Abstract
Sickle cell disease (SCD) is a complex genetic disorder associated with multiple clinical manifestations, including increased susceptibility to bacterial and viral infections. This review article presents a comprehensive analysis of the current literature obtained from various online databases focusing on the relationship between SCD and infections caused by specific pathogens, such as pneumonia- and influenza-causing pathogens, Escherichia coli, Staphylococcus aureus, parvovirus, and hepatitis viruses. We discuss the underlying mechanisms that contribute to the increased susceptibility of individuals with SCD to these infections, primarily related to the pathophysiology of variant hemoglobin (HbSS) and its impact on vascular occlusion, hemolysis, functional asplenia, and immune deficiency. Moreover, we highlight the significant burden of infections on SCD patients, particularly children under five years of age, where they are the leading cause of morbidity and mortality. Additionally, we address the challenges faced in attempts for reducing the global mortality rate associated with SCD, particularly in low-income countries, where factors such as increased pathogen exposure, co-morbidities like malnutrition, lower vaccination rates, and limited healthcare facilities contribute to the high disease burden. This review emphasizes the need for targeted interventions, improved healthcare access, vaccination programs, and infection prevention strategies to alleviate the impact of infections on individuals with SCD and reduce the global mortality rates associated with the disease.
1. Introduction
Sickle cell disease (SCD) is caused by a single amino acid substitution (Glu > Val) at codon six at the globin gene, which results in variant hemoglobin formation (HbSS). This causes an increase in viscosity and adhesion to vascular walls, resulting in obstructing the blood flow in small capillaries [1]. This primary pathophysiological condition has several clinical manifestations, such as vaso-occlusive crisis (VOC), splenomegaly, acute chest syndrome (ACS), ocular manifestation, hepatomegaly, pulmonary hypertension, leg ulcers, chronic kidney disease (CKD), and stroke, which leads to early mortality [2,3,4,5]. However, in the current scenario, hydroxyurea (HU) is the only SCD drug approved by the Food and Drug Administration (FDA) to increase fetal hemoglobin (HbF) levels [6]. Universally, it is found that more than 300,000 children are born with SCD each year, of which two-thirds are from Africa, while Nigeria, India, and the Democratic Republic of Congo bear half the global burden of SCD. It is expected that this number will rise to about 400,000 by 2050 [7].
The phenotypic manifestation of the disease is still poorly understood. However, environmental factors, including climate and air quality, infections, fetal hemoglobin levels, and other genetic factors, play a vital role in the progression of the disease [8,9]. The severity of the disease in pediatric SCD patients, particularly those under the age of five years, is increased for various reasons, including rapid sequestration of red blood cells in the spleen, failure of opsonization, and an inability to deal with infective encapsulated microorganisms after infection [10,11]. As a result, in children with SCD, infection is the second leading cause of death in their first ten years.
SCD patients in sub-Saharan Africa and the Eastern Mediterranean have a high infection rate, with obtrusive pneumococcal infection being the most common [12]. The global reports suggest implementing an effective management and systemic newborn screening (NBS) program is the first logical step in preventing disease [12]. The initial neonatal screening and the arrangement of immunization and prophylactic antimicrobial agents improve the quality of care for SCD patients, resulting in a significant decrease in mortality. However, in this review article, we discussed several aspects of infections in SCD children, the underlying mechanisms for susceptibility to specific pathogens, and how infection affects SCD outcomes. We also highlight the difficulties in reducing the global burden of SCD mortality.
2. Materials and Methods
Electronic databases, including PubMed, Web of Science, Google Scholar, and EMBASE were utilized to retrieve relevant published articles. A comprehensive search strategy was employed by combining keywords, including “sickle cell disease”/“sickle cell anemia”, AND “hemoglobinopathy”, “vaso-occlusive crisis”, “pathophysiology”, “clinical manifestations”, “bacterial infections”, “viral infections”, “parasitic infections”, “sepsis”, “pneumococcal infections”, “streptococcus infections/complications”, “pneumonia”, “osteomyelitis”, “meningitis”, bacteremia”, “sickle cell” AND “COVID-19, prevention, therapeutics, and management”. Furthermore, to identify additional pertinent studies, references from relevant original papers and review articles were examined.
| Keywords searched | ||
| “sickle cell disease”/“sickle cell anemia” | AND | “hemoglobinopathy”, “vaso-occlusive crisis”, “pathophysiology”, “clinical manifestations”, “bacterial infections”, “viral infections”, “parasitic infections”, “sepsis”, “pneumococcal infections”, “streptococcus infections/complications”, “pneumonia”, “osteomyelitis”, “meningitis”, “bacteremia” |
| “sickle cell” | AND | “COVID-19, prevention, therapeutics, and management” |
3. Results and Discussion
3.1. Immune Dysfunction and Susceptibility to Infection
In patients with SCD, the primary complications often arise from infections. The exact reason behind susceptibility to infections is complicated. A significant contributing factor is the impaired functioning of the spleen. SCD has been associated with abnormalities in processes such as opsonization, the alternate complement pathway, antibody production, leucocyte function, and cell-mediated immunity.
Individuals with SCD exhibit deficiencies in their innate immune system, primarily attributed to reduced splenic function, making them more susceptible to infections caused by encapsulated bacteria, and the compromised innate immune response stems from malfunctions in elements like chemotaxis, migration, and scavenging due to faulty neutrophils, alongside decreased splenic phagocytic filtration and a diminished opsonization capability [11]. Furthermore, the adaptive immune system in SCD is compromised, characterized by a reduced production of memory B cells and crucial IgM components, essential for the humoral immune system. This weakened immunity hinders the ability to combat viral infections, thereby worsening the condition in SCD patients, and the impact of infections contributes to multi-organ dysfunction [8].
3.2. Splenic Dysfunction
The spleen is primarily involved in the filtration of foreign microorganisms and the support of innate and adaptive immune functions. It serves various vital functions in immune response and plays a critical role in increasing vulnerability to most SCD patients’ infections. The spleen helps two essential tasks prevent bloodstream bacterial infections. First, it is a phagocytic filter that removes bacteria from the bloodstream. Second, the spleen hosts leucocytes, which produce an antibody response to polysaccharide antigens [13]. Still, most infections are caused by SCD complications, such as acidosis, hypoxia, and dehydration, which activate or exaggerate the sickle cell crises and cause vaso-occlusion and ischemia, ultimately harming the structure and function of the spleen [14].
Individuals with splenectomy and individuals with non- or partially functioning spleens (hypo or asplenism), as in SCD, are more vulnerable to infections. Hyposplenic and asplenic individuals lack IgM memory B cells (as stated above, the spleen is a major site of their generation or function). Thus, it cannot provide a rapid, precise response to encapsulated species of microorganisms, particularly Haemophilus influenzae type b (Hib), salmonellae, and pneumococcal infections [15]. Local infections can easily become systemic, which, combined with the loss of spleen filtering function, can contribute to the development of severe sepsis. The risk of infection in children under the age of five with SCD is nearly thirty to a hundred times higher than in healthy children, implying that SCD children are more vulnerable to invasive pneumococcal disease (IPD), including pneumonia, meningitis, and septicemia [16]. In addition, patients with SCD have a 25 times higher risk of salmonella infections, particularly in older children and adults [17]. The factors associated with increased SCD infections may be related or unrelated to the immune system. Few infections can occur as a result of treatment.
3.3. Opsonization
Splenic opsonization is compromised in SCD conditions due to a lack of production of the immunoglobins and opsonins required for bacterial destruction. The effective opsonization process is dependent on a complement cascade, which is activated by both classical and alternative pathways and destroys infectious microorganisms by creating holes in their cell membranes [18]. Lack of splenic IgM leads to decreased opsonization and impaired activation of the classical pathway. Another opsonin, tuftsin, formed in the spleen and required to activate cell-mediated immunity, is reduced in SCD patients, indicating that the spleen is involved in their synthesis [19]. Furthermore, a lack of C3b-binding protease enhances the alternative complement pathway, possibly due to depletion from clearing sickle shaped RBC caused by intravascular hemolysis, and ultimately reduces the alternative complement pathway’s immune function [20,21].
3.4. Lymphocytes
SCD patients are more susceptible to unusual pathogens, implying that other immune system defects compromise their immune systems. SCD reduces the production and function of B and T lymphocyte cells, resulting in impaired memory B-cell and anti-polysaccharide antibody formation [22,23,24]. Also, the IgM antibody response to an influenza virus vaccine is impaired [24]. There are also lower T-cell subsets CD4+ and CD8+ in SCD patients, which affect their divergence into mature lymphocytes [25]. Therefore, there is decreased production in both the Th1 (IFN-gamma, IL-2, and TNF-beta) and Th2 (IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13) responses of CD4+ T-helper cells in SCD [26].
3.5. Nutritional Deficiencies
Nutritional deficiencies also have a major impact on SCD children’s immune systems. SCD patients have macro-and micronutrient deficiencies due to pathways that include reduced calorie consumption, increased basal metabolic rate (BMR), increased RBC synthesis, increased protein turnover, dysregulated inflammatory response, and high myocardial energy requirements. Micronutrient deficiencies are associated with increased susceptibility to infection and a higher rate of SCD complications [27]. Low plasma zinc levels in malnourished children usually result in immune dysfunction. IL-2 is required for cell-mediated immunity maturation, and zinc deficiency causes lymphopenia, a decreased production of IL-2, which impairs the coordination of the innate and adaptive immune systems. Zinc deficiency is caused by insufficient food consumption, high protein turnover, and increased loss from the kidney due to insufficient reabsorption. Zinc supplementation improves somatic development in SCD children, and vitamins A, B, and magnesium have been shown to reduce inflammation, vaso-occlusive crisis, and hospitalizations [28,29,30,31,32].
3.6. Hereditary Influences
Despite having the same genetic defect, the severity of SCD varies widely among individuals because not all patients have identical pleiotropic genes. Some carriers have mutated genes, whichcan either improve or worsen the phenotype. This suggests that the phenotype of SCD is multigenic. Some other genes unrelated to the globin locus participate in relevant pathological events (rapid destruction of sickle cells, dense cell formation, endothelial adhesion) controlled by many, known as pleiotropic or secondary effector genes [33]. Polymorphisms in various immune-related genes have been proposed as a factor for increased vulnerability to infection in SCD patients. Previous findings show direct involvement of the HLA class II polymorphism in developing primary infections in SCD [34]. The increased risk of bacteremia may be controlled by the mannose-binding lectin receptor, Fc receptor, beta-S gene cluster haplotypes, IGF1R, and the TGF-beta/bone morphogenetic protein (BMP) pathway [35,36,37,38].
3.7. Mechanical Factors
Several mechanical factors may also play a role in a wide range of infections in SCD patients. Environmental factors can contribute to a variety of infections in SCD. The leading cause of respiratory infection in SCD is air pollution. Tobacco smoke exposure increases the risk of sickle cell crises in children with SCD due to chronic tissue hypoxia, vascular endothelial cell damage, and thrombus formation.
Furthermore, tobacco smoke may indirectly exacerbate asthma and acute chest syndrome in patients with SCD [39,40]. SCD patients are also predisposed to a variety of iatrogenic infections as a result of therapeutic interventions. Some of the complications treated by blood transfusion include vaso-occlusive crisis, splenic sequestration, acute chest syndrome, priapism, and strokes. Blood transfusions without pathogen screening can increase the risk of blood-borne infections, most notably hepatitis B and C, HIV, cytomegalovirus (CMV), and B19 parvovirus. Patients who are iron-overloaded and on deferoxamine medication due to frequent transfusions are especially vulnerable to Yersinia enterocolitica [41]. Patients with SCD who have a venous catheter in place for long-term blood transfusions are also at risk of bloodstream infections (BSI). Despite adequate antibiotic therapy, BSI, such as Staphylococcus aureus and Pneumococci, is hospital-acquired and primarily associated with venous catheters. These patients are at a high risk of developing an associated bone–joint infection if they are not adequately treated [42].
