. Explor. Neuroprot. Ther. 2021, 1, 159–172. [Google Scholar] [CrossRef]
- Lund, E.G.; Guileyardo, J.M.; Russell, D.W. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. USA 1999, 96, 7238–7243. [Google Scholar] [CrossRef] [PubMed]
- Miksys, S.L.; Cheung, C.; Gonzalez, F.J.; Tyndale, R.F. Human cyp2d6 and mouse cyp2ds: Organ distribution in a humanized mouse model. Drug Metab. Dispos. 2005, 33, 1495–1502. [Google Scholar] [CrossRef]
- Arguelles, N.; Miksys, S.; Tyndale, R.F. Sex and Estrous Cycle Differences in Analgesia and Brain Oxycodone Levels. Mol. Neurobiol. 2021, 58, 6540–6551. [Google Scholar] [CrossRef]
- Siegle, I.; Fritz, P.; Eckhardt, K.; Zanger, U.M.; Eichelbaum, M. Cellular localization and regional distribution of CYP2D6 mRNA and protein expression in human brain. Pharmacogenetics 2001, 11, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Miksys, S.; Rao, Y.; Hoffmann, E.; Mash, D.C.; Tyndale, R.F. Regional and cellular expression of CYP2D6 in human brain: Higher levels in alcoholics. J. Neurochem. 2002, 82, 1376–1387. [Google Scholar] [CrossRef]
- McFadyen, M.C.E.; Melvin, W.T.; Murray, G.I. Regional Distribution of Individual Forms of Cytochrome P450 mRNA in Normal Adult Human Brain. Biochem. Pharmacol. 1998, 55, 825–830. [Google Scholar] [CrossRef]
- Chinta, S.J.; Kommaddi, R.P.; Turman, C.M.; Strobel, H.W.; Ravindranath, V. Constitutive expression and localization of cytochrome P-450 1A1 in rat and human brain: Presence of a splice variant form in human brain. J. Neurochem. 2005, 93, 724–736. [Google Scholar] [CrossRef]
- Haduch, A.; Bromek, E.; Kuban, W.; Daniel, W.A. The Engagement of Cytochrome P450 Enzymes in Tryptophan Metabolism. Metabolites 2023, 13, 629. [Google Scholar] [CrossRef]
- Kuban, W.; Daniel, W.A. Cytochrome P450 expression and regulation in the brain. Drug Metab. Rev. 2021, 53, 1–29. [Google Scholar] [CrossRef]
- Granberg, L.; Östergren, A.; Brandt, I.; Brittebo, E.B. CYP1A1 and CYP1B1 in Blood-Brain Interfaces: CYP1A1-Dependent Bioactivation of 7,12-Dimethylbenz(a)anthracene in Endothelial Cells. Drug Metab. Dispos. 2003, 31, 259–265. [Google Scholar] [CrossRef]
- Kapoor, N.; Pant, A.B.; Dhawan, A.; Dwievedi, U.N.; Seth, P.K.; Parmar, D. Cytochrome P450 1A isoenzymes in brain cells: Expression and inducibility in cultured rat brain neuronal and glial cells. Life Sci. 2006, 79, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Kirchheiner, J.; Seeringer, A.; Godoy, A.L.; Ohmle, B.; Maier, C.; Beschoner, P.; Sim, E.-J.; Viviani, R. CYP2D6 in the brain: Genotype effects on resting brain perfusion. Mol. Psychiatry 2011, 16, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Lidin, E.; Sköld, M.K.; Angéria, M.; Davidsson, J.; Risling, M. Hippocampal Expression of Cytochrome P450 1B1 in Penetrating Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 722. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Tian, S.; Na, S.; Wei, H.; Wu, Y.; Yang, Y.; Shen, Z.; Ding, J.; Bao, S.; et al. CYP1B1 affects the integrity of the blood–brain barrier and oxidative stress in the striatum: An investigation of manganese-induced neurotoxicity. CNS Neurosci. Ther. 2024, 30, e14633. [Google Scholar] [CrossRef]
- Cheng, J.; Zhen, Y.; Miksys, S.; Beyoğlu, D.; Krausz, K.W.; Tyndale, R.F.; Yu, A.; Idle, J.R.; Gonzalez, F.J. Potential role of CYP2D6 in the central nervous system. Xenobiotica 2013, 43, 973–984. [Google Scholar] [CrossRef]
- Darney, K.; Lautz, L.S.; Béchaux, C.; Wiecek, W.; Testai, E.; Amzal, B.; Dorne, J.L.C.M. Human variability in polymorphic CYP2D6 metabolism: Implications for the risk assessment of chemicals in food and emerging designer drugs. Environ. Int. 2021, 156, 106760. [Google Scholar] [CrossRef]
- Just, K.S.; Dormann, H.; Freitag, M.; Schurig, M.; Böhme, M.; Steffens, M.; Scholl, C.; Seufferlein, T.; Graeff, I.; Schwab, M.; et al. CYP2D6 in the Brain: Potential Impact on Adverse Drug Reactions in the Central Nervous System—Results From the ADRED Study. Front. Pharmacol. 2021, 12, 624104. [Google Scholar] [CrossRef]
- Stingl, J.C.; Esslinger, C.; Tost, H.; Bilek, E.; Kirsch, P.; Ohmle, B.; Viviani, R.; Walter, H.; Rietschel, M.; Meyer-Lindenberg, A. Genetic variation in CYP2D6 impacts neural activation during cognitive tasks in humans. NeuroImage 2012, 59, 2818–2823. [Google Scholar] [CrossRef]
- Funae, Y.; Kishimoto, W.; Cho, T.; Niwa, T.; Hiroi, T. CYP2D in the Brain. Drug Metab. Pharmacokinet. 2003, 18, 337–349. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M.; Persson, A.; Jukic, M.M. Polymorphic Expression of Cyp2C19 and Cyp2D6 in the Developing and Adult Human Brain Causing Variability in Cognition, Risk for Depression and Suicide: The Search for the Endogenous Substrates. Pharmacogenomics 2014, 15, 1841–1844. [Google Scholar] [CrossRef]
- Naujokaitis, D.; Asmoniene, V.; Kadusevicius, E. Cytochrome P450 2C19 enzyme, Cytochrome P450 2C9 enzyme, and Cytochrome P450 2D6 enzyme allelic variants and its possible effect on drug metabolism: A retrospective study. Medicine 2021, 100, e24545. [Google Scholar] [CrossRef] [PubMed]
- Stingl, J.C.; Scholl, C.; Bosch, J.E.; Viviani, R. Genetic Polymorphism of CYP2C19 and Subcortical Variability in the Human Adult Brain. Transl. Psychiatry 2021, 11, 467. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-J.; Liu, N.; Yang, K.; Wang, A.-F.; Tan, Z.-R.; Li, X. Clinical application and importance of one-step human CYP2C19 genotype detection. J. Int. Med. Res. 2018, 46, 4965–4973. [Google Scholar] [CrossRef]
- Booth Depaz, I.M.; Toselli, F.; Wilce, P.A.; Gillam, E.M.J. Differential Expression of Human Cytochrome P450 Enzymes from the CYP3A Subfamily in the Brains of Alcoholic Subjects and Drug-Free Controls. Drug Metab. Dispos. 2013, 41, 1187–1194. [Google Scholar] [CrossRef]
- Fanni, D.; Manchia, M.; Lai, F.; Gerosa, C.; Ambu, R.; Faa, G. Immunohistochemical markers of CYP3A4 and CYP3A7: A new tool towards personalized pharmacotherapy of hepatocellular carcinoma. Eur. J. Histochem. EJH 2016, 60, 2614. [Google Scholar] [CrossRef]
- Alshammari, N. Content and Activity of Cytochrome P450 3A in Rat Brain Microsomes and Mitochondria. Master’s Thesis, Chapman University, Irvine, CA USA, 2022. Available online: https://digitalcommons.chapman.edu/pharmaceutical_sciences_theses/21/ (accessed on 16 February 2025).
- Brlek, P.; Bulić, L.; Glavaš Weinberger, D.; Bošnjak, J.; Pavlović, T.; Tomić, S.; Krivdić Dupan, Z.; Borić, I.; Primorac, D. Successful Treatment of a Rare Cholesterol Homeostasis Disorder Due to CYP27A1 Gene Mutation with Chenodeoxycholic Acid Therapy. Biomedicines 2023, 11, 1430. [Google Scholar] [CrossRef]
- Charvet, C.; Liao, W.-L.; Heo, G.-Y.; Laird, J.; Salomon, R.G.; Turko, I.V.; Pikuleva, I.A. Isolevuglandins and Mitochondrial Enzymes in the Retina. J. Biol. Chem. 2011, 286, 20413–20422. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Crick, P.J.; Meljon, A.; Theofilopoulos, S.; Abdel-Khalik, J.; Yutuc, E.; Parker, J.E.; Kelly, D.E.; Kelly, S.L.; Arenas, E.; et al. Additional pathways of sterol metabolism: Evidence from analysis of Cyp27a1−/− mouse brain and plasma. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2019, 1864, 191–211. [Google Scholar] [CrossRef]
- Liang, Z.; Jiao, W.; Wang, L.; Chen, Y.; Li, D.; Zhang, Z.; Zhang, Z.; Liang, Y.; Niu, H. CYP27A1 inhibits proliferation and migration of clear cell renal cell carcinoma via activation of LXRs/ABCA1. Exp. Cell Res. 2022, 419, 113279. [Google Scholar] [CrossRef]
- Liao, W.-L.; Heo, G.-Y.; Dodder, N.G.; Reem, R.E.; Mast, N.; Huang, S.; DiPatre, P.L.; Turko, I.V.; Pikuleva, I.A. Quantification of Cholesterol-Metabolizing P450s CYP27A1 and CYP46A1 in Neural Tissues Reveals a Lack of Enzyme−Product Correlations in Human Retina but Not Human Brain. J. Proteome Res. 2011, 10, 241–248. [Google Scholar] [CrossRef]
- Mast, N.; Butts, M.; Pikuleva, I.A. Unbiased insights into the multiplicity of the CYP46A1 brain effects in 5XFAD mice treated with low dose-efavirenz. J. Lipid Res. 2024, 65, 100555. [Google Scholar] [CrossRef]
- Moutinho, M.; Nunes, M.J.; Rodrigues, E. Cholesterol 24-hydroxylase: Brain cholesterol metabolism and beyond. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2016, 1861, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.M.; Pikuleva, I.A. Cholesterol 24-Hydroxylation by CYP46A1: Benefits of Modulation for Brain Diseases. Neurotherapeutics 2019, 16, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Daniel, W.A.; Bromek, E.; Danek, P.J.; Haduch, A. The mechanisms of interactions of psychotropic drugs with liver and brain cytochrome P450 and their significance for drug effect and drug-drug interactions. Biochem. Pharmacol. 2022, 199, 115006. [Google Scholar] [CrossRef]
- Hanin, A.; Baudin, P.; Demeret, S.; Roussel, D.; Lecas, S.; Teyssou, E.; Damiano, M.; Luis, D.; Lambrecq, V.; Frazzini, V.; et al. Disturbances of brain cholesterol metabolism: A new excitotoxic process associated with status epilepticus. Neurobiol. Dis. 2021, 154, 105346. [Google Scholar] [CrossRef] [PubMed]
- Anchisi, L.; Dessì, S.; Pani, A.; Mandas, A. Cholesterol homeostasis: A key to prevent or slow down neurodegeneration. Front. Physiol. 2013, 3, 486. [Google Scholar] [CrossRef]
- Li, X.; Dao, M.; Lykotrafitis, G.; Karniadakis, G.E. Biomechanics and biorheology of red blood cells in sickle cell anemia. J. Biomech. 2017, 50, 34–41. [Google Scholar] [CrossRef]
- Haider, A.; Zhao, C.; Wang, L.; Xiao, Z.; Rong, J.; Xia, X.; Chen, Z.; Pfister, S.K.; Mast, N.; Yutuc, E.; et al. Assessment of cholesterol homeostasis in the living human brain. Sci. Transl. Med. 2022, 14, eadc9967. [Google Scholar] [CrossRef]
- Pfrieger, F.W.; Ungerer, N. Cholesterol metabolism in neurons and astrocytes. Prog. Lipid Res. 2011, 50, 357–371. [Google Scholar] [CrossRef]
- Alavi, M.S.; Karimi, G.; Ghanimi, H.A.; Roohbakhsh, A. The potential of CYP46A1 as a novel therapeutic target for neurological disorders: An updated review of mechanisms. Eur. J. Pharmacol. 2023, 949, 175726. [Google Scholar] [CrossRef]
- Ahmed, H.; Wang, Y.; Griffiths, W.J.; Levey, A.I.; Pikuleva, I.; Liang, S.H.; Haider, A. Brain cholesterol and Alzheimer’s disease: Challenges and opportunities in probe and drug development. Brain 2024, 147, 1622–1635. [Google Scholar] [CrossRef]
