Abstract
Grapevine breeding programs face difficulties in preserving germplasm, especially from species and interspecific hybrids, since most collections are maintained in the field and exposed to biotic and abiotic stress, which can lead to material loss. The Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF) Grapevine Breeding Program faces similar challenges, limiting studies on hybrids resistant to the nematode Pratylenchus brachyurus and downy mildew (Plasmopara viticola), which are valuable for genetic improvement. This study aimed to implement in vitro conservation under minimal growth conditions for interspecific hybrids of Vitis spp. from the UENF program. The protocol followed a completely randomized design in a 2 × 2 × 3 factorial scheme: two hybrids (CH1.2 and CH1.3), two temperatures (18 ± 1 °C and 27 ± 2 °C), and three sucrose concentrations (10, 20, and 30 g L−1), over 180 days of in vitro culture. The results showed that conservation of the UENF hybrids is feasible using nodal segments as explants, at 18 ± 2 °C and 10 g L−1 of sucrose, for up to four months. This protocol may also be applied to other Vitis spp., contributing to the preservation and continued study of valuable germplasm.
1. Introduction
In 2023, Brazil harvested approximately 853 thousand tons of grapes, with the Northeast region contributing about 67% of this volume. The six states with the highest production volumes were Pernambuco (58.2%), São Paulo (17.9%), Bahia (8.5%), Santa Catarina (6.8%), Paraná (5.1%), and Minas Gerais (2.4%). In the same year, the São Francisco Valley produced around 554 thousand tons, representing a 40% increase compared to 2022 [1,2].
However, both Brazilian and global grape production face challenges primarily due to pests and diseases, which have caused a gradual decline in the productive vitality of grapevine cultivars [1,3,4,5,6]. In response, producers and breeders increasingly seek cultivars with high productivity, greater yield, resistance to pests and diseases, and tolerance to abiotic stresses, such as adaptation to Brazil’s high-temperature regions [7,8].
To meet these demands, breeding programs focus on genetic improvement through interspecific hybridization, the main method to transfer resistance genes from wild Vitis species to cultivated varieties [5]. In this context, the Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF) aims to develop new table grape cultivars adapted to semi-arid regions like the northern and northwestern regions of Rio de Janeiro and the Northeast, which currently lead national table grape production [4].
However, UENF’s interspecific hybrids are cultivated in greenhouses and face adaptation difficulties, along with pest and disease pressures, leading to losses of valuable genetic material. This problem is not unique to UENF but affects many grapevine collections maintained in the field. For example, UENF’s grapevine collection decreased from 81 interspecific hybrids in 2011 to only 63 by 2018, including both nematode (Pratylenchus brachyurus) and downy mildew-resistant and susceptible hybrids. As of 2025, there are 23 clonal plants from 6 hybrids that show resistance to nematodes and downy mildew [3,7,8,9,10,11,12,13].
Given this, in vitro conservation is essential. Slow growth in vitro cultivation is a viable strategy to preserve the genetic variability of grapevine germplasm banks. Various techniques can slow plant growth in vitro, such as reducing temperature and altering nutrient medium composition. Besides optimizing space, these collections are protected from climatic fluctuations and pest and disease attacks over the medium and long terms, reducing labor costs and facilitating access [14,15,16,17].
Suggested that reducing the temperature for tropical species to between 15 and 25 °C can promote slow in vitro growth. This temperature reduction decreases plant metabolism by altering enzyme content and activity, as well as cell membrane function [18]. Changes in leaf color to light green and yellowish, indicating the onset of senescence and leaf abscission, have also been observed and are related to environmental conditions of temperature and light [14,19,20].
Although minimal in vitro cultivation is promising for grapevine germplasm conservation, few studies detail protocols, including acclimatization and the effects of genotype on in vitro cultivation factors, which may vary among hybrids or species. Therefore, it is necessary to test culture media, temperature, osmotic regulators, light, and other conditions to determine the most effective protocol for each collection [21,22,23,24,25].
This study aimed, for the first time, to develop a protocol for the in vitro conservation of interspecific Vitis spp. hybrids from the UENF grapevine collection, using the slow growth method. For this purpose, the hybrids were cultivated for 180 days using three different sucrose concentrations and two temperatures, aiming to determine the best approach to prolong in vitro conservation without the need for subculturing.