3.8. Molecular Mechanism of Clinical Manifestations in SCD
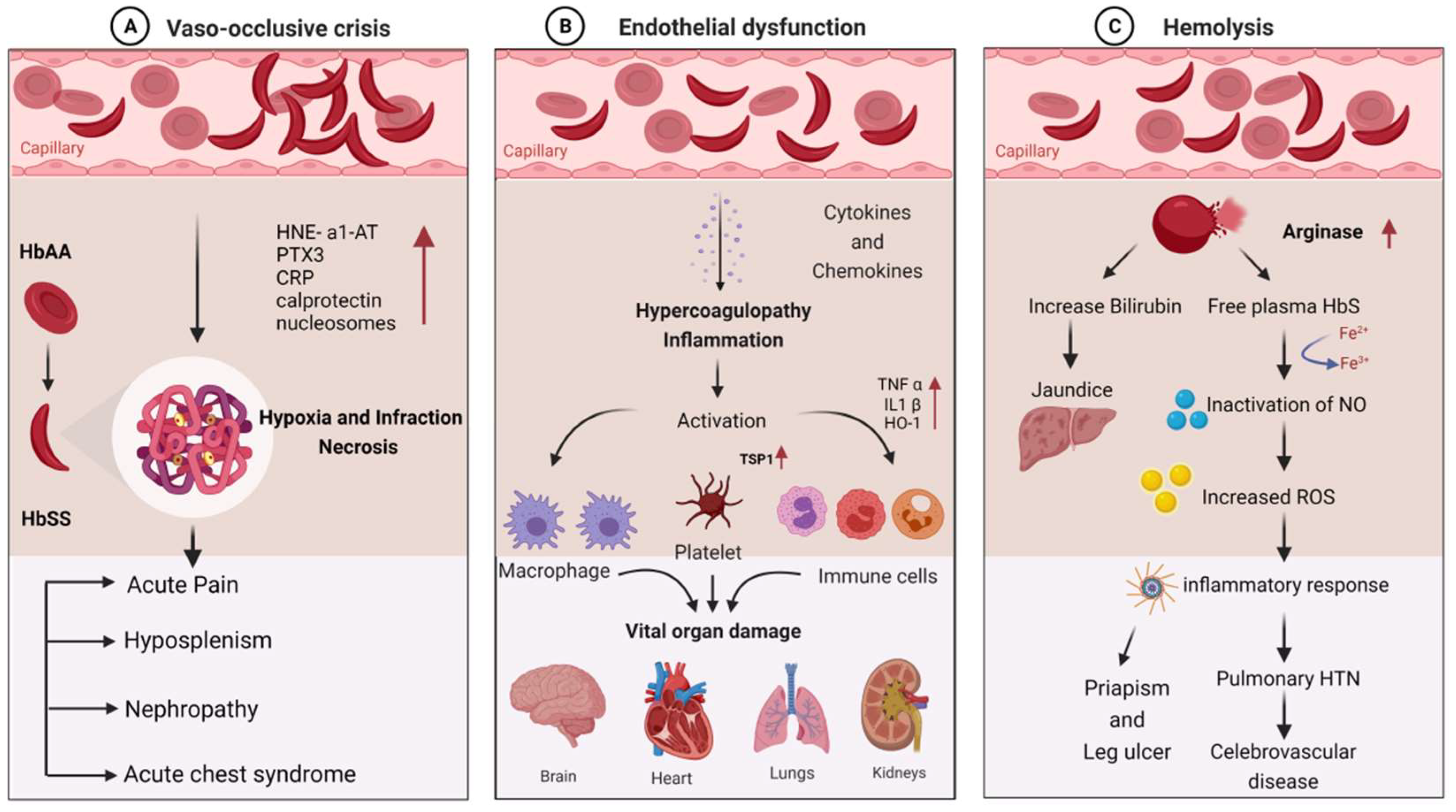
SCD patients are susceptible to infections due to a variety of immunological abnormalities and exposure to infectious agents. Pathophysiological mechanisms of the disease can explain the disease’s role in stimulating immune dysfunction. The clinical manifestations of infection in SCD are primarily vaso-occlusion, which causes endothelial dysfunction and hemolysis. The detailed molecular mechanism involved in the clinical manifestations of SCD is depicted in Figure 1.
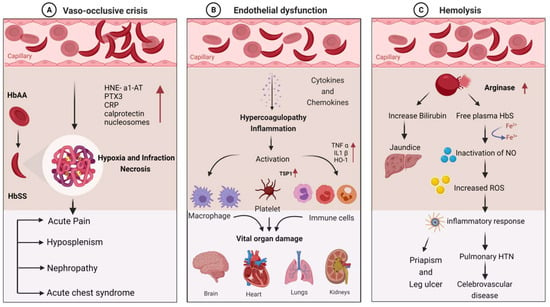
Figure 1.
Molecular mechanism involved in the clinical manifestations of SCD due to infections: (A) vaso-occlusive crisis; (B) endothelial dysfunction; (C) hemolysis.
Hematocrit, plasma viscosity, and erythrocyte deformability are some factors that affect blood rheology. Sickled RBCs become mechanically trapped in the microcirculation, promoting adhesive events among blood cells and resulting in chronic vaso-occlusion, causing frequent episodes of pain, hemolytic anemia, organ damage, and premature death [43,44]. Sickled RBCs also promote the exposure of adhesion molecules and binding motifs that are not generally found on RBCs’ outer membranes, such as phosphatidyl serine (PS), basal cell adhesion molecule-1 (B-CAM1), integrin-associated protein (IAP), and intercellular-adhesion-molecule-4 (ICAM-4). Sickled RBCs also have an integrin complex on their surface, which binds to fibronectin and vascular-cell adhesion molecule 1 (VCAM1), expressed on endothelial cells’ membrane and activated by inflammatory cytokines like tumor necrosis factor-alpha. C-reactive protein (CRP) is generated in response to interleukin-6 and pentraxin 3 (PTX3), generated mainly by neutrophils and endothelial cells as a result of an inflammatory response [45,46,47]. Thrombospondin, secreted by activated platelets, binds to endothelial cells and sickled RBC via CD36 and sulfated glycans found only on sickled RBC.
The interaction of sickled RBC with vascular endothelium may result in the formation of oxygen radicals by the endothelial cell and the transcription factor NF-kB by oxidants. When NF-kB is activated, the transcription of various genes of adhesion molecules, such as E- and P-selectin, VCAM-1, and ICAM-1, on the endothelial surface is increased [2]. It also increases the expression of major leukocyte chemo-attractants, such as interleukin-8 (IL-8) on endothelial cells [1,48]. Neutrophil-derived azurophilic protein elastase, with its inhibitor a1-antitrypsin (HNE-a1-AT), and neutrophil cytosolic protein calprotectin levels are increased with the activation of neutrophils. The inflammatory environment during VOC may also promote neutrophil, monocyte, and platelet activation, which increases their adhesion to each other and activated endothelial cells. These activated neutrophils, monocytes, and platelets do not act against pathogens but rather contribute to VOCs and act as sources of oxidative stress, impairing the immune response [49]. Activated neutrophils form neutrophil extracellular traps (NETs), and during NET formation, nucleosomes are squeezed out of the neutrophils, resulting in higher nucleosome levels during VOC [50,51]. Vaso-occlusion, on the other hand, contributes to ischemia–reperfusion injury, promoting chronic inflammation in SCD.
Hemolysis causes Hb to oxidize, resulting in the formation of heme and its oxidized form. The Fe3+ and Fe4+ states of hemoglobin formed in SCD are highly reactive in terms of encouraging oxidation. Hemolysis also results in red cell microparticles, which can deliver toxic heme to endothelial cells [52,53]. These hemolysis products are thought to be erythrocyte danger-associated molecular patterns (eDAMPs), which trigger innate immune responses and may play a role in sickle cell inflammation [54,55]. Hemin is also a potent TLR4 agonist, which contributes to a proinflammatory and procoagulant state in SCD. Because of the increased production of placental growth factor (PIGF) resulting from ischemia–reperfusion injury, monocytes are activated in response to PIGF, secreting more TNF-a, IL-1, and other chemokines. These increased oxidant production and leukocyte adhesion to the endothelium, followed by extravasation into the tissues and, finally, tissue damage [56]. Increased hemolysis and reperfusion promote chronic hypoxia, ROS production, microvasculature dysfunction, innate and adaptive immune response activation, and cell death, all of which contribute to ischemia–reperfusion injury. ROS-dependent degradation of cellular proteins, lipids, DNA, and ribonucleic acids triggers cell death programs, such as apoptosis, necrosis, and autophagy [57,58].
3.9. Infectious Complications Associated with SCD
Researchers previously identified bacterial infections as the leading cause of morbidity and mortality in SCD patients, and children are the most vulnerable age group [59,60,61,62]. SCD increases susceptibility to various microbial, viral, parasitic, and Protozoan infections (Figure 2). Bacteremia/sepsis is caused by both Gram-positive (S. pneumonia and S. aureus) and Gram-negative (E. coli, Klebsiella, and Bacteroides) bacteria, which is the most common infection reported [63]. The infections become more severe during severe anemia and recurrent VOC. The prevalence, susceptibility, and severity of the effect on disease depend on the age group. Children are more susceptible to sepsis and pneumococcal infection [64]. However, adults in their forties and fifties commonly report organ-specific infections, including pyelonephritis and osteomyelitis. Some of the infections that increase the risk of mortality are as follows.
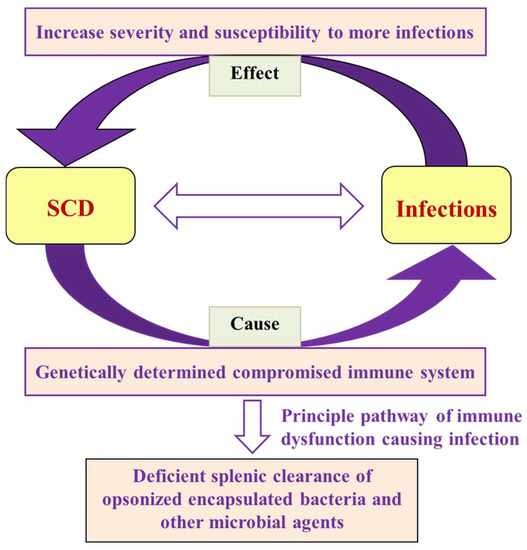
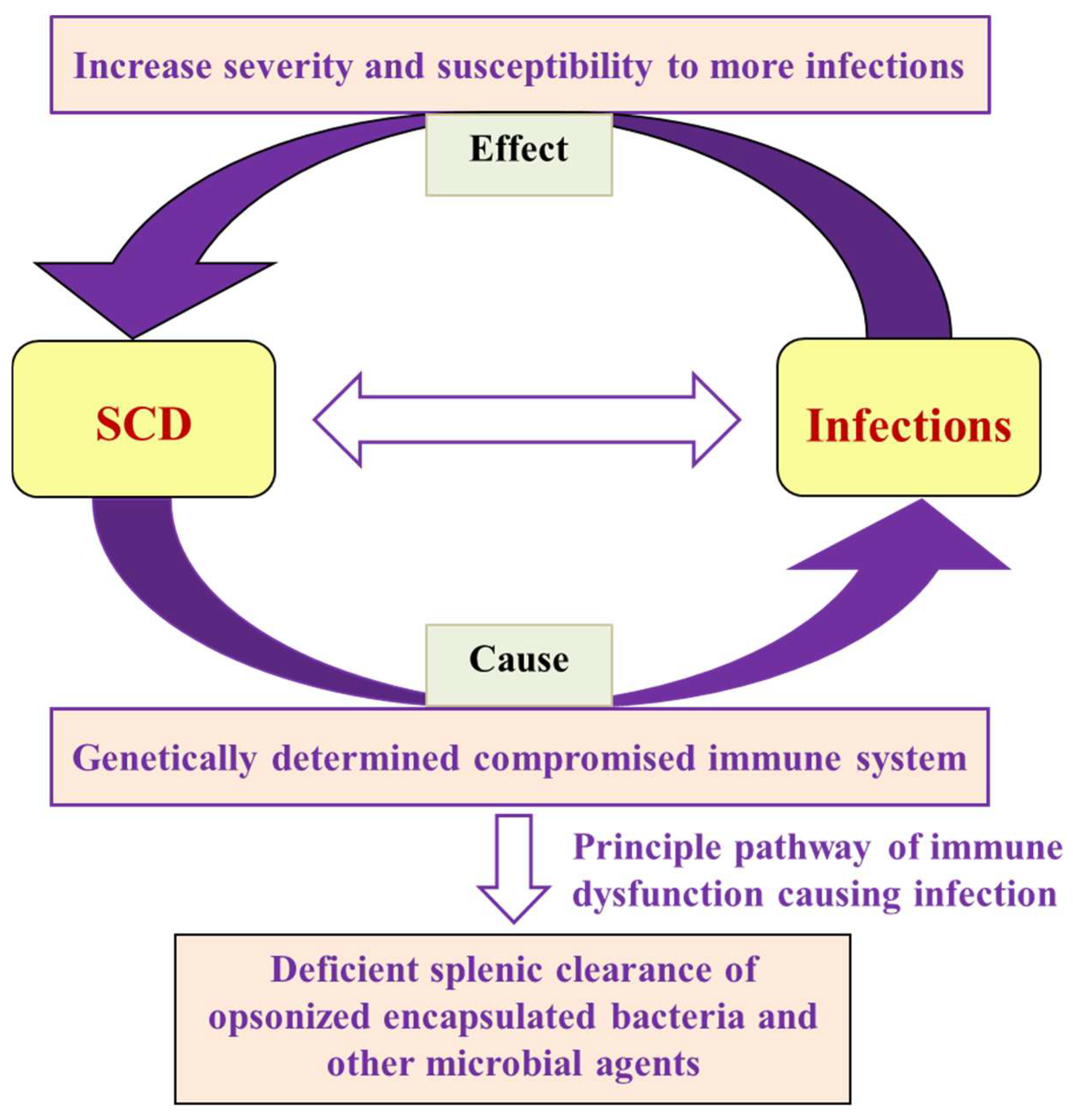
Figure 2.
Flow chart showing the interrelationship of SCD and infections in terms of cause and effect, along with the failure of the primary mechanism that leads to microbial and parasitic infections.