- Wang, H.-L.; Wang, Y.-Y.; Liu, X.-G.; Kuo, S.-H.; Liu, N.; Song, Q.-Y.; Wang, M.-W. Cholesterol, 24-Hydroxycholesterol, and 27-Hydroxycholesterol as Surrogate Biomarkers in Cerebrospinal Fluid in Mild Cognitive Impairment and Alzheimer’s Disease: A Meta-Analysis. J. Alzheimers Dis. 2016, 51, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Lövgren-Sandblom, A.; Leoni, V.; Meaney, S.; Brodin, L.; Salveson, L.; Winge, K.; Pålhagen, S.; Svenningsson, P. Oxysterols and Parkinson’s disease: Evidence that levels of 24S-hydroxycholesterol in cerebrospinal fluid correlates with the duration of the disease. Neurosci. Lett. 2013, 555, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Mitroi, D.N.; Pereyra-Gómez, G.; Soto-Huelin, B.; Senovilla, F.; Kobayashi, T.; Esteban, J.A.; Ledesma, M.D. NPC1 enables cholesterol mobilization during long-term potentiation that can be restored in Niemann-Pick disease type C by CYP46A1 activation. EMBO Rep. 2019, 20, e48143. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.-J.; Ziolkowski, L.H.; Salvatore, S.V.; Benz, A.M.; Wozniak, D.F.; Yuede, C.M.; Paul, S.M.; Zorumski, C.F.; Mennerick, S. Effects of Complete and Partial Loss of the 24S-Hydroxycholesterol-Generating Enzyme Cyp46a1 on Behavior and Hippocampal Transcription in Mouse. Biomolecules 2024, 14, 254. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Rajendran, P. The Multifarious Link between Cytochrome P450s and Cancer. Oxidative Med. Cell. Longev. 2020, 2020, 3028387. [Google Scholar] [CrossRef]
- .Han, M.; Wang, S.; Yang, N.; Wang, X.; Zhao, W.; Saed, H.S.; Daubon, T.; Huang, B.; Chen, A.; Li, G.; et al. Therapeutic implications of altered cholesterol homeostasis mediated by loss of CYP46A1 in human glioblastoma. EMBO Mol. Med. 2020, 12, e10924. [Google Scholar] [CrossRef]
- Bromek, E.; Haduch, A.; Daniel, W.A. The ability of cytochrome P450 2D isoforms to synthesize dopamine in the brain: An in vitro study. Eur. J. Pharmacol. 2010, 626, 171–178. [Google Scholar] [CrossRef]
- Bromek, E.; Haduch, A.; Gołembiowska, K.; Daniel, W.A. Cytochrome P450 mediates dopamine formation in the brain in vivo. J. Neurochem. 2011, 118, 806–815. [Google Scholar] [CrossRef]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef]
- Salvatore, M.F.; McInnis, T.R.; Cantu, M.A.; Apple, D.M.; Pruett, B.S. Tyrosine Hydroxylase Inhibition in Substantia Nigra Decreases Movement Frequency. Mol. Neurobiol. 2019, 56, 2728–2740. [Google Scholar] [CrossRef] [PubMed]
- Goswami, J.N.; Sankhyan, N.; Singhi, P.D. An Indian family with tyrosine hydroxylase deficiency. Indian Pediatr. 2017, 54, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin metabolism in the central nervous system. Curr. Neuropharmacol. 2010, 8, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Haduch, A.; Bromek, E.; Kot, M.; Kamińska, K.; Gołembiowska, K.; Daniel, W.A. The cytochrome P450 2D-mediated formation of serotonin from 5-methoxytryptamine in the brain in vivo: A microdialysis study. J. Neurochem. 2015, 133, 83–92. [Google Scholar] [CrossRef]
- Haduch, A.; Bromek, E.; Sadakierska-Chudy, A.; Wójcikowski, J.; Daniel, W.A. The catalytic competence of cytochrome P450 in the synthesis of serotonin from 5-methoxytryptamine in the brain: An in vitro study. Pharmacol. Res. 2013, 67, 53–59. [Google Scholar] [CrossRef]
- Haduch, A.; Bromek, E.; Wojcikowski, J.; embiowska, K.G.; Daniel, W.A. Melatonin Supports CYP2D-Mediated Serotonin Synthesis in the Brain. Drug Metab. Dispos. 2016, 44, 445–452. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Na, S.; Wu, J.; Yang, Z.; Xie, X.; Wan, Y.; Li, K.; Yue, J. The Involvement of PPARs in the Selective Regulation of Brain CYP2D by Growth Hormone. Neuroscience 2018, 379, 115–125. [Google Scholar] [CrossRef]
- Haduch, A.; Rysz, M.; Papp, M.; Daniel, W.A. The activity of brain and liver cytochrome P450 2D (CYP2D) is differently affected by antidepressants in the chronic mild stress (CMS) model of depression in the rat. Biochem. Pharmacol. 2018, 156, 398–405. [Google Scholar] [CrossRef]
- Sarparast, M.; Dattmore, D.; Alan, J.; Lee, K.S.S. Cytochrome p450 metabolism of polyunsaturated fatty acids and neurodegeneration. Nutrients 2020, 12, 3523. [Google Scholar] [CrossRef]
- Navarro-Mabarak, C.; Camacho-Carranza, R.; Espinosa-Aguirre, J.J. Cytochrome P450 in the central nervous system as a therapeutic target in neurodegenerative diseases. Drug Metab. Rev. 2018, 50, 95–108. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Kodani, S.D.; Morisseau, C. Role of epoxy-fatty acids and epoxide hydrolases in the pathology of neuro-inflammation. Biochimie 2019, 159, 59–65. [Google Scholar] [CrossRef]
- Isobe, Y.; Itagaki, M.; Ito, Y.; Naoe, S.; Kojima, K.; Ikeguchi, M.; Arita, M. Comprehensive analysis of the mouse cytochrome P450 family responsible for omega-3 epoxidation of eicosapentaenoic acid. Sci. Rep. 2018, 8, 7954. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, P.; Siest, G.; Visvikis-siest, S. Influence of inflammation on cardiovascular protective effects of cytochrome P450 epoxygenase-derived epoxyeicosatrienoic acids. Drug Metab. Rev. 2014, 46, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, A.I.; Waindok, P.; Schmidt, M.J.; Chiu, C.-Y.; Smyl, C.; Rohwer, N.; Weylandt, K.-H.; Schebb, N.H. Modulation of the endogenous omega-3 fatty acid and oxylipin profile in vivo—A comparison of the fat-1 transgenic mouse with C57BL/6 wildtype mice on an omega-3 fatty acid enriched diet. PLoS ONE 2017, 12, e0184470. [Google Scholar] [CrossRef]
- Ferdouse, A.; Leng, S.; Winter, T.; Aukema, H.M. The Brain Oxylipin Profile Is Resistant to Modulation by Dietary n-6 and n-3 Polyunsaturated Fatty Acids in Male and Female Rats. Lipids 2019, 54, 67–80. [Google Scholar] [CrossRef]
- Rey, C.; Delpech, J.C.; Madore, C.; Nadjar, A.; Greenhalgh, A.D.; Amadieu, C.; Aubert, A.; Pallet, V.; Vaysse, C.; Layé, S.; et al. Dietary n-3 long chain PUFA supplementation promotes a pro-resolving oxylipin profile in the brain. Brain Behav. Immun. 2019, 76, 17–27. [Google Scholar] [CrossRef]
- Taha, A.Y.; Blanchard, H.C.; Cheon, Y.; Ramadan, E.; Chen, M.; Chang, L.; Rapoport, S.I. Dietary Linoleic Acid Lowering Reduces Lipopolysaccharide-Induced Increase in Brain Arachidonic Acid Metabolism. Mol. Neurobiol. 2017, 54, 4303–4315. [Google Scholar] [CrossRef]
- Taha, A.Y.; Hennebelle, M.; Yang, J.; Zamora, D.; Rapoport, S.I.; Hammock, B.D.; Ramsden, C.E. Regulation of rat plasma and cerebral cortex oxylipin concentrations with increasing levels of dietary linoleic acid. Prostaglandins Leukot. Essent. Fat. Acids 2018, 138, 71–80. [Google Scholar] [CrossRef]
- McDougle, D.R.; Watson, J.E.; Abdeen, A.A.; Adili, R.; Caputo, M.P.; Krapf, J.E.; Johnson, R.W.; Kilian, K.A.; Holinstat, M.; Das, A. Anti-inflammatory ω-3 endocannabinoid epoxides. Proc. Natl. Acad. Sci. USA 2017, 114, E6034–E6043. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wu, J.; Hu, M.; Wu, J.; Zhu, Q.; Yang, Z.; Xie, X.; Feng, Y.-Q.; Yue, J. Glutamate affects the CYP1B1- and CYP2U1-mediated hydroxylation of arachidonic acid metabolism via astrocytic mGlu5 receptor. Int. J. Biochem. Cell Biol. 2019, 110, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Hadley, K.; Ryan, A.; Forsyth, S.; Gautier, S.; Salem, N. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Carver, K.A.; Lourim, D.; Tryba, A.K.; Harder, D.R. Rhythmic expression of cytochrome P450 epoxygenases CYP4x1 and CYP2c11 in the rat brain and vasculature. Am. J. Physiol.-Cell Physiol. 2014, 307, C989–C998. [Google Scholar] [CrossRef]
- Sarkar, P.; Zaja, I.; Bienengraeber, M.; Rarick, K.R.; Terashvili, M.; Canfield, S.; Falck, J.R.; Harder, D.R. Epoxyeicosatrienoic acids pretreatment improves amyloid β-induced mitochondrial dysfunction in cultured rat hippocampal astrocytes. Am. J. Physiol.-Heart Circ. Physiol. 2014, 306, H475–H484. [Google Scholar] [CrossRef]
- Lakkappa, N.; Krishnamurthy, P.T.; Hammock, B.D.; Velmurugan, D.; Bharath, M.M.S. Possible role of Epoxyeicosatrienoic acid in prevention of oxidative stress mediated neuroinflammation in Parkinson disorders. Med. Hypotheses 2016, 93, 161–165. [Google Scholar] [CrossRef]
- Huang, H.-J.; Wang, Y.-T.; Lin, H.-C.; Lee, Y.-H.; Lin, A.M.-Y. Soluble Epoxide Hydrolase Inhibition Attenuates MPTP-Induced Neurotoxicity in the Nigrostriatal Dopaminergic System: Involvement of α-Synuclein Aggregation and ER Stress. Mol. Neurobiol. 2018, 55, 138–144. [Google Scholar] [CrossRef]
- Deodhar, M.; Rihani, S.B.A.; Darakjian, L.; Turgeon, J.; Michaud, V. Assessing the Mechanism of Fluoxetine-Mediated CYP2D6 Inhibition. Pharmaceutics 2021, 13, 148. [Google Scholar] [CrossRef]
- Lu, J.; Yang, Y.; Lu, J.; Wang, Z.; He, Y.; Yan, Y.; Fu, K.; Jiang, W.; Xu, Y.; Wu, R.; et al. Effect of CYP2D6 polymorphisms on plasma concentration and therapeutic effect of risperidone. BMC Psychiatry 2021, 21, 70. [Google Scholar] [CrossRef]
- McMillan, D.M.; Tyndale, R.F. Inducing rat brain CYP2D with nicotine increases the rate of codeine tolerance; predicting the rate of tolerance from acute analgesic response. Biochem. Pharmacol. 2017, 145, 158–168. [Google Scholar] [CrossRef]
- McMillan, D.M.; El-Sherbeni, A.A.; Richards, J.; Tyndale, R.F. Centrally administered CYP2D inhibitors increase oral tramadol analgesia in rats. Brain Res. Bull. 2020, 164, 400–406. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.M.; Miksys, S.; Tyndale, R.F. Rat brain CYP2D activity alters in vivo central oxycodone metabolism, levels and resulting analgesia. Addict. Biol. 2019, 24, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, X.; Li, J.; Gao, X.; Wang, Y.; Liu, M.; Dong, G.; Yue, J. Regulation of cerebral CYP2D alters tramadol metabolism in the brain: Interactions of tramadol with propranolol and nicotine. Xenobiotica 2015, 45, 335–344. [Google Scholar] [CrossRef]
- Coates, S.; Lazarus, P. Hydrocodone, Oxycodone, and Morphine Metabolism and Drug–Drug Interactions. J. Pharmacol. Exp. Ther. 2023, 387, 150–169. [Google Scholar] [CrossRef]
- Tuet, W.Y.; Pierce, S.A.; Conroy, M.; Vignola, J.N.; Tressler, J.; diTargiani, R.C.; McCranor, B.J.; Wong, B. Metabolic clearance of select opioids and opioid antagonists using hepatic spheroids and recombinant cytochrome P450 enzymes. Pharmacol. Res. Perspect. 2022, 10, e01000. [Google Scholar] [CrossRef]