2. Material and Methods
2.1. Plant Material
Nodal segments of the selected interspecific hybrids CH1.2 and CH1.3, established in vitro and resistant to the nematode Pratylenchus brachyurus and downy mildew (Plasmopara viticola), were used. These hybrids belong to two different interspecific crosses of 06354-047 (V. vinifera × V. rotundifolia) × Cereza, which are the parents of hybrids CH1.2 and CH1.3 [3,11]. The grapevine collection is housed in a greenhouse at the Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF), located at the geographical coordinates latitude: −21.76233° (21° 45′ 44″ S) and longitude: −41.2897° (41° 17′ 23″ W).
These segments were cultivated in a medium containing half the MS mineral salts and White’s vitamin complex [26], 0.1 g L−1 myo-inositol, 30 g L−1 sucrose, 4.44 µmol L−1 gibberellic acid (GA3), and 4.44 µmol L−1 indole-butyric acid (IBA) [27] and solidified with 6.0 g L−1 of Sigma® bacteriological agar, pH 5.7. The medium was autoclaved for 15 min at 121 °C and 1.1 atm. They were grown in glass bottles (50 × 100 mm) in a growth chamber with a 16:8 h light photoperiod, a photosynthetically active radiation (PAR) of 50 µmol m2 s−1, and a temperature of 27 ± 2 °C, over a cultivation period of 60 days [10].
The in vitro low growth experiment was structured using a completely randomized design (CRD) within a 2 × 2 × 3 factorial arrangement. This included two interspecific hybrids of Vitis spp. (CH1.2 and CH1.3), two culture conditions (18 ± 1 °C and 27 ± 2 °C), and three sucrose concentrations (10, 20, and 30 g L−1). Evaluations were conducted at 60, 120, and 180 days of minimal growth, with 12 replicates per treatment. Each replicate consisted of three test tubes (25 mm × 150 mm) containing 10 mL of growth medium and one explant (nodal segment).
2.2. Slow In Vitro Growth in the Regeneration Phase of Nodal Segments of Interspecific Hybrids
Nodal segments, approximately 5 mm in length, were excised from plants that had been previously established in vitro and transferred to test tubes containing growth medium. This medium was composed of half the MS mineral salts, White’s vitamin complex, and three different concentrations of sucrose (10, 20, and 30 g L−1), supplemented with 4.44 µmol L−1 of benzyladenine (BA) and solidified with 6.0 g L−1 of Sigma®(XYZ Company, São Paulo, Brazil) bacteriological agar, pH 5.7. The medium was autoclaved for 15 min at 121 °C and 1.1 atm.
The nodal segments were maintained for 60, 120, and 180 days under two different temperatures. Temperature 1 involved a biochemical oxygen demand (BOD) chamber set at 18 ± 2 °C for six months, with a photoperiod of 16 h light/8 h dark and a light intensity of 25 µmol m−2 s−1 provided by OSRAM® daylight lamps (OSRAM GmbH, headquartered in Munich, Germany). Temperature 2 involved a growth room maintained at 27 ± 2 °C (measured using data logging equipment) with the same photoperiod and light conditions.
2.3. Elongation and Rooting Phase of Shoots of Hybrids Preserved In Vitro
After six months of cultivation, the shoots were transferred to a regeneration medium consisting of half the concentrations of MS mineral salts and White’s vitamin complex, supplemented with 0.1 g L−1 of myo-inositol, 30 g L−1 of sucrose, 4.44 µmol L−1 of GA3, and 4.44 µmol L−1 of IBA, with the pH adjusted to 5.7. The medium was solidified using 6 g L−1 of Sigma® bacteriological agar. Forty milliliters of this culture medium was distributed into each glass bottle (125 mm × 60 mm), and the bottles were autoclaved for 20 min at 121 °C and 1.1 atm.
The flasks were kept for 60 days in the growth room at a temperature of 27 ± 2 °C (photoperiod of 16:8 h of light/dark and brightness provided by OSRAM® daylight lamps with a light intensity of 54 µmol m−2 s−1). Then, the survival of the formed and acclimatized plants was evaluated.