3.10. Bacterial Infections
3.10.1. Bacteremia, Sepsis, and Pneumonia
Bacteremia and sepsis are common complications of SCD. Streptococcus pneumonia, Neisseria meningitides, Haemophilus influenza, and Escherichia coli cause sepsis [65]. Klebsiella pneumonia and Staphylococcus aureus are two other bacteria that can cause bacteremia and septicemia [66]. Salmonella species and Pantoea agglomerans enteric Gram-negative bacteria also negatively impact SCD patients [67,68]. An extensive data analysis of 550 SCD patients shows that the use of catheters during red cell exchange/transfusion increased the risk of bacteremia or thrombosis [69]. Cannas et al. noticed the rapid progression of infection and a high mortality rate in SCD children with bacteremia/sepsis or meningitis infections, irrespective of treatment with penicillin sodium ampicillin trihydrate [70].
Children with SCD have immune dysfunctions that cause severe sepsis symptoms, which is less common in normal children [71]. Sickling infants are more susceptible to Streptococcus pneumonia infections due to several factors, including the interference of antibody production, opsonophagocytosis, and functional asplenia, as well as defective splenic clearance [72]. On the other hand, children and adults are more susceptible to S. pneumonia infections due to a lack of IgG and IgM antibody response and impairments in splenic complement and opsonophagocytic functions [65]. Penicillin prophylaxis and vaccination against bacteremia are preventive measures for infections that have reduced childhood mortality [73]. Pneumonia is the most common chronic infection that causes death in children worldwide and is caused by encapsulated microbial species, the most common of which is Mycoplasma pneumoniae. Studies show that diplococcus pneumonia, salmonella species, and Hemophilus influenza cause more illness in children than their normal counterparts [74,75,76].
Among 2444 SCD patients, Yee et al. has shown that children up to five years of age have been more infected with S. pneumoniae and H. influenza [77]. SCD patients with bacterial pneumonia developed ACS, which causes pulmonary infarction in the young age group and emboli or thrombosis in older patients [78,79]. Pneumococcal polysaccharide vaccine 23 (PPSV23) was observed to be ineffective against some strain S. pneumonia in an adult SCD patient with functional asplenia and reduced IgG [80].
3.10.2. Acute Chest Syndrome (ACS)
Acute chest syndrome is one of the major clinical manifestations of SCD, leading to death. Primarily bacterial and viral infections cause ACS due to functional asplenia. Children under five years of age with SCD have been reported with high mortality rates because of Streptococcus pneumonia infection, responsible for ACS [81]. S. pneumonia is also a causative organism for severe anemia. It causes inflammation of the lungs by migrating from the upper respiratory tract to the lower respiratory tract [82].
3.10.3. Meningitis
Recently Chenou et al. observed that SCD patients are 300 times more likely to develop bacterial meningitis than the normal population, and 10% of affected children die due to the infection generated by meningitis and pneumococcus [83]. Previous research found that meningitis in SCD patients caused by various bacteria, including H. influenzae, H. meningitis, and E. coli [84], and Streptococcus pneumonia, was responsible for 70% of bacterial meningitis cases in SCD children, while H. influenza was responsible for 80% [83,85]. The recurrence of meningitis occurs more frequently in SCD patients [86]. The resulting pneumococcal sepsis and meningitis are common in adults, and reported cases have led to adult mortality. Treatment with a pneumococcal polysaccharide vaccine has been proposed to reduce infection, which has documented many co-morbidities, such as renal dysfunction and stroke, along with meningitis [87,88].
3.10.4. Osteomyelitis
Osteomyelitis is characterized by bone inflammation and is found in up to 61% of SCD patients, particularly in long bones such as the femur, humerus, and jaws. It is more common in SCD patients, and it is the second most affected tissue after the spleen [89]. The episodes of vaso-occlusive crisis ruptured the vasculature around the bones, allowing bacteria to infect the site, which is the primary cause of osteomyelitis [90]. Most SCD patients with osteomyelitis have Salmonella infections at multiple bone sites, which are most frequent in early childhood compared to non-SCD individuals [91,92] Furthermore, microbes such as Staphylococcus, Pneumococcus, and actinomycetes also cause osteomyelitis in SCD patients [93].
A meta-analysis shows that salmonellae are the most common bacterial pathogens of osteomyelitis in SCD in the USA and European populations. Meanwhile, Staphylococcus aureus is the most common pathogen in Sub-Saharan Africa and the Middle East region [94]. The predominance of Gram-negative bacteria has been linked to bowel ischemia and VOC [95]. Most of the symptoms of osteomyelitis are similar to VOC, but osteomyelitis affects fewer areas, specifically the diaphysis of a long bone [96]. As a result, physical examination, blood culture, or radiological examination are insufficient for detecting osteomyelitis in SCD, and ultrasonography or a higher imaging device for diagnosis of osteomyelitis has been suggested [97]. The clinical diagnosis of microbial osteomyelitis and bone infarction in early SCD patients is a major limitation. It has also been associated with Mycobacterium ulcers [98] and other causative agents, such as Haemophilus influenza, Escherichia coli, and Enterobacter sps [95]. However, early diagnosis has been suggested to prevent invasive surgical intervention and be treated through medicines/antibiotics.
3.10.5. Mycobacteria
It has been reported that iron overload is primarily known to cause organ dysfunction and also increases the risk for mycobacterial infection in SCD patients [99,100]. Shemisa et al. found that SCD Patients with iron overload were at an increased 17-fold higher risk to die from Mycobacterium tuberculosis in the sub-Saharan African population [101]. Thorell et al. reported that nontuberculous mycobacteria (NTM) infection, such as Mycobacterium fortuitum, causes frequent admissions for vaso-occlusive painful episodes in teenagers with SCD [102]. Edrees et al. subsequently confirmed an unusual Mycobacterium avium complex bacteremia infection in two SCD patients [103].
Another study found that the Mycobacterium terrae complex is a rare clinical pathogen known to cause pulmonary, bone and joint infections in a patient with febrile SCD [104]. Ashraf et al. also found Mycobacterium mucogenicum bloodstream infections in an outpatient setting in the USA [105]. The main risk factors of NTM in bloodstream infections are indwelling vascular catheters (IDVC), which are known to affect the immune system that causes relative immune deficiencies in SCD [106]. Recently, a Multicenter study suggests that Mycobacterium does not seem to be a risk factor for severe Tuberculosis (TB) and not a risk factor for mortality [107].
3.10.6. Urinary Tract Infection (UTI)
Urinary tract infection is a common cause of childhood morbidity and mortality in SCD. A high prevalence of UTIs with characteristics of pyelonephritis caused by Salmonella, Staphylococcus, Escherichia coli, and Enterobacter–Klebsiella was seen in SCD patients [108]. It was observed that scarring while healing the renal medulla and excretion of dilute urine were responsible for increased UTIs and pyelonephritis. A recent report on a longitudinal retrospective study of SCD children from Saudi Arabia showed most patients have UTIs due to bacteria and splenic dysfunction, followed by VOC. After the UTIs, compromised kidney function is a significant manifestation of SCD patients, increasing the asymptomatic bacteriuria infection in the African population [109,110].
A study revealed that homozygous HbSS has a higher rate of mortality with proteinuria than heterozygous HbAS. Furthermore, Staphylococcus species were the most common cause of infections in SCD patients with asymptomatic bacteriuria, followed by E. coli [111,112]. However, the early detection and treatment of UTIs are essential in patients with SCD because UTIs can lead to impaired kidney function, scarification, and severe septicemia [113]. Acute post-infectious glomerulonephritis is another clinical manifestation observed in SCD patients [114].
3.10.7. Gastrointestinal Infections
SCD patients have frequent hypoxia–reperfusion injuries caused by VOC in the intestinal vasculature, increasing gut permeability [115]. The accumulation of microbes and their products activates neutrophils, resulting in VOC [116]. The finding of ischemic colitis as an etiology of intestinal VOC has been observed in SCD patients [117]. Researchers suggest that the intestine is the primary entry point for non-typhoidal Salmonella infection because of its increased permeability, which causes gastroenteritis [118].
Non-typhoidal Salmonella and Pneumococcus were the most prevalent causative agents known to affect children and adults in Sub-Saharan Africa with SCD [119]. The report suggested that SCD children are more likely to become infected with non-typhoidal Salmonella than non-SCD children, exaggerating the severity [120]. However, these studies indicate that changing the density of the gut microbiota could improve the intestinal health of SCD patients.
3.11. Viral Infection
3.11.1. Respiratory Infections (RI)
Lung function abnormalities, or RI, are common in children and adults with SCD and may lead to a progressive decline in lung function with age [121,122]. The respiratory system is affected by several syncytial viruses, such as influenza, rhinoviruses, human metapneumo, and para-influenza, resulting in respiratory failure, which causes ACS [123]. The symptoms and severity are similar to seasonal influenza reported in SCD children and teenagers [124]. More hospitalization rates have been observed due to seasonal influenza infection being observed more in SCD children than normal [125].
Further, children infected with the influenza virus are more prone to secondary bacterial infections, which lead to ACS [78]. The severity of asthma is one of the common comorbid factors that increase in SCD children [126]. It also observed that patients suffering from repetitive episodes of ACS could develop scattered areas of lung fibrosis, and leads to advanced chronic lung disease in SCD [127].
3.11.2. Anemia Associated with Viral Infections
Severe anemia due to a transient aplastic crisis caused by B19 parvovirus belonging to the genus Erythrovirus (family Parvoviridae) was observed in 65–80% of SCD patients. It infects the erythroid progenitor cells and obstructs erythropoiesis [128]. B19 parvovirus has been associated with ACS, splenic and hepatic sequestration, bone marrow necrosis, pain crisis, and stroke [129]. In addition, Epstein–Barr virus is also responsible for hemolytic anemia, along with splenic rupture, thrombocytopenia, agranulocytosis, hemolytic anemia, and hemophagocytic lymphohistiocytosis in patients with SCD [63].
3.11.3. Hepatitis B and C Infections
Both hepatitis B virus (HBV) and hepatitis C virus (HCV) infections have a common transmission mode and can cause chronic liver infection. The prevalence of hepatitis B infections is globally higher than hepatitis C, and the infection rate is higher in males than in females [130]. Several reports suggest that SCD children are more frequently infected with hepatitis C in African countries due to multiple transfusions [131,132]. About 10% of adults with liver dysfunction documented to be infected with hepatitis C virus [133].
Iron overload following blood transfusions is additive to the liver damage caused by HCV infection. The administration of ribavirin used to treat hepatitis C virus has been known to cause hemolysis in SCD patients, which in turn increases the complications of the disease [134]. Some studies found that the percentage of hepatitis B was lower in vaccinated children than in unvaccinated children, indicating that vaccination can prevent hepatitis in SCD children [135,136].
3.11.4. HIV Infections
There are many crosstalks between HIV and SCD; thus, the coexistence of HIV and SCD has a synergistic effect. HIV infection was found in 0–11.5% of SCD patients [137]. HIV infection also creates a favorable environment for pneumococcal infection, which can be fatal, resulting in severe pneumonia or meningitis in SCD adults [138]. A nested case-control study by Belisário et al. showed that HIV infection increased the severity of SCD-related symptoms such as ACS/pneumonia, sepsis/bacteremia, pyelonephritis, ischemic stroke, hemorrhagic stroke, abnormal transcranial doppler, and pulmonary hypertension [139]. Furthermore, National Hospital Discharge Survey data in the period of 1997–2009 showed that SCD is associated with decreased HIV but higher HBV and HCV co-morbidities in adult African-Americans [140]. Odera et al. found that children with HIV and SCD faced significant life-threatening conditions and developed a severe hypersensitivity reaction during first-line treatment [141]. Despite various reports demonstrating the impact of HIV on the clinical outcome of individuals with SCD, the literature also shows controversial data. Studies between 1995 and 2009 by Neto et al. observed no direct link between HIV and SCD complications [142]. In addition, according to another report, SCD patients are more resistant to HIV infection, and the progression of AIDS is slower in SCD patients than in non-SCD controls [143].
3.11.5. Dengue Virus
Dengue virus is a mosquito-borne flavivirus that is most common in tropical and subtropical areas [144]. Dengue causes headaches, fever, abdominal pain, bleeding, myalgias, and loss of capillary integrity and increased mortality by up to 12.5% in SCD patients [145,146,147], due to their compromised immune systems, which make them more susceptible to hypovolemia and endothelial cell activation. Several reports show that the prevalence of dengue is high in geographic areas where SCD is endemic, such as the Caribbean, Central and South America, areas of Africa and the Middle East, Asia, and Oceania [63,148]. Two case studies on ten and nineteen-year-old female SCD patients with severe dengue revealed multi-organ dysfunction syndrome (MODS) and impending death [149]. A recent retrospective study found that children with the HbSC genotype had a higher rate of severe dengue and death than those with the SS genotype [150].