- Romand, S.; Spaggiari, D.; Marsousi, N.; Samer, C.; Desmeules, J.; Daali, Y.; Rudaz, S. Characterization of oxycodone in vitro metabolism by human cytochromes P450 and UDP-glucuronosyltransferases. J. Pharm. Biomed. Anal. 2017, 144, 129–137. [Google Scholar] [CrossRef]
- Kapil, R.P.; Friedman, K.; Cipriano, A.; Michels, G.; Shet, M.; Mondal, S.A.; Harris, S.C. Effects of Paroxetine, a CYP2D6 Inhibitor, on the Pharmacokinetic Properties of Hydrocodone After Coadministration with a Single-entity, Once-daily, Extended-release Hydrocodone Tablet. Clin. Ther. 2015, 37, 2286–2296. [Google Scholar] [CrossRef]
- Volkow, N.D.; Koob, G.F.; McLellan, A.T. Neurobiologic Advances from the Brain Disease Model of Addiction. N. Engl. J. Med. 2016, 374, 363–371. [Google Scholar] [CrossRef]
- Volkow, N.D.; McLellan, A.T. Opioid Abuse in Chronic Pain—Misconceptions and Mitigation Strategies. N. Engl. J. Med. 2016, 374, 1253–1263. [Google Scholar] [CrossRef]
- Karsy, M.; Bowers, C.A.; Scoville, J.; Kundu, B.; Azab, M.A.; Gee, J.M.; Guan, J.; Couldwell, W.T. Evaluation of Complications and Costs During Overlapping Transsphenoidal Surgery in the Treatment of Pituitary Adenoma. Neurosurgery 2019, 84, 1104–1111. [Google Scholar] [CrossRef]
- .Wang, S.-C.; Chen, Y.-C.; Lee, C.-H.; Cheng, C.-M. Opioid Addiction, Genetic Susceptibility, and Medical Treatments: A Review. Int. J. Mol. Sci. 2019, 20, 4294. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.; Muriel, J.; Peiró, A.M. CYP2D6 phenotypes and opioid metabolism: The path to personalized analgesia. Expert Opin. Drug Metab. Toxicol. 2022, 18, 261–275. [Google Scholar] [CrossRef]
- Langman, L.J.; Korman, E.; Stauble, M.E.; Boswell, M.V.; Baumgartner, R.N.; Jortani, S.A. Therapeutic Monitoring of Opioids. Ther. Drug Monit. 2013, 35, 352–359. [Google Scholar] [CrossRef]
- Ko, T.-M.; Wong, C.-S.; Wu, J.-Y.; Chen, Y.-T. Pharmacogenomics for personalized pain medicine. Acta Anaesthesiol. Taiwan. 2016, 54, 24–30. [Google Scholar] [CrossRef]
- Huddart, R.; Clarke, M.; Altman, R.B.; Klein, T.E. PharmGKB summary: Oxycodone pathway, pharmacokinetics. Pharmacogenet. Genomics 2018, 28, 230–237. [Google Scholar] [CrossRef]
- Kinnunen, M.; Piirainen, P.; Kokki, H.; Lammi, P.; Kokki, M. Updated Clinical Pharmacokinetics and Pharmacodynamics of Oxycodone. Clin. Pharmacokinet. 2019, 58, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Takashina, Y.; Yamamoto, K.; Tashiro, M.; Ohnishi, K.; Kagawa, Y.; Kawakami, J. CYP3A5*3 Affects Plasma Disposition of Noroxycodone and Dose Escalation in Cancer Patients Receiving Oxycodone. J. Clin. Pharmacol. 2011, 51, 1529–1538. [Google Scholar] [CrossRef]
- Stamer, U.M.; Zhang, L.; Book, M.; Lehmann, L.E.; Stuber, F.; Musshoff, F. CYP2D6 Genotype Dependent Oxycodone Metabolism in Postoperative Patients. PLoS ONE 2013, 8, e60239. [Google Scholar] [CrossRef]
- Drewes, A.M.; Jensen, R.D.; Nielsen, L.M.; Droney, J.; Christrup, L.L.; Arendt-Nielsen, L.; Riley, J.; Dahan, A. Differences between opioids: Pharmacological, experimental, clinical and economical perspectives. Br. J. Clin. Pharmacol. 2013, 75, 60–78. [Google Scholar] [CrossRef]
- Babalonis, S.; Comer, S.D.; Jones, J.D.; Nuzzo, P.; Lofwall, M.R.; Manubay, J.; Hatton, K.W.; Whittington, R.A.; Walsh, S.L. Relative potency of intravenous oxymorphone compared to other µ opioid agonists in humans—Pilot study outcomes. Psychopharmacology 2021, 238, 2503–2514. [Google Scholar] [CrossRef]
- Kharasch, E.D.; Stubbert, K. Role of Cytochrome P4502B6 in Methadone Metabolism and Clearance. J. Clin. Pharmacol. 2013, 53, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Gabel, F.; Hovhannisyan, V.; Berkati, A.-K.; Goumon, Y. Morphine-3-Glucuronide, Physiology and Behavior. Front. Mol. Neurosci. 2022, 15, 882443. [Google Scholar] [CrossRef] [PubMed]
- Kramlinger, V.M.; Rojas, M.A.; Kanamori, T.; Guengerich, F.P. Cytochrome P450 3A Enzymes Catalyze the O6-Demethylation of Thebaine, a Key Step in Endogenous Mammalian Morphine Biosynthesis. J. Biol. Chem. 2015, 290, 20200–20210. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.J. The Clinical Implications of Cytochrome P450 Interactions with Opioids and Strategies for Pain Management. J. Pain Symptom Manag. 2012, 44, S15–S22. [Google Scholar] [CrossRef]
- Ing Lorenzini, K.; Daali, Y.; Dayer, P.; Desmeules, J. Pharmacokinetic-pharmacodynamic modelling of opioids in healthy human volunteers. a minireview. Basic Clin. Pharmacol. Toxicol. 2012, 110, 219–226. [Google Scholar] [CrossRef]
- Seleman, M.; Chapy, H.; Cisternino, S.; Courtin, C.; Smirnova, M.; Schlatter, J.; Chiadmi, F.; Scherrmann, J.-M.; Noble, F.; Marie-Claire, C. Impact of P-glycoprotein at the blood-brain barrier on the uptake of heroin and its main metabolites: Behavioral effects and consequences on the transcriptional responses and reinforcing properties. Psychopharmacology 2014, 231, 3139–3149. [Google Scholar] [CrossRef]
- Roeckel, L.-A.; Utard, V.; Reiss, D.; Mouheiche, J.; Maurin, H.; Robé, A.; Audouard, E.; Wood, J.N.; Goumon, Y.; Simonin, F.; et al. Morphine-induced hyperalgesia involves mu opioid receptors and the metabolite morphine-3-glucuronide. Sci. Rep. 2017, 7, 10406. [Google Scholar] [CrossRef]
- Peñas-Lledó, E.M.; Llerena, A. CYP2D6 variation, behaviour and psychopathology: Implications for pharmacogenomics-guided clinical trials. Br. J. Clin. Pharmacol. 2014, 77, 673–683. [Google Scholar] [CrossRef]
- Cojocaru, A.; Braha, A.; Jeleriu, R.; Andreescu, N.I.; Puiu, M.; Ageu, L.; Folescu, R.; Zamfir, C.L.; Nussbaum, L.A. The Implications of Cytochrome P450 2D6/CYP2D6 Polymorphism in the Therapeutic Response of Atypical Antipsychotics in Adolescents with Psychosis—A Prospective Study. Biomedicines 2024, 12, 494. [Google Scholar] [CrossRef]
- Doohan, P.T.; Oldfield, L.D.; Arnold, J.C.; Anderson, L.L. Cannabinoid Interactions with Cytochrome P450 Drug Metabolism: A Full-Spectrum Characterization. AAPS J. 2021, 23, 91. [Google Scholar] [CrossRef]
- Chayasirisobhon, S. Mechanisms of Action and Pharmacokinetics of Cannabis. Perm. J. 2021, 25, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kuzin, M.; Xepapadakos, F.; Scharrer, I.; Augsburger, M.; Eap, C.; Schoretsanitis, G. The role of cytochrome P450 enzyme genetic variants in cannabis hyperemesis syndrome—A case report. Basic Clin. Pharmacol. Toxicol. 2021, 129, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.T.; Gruber, S.A. Contemplating cannabis? The complex relationship between cannabinoids and hepatic metabolism resulting in the potential for drug-drug interactions. Front. Psychiatry 2023, 13, 1055481. [Google Scholar] [CrossRef] [PubMed]
- Yabut, K.C.B.; Winnie Wen, Y.; Simon, K.T.; Isoherranen, N. CYP2C9, CYP3A and CYP2C19 metabolize Δ9-tetrahydrocannabinol to multiple metabolites but metabolism is affected by human liver fatty acid binding protein (FABP1). Biochem. Pharmacol. 2024, 228, 116191. [Google Scholar] [CrossRef]
- Caicedo, D.A.; Pérez-Mañá, C.; Farré, M.; Papaseit, E. An Overview of the Potential for Pharmacokinetic Interactions Between Drugs and Cannabis Products in Humans. Pharmaceutics 2025, 17, 319. [Google Scholar] [CrossRef]
- Davis, C.N.; Markowitz, J.S.; Squeglia, L.M.; Ellingson, J.M.; McRae-Clark, A.L.; Gray, K.M.; Kretschmer, D.; Tomko, R.L. Evidence for sex differences in the impact of cytochrome P450 genotypes on early subjective effects of cannabis. Addict. Behav. 2024, 153, 107996. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- Vučković, S.; Srebro, D.; Vujović, K.S.; Vučetić, Č.; Prostran, M. Cannabinoids and Pain: New Insights From Old Molecules. Front. Pharmacol. 2018, 9, 1259. [Google Scholar] [CrossRef]
- Mun, C.J.; Nordeck, C.; Goodell, E.M.A.; Vandrey, R.; Zipunnikov, V.; Dunn, K.E.; Finan, P.H.; Thrul, J. Real-Time Monitoring of Cannabis and Prescription Opioid Co-Use Patterns, Analgesic Effectiveness, and the Opioid-Sparing Effect of Cannabis in Individuals with Chronic Pain. J. Pain 2022, 23, 1799–1810. [Google Scholar] [CrossRef]
- Herdegen, T.; Cascorbi, I. Drug Interactions of Tetrahydrocannabinol and Cannabidiol in Cannabinoid Drugs. Dtsch. Arztebl. Int. 2023, 120, 833–840. [Google Scholar] [CrossRef]
- Al-Khazaleh, A.K.; Zhou, X.; Bhuyan, D.J.; Münch, G.W.; Al-Dalabeeh, E.A.; Jaye, K.; Chang, D. The Neurotherapeutic Arsenal in Cannabis sativa: Insights into Anti-Neuroinflammatory and Neuroprotective Activity and Potential Entourage Effects. Molecules 2024, 29, 410. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.; Glaser, T.; Villegas, C.; Burgos, V.; Ulrich, H.; Paz, C. Therapeutic Effects of Cannabinoids and Their Applications in COVID-19 Treatment. Life 2022, 12, 2117. [Google Scholar] [CrossRef] [PubMed]
- Bietar, B.; Tanner, S.; Lehmann, C. Neuroprotection and Beyond: The Central Role of CB1 and CB2 Receptors in Stroke Recovery. Int. J. Mol. Sci. 2023, 24, 16728. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Carmona, N.E.; Lee, Y.L.; Ragguett, R.-M.; Pan, Z.; Rosenblat, J.D.; Subramaniapillai, M.; Shekotikhina, M.; Almatham, F.; Alageel, A.; et al. Drug-drug interactions as a result of co-administering Δ9 -THC and CBD with other psychotropic agents. Expert Opin. Drug Saf. 2018, 17, 51–54. [Google Scholar] [CrossRef]
- Wright, J.A.; Huang, L.; Katamesh, B.E.; Yadav, S.; Singla, A.; Vincent, A. Hypothesized pharmacogenomic and medication influences on tetrahydrocannabinol and cannabidiol metabolism in a cohort of unselected oral cannabis users. J. Cannabis Res. 2025, 7, 1. [Google Scholar] [CrossRef]
- Tanner, J.-A.; Tyndale, R.F. Variation in CYP2A6 Activity and Personalized Medicine. J. Pers. Med. 2017, 7, 18. [Google Scholar] [CrossRef]
- Gao, Y.; Miksys, S.; Palmour, R.; Tyndale, R. The Influence of Tobacco Smoke/Nicotine on CYP2A Expression in Human and African Green Monkey Lungs. Preprints 2020. Available online: https://www.authorea.com/users/335041/articles/460952-the-influence-of-tobacco-smoke-nicotine-on-cyp2a-expression-in-human-and-african-green-monkey-lungs?commit=5ef8fb5b9f5d8952f9ae2e6b2b19b650ea939f58 (accessed on 21 February 2025).