2.4. Characteristics Observed in the Slow Growth of the Vines
The plants were evaluated at 60, 120, and 180 days for survival, number of leaves, root presence, and shoot length (mm). Additionally, plant color was assessed using a color scale (1: dark green, 2: light green, 3: yellow, 4: brown) [15] (Figure 1). The coloration of the grapevine leaves was categorized as follows: means from 1 to 1.5 were considered dark green, from 1.6 to 2.5 as light green, from 2.6 to 3.5 as yellow, and from 3.6 to 4.5 as brown (Figure 1).
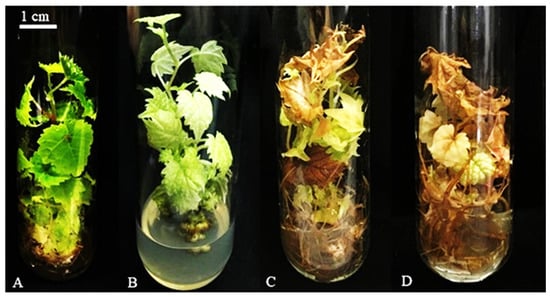
Figure 1.
Scale for evaluating the color of the nodal segments of interspecific hybrids of Vitis spp. during slow in vitro growth (A) 1 = dark green leaves, (B) 2 = light green leaves, (C) 3 = yellowish leaves (chlorosis) (D) 4 = brown leaves (necrosis).
2.5. Acclimatization Phase of Interspecific Hybrids
The rooted hybrids and survivors of the elongation and rooting phase were acclimatized in polypropylene trays in Basaplant© substrate (Oba Hortifruti (Grupo Fartura de Hortifrut S.A.), headquartered in Sumaré, São Paulo, Brazil). They remained for 60 days in a greenhouse at a minimum temperature of 25 °C and a maximum of 32 °C over the 30-day period and a relative humidity of 68.5% (data logger). The plants were irrigated twice a day, the first at 6:00 in the morning and the second at 18:00 in the afternoon, via sprinkler.
2.6. Statistical Analysis
The observed data were initially subjected to tests of homogeneity and normality using Bartlett’s and Shapiro–Wilk tests, respectively. A square root transformation (√y + 1) was applied to the survival variable during plant regeneration. Subsequently, an analysis of variance (ANOVA) was performed, and the degrees of freedom for treatments and their interactions were evaluated using the F-test. Means were compared using Tukey’s test at a p ≤ 0.05 significance level, with the aid of the statistical software (Version 4.5.1 “Great Square Root) R© [28].
3. Results
This work presents unprecedented results of the in vitro conservation of grapevines of interspecific hybrids from the grapevine collection of the Universidade Estadual do Norte Fluminense Darcy Ribeiro, which contribute new evidence in the in vitro cultivation of species, cultivars, and hybrids of Vitis spp.
3.1. In Vitro Regeneration of Nodal Segments of Interspecific Hybrids After 60 Days of Slow Growth
After 60 days of cultivation, evaluations revealed significant results for the traits analyzed within each factor. ANOVA showed that shoot height was significantly affected by all factors tested (Table S1). Significant interactions were found between Hybrid × Sucrose and Environment × Sucrose for the number of leaves and leaf coloration. Additionally, leaf color was influenced by the interaction between Hybrid × Environment. No significant differences were observed in survival rates. At 60 days, the hybrids had not yet developed roots.
Hybrid CH1.3 exhibited the greatest shoot height, averaging 22.86 mm, compared to 17.43 mm for CH1.2. Different sucrose concentrations influenced growth, with the 10 g L−1 concentration resulting in reduced growth and shorter shoots. The cooler environment (18 ± 1 °C) also affected shoot height, with plants reaching 19.01 mm, which was lower than the height observed in the warmer environment (27 ± 2 °C). Survival rates ranged from 97% to 100% across treatments. (Table 1).

Table 1.
Mean values of shoot height after 60 days of conservation by slow in vitro growth of Vitis spp. hybrids.
For the number of leaves, significant interactions were observed between the factors Hybrid × Sucrose and Environment × Sucrose (Table 2). Hybrid CH1.3 showed the highest mean for this trait. In contrast, hybrid CH1.2 had the lowest number of leaves when grown with 10 g L−1 sucrose. At 27 ± 2 °C, this low sucrose concentration also reduced the number of leaves in both hybrids. Conversely, under the milder temperature of 18 ± 1 °C, the number of leaves remained more stable, ranging from 12.41 to 13.22, regardless of sucrose concentration (Table 2).