3.11.6. Coronavirus Disease (SARS-CoV-2)
Since 2019, SARS-CoV-2 coronavirus (COVID-19) infection has been a worldwide concern. Individuals with co-morbidities are more likely to become infected with the coronavirus. Individuals with HbSS hemoglobin have hypoxia or ACS. They are thought to be more susceptible to SARS-CoV-2 infection, which affects the lungs and respiratory tract. Case reports confirmed ACS and VOC complications in SCD patients with weakened immunity and infected with the novel coronavirus [151]. However, reports on the impact of COVID-19 in terms of severity and mortality are contradictory. Some African countries reported ACS and other co-morbidities in SCD patients with no casualties [152]. According to a survey of COVID-19 in hemoglobinopathy from the United Kingdom, the majority of patients hospitalized due to SARS-CoV-2 coronavirus infections had SCD (85.1%) and were primarily homozygous (Hb SS) patients (64.1%) [153]. Minniti et al. found that SCD patients infected with the coronavirus had a higher severity of disease (10%) and a higher risk of mortality without hydroxyurea therapy, compared to approximately 3% of the general population. The reason for the conflicting reports has been argued to be age, which could be one of the factors influencing severity and mortality, as children and young adult patients (45–50 years) have a low risk of mortality. In contrast, older SCD patients have a high risk [154]. Panepinto et al. found that SARS-CoV-2 increased the risk of severe symptoms in 69% of SCD patients [155]. However, studies in France and the United Kingdom found that patients with SCD infected with the coronavirus had a low severity and mortality rate [156,157]. Second, females died at a higher rate due to SARS-CoV-2 infections than males [158]
Researchers reported the hospitalization rate was high for SCD patients over 40 years. However, Ramachandran et al. also did not find any increased risk of severity or mortality in SCD patients with the coronavirus infection [159]. A recent study showed that SARS-CoV-2 infection increased disease severity in patients with SCD in a different population [157,160,161,162]. Still, the overall effect of the coronavirus needs to be elucidated through multicenter reports; the existing reports are mostly speculation rather than ground-level research. Later reports showed that remdesivir injections given with blood transfusion were an effective therapy that does not require immunosuppressive or steroid treatments [163,164].
Hydroxyurea administration may be one of the most effective ways to reduce complications such as VOC during SARS-CoV-2. The HBG2 rs7482144 (C > T) polymorphism, on the other hand, is linked to HbF levels but not to the severity of SCD [165]. The use of antimalarial drugs, specifically hydroxychloroquine, has been linked to side effects in SCD patients, such as ventricular arrhythmias and cardiac toxicity, as reported in other COVID-19 patients [166].
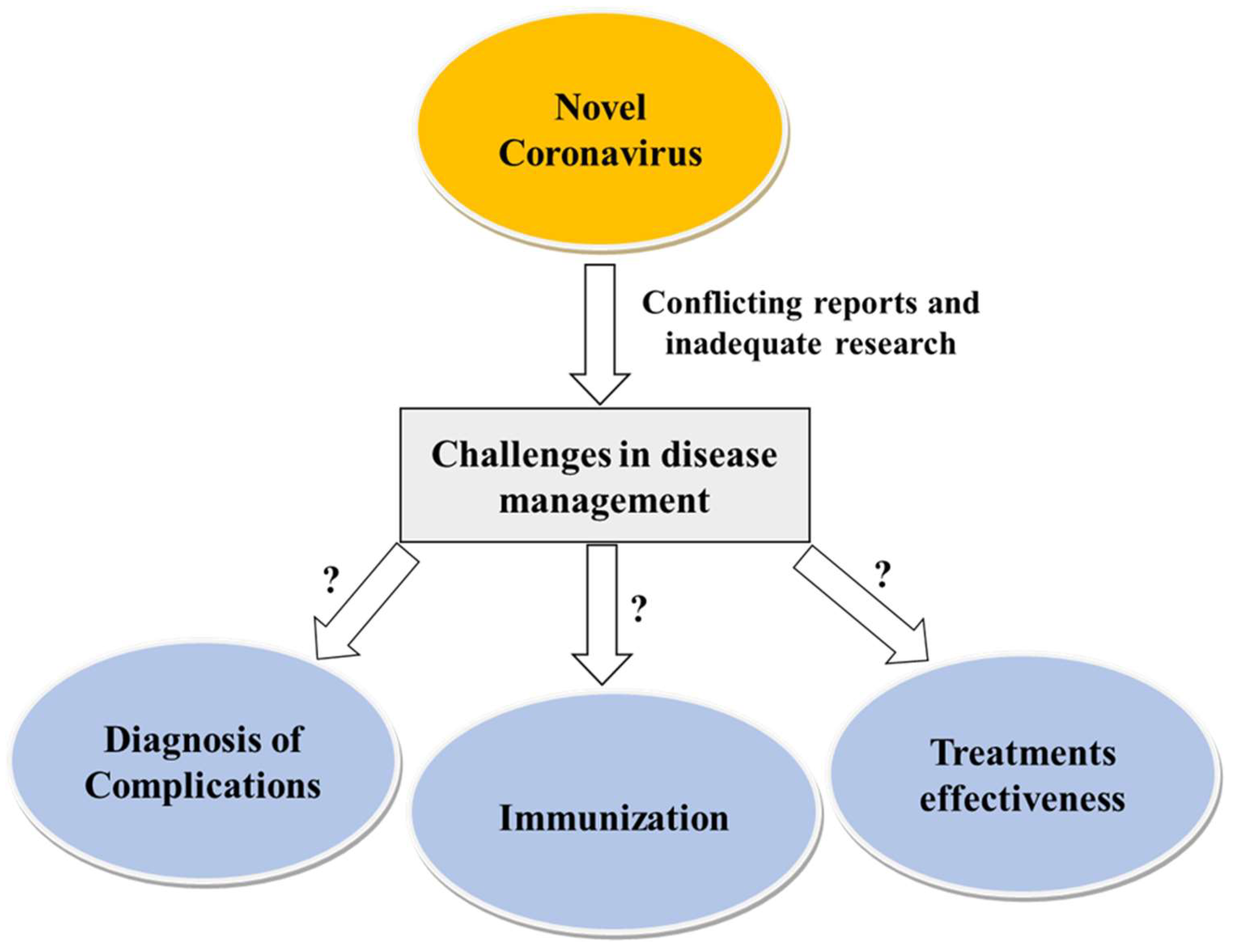
However, there is no evidence of the extent of the side effects or complications in SCD patients. Figure 3 depicts SARS-CoV-2 infection and the challenges of its management in SCD.
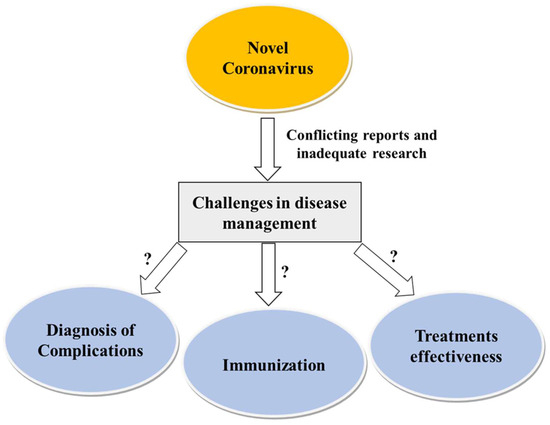
Figure 3.
SARS-CoV-2 infection and challenges of its management in SCD.
3.12. Parasitic Infections
3.12.1. Malaria
Malaria is a highly vulnerable parasitic infection exhibiting fatal clinical manifestations, such as impaired splenic function in homozygous (HbSS) patients compared to HbAS or normal individuals. Individuals with HbAS show a protective effect and a lower frequency of malaria infection compared to normal individuals due to the HbAS making an unfavorable environment for malaria parasites. Moreover, the phagocytic action of infected RBC macrophages reduces infection rates [167,168,169,170,171]. Therefore, heterozygote individuals with the sickle cell gene are malaria-resistant compared to normal individuals. P. falciparum infection is also lower due to impaired erythrocyte membrane protein 1 (PfEMP 1) binding [172].
In SCD homozygous patients, inadequate splenic functions increase the risk of P. falciparum infections, leading to death in children by increasing VOC [173]. However, the effect of malaria parasite infection on HbSS is fatal due to the increased severity of the already existing anemic condition and the associated pain [174]. Further severe oxidative stress is another consequence of P. falciparum infection in SCD patients due to increased intravascular hemolysis, ischemia–reperfusion injury, and chronic inflammation [175].
3.12.2. Other Parasitic Infections
In addition to Plasmodium, intestinal parasites such as Entamoeba histolytica, Entamoeba coli, and Giardia lamblia, and some helminths, such as Ascaris lumbricoides, Ancylostoma duodenale, Trichuris trichiura, and Strongyloides stercoralis, cause severe anemia in SCD patients. A study in Nigerian patients found that patients without parasitic infections had a significantly higher mean hematocrit level than patients with parasitic infections [176].
Mahdi et al. documented Blastocystis hominis and Giardia lamblia as the most common intestinal parasites in patients with SCD in Iraq and the Mediterranean [177]. Furthermore, SCD patients with schistosomiasis was found with elevated reticulocyte counts, lowered hematocrits, and augmented bacterial UTIs and their severity [178].
3.13. Treatment of Infections in SCD
Bacterial infection and sepsis are common in SCD patients as their immune systems are compromised. These should be treated with broad-spectrum antibiotics, including third-generation cephalosporins like ceftriaxone, cefotaxime oxacillin, nafcillin, or cefazolin vancomycin, and clindamycin. Penicillin, beta-lactam inhibitors, and amphotericin B deoxycholate, along with third-generation cephalosporins should be used for meningitis. For Mycobacterium tuberculosis, the first two months of treatment include rifampin, isoniazid, pyrazinamide, and ethambutol, followed by isoniazid and rifampin for the remaining four months. Gastrointestinal infections are caused by enteric Gram-negative pathogens, including Typhi and non-Typhi Salmonella, Enterococci, and anaerobic bacteria. These can be treated by with piperacillin–tazobactam, a carbapenem, and cholecystectomy procedures should be performed if the situation worsens. Influenza, rhinoviruses, para-influenza, S. pneumonia, H. influenza, Chlamydophila pneumonia, Mycoplasma pneumonia, and S. aureus are common pathogens that cause respiratory tract infections. The treatment options for these infections are oseltamivir, inhaled zanamivir, third-generation cephalosporins, vancomycin, clindamycin, macrolides, and quinolones. For UTIs, third-generation cephalosporins are used. Mosquitoes cause dengue and malaria, yet their causative agents are different. Non-steroidal anti-inflammatory products, like ibuprofen and acetaminophen, are given for pain and fever with a high intake of fluids via oral or intravenous fluids. Anti-viral medicines, like chloroquine, favipiravir, and celgosivir, are continued until platelets rise to the normal range. On the other hand, intravenous quinidine is given to SCD patients infected with malaria until the parasite density is <1%.
Moreover, oral therapies are also given based on infecting parasite species. SCD patients are also very susceptible to different parasitic infections; common medicines, like albendazole, mebendazole, doxycycline, and ivermectin are given. Viral infections are very dangerous for SCD patients, as they are already suffering from other disease complications. Treatment options for viral diseases like HIV vary with antiretroviral drug resistance and adverse event profiles, and consultation with an HIV expert is recommended. The emergence of COVID-19 from SARS-CoV-2 also put SCD patients into life-threatening conditions. Hydroxyurea, L-glutamine, voxelotor, and crizanlizumab are the primary medications for preventing hemolysis and VOC. Mechanical ventilation is given if oxygen saturation decreases; supplementation with vitamins D and C and zinc also shows effective results. Convalescent plasma infusion and remdesivir with intravenous dexamethasone are given if the severity increases. Infection prevention and detailed treatment strategies are included in Table 1.

Table 1.
Most common microorganisms, prophylaxis, and treatment associated with infection in sickle cell disease with underlying mechanisms for predisposition; updated from [67].
4. Future Perspective and Roadmap for Research and Implementation
In a nutshell, a roadmap for SCD research can be developed to diagnose symptoms and design effective therapies to reduce complications in sickling patients. The goal of treatment is to reduce the complications of symptoms that lead to hospitalization, suffering, and early death, particularly in children. Research is being conducted to target various pathways and mechanisms that lead to SCD complexity extensively. Rapid test kits for SCD, such as the rapid Sickle SCAN® point-of-care (POC) test, are a less expensive, more affordable, and more accurate early mode of diagnosis in economically underdeveloped areas, where previously available SCD diagnostic methods required a well-equipped laboratory and were time-consuming. Therefore, high-accuracy kits could be promoted in low-income countries with endemic SCD.
A technology- and algorithm-based cost-effective screening of SCD (smartphone-based microscope and deep-learning) has been proposed; similarly, research on the diagnosis of infections could be a better non-invasive strategy. Physical examination and blood culture determination of the extent of infections are insufficient, and the invasive methods are painful. As a result, researchers advocate for using advanced non-invasive techniques, such as ultrasonography and imaging techniques, to thoroughly diagnose the severity of the infection and its clinical manifestation when the first symptoms of infection appear and to plan appropriate treatment. Treatments with antibiotics to suppress the infection are not sufficient for patients with high risk.