- Schaefer, K.R.; Avey, J.P.; Todd, M.R.; Beans, J.A.; Dillard, D.A.; Shireman, L.M.; Thornton, T.A.; Tyndale, R.F.; Thummel, K.E.; Robinson, R.F.; et al. Nicotine metabolism and its association with CYP2A6 genotype among Indigenous people in Alaska who smoke. Clin. Transl. Sci. 2021, 14, 2474–2486. [Google Scholar] [CrossRef]
- Tanner, J.-A.; Chenoweth, M.J.; Tyndale, R.F. Pharmacogenetics of nicotine and associated smoking behaviors. Curr. Top. Behav. Neurosci. 2015, 23, 37–86. [Google Scholar] [CrossRef]
- Tanner, J.-A.; Novalen, M.; Jatlow, P.; Huestis, M.A.; Murphy, S.E.; Kaprio, J.; Kankaanpää, A.; Galanti, L.; Stefan, C.; George, T.P.; et al. Nicotine metabolite ratio (3-hydroxycotinine/cotinine) in plasma and urine by different analytical methods and laboratories: Implications for clinical implementation. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2015, 24, 1239–1246. [Google Scholar] [CrossRef]
- Sofuoglu, M.; Herman, A.I.; Nadim, H.; Jatlow, P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology 2012, 37, 1509–1516. [Google Scholar] [CrossRef]
- Weng, M.-T.; Ratsch, A.; Miles, J.A.; Zheng, Q.; Steadman, K.J. The impact of rate of nicotine metabolism, as measured by the nicotine metabolite ratio (NMR), on smoking behaviours during pregnancy: A scoping review. Health Sci. Rev. 2024, 12, 100186. [Google Scholar] [CrossRef]
- Pan, L.; Yang, X.; Li, S.; Jia, C. Association of CYP2A6 gene polymorphisms with cigarette consumption: A meta-analysis. Drug Alcohol Depend. 2015, 149, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Shahab, L.; Bauld, L.; McNeill, A.; Tyndale, R.F. Does the nicotine metabolite ratio moderate smoking cessation treatment outcomes in real-world settings? A prospective study. Addiction 2019, 114, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, P.R.X.; Gonçalves, T.S.; Santos, J.R.; Scholz, J.; Abe, T.O.; Gaya, P.V.; Figueiredo, E.C.; De Faria, H.D.; Martins, I.; Pego, A.M.F.; et al. Evaluation of the nicotine metabolite ratio in smoking patients treated with varenicline and bupropion. Front. Pharmacol. 2022, 13, 900112. [Google Scholar] [CrossRef]
- Ou, W.-C.; Huang, Y.-C.; Huang, C.-L.; Lin, M.-H.; Chen, Y.-C.; Chen, Y.-J.; Liu, C.-N.; Chen, M.-C.; Huang, C.-S.; Chen, P.-L. Interaction between cytochrome P450 2A6 and Catechol-O-Methyltransferase genes and their association with smoking risk in young men. Behav. Brain Funct. BBF 2017, 13, 8. [Google Scholar] [CrossRef]
- Garcia, K.L.P.; Lê, A.D.; Tyndale, R.F. Brain CYP2B induction can decrease nicotine levels in the brain. Addict. Biol. 2017, 22, 1257–1266. [Google Scholar] [CrossRef]
- Garcia, K.L.P.; Coen, K.; Miksys, S.; Lê, A.D.; Tyndale, R.F. Effect of Brain CYP2B Inhibition on Brain Nicotine Levels and Nicotine Self-Administration. Neuropsychopharmacology 2015, 40, 1910–1918. [Google Scholar] [CrossRef]
- Tan, X.; Vrana, K.; Ding, Z.-M. Cotinine: Pharmacologically Active Metabolite of Nicotine and Neural Mechanisms for Its Actions. Front. Behav. Neurosci. 2021, 15, 758252. [Google Scholar] [CrossRef]
- Li, Y.; Mao, J.; Chai, G.; Zheng, R.; Liu, X.; Xie, J. Neurobiological mechanisms of nicotine’s effects on feeding and body weight. Neurosci. Biobehav. Rev. 2025, 169, 106021. [Google Scholar] [CrossRef]
- Perez-Paramo, Y.X.; Watson, C.J.W.; Chen, G.; Thomas, C.E.; Adams-Haduch, J.; Wang, R.; Khor, C.C.; Koh, W.-P.; Nelson, H.H.; Yuan, J.-M.; et al. Impact of Genetic Variants in the Nicotine Metabolism Pathway on Nicotine Metabolite Levels in Smokers. Cancer Epidemiol. Biomark. Prev. 2023, 32, 54–65. [Google Scholar] [CrossRef]
- Ohmoto, M.; Takahashi, T.; Kubota, Y.; Kobayashi, S.; Mitsumoto, Y. Genetic influence of dopamine receptor, dopamine transporter, and nicotine metabolism on smoking cessation and nicotine dependence in a Japanese population. BMC Genet. 2014, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.D.L.; Barata-Silva, C.; Moreira, J.C.; Mitri, S. Genetic variability in the neurobiology of nicotine dependence: Effects on smoking behavior. Cad. Saúde Coletiva 2023, 31, e31010250. [Google Scholar] [CrossRef]
- Wassenaar, C.A.; Dong, Q.; Wei, Q.; Amos, C.I.; Spitz, M.R.; Tyndale, R.F. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J. Natl. Cancer Inst. 2011, 103, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.T.; Miksys, S.; Hoffmann, E.; Tyndale, R.F. Ethanol self-administration and nicotine treatment increase brain levels of CYP2D in African green monkeys. Br. J. Pharmacol. 2014, 171, 3077–3088. [Google Scholar] [CrossRef]
- Ferguson, C.S.; Miksys, S.; Palmour, R.M.; Tyndale, R.F. Ethanol self-administration and nicotine treatment induce brain levels of CYP2B6 and CYP2E1 in African green monkeys. Neuropharmacology 2013, 72, 74–81. [Google Scholar] [CrossRef]
- Na, S.; Li, J.; Zhang, H.; Li, Y.; Yang, Z.; Zhong, Y.; Dong, G.; Yang, J.; Yue, J. The induction of cytochrome P450 2E1 by ethanol leads to the loss of synaptic proteins via PPARα down-regulation. Toxicology 2017, 385, 18–27. [Google Scholar] [CrossRef]
- Stocco, M.R.; El-Sherbeni, A.A.; Zhao, B.; Novalen, M.; Tyndale, R.F. The role of CYP2D in rat brain in methamphetamine-induced striatal dopamine and serotonin release and behavioral sensitization. Psychopharmacology 2021, 238, 1791–1804. [Google Scholar] [CrossRef]
- Haufroid, V.; Hantson, P. CYP2D6 genetic polymorphisms and their relevance for poisoning due to amfetamines, opioid analgesics and antidepressants. Clin. Toxicol. 2015, 53, 501–510. [Google Scholar] [CrossRef]
- Mappin-Kasirer, B.; Pan, H.; Lewington, S.; Kizza, J.; Gray, R.; Clarke, R.; Peto, R. Tobacco smoking and the risk of Parkinson disease: A 65-year follow-up of 30,000 male British doctors. Neurology 2020, 94, E2132–E2138. [Google Scholar] [CrossRef]
- Lee, P.-C.; Ahmed, I.; Loriot, M.-A.; Mulot, C.; Paul, K.C.; Bronstein, J.M.; Ritz, B.; Elbaz, A. Smoking and Parkinson disease: Evidence for gene-by-smoking interactions. Neurology 2018, 90, e583–e592. [Google Scholar] [CrossRef]
- Quik, M.; Boyd, J.T.; Bordia, T.; Perez, X. Potential Therapeutic Application for Nicotinic Receptor Drugs in Movement Disorders. Nicotine Tob. Res. 2019, 21, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Oertel, W.H.; Müller, H.-H.; Unger, M.M.; Schade-Brittinger, C.; Balthasar, K.; Articus, K.; Brinkman, M.; Venuto, C.S.; Tracik, F.; Eberling, J.; et al. Transdermal Nicotine Treatment and Progression of Early Parkinson’s Disease. NEJM Evid. 2023, 2, EVIDoa2200311. [Google Scholar] [CrossRef]
- Villafane, G.; Thiriez, C.; Audureau, E.; Straczek, C.; Kerschen, P.; Cormier-Dequaire, F.; Van Der Gucht, A.; Gurruchaga, J.-M.; Quéré-Carne, M.; Evangelista, E.; et al. High-dose transdermal nicotine in Parkinson’s disease patients: A randomized, open-label, blinded-endpoint evaluation phase 2 study. Eur. J. Neurol. 2018, 25, 120–127. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, C.; Guo, T.; Huang, P.; Xu, X.; Zhang, M. Association between cigarette smoking and Parkinson’s disease: A neuroimaging study. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221092566. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Park, Y.H.; Lee, S.C.; Suh, J.H.; Yang, S.N.; Kang, D.R.; Kim, Y.W. Association between smoking and all-cause mortality in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 59. [Google Scholar] [CrossRef]
- Rose, K.N.; Schwarzschild, M.A.; Gomperts, S.N. Clearing the Smoke: What Protects Smokers from Parkinson’s Disease? Mov. Disord. 2024, 39, 267–272. [Google Scholar] [CrossRef]
- Haduch, A.; Danek, P.J.; Kuban, W.; Pukło, R.; Alenina, N.; Gołębiowska, J.; Popik, P.; Bader, M.; Daniel, W.A. Cytochrome P450 2D (CYP2D) enzyme dysfunction associated with aging and serotonin deficiency in the brain and liver of female Dark Agouti rats. Neurochem. Int. 2022, 152, 105223. [Google Scholar] [CrossRef]
- Pennazio, F.; Brasso, C.; Villari, V.; Rocca, P. Current Status of Therapeutic Drug Monitoring in Mental Health Treatment: A Review. Pharmaceutics 2022, 14, 2674. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, e1. [Google Scholar] [CrossRef]
- Luethi, D.; Hoener, M.C.; Krähenbühl, S.; Liechti, M.E.; Duthaler, U. Cytochrome P450 enzymes contribute to the metabolism of LSD to nor-LSD and 2-oxo-3-hydroxy-LSD: Implications for clinical LSD use. Biochem. Pharmacol. 2019, 164, 129–138. [Google Scholar] [CrossRef]
- Vizeli, P.; Straumann, I.; Holze, F.; Schmid, Y.; Dolder, P.C.; Liechti, M.E. Genetic influence of CYP2D6 on pharmacokinetics and acute subjective effects of LSD in a pooled analysis. Sci. Rep. 2021, 11, 10851. [Google Scholar] [CrossRef]
- McMillan, D.M.; Tyndale, R.F. Nicotine Increases Codeine Analgesia Through the Induction of Brain CYP2D and Central Activation of Codeine to Morphine. Neuropsychopharmacology 2015, 40, 1804–1812. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.Y.; Karm, M.-H.; Bae, H.-Y.; Lee, J.-Y.; Ahn, H.S.; Lee, K.; Leem, J.G. Smoking May Increase Postoperative Opioid Consumption in Patients Who Underwent Distal Gastrectomy with Gastroduodenostomy for Early Stomach Cancer. Clin. J. Pain 2017, 33, 905–911. [Google Scholar] [CrossRef]
- Miksys, S.; Wadji, F.B.; Tolledo, E.C.; Remington, G.; Nobrega, J.N.; Tyndale, R.F. Rat brain CYP2D enzymatic metabolism alters acute and chronic haloperidol side-effects by different mechanisms. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 78, 140–148. [Google Scholar] [CrossRef]
- Khokhar, J.Y.; Tyndale, R.F. Drug Metabolism within the Brain Changes Drug Response: Selective Manipulation of Brain CYP2B Alters Propofol Effects. Neuropsychopharmacology 2011, 36, 692–700. [Google Scholar] [CrossRef]
- Wang, Z.; Bao, Y.; Yan, S.; Lian, Z.; Jia, Z.; Liu, Z. An Investigation of Cigarettes Smoking Behavior and Nicotine Dependence among Chinese Methamphetamine Users in Two Provinces. BioMed Res. Int. 2014, 2014, 175205. [Google Scholar] [CrossRef]