Table 2.
Interaction decomposition for number of leaves in each hybrid according to sucrose level and ambient temperature.
The Hybrid × Environment interaction revealed differences in leaf coloration: CH1.3 showed lighter leaves than CH1.2, with a greener hue at 27 ± 2 °C (Table 3). In the cooler environment (18 ± 1 °C), chlorosis occurred at higher sucrose concentrations (20 and 30 g L−1). In contrast, sucrose had no effect on leaf color at 27 ± 2 °C, and leaves remained green throughout the 60-day period.

Table 3.
Interaction decomposition for leaf color in each hybrid according to sucrose level and ambient temperature.
Leaf color varied between sucrose levels, with intensity shifting from green to light green (Table 3). Green leaves represent the live and healthy plants within the cultivation environment. Hybrid CH1.3 showed a more intense green, with the greatest intensity achieved at the lowest sucrose concentration (10 g L−1), averaging 1.29. This intensity decreased to light green as sucrose levels increased to 20 and 30 g L−1 (Table 3).
3.2. In Vitro Regeneration of Nodal Segments of Interspecific Hybrids After 120 Days of Slow Growth
After 120 days of in vitro culture, changes were observed in the characteristics of the vines under the different conditions analyzed in this study. There were interactions for shoot height between Hybrids × Sucrose Levels and Hybrids × Temperature. Additionally, an interaction effect for the “survival” trait was detected between Environment × Sucrose. For the number of leaves, a Hybrid × Environment interaction was noted. A triple interaction that influenced the presence of roots was identified. For leaf color, a significant interaction was observed between Hybrids × Sucrose Levels (Table S2).
For shoot height (Table 4), it was observed that sucrose concentrations of 10 and 20 g L−1 contributed to the minimal growth of hybrid CH1.2, which reached a height of 18.81, in contrast to those grown in a medium containing 30 g L−1 of sucrose, which averaged 32.23 in height. At 120 days, no differences in height were observed between sucrose concentrations for hybrid CH1.3. Analysis of the Hybrid × Environment interaction revealed that the environment at 18 ± 1 °C had the lowest growth means, thus being indicated for the conservation of interspecific Vitis spp. hybrids. In this environment, the average heights were 27.59 for CH1.3 and 21.0 for CH1.2, respectively (Table 4). Leaf color was influenced by the hybrid factor; CH1.3 exhibited plants with light green, chlorophyllous leaves, whereas CH1.2 did not maintain green leaves beyond 180 days of in vitro culture, showing only light green leaves (Table 4).

Table 4.
Interaction decomposition for shoot height and leaf color in each hybrid according to sucrose level and ambient temperature, at 120 days.
As observed in Table 5, sucrose concentrations did not have a significant effect on the number of leaves in hybrid CH1.2. However, in hybrid CH1.3, significant differences were found among the tested concentrations, with 10 g L−1 resulting in the highest leaf number, while 20 and 30 g L−1 concentrations showed lower averages. Additionally, a difference in leaf number between the two hybrids was noted, with CH1.3 exhibiting higher averages at 10 and 20 g L−1, differing significantly only from the 30 g L−1 concentration.

Table 5.
Interaction decomposition for number of leaves in each hybrid according to sucrose level and ambient temperature, at 120 days.
Regarding the influence of temperature, Table 5 shows that the temperature of 18 ± 1 °C led to a lower number of leaves in hybrid CH1.3 when compared to 27 ± 2 °C. In addition, the hybrids also showed significant differences in leaf number under the 27 ± 2 °C condition.
Decomposition analysis for survival rates of the vines across different environments and sucrose levels (Table 6) revealed that plants grown at 18 ± 1 °C with 10 g L−1 of sucrose exhibited higher survival rates. Additionally, these plants also showed a greater presence of roots compared to those grown at 27 ± 2 °C with the same sucrose concentration. This pattern suggests that milder environments not only enhance root development for improved survival but also moderate growth by promoting bud dormancy, contributing to the slow in vitro growth of the plants.