Consequently, many therapeutic strategies targeting the various pathways leading to complications in SCD are currently in use. At the molecular level, medication based on antibiotics or antibodies has proven to be a better therapeutic approach that reduces mortality while decreasing pain in SCD patients. Antibiotics such as voxelotor have recently been shown to prevent hemoglobin polymerization in SCD patients. Its concentration rises in the blood, allowing oxygen to circulate throughout the body aiding. Oral administration of pharmaceutical-grade l-glutamine has also been reported to reduce oxidative stress, and thus, pain, with minimal or no toxic effect on patients, as has the administration of the anti-P-selectin (P-selectin binds on endothelial cells and platelets) monoclonal antibody crizanlizumab to reduce and delay the first vaso-occlusion crisis and the resulting pain. Gene therapy or hematopoietic stem cell transplantation to form fetal hemoglobin to restore normal hemoglobin in SCD patients could be one method of implementing a systematic approach. The screening and resistance to antibiotics of new variants or mutant genetic strains, such as the self-mutable highly pandemic coronavirus severe acute respiratory syndrome coronavirus 2 on SCD patients, are currently some of the significant challenges. As mutant variants have different degrees of resistance to antibiotics, the effect of immunization against the coronavirus on SCD patients of varying age groups with existing co-morbidities must be monitored carefully and meticulously. Research on many aspects, such as incubation time and recovery period during viral or any other microbial infections, are of great concern. Thus, research on molecular, antigen, serological, and internal physical testing to determine the extent of the complications on different biological systems, the effectiveness of immunization, and therapy is a priority.
5. Conclusions
Pneumonia, meningitis, osteomyelitis, and UTIs are prevalent among individuals with SCD in developing countries, constituting the leading factors behind its morbidity and mortality. It has also been observed that the primary infectious symptoms occur in children under the age of five, necessitating the need for immediate treatment. Priorities for the future management of infectious complications in SCD vary according to geography and socioeconomic status. Susceptibility to infection varies among individuals and across age groups. Some patients have chronic hemolytic anemia with vital organ failure due to infarctive damage, while others have no or few medical problems. A better understanding of the mechanisms underlying the increased susceptibility of these patients to infection may lead to interventions that address the underlying cause in the future. Meanwhile, the early detection and treatment of infections with hydroxyurea and blood transfusions can help to avoid severe complications and splenic dysfunction. Simultaneously, primary interventions, such as penicillin prophylaxis and vaccinations, result in significant improvements in SCD patients.
Nonetheless, these therapies are associated with substantial risks, making their routine use inappropriate. However, if a more precise and comprehensive analysis of the genetic variations conferring an increased risk of infection and testing could help stratify patients into high-risk groups, the frequency of crises could be reduced by improving the QOL of SCD patients. Additionally, it could help to postpone long-term challenges, thus also enhancing life expectancy.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaul, D.K.; Finnegan, E.; Barabino, G.A. Sickle Red Cell–Endothelium Interactions. Microcirculation 2009, 16, 97–111. [Google Scholar] [CrossRef]
- Sundd, P.; Gladwin, M.T.; Novelli, E.M. Pathophysiology of Sickle Cell Disease. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 263–292. [Google Scholar] [CrossRef]
- Lakkakula, B.V.; Sahoo, R.; Verma, H.; Lakkakula, S. Pain Management Issues as Part of the Comprehensive Care of Patients with Sickle Cell Disease. Pain Manag. Nurs. 2018, 19, 558–572. [Google Scholar] [CrossRef]
- Patra, P.K.; Lakkakula, B.V.K.S.; Verma, H.K.; Choubey, M.; Patra, S.; Khodiar, P.K. Assessment of renal function in Indian patients with sickle cell disease. Saudi J. Kidney Dis. Transplant. 2017, 28, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, L.V.K.S.; Shukla, P.; Verma, H.; Patel, S.; Patra, P. Ocular manifestations of sickle cell disease and genetic susceptibility for refractive errors. Taiwan J. Ophthalmol. 2017, 7, 89–93. [Google Scholar] [CrossRef]
- Verma, H.K.; Lakkakula, S.; Lakkakula, B.V. Retrospection of the effect of hydroxyurea treatment in patients with sickle cell disease. Acta Haematol. Pol. 2018, 49, 1–8. [Google Scholar] [CrossRef]
- Jain, D.; Odame, I. Sickle cell disease: Progress made & challenges ahead. Indian J. Med Res. 2020, 151, 505–508. [Google Scholar] [CrossRef]
- Jha, A.N.; Mishra, H.; Verma, H.K.; Pandey, I. Compound Heterozygosity of beta-Thalassemia and the Sickle Cell Hemoglobin in Various Populations of Chhattisgarh Statel, India. Hemoglobin 2018, 2, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bhanushali, A.A.; Patra, P.; Nair, D.; Verma, H.; Das, B. Genetic variant in the BCL11A (rs1427407), but not HBS1-MYB (rs6934903) loci associate with fetal hemoglobin levels in Indian sickle cell disease patients. Blood Cells Mol. Dis. 2015, 54, 4–8. [Google Scholar] [CrossRef]
- Al-Salem, A.H. Splenic Complications of Sickle Cell Anemia and the Role of Splenectomy. ISRN Hematol. 2011, 2011, 864257. [Google Scholar] [CrossRef]
- Monaco, C.P.; Fonseca, P.B.B.; Braga, J.A.P. Infectious complications after surgical splenectomy in children with sickle cell disease. Rev. Paul. Pediatr. (Engl. Ed.) 2015, 33, 150–153. [Google Scholar] [CrossRef]
- Hsu, L.; E Nnodu, O.; Brown, B.J.; Tluway, F.; King, S.; Dogara, L.G.; Patil, C.; Shevkoplyas, S.S.; Lettre, G.; Cooper, R.S.; et al. White Paper: Pathways to Progress in Newborn Screening for Sickle Cell Disease in Sub-Saharan Africa. J. Trop. Dis. 2018, 6, 260. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, J.F.; Brown, E.J. The role of the spleen in resistance to infection. Annu. Rev. Med. 1986, 37, 49–59. [Google Scholar] [CrossRef]
- Ansari, J.; Gavins, F.N.E. Ischemia-Reperfusion Injury in Sickle Cell Disease: From Basics to Therapeutics. Am. J. Pathol. 2019, 189, 706–718. [Google Scholar] [CrossRef]
- West, T.B.; West, D.W.; Ohene-Frempong, K. The presentation, frequency, and outcome of bacteremia among children with sickle cell disease and fever. Pediatr. Emerg. Care 1994, 10, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Halasa, N.B.; Shankar, S.M.; Talbot, T.R.; Arbogast, P.G.; Mitchel, E.F.; Wang, W.C.; Schaffner, W.; Craig, A.S.; Griffin, M.R. Incidence of Invasive Pneumococcal Disease among Individuals with Sickle Cell Disease before and after the Introduction of the Pneumococcal Conjugate Vaccine. Clin. Infect. Dis. 2007, 44, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Ware, R.E. Salmonella infection in sickle cell disease: A clear and present danger. J. Pediatr. 1997, 130, 350–351. [Google Scholar]
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Spirer, Z.; Weisman, Y.; Zakuth, V.; Fridkin, M.; Bogair, N. Decreased serum tuftsin concentrations in sickle cell disease. Arch. Dis. Child. 1980, 55, 566–567. [Google Scholar] [CrossRef]
- Deceulaer, K.; Wilson, W.A.; Morgan, A.G.; Serjeant, G.R. Plasma haemoglobin and complement activation in sickle cell disease. J. Clin. Lab. Immunol. 1981, 6, 57–60. [Google Scholar]
- Wilson, W.A.; Thomas, E.J. Activation of the alternative pathway of human complement by haemoglobin. Clin. Exp. Immunol. 1979, 36, 140–144. [Google Scholar]
- Cameron, P.U.; Jones, P.; Gorniak, M.; Dunster, K.; Paul, E.; Lewin, S.; Woolley, I.; Spelman, D. Splenectomy Associated Changes in IgM Memory B Cells in an Adult Spleen Registry Cohort. PLoS ONE 2011, 6, e23164. [Google Scholar] [CrossRef]
- Weller, S. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 2004, 104, 3647–3654. [Google Scholar] [CrossRef] [PubMed]
- Ballester, O.F.; Abdallah, J.M.; Prasad, A.S. Impaired IgM antibody responses to an influenza virus vaccine in adults with sickle cell anemia. Am. J. Hematol. 1985, 20, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Koffi, K.G.; Sawadogo, D.; Meite, M.; Nanho, D.C.; Tanoh, E.S.; Attia, A.K.; Sanogo, I.; Sangare, A. Reduced levels of T-cell subsets CD4+ and CD8+ in homozygous sickle cell anaemia patients with splenic defects. Hematol. J. 2003, 4, 363–365. [Google Scholar] [CrossRef]
- Romagnani, S. T-cell subsets (Th1 versus Th2). Annals of allergy, asthma & immunology: Official publication of the American College of Allergy. Asthma Immunol. 2000, 85, 9–18. [Google Scholar]
- Hibbert, J.M.; Creary, M.S.; E Gee, B.; Buchanan, I.D.; Quarshie, A.; Hsu, L.L. Erythropoiesis and Myocardial Energy Requirements Contribute to the Hypermetabolism of Childhood Sickle Cell Anemia. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 680–687. [Google Scholar] [CrossRef]
- Prasad, A.S.; Schoomaker, E.B.; Ortega, J.; Brewer, G.J.; Oberleas, D.; Oelshlegel, F.J. Zinc Deficiency in Sickle Cell Disease. Clin. Chem. 1975, 21, 582–587. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc deficiency and effects of zinc supplementation on sickle cell anemia subjects. Prog. Clin. Biol. Res. 1981, 55, 99–122. [Google Scholar]
- Fraker, P.J.; King, L.E.; Laakko, T.; Vollmer, T.L. The Dynamic Link between the Integrity of the Immune System and Zinc Status. J. Nutr. 2000, 130, 1399S–1406S. [Google Scholar] [CrossRef]
- De Franceschi, L.; Bachir, D.; Galacteros, F.; Tchernia, G.; Cynober, Y.; Neuberg, D.; Beuzard, Y.; Brugnara, C. Oral magnesium pidolate: Effects of long-term administration in patients with sickle cell disease. Br. J. Haematol. 2000, 108, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Schall, J.I.; Zemel, B.S.; Kawchak, D.A.; Ohene-Frempong, K.; Stallings, V.A. Vitamin A status, hospitalizations, and other outcomes in young children with sickle cell disease. J. Pediatr. 2004, 145, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.J.; Nagel, R.L. Sickle-cell disease. Lancet 2004, 9442, 1343–1360. [Google Scholar] [CrossRef]
- Tamouza, R.; Neonato, M.-G.; Busson, M.; Marzais, F.; Girot, R.; Labie, D.; Elion, J.; Charron, D. Infectious complications in sickle cell disease are influenced by HLA class II alleles. Hum. Immunol. 2002, 63, 194–199. [Google Scholar] [CrossRef]
- Neonato, M.-G.; Lu, C.Y.; Guilloud-Bataille, M.; Lapouméroulie, C.; Nabeel-Jassim, H.; Dabit, D.; Girot, R.; Krishnamoorthy, R.; Feingold, J.; Besmond, C.; et al. Genetic polymorphism of the mannose-binding protein gene in children with sickle cell disease: Identification of three new variant alleles and relationship to infections. Eur. J. Hum. Genet. 1999, 7, 679–686. [Google Scholar] [CrossRef][Green Version]