- Jukic, M.M.; Smith, R.L.; Haslemo, T.; Molden, E.; Ingelman-Sundberg, M. Effect of CYP2D6 genotype on exposure and efficacy of risperidone and aripiprazole: A retrospective, cohort study. Lancet Psychiatry 2019, 6, 418–426. [Google Scholar] [CrossRef]
- Belmonte, C.; Ochoa, D.; Román, M.; Saiz-Rodríguez, M.; Wojnicz, A.; Gómez-Sánchez, C.I.; Martín-Vílchez, S.; Abad-Santos, F. Influence of CYP2D6, CYP3A4, CYP3A5 and ABCB1 Polymorphisms on Pharmacokinetics and Safety of Aripiprazole in Healthy Volunteers. Basic Clin. Pharmacol. Toxicol. 2018, 122, 596–605. [Google Scholar] [CrossRef]
- Dean, A.C.; Nurmi, E.L.; Morales, A.M.; Cho, A.K.; Seaman, L.C.; London, E.D. CYP2D6 genotype may moderate measures of brain structure in methamphetamine users. Addict. Biol. 2021, 26, e12950. [Google Scholar] [CrossRef]
- Morgan, E.T. Impact of Infectious and Inflammatory Disease on Cytochrome P450–Mediated Drug Metabolism and Pharmacokinetics. Clin. Pharmacol. Ther. 2009, 85, 434–438. [Google Scholar] [CrossRef]
- Mast, N.; Petrov, A.M.; Prendergast, E.; Bederman, I.; Pikuleva, I.A. Brain Acetyl-CoA Production and Phosphorylation of Cytoskeletal Proteins Are Targets of CYP46A1 Activity Modulation and Altered Sterol Flux. Neurotherapeutics 2021, 18, 2040–2060. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Dementia Forecasting Collaborators Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [CrossRef] [PubMed]
- Li, X.; Feng, X.; Sun, X.; Hou, N.; Han, F.; Liu, Y. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2019. Front. Aging Neurosci. 2022, 14, 937486. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Seeher, K.M.; Schiess, N.; Nichols, E.; Cao, B.; Servili, C.; Cavallera, V.; Cousin, E.; Hagins, H.; Moberg, M.E.; et al. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; Wilson, R.S.; Evans, D.A. Prevalence and incidence of clinically diagnosed Alzheimer’s disease dementia from 1994 to 2012 in a population study. Alzheimers Dement. J. Alzheimers Assoc. 2019, 15, 1–7. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Vishweswaraiah, S.; Turkoglu, O.; Graham, S.F.; Radhakrishna, U. Alzheimer’s Precision Neurology: Epigenetics of Cytochrome P450 Genes in Circulating Cell-Free DNA for Disease Prediction and Mechanism. Int. J. Mol. Sci. 2023, 24, 2876. [Google Scholar] [CrossRef]
- Jia, F.; Liu, Z.; Song, N.; Du, X.; Xie, J.; Jiang, H. The association between CYP46A1 rs4900442 polymorphism and the risk of Alzheimer’s disease: A meta-analysis. Neurosci. Lett. 2016, 620, 83–87. [Google Scholar] [CrossRef]
- Mast, N.; Verwilst, P.; Wilkey, C.J.; Guengerich, F.P.; Pikuleva, I.A. In Vitro Activation of Cytochrome P450 46A1 (CYP46A1) by Efavirenz-Related Compounds. J. Med. Chem. 2020, 63, 6477–6488. [Google Scholar] [CrossRef]
- Lerner, A.J.; Arnold, S.E.; Maxfield, E.; Koenig, A.; Toth, M.E.; Fortin, B.; Mast, N.; Trombetta, B.A.; Denker, J.; Pieper, A.A.; et al. CYP46A1 activation by low-dose efavirenz enhances brain cholesterol metabolism in subjects with early Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 198. [Google Scholar] [CrossRef]
- Anderson, K.W.; Mast, N.; Hudgens, J.W.; Lin, J.B.; Turko, I.V.; Pikuleva, I.A. Mapping of the Allosteric Site in Cholesterol Hydroxylase CYP46A1 for Efavirenz, a Drug That Stimulates Enzyme Activity. J. Biol. Chem. 2016, 291, 11876–11886. [Google Scholar] [CrossRef]
- Petrov, A.M.; Mast, N.; Li, Y.; Pikuleva, I.A. The key genes, phosphoproteins, processes, and pathways affected by efavirenz-activated CYP46A1 in the amyloid-decreasing paradigm of efavirenz treatment. FASEB J. 2019, 33, 8782–8798. [Google Scholar] [CrossRef]
- Petrov, A.M.; Lam, M.; Mast, N.; Moon, J.; Li, Y.; Maxfield, E.; Pikuleva, I.A. CYP46A1 Activation by Efavirenz Leads to Behavioral Improvement without Significant Changes in Amyloid Plaque Load in the Brain of 5XFAD Mice. Neurother. J. Am. Soc. Exp. Neurother. 2019, 16, 710–724. [Google Scholar] [CrossRef] [PubMed]
- van der Kant, R.; Langness, V.F.; Herrera, C.M.; Williams, D.A.; Fong, L.K.; Leestemaker, Y.; Steenvoorden, E.; Rynearson, K.D.; Brouwers, J.F.; Helms, J.B.; et al. Cholesterol Metabolism Is a Druggable Axis that Independently Regulates Tau and Amyloid-β in iPSC-Derived Alzheimer’s Disease Neurons. Cell Stem Cell 2019, 24, 363–375.e9. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Yoshikawa, M.; Ando, H.K.; Farnaby, W.; Nishi, T.; Watanabe, E.; Yano, J.; Miyamoto, M.; Kondo, S.; Ishii, T.; et al. Discovery of Soticlestat, a Potent and Selective Inhibitor for Cholesterol 24-Hydroxylase (CH24H). J. Med. Chem. 2021, 64, 12228–12244. [Google Scholar] [CrossRef]
- Latorre-Leal, M.; Rodriguez-Rodriguez, P.; Franchini, L.; Nikolidakis, O.; Daniilidou, M.; Delac, L.; Varshney, M.K.; Arroyo-García, L.E.; Eroli, F.; Winblad, B.; et al. CYP46A1-mediated cholesterol turnover induces sex-specific changes in cognition and counteracts memory loss in ovariectomized mice. Sci. Adv. 2024, 10, eadj1354. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Narayanan, J.; Harder, D.R. Differential effect of amyloid beta on the cytochrome P450 epoxygenase activity in rat brain. Neuroscience 2011, 194, 241–249. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, S.; Sanders, A.R.; Duan, J. Brain Lipids and Lipid Droplet Dysregulation in Alzheimer’s Disease and Neuropsychiatric Disorders. Complex Psychiatry 2023, 9, 154–171. [Google Scholar] [CrossRef]
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived with Disability of Parkinson’s Disease in 204 Countries/Territories From 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- GBD 2016 Neurology Collaborators Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [CrossRef]
- Baltazar, M.T.; Dinis-Oliveira, R.J.; Bastos, M. de L.; Tsatsakis, A.M.; Duarte, J.A.; Carvalho, F. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases—A mechanistic approach. Toxicol. Lett. 2014, 230, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.J.; Hoek, S.; Fon, E.A.; Wade-Martins, R. Mitochondrial dysfunction and mitophagy in Parkinson’s: From familial to sporadic disease. Trends Biochem. Sci. 2015, 40, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Trinh, J.; Farrer, M. Advances in the genetics of Parkinson disease. Nat. Rev. Neurol. 2013, 9, 445–454. [Google Scholar] [CrossRef]
- Anwarullah; Aslam, M.; Badshah, M.; Abbasi, R.; Sultan, A.; Khan, K.; Ahmad, N.; von Engelhardt, J. Further evidence for the association of CYP2D6*4 gene polymorphism with Parkinson’s disease: A case control study. Genes Environ. 2017, 39, 18. [Google Scholar] [CrossRef]
- Lu, Y.; Peng, Q.; Zeng, Z.; Wang, J.; Deng, Y.; Xie, L.; Mo, C.; Zeng, J.; Qin, X.; Li, S. CYP2D6 phenotypes and Parkinson’s disease risk: A meta-analysis. J. Neurol. Sci. 2014, 336, 161–168. [Google Scholar] [CrossRef]
- Srivastava, G.; Dixit, A.; Yadav, S.; Patel, D.K.; Prakash, O.; Singh, M.P. Resveratrol potentiates cytochrome P450 2d22-mediated neuroprotection in maneb- and paraquat-induced parkinsonism in the mouse. Free Radic. Biol. Med. 2012, 52, 1294–1306. [Google Scholar] [CrossRef]
- Fernandez-Abascal, J.; Ripullone, M.; Valeri, A.; Leone, C.; Valoti, M. β-Naphtoflavone and Ethanol Induce Cytochrome P450 and Protect towards MPP+ Toxicity in Human Neuroblastoma SH-SY5Y Cells. Int. J. Mol. Sci. 2018, 19, 3369. [Google Scholar] [CrossRef]
- Mann, A.; Tyndale, R.F. Cytochrome P450 2D6 enzyme neuroprotects against 1-methyl-4-phenylpyridinium toxicity in SH-SY5Y neuronal cells. Eur. J. Neurosci. 2010, 31, 1185–1193. [Google Scholar] [CrossRef]
- Stocco, M.R.; Tolledo, C.; Wadji, F.B.; Gonzalez, F.J.; Miksys, S.; Tyndale, R.F. Human CYP2D6 in the Brain Is Protective Against Harmine-Induced Neurotoxicity: Evidence from Humanized CYP2D6 Transgenic Mice. Mol. Neurobiol. 2020, 57, 4608–4621. [Google Scholar] [CrossRef]
- Christensen, C.; Þorsteinsson, H.; Maier, V.H.; Karlsson, K.Æ. Multi-parameter Behavioral Phenotyping of the MPP+ Model of Parkinson’s Disease in Zebrafish. Front. Behav. Neurosci. 2020, 14, 623924. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.; Chowdhury, A.R.; Feng, T.; Assenmacher, C.A.; Radaelli, E.; Guengerich, F.P.; Avadhani, N.G. Mitochondrially targeted cytochrome P450 2D6 is involved in monomethylamine-induced neuronal damage in mouse models. J. Biol. Chem. 2019, 294, 10336–10348. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, H.N.; Andersson, D.R.; Nissbrandt, H. Cytochrome P450 2E1 in the substantia nigra: Relevance for dopaminergic neurotransmission and free radical production. Synapse 2008, 62, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Vaglini, F.; Viaggi, C.; Piro, V.; Pardini, C.; Gerace, C.; Scarselli, M.; Corsini, G.U. Acetaldehyde and parkinsonism: Role of CYP450 2E1. Front. Behav. Neurosci. 2013, 7, 71. [Google Scholar] [CrossRef]
- Moyano, P.; Ruiz, M.; García, J.M.; Frejo, M.T.; Baselga, M.J.A.; Lobo, M.; García, J.; Pino, J.D. Oxidative stress and cell death induction by amitraz and its metabolite BTS-27271 mediated through cytochrome P450 and NRF2 pathway alteration in primary hippocampal cell. Food Chem. Toxicol. 2019, 129, 87–96. [Google Scholar] [CrossRef]
- Kaut, O.; Schmitt, I.; Wüllner, U. Genome-scale methylation analysis of Parkinson’s disease patients’ brains reveals DNA hypomethylation and increased mRNA expression of cytochrome P450 2E1. Neurogenetics 2012, 13, 87–91. [Google Scholar] [CrossRef]
- Popat, R.A.; Eeden, S.K.V.D.; Tanner, C.M.; Kamel, F.; Umbach, D.M.; Marder, K.; Mayeux, R.; Ritz, B.; Ross, G.W.; Petrovitch, H.; et al. Coffee, ADORA2A, and CYP1A2: The caffeine connection in Parkinson’s disease. Eur. J. Neurol. 2011, 18, 756–765. [Google Scholar] [CrossRef]