Table 6.
Survival rate of hybrids under different sucrose levels, and triple interaction of the analyzed factors for root presence, at 120 days.
Furthermore, the triple interaction among specific factors affecting root presence was identified. Plants of the CH1.2 genotype grown in 10 g L−1 of sucrose demonstrated a reduced root presence across various environments. The environmental influence was also noted for CH1.3, which showed less root presence when cultivated in 10 g L−1 of sucrose at 27 ± 2 °C.
3.3. In Vitro Regeneration of Nodal Segments of Interspecific Hybrids After 180 Days of Slow Growth
After 180 days of cultivation, it was possible to evaluate the characteristics influenced by each factor: Hybrid × Sucrose × Environment (Table S3).
The decomposition of the interaction between hybrids, sucrose levels, and environment after 180 days indicated a significant triple interaction affecting shoot height. Concentrations of 10 and 20 g L−1 were optimal for promoting slow growth in the vines. Furthermore, it was noted that plants cultivated with 30 g L−1 of sucrose in an environment at 27 ± 2 °C began the senescence process, leading to a reduction in shoot height, as depicted in Figure 2 and Figure 3, with an average height of 7.47 (Table 7). The environmental conditions significantly influenced the shoot heights of the hybrids, with the lowest values observed at 18 ± 1 °C (Table 7). This environment is thus ideal for preserving vines in vitro for extended periods.

Figure 2.
Slow in vitro growth after 180 days of culture of the two interspecific hybrids evaluated. (A–C) = Hybrid CH1.2 cultivated in 10, 20, and 30 g L−1 of sucrose, respectively. (D–F) = Hybrid CH1.3 in 10, 20, and 30 g L−1 of sucrose, in a growth room at 27 ± 2 °C.
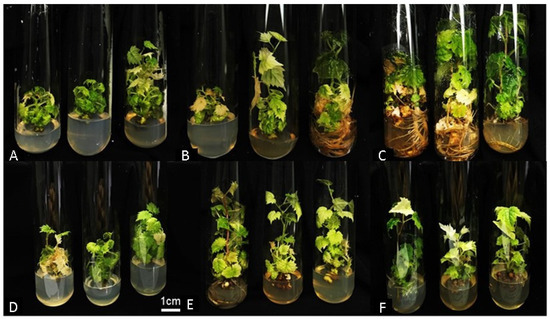
Figure 3.
Slow in vitro growth after 180 days of culture of the two interspecific hybrids evaluated. (A–C) = Hybrid CH1.2 cultivated in 10, 20, and 30 g L−1 of sucrose, respectively. (D–F) = Hybrid CH1.3 in 10, 20, and 30 g L−1 of sucrose, in a BOD chamber at a temperature of 18 ± 1 °C.

Table 7.
Triple decomposition analysis of the “hybrid”, “sucrose”, and “environment” factors on shoot height at 180 days.
The highest survival rate was observed in hybrid CH1.3 grown with 10 g L−1 of sucrose, reaching 62.5%. A genotypic influence on survival was also evident, as CH1.2 did not show significant survival differences across sucrose concentrations of 10 and 30 g L−1, with survival rates ranging from 52% to 40% (Table 8).

Table 8.
Decomposition analysis between hybrids and sucrose for survival rate, number of leaves, leaf color, and root presence.
The number of leaves declined sharply in the CH1.3 hybrid at sucrose concentrations of 20 and 30 g L−1. This reduction is attributed to leaf senescence that commenced at 180 days of in vitro culture (Figure 2 and Figure 3). However, vines grown at 10 g L−1 of sucrose exhibited a higher leaf count, demonstrating the beneficial effect of slow growth at lower sucrose concentrations. Leaf senescence was further indicated by the change in leaf color to light green and chlorotic at 180 days, reflecting reduced chlorophyll presence (Table 8).
At 180 days, the influence of genotype on root presence under different sucrose levels was evident. Hybrid CH1.2 showed a low rooting percentage (3.96%) with 10 g L−1 of sucrose, whereas the same conditions resulted in 43% rooting in CH1.3. At higher sucrose concentrations (20 and 30 g L−1), CH1.2 showed 11.04% and 18.69% rooting, respectively, while CH1.3 presented 19.29% and 5.54%, respectively (Table 8; Figure 2 and Figure 3).