- Norris, C.F.; Surrey, S.; Bunin, G.R.; Schwartz, E.; Buchanan, G.R.; McKenzie, S.E. Relationship between Fc receptor IIA polymorphism and infection in children with sickle cell disease. J. Pediatr. 1996, 128, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Powars, D.R.; Chan, L.; Schroeder, W.A. Beta S-gene-cluster haplotypes in sickle cell anemia: Clinical implications. Am. J. Pediatr. Hematol. Oncol. 1990, 12, 367–374. [Google Scholar] [CrossRef]
- Adewoye, A.H.; Nolan, V.G.; Ma, Q.; Baldwin, C.; Wyszynski, D.F.; Farrell, J.J.; Farrer, L.A.; Steinberg, M.H. Association of polymorphisms of IGF1R and genes in the transforming growth factor-beta/bone morphogenetic protein pathway with bacteremia in sickle cell anemia. Clin. Infect. Dis.Off. Publ. Infect. Dis. Soc. Am. 2006, 43, 593–598. [Google Scholar] [CrossRef]
- West, D.C.; Romano, P.S.; Azari, R.; Rudominer, A.; Holman, M.; Sandhu, S. Impact of Environmental Tobacco Smoke on Children With Sickle Cell Disease. Arch. Pediatr. Adolesc. Med. 2003, 157, 1197–1201. [Google Scholar] [CrossRef]
- Cohen, R.T.; DeBaun, M.R.; Blinder, M.A.; Strunk, R.C.; Field, J.J. Smoking is associated with an increased risk of acute chest syndrome and pain among adults with sickle cell disease. Blood 2010, 115, 3852–3854. [Google Scholar] [CrossRef]
- Vichinsky, E.P. Current issues with blood transfusions in sickle cell disease. Semin. Hematol. 2001, 38 (Suppl. 1), 14–22. [Google Scholar] [CrossRef]
- Zarrouk, V.; Habibi, A.; Zahar, J.P.; Roudot-Thoraval, F.; Bachir, D.; Brun-Buisson, C.; Legrand, P.; Godeau, B.; Galacteros, F.; Lesprit, P. Bloodstream infection in adults with sickle cell disease: Association with venous catheters, Staphylococcus aureus, and bone-joint infections. Medicine 2006, 85, 43–48. [Google Scholar] [CrossRef]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef]
- Cisneros, G.S.; Thein, S.L. Recent Advances in the Treatment of Sickle Cell Disease. Front. Physiol. 2020, 11, 435. [Google Scholar] [CrossRef]
- Krishnan, S.; Setty, Y.; Betal, S.G.; Vijender, V.; Rao, K.; Dampier, C.; Stuart, M. Increased levels of the inflammatory biomarker C-reactive protein at baseline are associated with childhood sickle cell vasocclusive crises. Br. J. Haematol. 2010, 148, 797–804. [Google Scholar] [CrossRef]
- Bargoma, E.M.; Mitsuyoshi, J.K.; Larkin, S.K.; Styles, L.A.; Kuypers, F.A.; Test, S.T. Serum C-reactive protein parallels secretory phospholipase A2 in sickle cell disease patients with vasoocclusive crisis or acute chest syndrome. Blood 2005, 105, 3384–3385. [Google Scholar] [CrossRef]
- Jaillon, S.; Peri, G.; Delneste, Y.; Frémaux, I.; Doni, A.; Moalli, F.; Garlanda, C.; Romani, L.; Gascan, H.; Bellocchio, S.; et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J. Exp. Med. 2007, 204, 793–804. [Google Scholar] [CrossRef]
- Kato, G.J.; Steinberg, M.H.; Gladwin, M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Investig. 2017, 127, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, M.; Luken, B.M.; Nur, E.; van Tuijn, C.F.J.; Sins, J.W.; Brandjes, D.P.M.; Zeerleder, S.S.; Biemond, B.J. Inflammatory and endothelial markers during vaso-occlusive crisis and acute chest syndrome in sickle cell disease. Am. J. Hematol. 2017, 92, E634–E636. [Google Scholar] [CrossRef] [PubMed]
- Zeerleder, S.; Stephan, F.; Emonts, M.; de Kleijn, E.D.; Esmon, C.T.; Varadi, K.; Hack, C.E.; Hazelzet, J.A. Circulating nucleosomes and severity of illness in children suffering from meningococcal sepsis treated with protein C. Crit. Care Med. 2012, 40, 3224–3229. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, M.; Nur, E.; Biemond, B.J.; van Mierlo, G.J.; Solati, S.; Brandjes, D.P.; Otten, H.M.; Schnog, J.J.; Zeerleder, S. Nucleosomes and neutrophil activation in sickle cell disease painful crisis. Haematologica 2013, 98, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Camus, S.M.; De Moraes, J.A.; Bonnin, P.; Abbyad, P.; Le Jeune, S.; Lionnet, F.; Loufrani, L.; Grimaud, L.; Lambry, J.-C.; Charue, D.; et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood 2015, 125, 3805–3814. [Google Scholar] [CrossRef] [PubMed]
- Reeder, B.J.; Andersen, C.B.F.; Stødkilde, K.; Sæderup, K.L.; Kuhlee, A.; Raunser, S.; Graversen, J.H.; Moestrup, S.K.; Strader, M.B.; Alayash, A.I.; et al. The Redox Activity of Hemoglobins: From Physiologic Functions to Pathologic Mechanisms. Antioxid. Redox Signal. 2010, 13, 1087–1123. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Ofori-Acquah, S.F. Erythroid DAMPs drive inflammation in SCD. Blood 2014, 123, 3689–3690. [Google Scholar] [CrossRef]
- Mendonça, R.; Silveira, A.A.A.; Conran, N. Red cell DAMPs and inflammation. Inflamm. Res. 2016, 65, 665–678. [Google Scholar] [CrossRef]
- Mpollo, M.-S.E.M.; Brandt, E.B.; Shanmukhappa, S.K.; Arumugam, P.I.; Tiwari, S.; Loberg, A.; Pillis, D.; Rizvi, T.; Lindsey, M.; Jonck, B.; et al. Placenta growth factor augments airway hyperresponsiveness via leukotrienes and IL-13. J. Clin. Investig. 2015, 126, 571–584. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Strasser, A.; McDunn, J.E.; Swanson, P.E. Cell death. N. Engl. J. Med. 2009, 361, 1570–1583. [Google Scholar] [CrossRef]
- Onwubalili, J.K. Sickle cell disease and infection. J. Infect. 1983, 7, 2–20. [Google Scholar] [CrossRef]
- Adeyokunnu, A.; Hendrickse, G. Salmonella osteomyelitis in childhood. Arch. Dis. Child. 1980, 55, 175–184. [Google Scholar] [CrossRef]
- El-Hazmi, M. Infections in Sickle Cell Disease. Ann. Saudi Med. 1986, 6, 33–40. [Google Scholar] [CrossRef]
- Powars, D.; Overturf, G.; Turner, E. Is There an Increased Risk of Haemophilus influenzae Septicemia in Children with Sickle Cell Anemia? Pediatrics 1983, 71, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Ochocinski, D.; Dalal, M.; Black, L.V.; Carr, S.; Lew, J.; Sullivan, K.; Kissoon, N. Life-Threatening Infectious Complications in Sickle Cell Disease: A Concise Narrative Review. Front. Pediatr. 2020, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.G. The Role of Infection in the Pathogenesis of Vaso-Occlusive Crisis in Patients with Sickle Cell Disease. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e2011028. [Google Scholar] [CrossRef]
- Gonzales, J.; Chakraborty, T.; Romero, M.; Abu Mraheil, M.; Kutlar, A.; Pace, B.; Lucas, R. Streptococcus pneumoniae and Its Virulence Factors H2O2 and Pneumolysin Are Potent Mediators of the Acute Chest Syndrome in Sickle Cell Disease. Toxins 2021, 13, 157. [Google Scholar] [CrossRef]
- Brown, B.; Dada-adegbola, H.; Trippe, C.; Olopade, O. Prevalence and Etiology of Bacteremia in Febrile Children with Sickle Cell Disease at a Nigeria Tertiary Hospital. Mediterr. J. Hematol. Infect. Dis. 2017, 9, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wierenga, K.J.J.; Hambleton, I.R.; Wilson, R.M.; Alexander, H.; E Serjeant, B.; Serjeant, G.R. Significance of fever in Jamaican patients with homozygous sickle cell disease. Arch. Dis. Child. 2001, 84, 156–159. [Google Scholar] [CrossRef]
- Al Achkar, M.; Rogers, J.S.; Muszynski, M.J. Pantoea species sepsis associated with sickle cell crisis in a pregnant woman with a history of pica. Am. J. Case Rep. 2012, 13, 26–28. [Google Scholar] [CrossRef]
- Alkindi, S.; Matwani, S.; Al-Maawali, A.; Al-Maskari, B.; Pathare, A. Complications of PORT-A-CATH® in patients with sickle cell disease. J. Infect. Public Heal. 2012, 5, 57–62. [Google Scholar] [CrossRef]
- Cannas, G.; Merazga, S.; Virot, E. Sickle Cell Disease and Infections in High- and Low-Income Countries. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019042. [Google Scholar] [CrossRef]
- Quinn, C.T. Sickle cell disease in childhood: From newborn screening through transition to adult medical care. Pediatr. Clin. North. Am. 2013, 60, 1363–1381. [Google Scholar] [CrossRef]
- Battersby, A.J.; Knox-Macaulay, H.H.; Carrol, E.D. Susceptibility to invasive bacterial infections in children with sickle cell disease. Pediatr. Blood Cancer 2010, 55, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Rincón-López, E.M.; Gómez, M.L.N.; Matos, T.H.; Saavedra-Lozano, J.; de la Red, Y.A.; Rupérez, B.H.; de Julián, E.C. RETRO-DREP Study Group Low-risk factors for severe bacterial infection and acute chest syndrome in children with sickle cell disease. Pediatr. Blood Cancer 2019, 66, e27667. [Google Scholar] [CrossRef] [PubMed]
- BiscevicTokic, J.; Tokic, N.; Musanovic, A. Pneumonia as the Most Common Lower Respiratory Tract Infection. Med. Arch. 2013, 67, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Suarez, V.; Michel, F.; Toscano, C.M.; Bierrenbach, A.L.; Gonzales, M.; Alencar, A.P.; Matus, C.R.; Andrus, J.K.; de Oliveira, L.H. Impact of pneumococcal conjugate vaccine in children morbidity and mortality in Peru: Time series analyses. Vaccine 2016, 34, 4738–4743. [Google Scholar] [CrossRef]
- Yousif, T.I.; Elnazir, B. Approach to a child with recurrent pneumonia. Sudan. J. Paediatr. 2015, 15, 71–77. [Google Scholar]
- Yee, M.E.; Bakshi, N.; Graciaa, S.H.; Lane, P.A.; Jerris, R.C.; Wang, Y.F.; Yildirim, I. Incidence of invasive Haemophilus influenzae infections in children with sickle cell disease. Pediatr. Blood Cancer 2019, 66, e27642. [Google Scholar] [CrossRef]
- Jain, S.; Bakshi, N.; Krishnamurti, L. Acute Chest Syndrome in Children with Sickle Cell Disease. Pediatr. Allergy Immunol. Pulmonol. 2017, 30, 191–201. [Google Scholar] [CrossRef]
- Howard, J.; Hart, N.; Roberts-Harewood, M.; Cummins, M.; Awogbade, M.; Davis, B.; BCSH Committee. Guideline on the management of acute chest syndrome in sickle cell disease. Br. J. Haematol. 2015, 169, 492–505. [Google Scholar] [CrossRef]
- Clay, E.L.J.; Burrell, T.; Belhorn, T.; Redding-Lallinger, R. Immunogenicity of pneumococcal vaccination in a patient with sickle hemoglobinopathy: A case report. Clin. Case Rep. 2015, 3, 618–621. [Google Scholar] [CrossRef]
- Wahl, B.; Brien, K.L.O.; Greenbaum, A.; Majumder, A.; Liu, L.; Chu, Y.; Lukšić, I.; Nair, H.; McAllister, D.A.; Campbell, H.; et al. Articles Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for. Lancet Glob. Health 2018, 6, e744–e757. [Google Scholar] [CrossRef]
- Mani, C.S. Acute Pneumonia and Its Complications. Princ. Pract. Pediatr. Infect. Dis. 2018, 238–249.e4. [Google Scholar] [CrossRef]
- Chenou, F.; Azevedo, J.; Leal, H.F.; Gonçalves, M.d.S.; Reis, J.N. Bacterial meningitis in patients with sickle cell anemia in Salvador, Bahia, Brazil: A report on ten cases. Hematol. Transfus. Cell Ther. 2020, 42, 139–144. [Google Scholar] [CrossRef]
- Kiriazopulos, D.; Pedroni, P.; Occhi, G.; Sassi, G.; Corna, A.; Cieri, F.; Cipolletta, E.; Orobello, M.; Colombo, M. Pneumococcal Meningitis in a Child With Sickle Cell Anemia: A Case Report. Int. J. Clin. Pediatr. 2015, 4, 168–170. [Google Scholar] [CrossRef][Green Version]
- Nottidge, V.A. Pneumococcal Meningitis in Sickle Cell Disease in Childhood. Arch. Pediatr. Adolesc. Med. 1983, 137, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P. Overview of Acute and Chronic Meningitis. Neurol. Clin. 1999, 17, 691–710. [Google Scholar] [CrossRef] [PubMed]
- Rankine-Mullings, A.E.; Owusu-Ofori, S. Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease. Cochrane Database Syst. Rev. 2017, 10, CD003427. [Google Scholar] [CrossRef]
- Thompson, A.A. Primary Prophylaxis in Sickle Cell Disease: Is It Feasible? Is It Effective? Hematology 2011, 2011, 434–439. [Google Scholar] [CrossRef]