- Sbrini, G.; Mutti, V.; Bono, F.; Tomasoni, Z.; Fadel, D.; Missale, C.; Fiorentini, C. 17-β-estradiol potentiates the neurotrophic and neuroprotective effects mediated by the dopamine D3/acetylcholine nicotinic receptor heteromer in dopaminergic neurons. Eur. J. Pharmacol. 2024, 976, 176678. [Google Scholar] [CrossRef]
- Domenighetti, C.; Sugier, P.-E.; Sreelatha, A.A.K.; Schulte, C.; Grover, S.; Mohamed, O.; Portugal, B.; May, P.; Bobbili, D.R.; Radivojkov-Blagojevic, M.; et al. Mendelian Randomisation Study of Smoking, Alcohol, and Coffee Drinking in Relation to Parkinson’s Disease. J. Park. Dis. 2022, 12, 267–282. [Google Scholar] [CrossRef]
- Gallo, V.; Vineis, P.; Cancellieri, M.; Chiodini, P.; Barker, R.A.; Brayne, C.; Pearce, N.; Vermeulen, R.; Panico, S.; Bueno-de-Mesquita, B.; et al. Exploring causality of the association between smoking and Parkinson’s disease. Int. J. Epidemiol. 2019, 48, 912–925. [Google Scholar] [CrossRef]
- Kacher, R.; Lamazière, A.; Heck, N.; Kappes, V.; Mounier, C.; Despres, G.; Dembitskaya, Y.; Perrin, E.; Christaller, W.; Sasidharan Nair, S.; et al. CYP46A1 gene therapy deciphers the role of brain cholesterol metabolism in Huntington’s disease. Brain J. Neurol. 2019, 142, 2432–2450. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Mahjoub, Y.; Shaver, L.; Pringsheim, T. Prevalence and Incidence of Huntington’s Disease: An Updated Systematic Review and Meta-Analysis. Mov. Disord. 2022, 37, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Exuzides, A.; Reddy, S.R.; Chang, E.; Ta, J.T.; Patel, A.M.; Paydar, C.; Yohrling, G.J. Epidemiology of Huntington’s Disease in the United States Medicare and Medicaid Populations. Neuroepidemiology 2022, 56, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kreilaus, F.; Spiro, A.S.; McLean, C.A.; Garner, B.; Jenner, A.M. Evidence for altered cholesterol metabolism in Huntington’s disease post mortem brain tissue. Neuropathol. Appl. Neurobiol. 2016, 42, 535–546. [Google Scholar] [CrossRef]
- Birolini, G.; Valenza, M.; Ottonelli, I.; Talpo, F.; Minoli, L.; Cappelleri, A.; Bombaci, M.; Caccia, C.; Canevari, C.; Trucco, A.; et al. Chronic cholesterol administration to the brain supports complete and long-lasting cognitive and motor amelioration in Huntington’s disease. Pharmacol. Res. 2023, 194, 106823. [Google Scholar] [CrossRef]
- Valenza, M.; Marullo, M.; Di Paolo, E.; Cesana, E.; Zuccato, C.; Biella, G.; Cattaneo, E. Disruption of astrocyte-neuron cholesterol cross talk affects neuronal function in Huntington’s disease. Cell Death Differ. 2015, 22, 690–702. [Google Scholar] [CrossRef]
- Benraiss, A.; Mariani, J.N.; Osipovitch, M.; Cornwell, A.; Windrem, M.S.; Villanueva, C.B.; Chandler-Militello, D.; Goldman, S.A. Cell-intrinsic glial pathology is conserved across human and murine models of Huntington’s disease. Cell Rep. 2021, 36, 109308. [Google Scholar] [CrossRef]
- Maioli, S.; Båvner, A.; Ali, Z.; Heverin, M.; Ismail, M.-A.-M.; Puerta, E.; Olin, M.; Saeed, A.; Shafaati, M.; Parini, P.; et al. Is it possible to improve memory function by upregulation of the cholesterol 24S-hydroxylase (CYP46A1) in the brain? PLoS ONE 2013, 8, e68534. [Google Scholar] [CrossRef]
- Moutinho, M.; Nunes, M.J.; Correia, J.C.; Gama, M.J.; Castro-Caldas, M.; Cedazo-Minguez, A.; Rodrigues, C.M.P.; Björkhem, I.; Ruas, J.L.; Rodrigues, E. Neuronal cholesterol metabolism increases dendritic outgrowth and synaptic markers via a concerted action of GGTase-I and Trk. Sci. Rep. 2016, 6, 30928. [Google Scholar] [CrossRef]
- Piguet, F.; de Saint Denis, T.; Audouard, E.; Beccaria, K.; André, A.; Wurtz, G.; Schatz, R.; Alves, S.; Sevin, C.; Zerah, M.; et al. The Challenge of Gene Therapy for Neurological Diseases: Strategies and Tools to Achieve Efficient Delivery to the Central Nervous System. Hum. Gene Ther. 2021, 32, 349–374. [Google Scholar] [CrossRef]
- Danek, P.J.; Daniel, W.A. The Atypical Antipsychotic Lurasidone Affects Brain but Not Liver Cytochrome P450 2D (CYP2D) Activity. A Comparison with Other Novel Neuroleptics and Significance for Drug Treatment of Schizophrenia. Cells 2022, 11, 3513. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Bernini de Brito, R.; Ghedini, P.C. CYP2C19 polymorphisms and outcomes of Escitalopram treatment in Brazilians with major depression. Heliyon 2020, 6, e04015. [Google Scholar] [CrossRef]
- Zastrozhin, M.S.; Skryabin, V.; Rwere, F.; Petukhov, A.E.; Pankratenko, E.P.; Pozdniakov, S.A.; Ivanchenko, V.A.; Noskov, V.V.; Zaytsev, I.A.; Vinokurova, N.V.; et al. Influence of CYP2C19*17 Genetic Polymorphism on the Steady-State Concentration of Escitalopram in Patients with Recurrent Depressive Disorder. Psychopharmacol. Bull. 2022, 52, 8–19. [Google Scholar]
- Świechowski, R.; Jeleń, A.; Pietrzak, J.; Gałecki, P.; Szmajda-Krygier, D.; Balcerczak, E. The influence of CYP2C19*2 and CYP3A5*3 variants on the development of depression and effectiveness of therapy: A preliminary study. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 142, 112055. [Google Scholar] [CrossRef]
- Zemanova, N.; Anzenbacher, P.; Anzenbacherova, E. The role of cytochromes P450 in the metabolism of selected antidepressants and anxiolytics under psychological stress. Biomed. Pap. 2022, 166, 140–149. [Google Scholar] [CrossRef]
- Buathong, N.; Kalayasiri, R.; Chaliyavilaskul, P.; Phetnoi, K.; Ratananupong, T. Association of cytochrome P450 2A6 polymorphism, anxiety, and environmental factors with cigarette smoking by Thai adults. Asian Biomed. 2017, 10, 147–154. [Google Scholar] [CrossRef]
- Grayaa, S.; Zerbinati, C.; Messedi, M.; HadjKacem, I.; Chtourou, M.; Ben Touhemi, D.; Naifar, M.; Ayadi, H.; Ayedi, F.; Iuliano, L. Plasma oxysterol profiling in children reveals 24-hydroxycholesterol as a potential marker for Autism Spectrum Disorders. Biochimie 2018, 153, 80–85. [Google Scholar] [CrossRef]
- Nóbrega, C.; Mendonça, L.; Marcelo, A.; Lamazière, A.; Tomé, S.; Despres, G.; Matos, C.A.; Mechmet, F.; Langui, D.; den Dunnen, W.; et al. Restoring brain cholesterol turnover improves autophagy and has therapeutic potential in mouse models of spinocerebellar ataxia. Acta Neuropathol. 2019, 138, 837–858. [Google Scholar] [CrossRef]
- Odnoshivkina, U.G.; Kuznetsova, E.A.; Petrov, A.M. 25-Hydroxycholesterol as a Signaling Molecule of the Nervous System. Biochem. Biokhimiia 2022, 87, 524–537. [Google Scholar] [CrossRef]
- Chen, Z.; Ge, R.; Wang, C.; Elazab, A.; Fu, X.; Min, W.; Qin, F.; Jia, G.; Fan, X. Identification of important gene signatures in schizophrenia through feature fusion and genetic algorithm. Mamm. Genome 2024, 35, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.M.; Galea, S. Key considerations for the future of mental health epidemiology. Am. J. Epidemiol. 2024, 193, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Seitidis, G.; Mavridis, D.; Correll, C.U.; Dragioti, E.; Guimond, S.; Tuominen, L.; Dargél, A.; Carvalho, A.F.; Fornaro, M.; et al. Incidence, prevalence, and global burden of schizophrenia—Data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol. Psychiatry 2023, 28, 5319–5327. [Google Scholar] [CrossRef]
- Pardiñas, A.F.; Holmans, P.; Pocklington, A.J.; Escott-Price, V.; Ripke, S.; Carrera, N.; Legge, S.E.; Bishop, S.; Cameron, D.; Hamshere, M.L.; et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat. Genet. 2018, 50, 381–389. [Google Scholar] [CrossRef]
- Ehrenberg, A.J.; Khatun, A.; Coomans, E.; Betts, M.J.; Capraro, F.; Thijssen, E.H.; Senkevich, K.; Bharucha, T.; Jafarpour, M.; Young, P.N.E.; et al. Relevance of biomarkers across different neurodegenerative diseases. Alzheimers Res. Ther. 2020, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, K.; Pedersen, T.L.; Seyfried, N.T.; Lah, J.J.; Levey, A.I.; Hales, C.M.; Dammer, E.B.; Blach, C.; Louie, G.; Kaddurah-Daouk, R.; et al. Association of plasma and CSF cytochrome P450, soluble epoxide hydrolase, and ethanolamide metabolism with Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 149. [Google Scholar] [CrossRef]
- Leoni, V.; Caccia, C. Potential diagnostic applications of side chain oxysterols analysis in plasma and cerebrospinal fluid. Biochem. Pharmacol. 2013, 86, 26–36. [Google Scholar] [CrossRef]
- Ogura, H.; Hatip-Al-Khatib, I.; Suenaga, M.; Hatip, F.B.; Mishima, T.; Fujioka, S.; Ouma, S.; Matsunaga, Y.; Tsuboi, Y. Circulatory 25(OH)D and 1,25(OH)2D as differential biomarkers between multiple system atrophy and Parkinson’s disease patients. eNeurologicalSci 2021, 25, 100369. [Google Scholar] [CrossRef]
- Kakimoto, A.; Ogura, H.; Suenaga, M.; Mishima, T.; Fujioka, S.; Ouma, S.; Matsunaga, Y.; Tsuboi, Y. Role of cytochrome P450 for vitamin D metabolisms in patients with neurodegenerative disorders. Clin. Park. Relat. Disord. 2022, 7, 100162. [Google Scholar] [CrossRef]
- Ouma, S.; Suenaga, M.; Bölükbaşı Hatip, F.F.; Hatip-Al-Khatib, I.; Tsuboi, Y.; Matsunaga, Y. Serum vitamin D in patients with mild cognitive impairment and Alzheimer’s disease. Brain Behav. 2018, 8, e00936. [Google Scholar] [CrossRef]
- de Jong, L.M.; Jiskoot, W.; Swen, J.J.; Manson, M.L. Distinct Effects of Inflammation on Cytochrome P450 Regulation and Drug Metabolism: Lessons from Experimental Models and a Potential Role for Pharmacogenetics. Genes 2020, 11, 1509. [Google Scholar] [CrossRef] [PubMed]
- Ihara, K.; Oguro, A.; Imaishi, H. Diagnosis of Parkinson’s disease by investigating the inhibitory effect of serum components on P450 inhibition assay. Sci. Rep. 2022, 12, 6622. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B. Artificial Intelligence as a Tool for the Diagnosis and Treatment of Neurodegenerative Diseases. Brain Sci. 2023, 13, 938. [Google Scholar] [CrossRef]
- Feng, T. Applications of Artificial Intelligence to Diagnosis of Neurodegenerative Diseases. In Studies in Health Technology and Informatics; Yu, Y., Nguyen, B.P., Sang, J., Eds.; IOS Press: Amsterdam, The Netherlands, 2023; Available online: https://ebooks.iospress.nl/doi/10.3233/SHTI230896 (accessed on 17 February 2025).