- Junior, G.B.D.S.; Daher, E.D.F.; Da Rocha, F.A.C. Osteoarticular involvement in sickle cell disease. Rev. Bras. Hematol. E Hemoter. 2012, 34, 156–164. [Google Scholar] [CrossRef]
- Mary, P. Sickle cell disease as a cause of osteoarthritis. Arch. Pediatr. 2008, 15, 639–641. [Google Scholar] [CrossRef]
- Anand, A.J.; Glatt, A.E. Salmonella osteomyelitis and arthritis in sickle cell disease. Semin. Arthritis Rheum. 1994, 24, 211–221. [Google Scholar] [CrossRef] [PubMed]
- AlFawaz, T.; Alzumar, O.; AlShahrani, D.; Alshehri, M. Severity of Salmonella infection among sickle cell diseases pediatric patients: Description of the infection pattern. Int. J. Pediatr. Adolesc. Med. 2019, 6, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Fontalis, A.; Hughes, K.; Nguyen, M.P.; Williamson, M.; Yeo, A.; Lui, D.; Gelfer, Y. The challenge of differentiating vaso-occlusive crises from osteomyelitis in children with sickle cell disease and bone pain: A 15-year retrospective review. J. Child. Orthop. 2019, 13, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Thanni, L.O.A. Bacterial osteomyelitis in major sickling haemoglobinopathies: Geographic difference in pathogen prevalence. Afr. Health Sci. 2006, 6, 236–239. [Google Scholar]
- Almeida, A.; Roberts, I. Bone involvement in sickle cell disease. Br. J. Haematol. 2005, 129, 482–490. [Google Scholar] [CrossRef]
- Calhoun, J.H.; Manring, M.M.; Shirtliff, M. Osteomyelitis of the long bones. Semin. Plast. Surg. 2009, 23, 59–72. [Google Scholar] [CrossRef]
- Burnett, M.W.; Bass, J.W.; Cook, B.A. Etiology of Osteomyelitis Complicating Sickle Cell Disease. Pediatrics 1998, 101, 296–297. [Google Scholar] [CrossRef]
- Pszolla, N.; Sarkar, M.R.; Strecker, W.; Kern, P.; Kinzl, L.; Meyers, W.M.; Portaels, F. Buruli Ulcer: A Systemic Disease. Clin. Infect. Dis. 2003, 37, e78–e82. [Google Scholar] [CrossRef]
- Coates, T.D.; Wood, J.C. How we manage iron overload in sickle cell patients. Br. J. Haematol. 2017, 177, 703–716. [Google Scholar] [CrossRef]
- Raghupathy, R.; Manwani, D.; Little, J.A. Iron Overload in Sickle Cell Disease. Adv. Hematol. 2010, 2010, 272940. [Google Scholar] [CrossRef]
- Shemisa, K.; Jafferjee, N.; Thomas, D.; Jacobs, G.; Meyerson, H.J. Mycobacterium avium Complex Infection in a Patient with Sickle Cell Disease and Severe Iron Overload. Case Rep. Infect. Dis. 2014, 2014, 405323. [Google Scholar] [CrossRef] [PubMed]
- Thorell, E.A.; Sharma, M.; Jackson, M.A.; Selvarangan, R.; Woods, G.M. Disseminated Nontuberculous Mycobacterial Infections in Sickle Cell Anemia Patients. J. Pediatr. Hematol. 2006, 28, 678–681. [Google Scholar] [CrossRef]
- Edrees, N.; Howard, T.H. Unusual Presentation for Unusual Infection: Disseminated Mycobacterium Avium-Intracellulare complex (MAC) in Patient with Sickle Cell Anemia Mimicking Blood Transfusion Reaction. Blood 2014, 124, 4980. [Google Scholar] [CrossRef]
- Esnakula, A.K.; Mummidi, S.K.; Oneal, P.A.; Naab, T.J. Sepsis caused by Mycobacterium terrae complex in a patient with sickle cell disease. BMJ Case Rep. 2013, 2013, bcr2013009159. [Google Scholar] [CrossRef]
- Ashraf, M.S.; Swinker, M.; Augustino, K.L.; Nobles, D.; Knupp, C.; Liles, D.; Christie, J.; Ramsey, K.M. Outbreak of Mycobacterium mucogenicum Bloodstream Infections among Patients with Sickle Cell Disease in an Outpatient Setting. Infect. Control Hosp. Epidemiol. 2012, 33, 1132–1136. [Google Scholar] [CrossRef]
- Edun, B.; Shah, A.; Durkin, M.; Whitmire, M.; Williams, S.P.; Albrecht, H.; Al-Hasan, M.; Weissman, S. Non-tuberculous mycobacterial bloodstream infections in patients with indwelling vascular catheters—The role of sickle cell anaemia. Infect. Dis. 2017, 49, 341–346. [Google Scholar] [CrossRef]
- Droz, N.; De Lauzanne, A.; Holvoet, L.; Missud, F.; Benkerrou, M.; Brousse, V.; Odièvre, M.-H.; Faye, A.; Koehl, B. Tuberculosis in children with sickle cell anaemia: A retrospective study in French tertiary care centres. Eur. J. Pediatr. 2017, 176, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Yanda, A.N.A.; Nansseu, J.R.N.; Awa, H.D.M.; Tatah, S.A.; Seungue, J.; Eposse, C.; Koki, P.O.N. Burden and spectrum of bacterial infections among sickle cell disease children living in Cameroon. BMC Infect. Dis. 2017, 17, 211. [Google Scholar] [CrossRef]
- Musonda, T.; Zulu, M.; Samutela, M.; Kalonda, A.; Mantina, H.; Okuku, P.; Sinkala, M.; Nkhoma, P. Leucocytosis and Asymptomatic Urinary Tract Infections in Sickle Cell Patients at a Tertiary Hospital in Zambia. Anemia 2020, 2020, 3792728. [Google Scholar] [CrossRef] [PubMed]
- Chukwu, B.F.; Okafor, H.U.; Ikefuna, A.N. Asymptomatic bacteriuria in children with sickle cell anemia at The University of Nigeria teaching hospital, Enugu, South East, Nigeria. Ital. J. Pediatr. 2011, 37, 45. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S.; Osei, J.A.; Anim-Baidoo, I.; Darkwah, S. Risk of Asymptomatic Bacteriuria among People with Sickle Cell Disease in Accra, Ghana. Diseases 2017, 5, 4. [Google Scholar] [CrossRef]
- Cumming, V.; Ali, S.; Forrester, T.; Roye-Green, K.; Reid, M. Asymptomatic bacteriuria in sickle cell disease: A cross-sectional study. BMC Infect. Dis. 2006, 6, 46. [Google Scholar] [CrossRef]
- Mava, Y.; Bello, M.; Ambe, J.; Zailani, S. Antimicrobial sensitivity pattern of organisms causing urinary tract infection in children with sickle cell anemia in Maiduguri, Nigeria. Niger. J. Clin. Pr. 2012, 15, 420–423. [Google Scholar] [CrossRef]
- Neves, P.D.M.d.M.; Reichert, B.V.; Bridi, R.A.; Yu, L.; Dias, C.B.; Pinheiro, R.B.B.; Testagrossa, L.d.A.; Cavalcante, L.B.; Malheiros, D.M.A.C.; Jorge, L.B.; et al. Atypical presentation of acute post-infectious glomerulonephritis in patients with sickle cell disease: Report of two cases. BMC Nephrol. 2020, 21, 56. [Google Scholar] [CrossRef]
- Dutta, D.; Methe, B.; Amar, S.; Morris, A.; Lim, S.H. Intestinal injury and gut permeability in sickle cell disease. J. Transl. Med. 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Fast, L.; Morris, A. Sickle cell vaso-occlusive crisis: It’s a gut feeling. J. Transl. Med. 2016, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- Green, B.T.; Branch, M.S. Ischemic colitis in a young adult during sickle cell crisis: Case report and review. Gastrointest. Endosc. 2003, 57, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Methé, B.A.; Knoll, B.M.; Morris, A.; Obaro, S.K. Invasive non-typhoidal Salmonella in sickle cell disease in Africa: Is increased gut permeability the missing link? J. Transl. Med. 2018, 16, 239. [Google Scholar] [CrossRef]
- Sigaúque, B.; Roca, A.M.; Mandomando, I.D.; Morais, L.D.; Quintó, L.; Sacarlal, J.; Macete, E.; Nhamposa, T.; Machevo, S.; Aide, P.; et al. Community-Acquired Bacteremia Among Children Admitted to a Rural Hospital in Mozambique. Pediatr. Infect. Dis. J. 2009, 28, 108–113. [Google Scholar] [CrossRef]
- Obaro, S.K.; Hassan-hanga, F.; Olateju, E.K.; Umoru, D.; Lawson, L.; Olanipekun, G.; Ibrahim, S.; Munir, H.; Ihesiolor, G.; Maduekwe, A.; et al. Salmonella Bacteremia Among Children in Central and Northwest Nigeria, 2008–2015. Clin. Infect. Dis. 2015, 61 (Suppl. 4), 325–331. [Google Scholar] [CrossRef]
- MacLean, J.E.; Atenafu, E.; Kirby-Allen, M.; MacLusky, I.B.; Stephens, D.; Grasemann, H.; Subbarao, P. Longitudinal decline in lung volume in a population of children with sickle cell disease. Am. J. Respir. Crit. Care Med. 2009, 178, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Lunt, A.; McGhee, E.; Sylvester, K.; Rafferty, G.; Dick, M.; Rees, D.; Height, S.; Thein, S.L.; Greenough, A. Longitudinal assessment of lung function in children with sickle cell disease. Pediatr. Pulmonol. 2015, 51, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Claudio, A.M.; Foltanski, L.; Delay, T.; Britell, A.; Duckett, A.; Weeda, E.R.; Bohm, N. Antibiotic Use and Respiratory Pathogens in Adults With Sickle Cell Disease and Acute Chest Syndrome. Ann. Pharmacother. 2019, 53, 991–996. [Google Scholar] [CrossRef]
- Alkindi, S.; Al-Yahyai, T.; Raniga, S.; Boulassel, M.R.; Pathare, A. Respiratory Viral Infections in Sickle Cell Anemia: Special Emphasis on H1N1 Co-infection. Oman Med. J. 2020, 35, e197. [Google Scholar] [CrossRef]
- Bundy, D.G.; Strouse, J.J.; Casella, J.F.; Miller, M.R. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics 2010, 125, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Morris, C.R. Asthma Management in Sickle Cell Disease. BioMed Res. Int. 2013, 2013, 604140. [Google Scholar] [CrossRef]
- Powars, D.; A Weidman, J.; Odom-Maryon, T.; Niland, J.C.; Johnson, C. Sickle cell chronic lung disease: Prior morbidity and the risk of pulmonary failure. Medicine 1988, 67, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Slavov, S.N.; Kashima, S.; Pinto, A.C.S.; Covas, D.T. Human parvovirus B19: General considerations and impact on patients with sickle-cell disease and thalassemia and on blood transfusions. FEMS Immunol. Med. Microbiol. 2011, 62, 247–262. [Google Scholar] [CrossRef]
- Novelli, E.M.; Gladwin, M.T. Crises in Sickle Cell Disease. Chest 2016, 149, 1082–1093. [Google Scholar] [CrossRef]
- Bakarey, A.S.; Akinboade, I.O.; Aken’ova, Y.A. Transmission transmissible hepatitis B virus markers of infection among sickle cell disease patients receiving care at a tertiary health facility in Ibadan, southwest Nigeria. J. Immunoass. Immunochem. 2018, 39, 416–427. [Google Scholar] [CrossRef]
- Sonderup, M.W.; Afihene, M.; Ally, R.; Apica, B.; Awuku, Y.; Cunha, L.; Dusheiko, G.; Gogela, N.; Lohouès-Kouacou, M.-J.; Lam, P.; et al. Hepatitis C in sub-Saharan Africa: The current status and recommendations for achieving elimination by 2030. Lancet Gastroenterol. Hepatol. 2017, 2, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Mulumba, L.L.; Wilson, L. Sickle cell disease among children in Africa: An integrative literature review and global recommendations. Int. J. Afr. Nurs. Sci. 2015, 3, 56–64. [Google Scholar] [CrossRef]
- Baseke, J.; Musenero, M.; Mayanja-Kizza, H. Prevalence of hepatitis B and C and relationship to liver damage in HIV infected patients attending Joint Clinical Research Centre Clinic (JCRC), Kampala, Uganda. Afr. Health Sci. 2015, 15, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Swaim, M.W.; Agarwal, S.; Rosse, W.F. Successful treatment of hepatitis C in sickle-cell disease. Ann. Intern. Med. 2000, 133, 750–751. [Google Scholar] [CrossRef]
- Babatola, A.O.; Olatunya, O.S.; Faboya, A.O.; Ojo, T.O.; Kayode, S.T.; Komolafe, A.K.; Oyelami, O.A.; Ajayi, O.A. Hepatitis B and C Infections Among Pediatric Patients with Sickle Cell Disease at a Tertiary Hospital in Nigeria. Arch. Pediatr. Infect. Dis. 2020, 8, e101632. [Google Scholar] [CrossRef]
- Mora, N.; Adams, W.H.; Kliethermes, S.; Dugas, L.; Balasubramanian, N.; Sandhu, J.; Nde, H.; Small, C.; Jose, J.; Scaglione, S.; et al. A Synthesis of Hepatitis C prevalence estimates in Sub-Saharan Africa: 2000–2013. BMC Infect. Dis. 2016, 16, 283. [Google Scholar] [CrossRef]