- Tăuţan, A.-M.; Ionescu, B.; Santarnecchi, E. Artificial intelligence in neurodegenerative diseases: A review of available tools with a focus on machine learning techniques. Artif. Intell. Med. 2021, 117, 102081. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Hartz, P.; Fehlmann, T.; Wagenpfeil, G.; Unger, M.M.; Bernhardt, R. A CYPome-wide study reveals new potential players in the pathogenesis of Parkinson’s disease. Front. Pharmacol. 2023, 13, 1094265. [Google Scholar] [CrossRef]
- Chen, Z.-D.; Zhao, L.; Chen, H.-Y.; Gong, J.-N.; Chen, X.; Chen, C.Y.-C. A novel artificial intelligence protocol to investigate potential leads for Parkinson’s disease. RSC Adv. 2020, 10, 22939–22958. [Google Scholar] [CrossRef]
- Gong, C.; Feng, Y.; Zhu, J.; Liu, G.; Tang, Y.; Li, W. Evaluation of machine learning models for cytochrome P450 3A4, 2D6, and 2C9 inhibition. J. Appl. Toxicol. 2024, 44, 1050–1066. [Google Scholar] [CrossRef]
- Adebesin, A.M.; Roman, R.J.; Campbell, W.B.; Seubert, J.M.; Totah, R.A. Editorial: Cytochromes P450, their modulators and metabolites in cardiovascular function and disease. Front. Pharmacol. 2024, 15, 1531166. [Google Scholar] [CrossRef]
- Jiang, S.; Han, S.; Wang, D.W. The involvement of soluble epoxide hydrolase in the development of cardiovascular diseases through epoxyeicosatrienoic acids. Front. Pharmacol. 2024, 15, 1358256. [Google Scholar] [CrossRef]
- Imig, J.D. Epoxyeicosanoids in hypertension. Physiol. Res. 2019, 68, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Yang, X.; Li, J.; Dong, W.; Zhang, X.; Liu, N.; Gao, S.; Wang, J.; Zhang, L.; Lu, D. New Molecular Mechanism Underlying Myc-Mediated Cytochrome P450 2E1 Upregulation in Apoptosis and Energy Metabolism in the Myocardium. J. Am. Heart Assoc. 