- Owusu, E.D.A.; Visser, B.J.; Nagel, I.M.; Mens, P.F.; Grobusch, M.P. The Interaction Between Sickle Cell Disease and HIV Infection: A Systematic Review. Clin. Infect. Dis. 2014, 60, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.M.; Lanzkron, S.M. Standard Definitions of Pneumococcal Immunity May Not Accurately Predict Protection in Adults with Sickle Cell Disease. Blood 2019, 134, 1014. [Google Scholar] [CrossRef]
- Belisário, A.R.; Blatyta, P.F.; Vivanco, D.; Oliveira, C.D.L.; Carneiro-Proietti, A.B.; Sabino, E.C.; de Almeida-Neto, C.; Loureiro, P.; Mateos, S.d.O.G.; Flor-Park, M.V.; et al. Association of HIV infection with clinical and laboratory characteristics of sickle cell disease. BMC Infect. Dis. 2020, 20, 638. [Google Scholar] [CrossRef]
- Nouraie, M.; Nekhai, S.; Gordeuk, V.R. Sickle cell disease is associated with decreased HIV but higher HBV and HCV comorbidities in U.S. hospital discharge records: A cross-sectional study. Sex. Transm. Infect. 2012, 88, 528–533. [Google Scholar] [CrossRef]
- Odera, E.B.; Kwobah, C.; Stone, G.; Some, F.; Vreeman, R.C. Sickle cell disease and HIV: A case highlighting management challenges for children in a resource-limited setting. J. Int. Assoc. Provid. AIDS Care 2014, 13, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.P.M.; Lyra, I.M.; Reis, M.G.; Goncalves, M.S. The association of infection and clinical severity in sickle cell anaemia patients. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 121–126. [Google Scholar] [CrossRef]
- Kelly, S.; Jacobs, E.S.; Stone, M.; Keating, S.M.; Lee, T.-H.; Chafets, D.; Heitman, J.; Dimapasoc, M.; Operskalski, E.; Hagar, W.; et al. Influence of sickle cell disease on susceptibility to HIV infection. PLoS ONE 2020, 15, e0218880. [Google Scholar] [CrossRef]
- Halstead, S.; Wilder-Smith, A. Severe dengue in travellers: Pathogenesis, risk and clinical management. J. Travel Med. 2019, 26, taz062. [Google Scholar] [CrossRef]
- Martina, B.E.E.; Koraka, P.; Osterhaus, A.D.M.E. Dengue Virus Pathogenesis: An Integrated View. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef]
- Spiropoulou, C.F.; Srikiatkhachorn, A. The role of endothelial activation in dengue hemorrhagic fever and hantavirus pulmonary syndrome. Virulence 2013, 4, 525–536. [Google Scholar] [CrossRef]
- Rankine-mullings, A.; Reid, M.E.; Moo, M.; Richards-dawson, M.; Knight, J.M. A Retrospective Analysis of the Signi fi cance of Haemoglobin SS and SC in Disease Outcome in Patients With Sickle Cell Disease and Dengue Fever. EBioMedicine 2015, 2, 937–941. [Google Scholar] [CrossRef]
- Jentes, E.S.; Lash, R.R.; Johansson, M.A.; Sharp, T.M.; Henry, R.; Brady, O.J.; Sotir, M.J.; Hay, S.I.; Margolis, H.S.; Brunette, G.W. Evidence-based risk assessment and communication: A new global dengue-risk map for travellers and clinicians. J. Travel Med. 2016, 23, taw062. [Google Scholar] [CrossRef]
- Moesker, F.M.; Muskiet, F.D.; Koeijers, J.J.; Fraaij, P.L.A.; Gerstenbluth, I.; van Gorp, E.C.M.; Osterhaus, A.D.M.E. Fatal Dengue in Patients with Sickle Cell Disease or Sickle Cell Anemia in Curaçao: Two Case Reports. PLoS Negl. Trop. Dis. 2013, 7, e2203. [Google Scholar] [CrossRef] [PubMed]
- Elenga, N.; Celicourt, D.; Muanza, B.; Elana, G.; Hocquelet, S.; Tarer, V.; Maillard, F.; Sibille, G.; Doumdo, L.D.; Petras, M.; et al. Dengue in hospitalized children with sickle cell disease: A retrospective cohort study in the French departments of America. J. Infect. Public Health 2019, 13, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Elia, G.M.; Angel, A.; Regacini, R.; Nais, R.P.; dos Santos, A.R.A.; Vieira, P.P.M.G.; Braga, J.A.P. Acute chest syndrome and COVID-19 in sickle cell disease pediatric patients. Hematol. Transfus. Cell Ther. 2020, 43, 104–108. [Google Scholar] [CrossRef]
- Garg, S.; Kim, L.; Whitaker, M.; O’Halloran, A.; Cummings, C.; Holstein, R.; Prill, M.; Chai, S.J.; Kirley, P.D.; Alden, N.B.; et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 458–464. [Google Scholar] [CrossRef]
- Telfer, P.; De La Fuente, J.; Sohal, M.; Brown, R.; Eleftheriou, P.; Roy, N.; Piel, F.B.; Chakravorty, S.; Gardner, K.; Velangi, M.; et al. Real-time national survey of COVID-19 in hemoglobinopathy and rare inherited anemia patients. Haematologica 2020, 105, 2651–2654. [Google Scholar] [CrossRef]
- Minniti, C.P.; Zaidi, A.U.; Nouraie, M.; Manwani, D.; Crouch, G.D.; Crouch, A.S.; Callaghan, M.U.; Carpenter, S.; Jacobs, C.; Han, J.; et al. Clinical predictors of poor outcomes in patients with sickle cell disease and COVID-19 infection. Blood Adv. 2021, 5, 207–215. [Google Scholar] [CrossRef]
- Panepintoa, J.A.; Brandow, A.; Mucalo, L.; Yusuf, F.; Singh, A.; Taylor, B.; Woods, K.; Payne, A.B.; Peacock, G.; Schieve, L.A. Coronavirus Disease among Persons with Sickle Cell Disease, United States, March 20–May 21, 2020. Emerg. Infect. Dis. 2020, 26, 2473. [Google Scholar] [CrossRef]
- Chakravorty, S.; Padmore-Payne, G.; Ike, F.; Tshibangu, V.; Graham, C.; Rees, D.; Stuart-Smith, S. COVID-19 in patients with sickle cell disease—A case series from a UK Tertiary Hospital. Haematologica 2020, 105, 2691–2693. [Google Scholar] [CrossRef] [PubMed]
- Arlet, J.-B.; de Luna, G.; Khimoud, D.; Odièvre, M.-H.; de Montalembert, M.; Joseph, L.; Chantalat-Auger, C.; Flamarion, E.; Bartolucci, P.; Lionnet, F.; et al. Prognosis of patients with sickle cell disease and COVID-19: A French experience. Lancet Haematol. 2020, 7, e632–e634. [Google Scholar] [CrossRef] [PubMed]
- Menapace, L.A.; Thein, S.L. COVID-19 and sickle cell disease. Haematologica 2020, 105, 2501–2504. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Perisetti, A.; Kathirvelu, B.; Gajendran, M.; Ghanta, S.; Onukogu, I.; Lao, T.; Anwer, F. Low morbidity and mortality with COVID-19 in sickle cell disease: A single center experience. Ejhaem 2020, 1, 608–614. [Google Scholar] [CrossRef]
- AbdulRahman, A.; AlAli, S.; Yaghi, O.; Shabaan, M.; Otoom, S.; Atkin, S.L.; AlQahtani, M. COVID-19 and sickle cell disease in Bahrain. Int. J. Infect. Dis. 2020, 101, 14–16. [Google Scholar] [CrossRef]
- Hussain, F.A.; Njoku, F.U.; Saraf, S.L.; Molokie, R.E.; Gordeuk, V.R.; Han, J. COVID-19 infection in patients with sickle cell disease. Br. J. Haematol. 2020, 189, 851–852. [Google Scholar] [CrossRef]
- Balanchivadze, N.; Kudirka, A.A.; Askar, S.; Almadhoun, K.; Kuriakose, P.; Fadel, R.; Dabak, V. Impact of COVID-19 Infection on 24 Patients with Sickle Cell Disease. One Center Urban Experience, Detroit, MI, USA. Hemoglobin 2020, 44, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.C.; Murphy, M.L.; Hendricks, H.; Dulek, D.E.; Volanakis, E.J.; Borinstein, S.C. COVID-19 pneumonia in a pediatric sickle cell patient requiring red blood cell exchange. Clin. Case Rep. 2021, 9, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Sahu, K.K.; George, L.; Jones, N.; Mangla, A. COVID-19 in patients with sickle cell disease: A single center experience from Ohio, United States. J. Med. Virol. 2021, 93, 2591–2594. [Google Scholar] [CrossRef] [PubMed]
- Lakkakula, B.V.K.S.; Pattnaik, S. The HBG2 rs7482144 (C > T) Polymorphism is Linked to HbF Levels but not to the Severity of Sickle Cell Anemia. J. Pediatr. Genet. 2021, 12, 129–134. [Google Scholar] [CrossRef]
- Yazdany, J.; Kim, A.H.J. Use of Hydroxychloroquine and Chloroquine During the COVID-19 Pandemic: What Every Clinician Should Know. Ann. Intern. Med. 2020, 172, 754–755. [Google Scholar] [CrossRef]
- Luzzatto, L. Sickle Cell Anaemia and Malaria. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012065. [Google Scholar] [CrossRef]
- Mwaiswelo, R.O.; Mawala, W.; Iversen, P.O.; de Montalembert, M.; Luzzatto, L.; Makani, J. Sickle cell disease and malaria: Decreased exposure and asplenia can modulate the risk from Plasmodium falciparum. Malar. J. 2020, 19, 165. [Google Scholar] [CrossRef]
- Achkar, M.A.; Rogers, J.S.; Muszynski, M.J. Resistance to Plasmodium falciparum in sickle cell trait erythrocytes is driven by oxygen-dependent growth inhibition. Proc. Natl. Acad. Sci. USA 2018, 115, 7350–7355. [Google Scholar]
- Dada-Adegbola, H.O.; Brown, B.J.; Labaeka, A.A. Prevalence of malaria and performance of a rapid diagnostic test for malaria in febrile children with sickle cell disease. Pediatr. Hematol. Oncol. J. 2018, 3, 42–45. [Google Scholar] [CrossRef]
- Goheen, M.; Wegmüller, R.; Bah, A.; Darboe, B.; Danso, E.; Affara, M.; Gardner, D.; Patel, J.; Prentice, A.; Cerami, C. Anemia Offers Stronger Protection Than Sickle Cell Trait Against the Erythrocytic Stage of Falciparum Malaria and This Protection Is Reversed by Iron Supplementation. EBioMedicine 2016, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Cholera, R.; Brittain, N.J.; Gillrie, M.R.; Lopera-Mesa, T.M.; Diakité, S.A.S.; Arie, T.; Krause, M.A.; Guindo, A.; Tubman, A.; Fujioka, H.; et al. Impaired cytoadherence of Plasmodium falciparum -infected erythrocytes containing sickle hemoglobin. Proc. Natl. Acad. Sci. USA 2008, 105, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Eleonore, N.L.E.; Cumber, S.N.; Charlotte, E.E.; Lucas, E.E.; Edgar, M.M.L.; Nkfusai, C.N.; Geh, M.M.; Ngenge, B.M.; Bede, F.; Fomukong, N.H.; et al. Malaria in patients with sickle cell anaemia: Burden, risk factors and outcome at the Laquintinie hospital, Cameroon. BMC Infect. Dis. 2020, 20, 40. [Google Scholar] [CrossRef]
- White, N.J. Anaemia and malaria. Malar. J. 2018, 17, 371. [Google Scholar] [CrossRef]
- Atiku, S.M.; Louise, N.; Kasozi, D.M. Severe oxidative stress in sickle cell disease patients with uncomplicated Plasmodium falciparum malaria in Kampala, Uganda. BMC Infect. Dis. 2019, 19, 600. [Google Scholar] [CrossRef]
- Ahmed, S.; Uraka, J. Impact of intestinal parasites on haematological parameters of sickle-cell anaemia patients in Nigeria. East. Mediterr. Heal. J. 2011, 17, 710–713. [Google Scholar] [CrossRef]
- Mahdi, N.K.; Ali, N. Intestinal parasites, including Cryptosporidium species, in Iraqi patients with sickle-cell anaemia. East. Mediterr. Heal. J. 2002, 8, 345–349. [Google Scholar] [CrossRef]
- Ahmed, S.G.; Ibrahim, U. A compendium of pathophysiologic basis of etiologic risk factors for painful vaso-occlusive crisis in sickle cell disease. Niger. J. Basic Clin. Sci. 2017, 14, 57. [Google Scholar] [CrossRef]
- Hernigou, P.; Daltro, G.; Flouzat-Lachaniette, C.-H.; Roussignol, X.; Poignard, A. Septic Arthritis in Adults with Sickle Cell Disease Often is Associated with Osteomyelitis or Osteonecrosis. Clin. Orthop. Relat. Res. 2010, 468, 1676–1681. [Google Scholar] [CrossRef]
- Guabiraba, R.; Ryffel, B. Dengue virus infection: Current concepts in immune mechanisms and lessons from murine models. Immunology 2014, 141, 143–156. [Google Scholar] [CrossRef]
- Motran, C.C.; Silvane, L.; Chiapello, L.S.; Theumer, M.G.; Ambrosio, L.F.; Volpini, X.; Celias, D.P.; Cervi, L. Helminth Infections: Recognition and Modulation of the Immune Response by Innate Immune Cells. Front. Immunol. 2018, 9, 664. [Google Scholar] [CrossRef] [PubMed]
- Seed, J.R. Protozoa: Pathogenesis and Defenses. In Medical Microbiology, 4th ed.; Baron, S., Ed.; The University of Texas Medical Branch: Galveston, TX, USA, 1996. [Google Scholar]
- Alsayegh, F.; Mousa, S.A. Challenges in the Management of Sickle Cell Disease During SARS-CoV-2 Pandemic. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620955240. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).