2019, 8, e009871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ran, Q.; Yin, K.; Wang, Y.; Liu, J.; Zong, Y.; Wang, Y.; Cao, Y. The effects of CYP2C19 genotype polymorphism and clopidogrel resistance on ischemic event occurrence in patients with peripheral arterial disease undergoing revascularization: A prospective cohort study. Thromb. Res. 2024, 236, 37–50. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zhan, M.; Liu, Y.; Li, Z.; Li, J.; Cheng, G.; Teng, G.; Lu, L. Astrocytic cytochrome P450 4A/20-hydroxyeicosatetraenoic acid contributes to angiogenesis in the experimental ischemic stroke. Brain Res. 2019, 1708, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Davis, C.M.; Alkayed, N.J. P450 Eicosanoids and Reactive Oxygen Species Interplay in Brain Injury and Neuroprotection. Antioxid. Redox Signal. 2018, 28, 987–1007. [Google Scholar] [CrossRef]

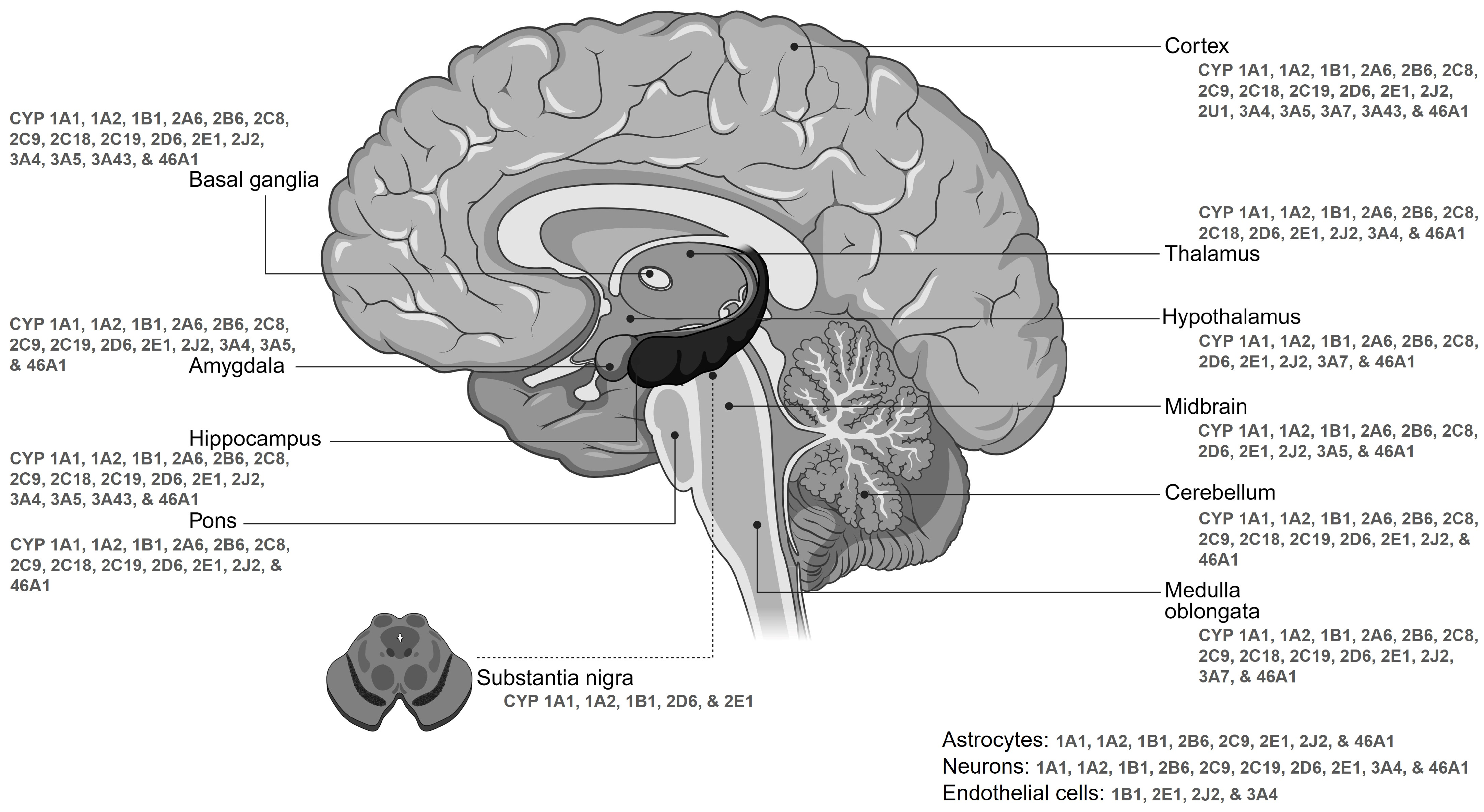
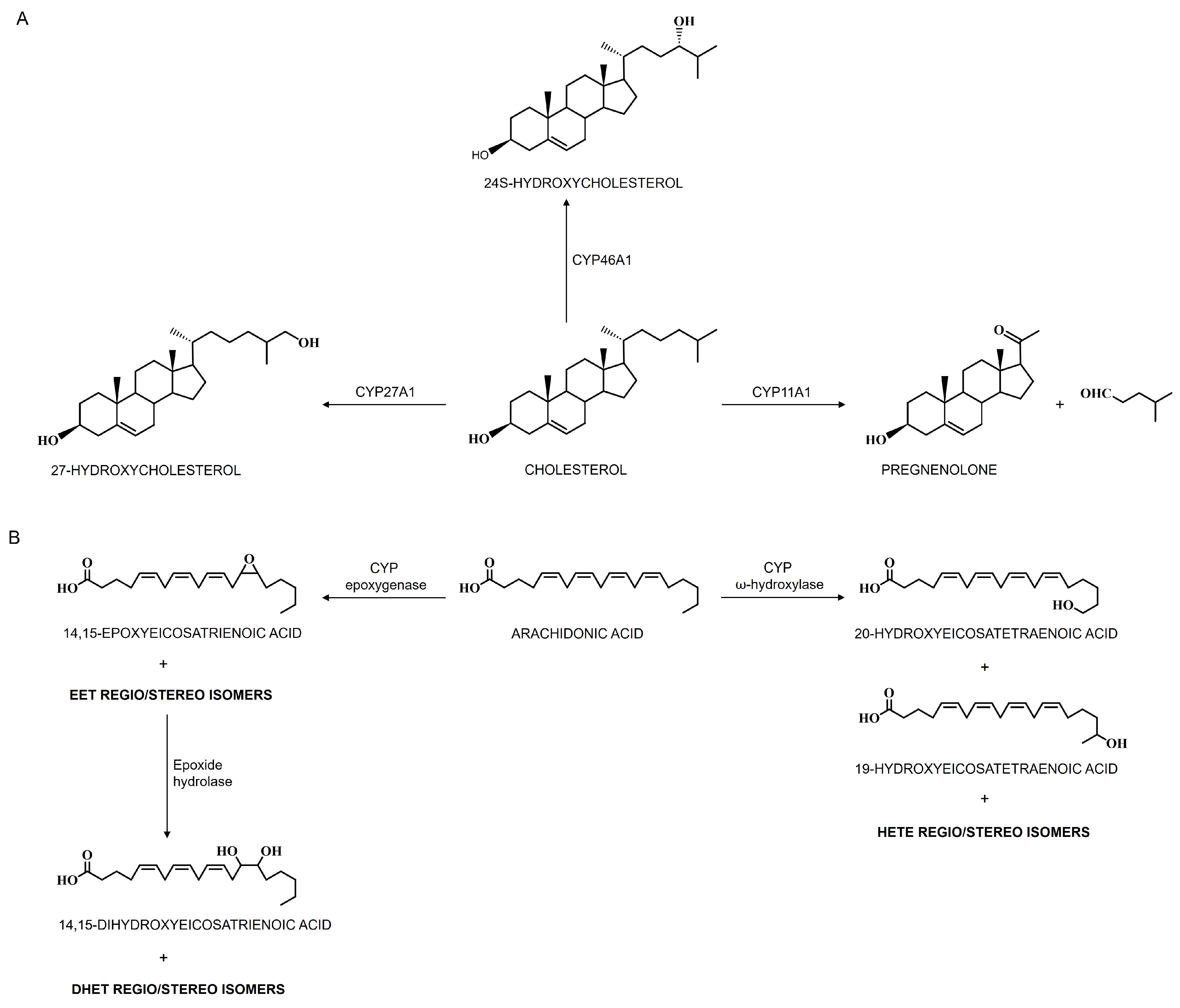
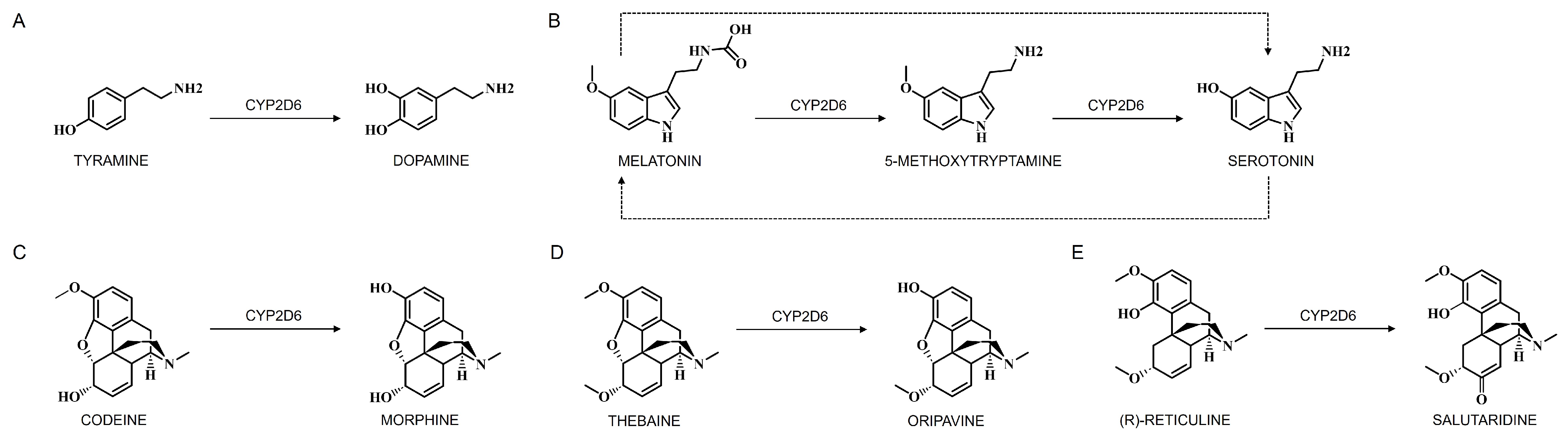

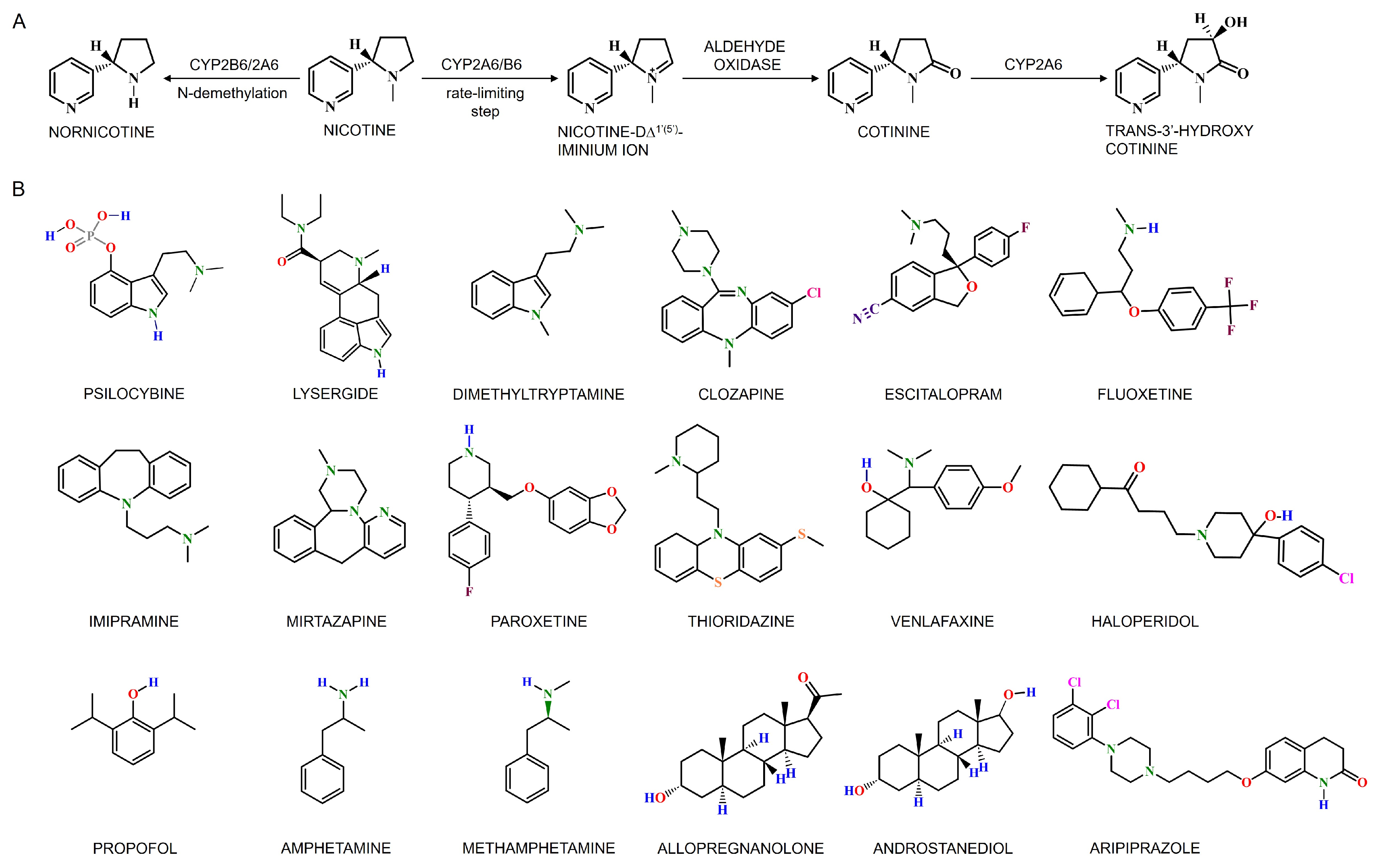
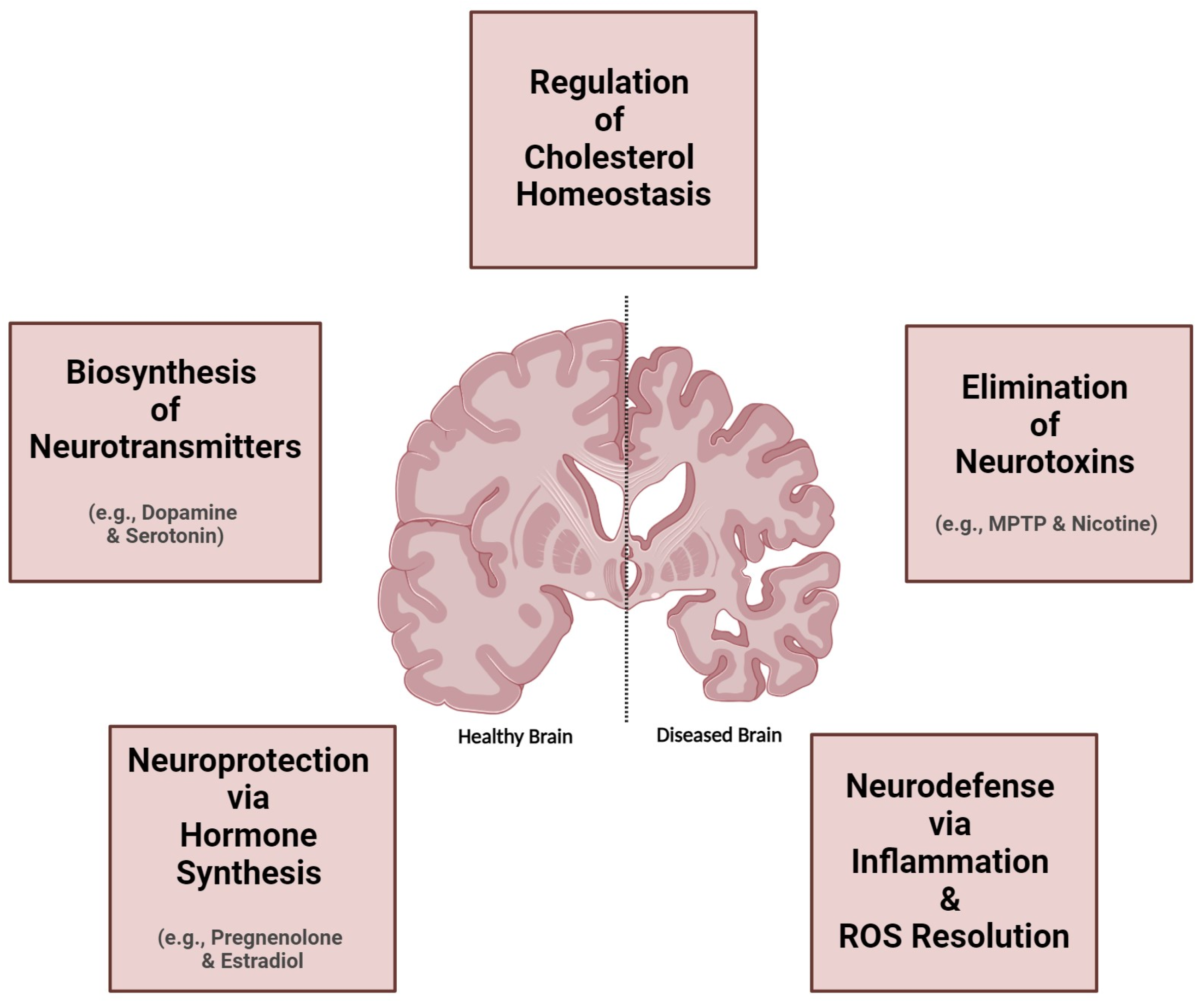
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durairaj, P.; Liu, Z.L. Brain Cytochrome P450: Navigating Neurological Health and Metabolic Regulation. J. Xenobiot. 2025, 15, 44. https://doi.org/10.3390/jox15020044
Durairaj P, Liu ZL. Brain Cytochrome P450: Navigating Neurological Health and Metabolic Regulation. Journal of Xenobiotics. 2025; 15(2):44. https://doi.org/10.3390/jox15020044
Chicago/Turabian StyleDurairaj, Pradeepraj, and Zixiang Leonardo Liu. 2025. "Brain Cytochrome P450: Navigating Neurological Health and Metabolic Regulation" Journal of Xenobiotics 15, no. 2: 44. https://doi.org/10.3390/jox15020044
APA StyleDurairaj, P., & Liu, Z. L. (2025). Brain Cytochrome P450: Navigating Neurological Health and Metabolic Regulation. Journal of Xenobiotics, 15(2), 44. https://doi.org/10.3390/jox15020044