At 120 days, plants grown at 18 ± 1 °C exhibited leaf color values ranging from 1.6 to 2.5, corresponding to shades of green to light green (Figure 3). Under these conditions, a broader variation in leaf coloration was also observed, with values from 2.6 to 3.5 indicating yellowish leaves and from 3.6 to 4.5 corresponding to brown leaves (Figure 2).
3.4. Elongation Phase and In Vitro Rooting of Shoots Maintained in Slow Growth for 180 Days
At 180 days of cultivation, a triple interaction among the analyzed factors was observed regarding the survival of grapevines grown in vitro (Table S4).
Table 9 shows the combined influence of the analyzed factors. Sucrose levels were critical at this stage: CH1.3 plants achieved 100% survival when cultivated with 10 g L−1 of sucrose at 18 ± 1 °C. CH1.2, on the other hand, exhibited higher survival rates regardless of sucrose concentration when compared to CH1.3. However, when survival rates were analyzed within each sucrose level, the 10 g L−1 concentration stood out once again, presenting the highest average survival at 18 ± 1 °C. These conditions proved to be the most favorable for plant survival after 180 days of cultivation.

Table 9.
Decomposition analysis of the factors for survival rate after in vitro regeneration of hybrids preserved in vitro, under different sucrose levels and temperatures, after 180 days.
3.5. Acclimatization of Surviving Hybrids After In Vitro Elongation and Rooting
The average greenhouse temperature over this period was 30 ± 2 °C with a relative humidity of 68.5% (data logger), ranging between a minimum of 25 °C and a maximum of 32 °C. Following this period, it was noted that the acclimatized hybrids did not survive.
4. Discussion
At 120 days, the results demonstrated that the cultivation conditions had a more direct influence on the hybrids than their genetic and physiological characteristics. Plants in lower sucrose concentrations exhibited smaller shoots but a greater presence of roots and leaves. These findings suggest that reduced or absent sucrose in the culture medium contributes to plant growth stability, resulting in slower growth and a higher number of leaves, which indicates an increased need for energy through photosynthesis [6,11,17,20,29,30]. This energy demand facilitates easier root production compared to plants in environments with higher sucrose concentrations [2,14,21,29].
Sucrose alters the osmolarity of the culture medium; thus, higher concentrations can interfere with the absorption of mineral salts and vitamins available to the plants [14,19,31]. As an additive in the culture medium, sucrose significantly impacts plant growth in vitro by serving both as an energy source and an osmotic regulator. Depending on the concentration, it can lead to the expulsion of excess intracellular water through an osmotic gradient, consequently slowing growth [11,12,25].
Additionally, shoot growth is influenced by the environmental conditions of in vitro cultivation. Plants grown in environments with lower temperatures and light intensities exhibit smaller shoots. Reducing incubation temperature and light intensity is a strategic approach for tropical and subtropical species, resulting in a significant decrease in plant metabolism [19,20,27,32,33].
At 180 days of cultivation, a reduction in shoot length was observed, especially in hybrid CH1.3. This decline is associated with leaf senescence, intensified by increased ethylene production, which promotes leaf abscission and stem reduction. Ethylene is a gaseous hormone that can accumulate inside culture vessels, promoting foliar abscission and accelerating plant aging [34]. The cascading effect of ethylene can be visualized in Figure 2 and Figure 3, which show in vitro plantlets at 180 days of cultivation.
In general, there is already evidence that in vitro cultivation under different temperature conditions and sucrose levels favors conservation through slow growth, as each species has optimal temperature and light intensity requirements for development. Tropical and subtropical plants perform better when maintained at temperatures between 15 °C and 20 °C. The results of this study demonstrate that grapevines, regardless of the hybrid, showed higher survival rates when cultivated at 18 ± 1 °C and under reduced sucrose concentrations (10 to 20 g L−1).
The literature remains scarce regarding studies on in vitro conservation by minimal growth of grapevine species and hybrids. Some studies report storage temperatures as low as 10 °C with 100% survival. However, it is essential to consider the specific characteristics of the cultivar, species, or hybrid being studied, as well as the regional climate and the conditions under which the plants were acclimatized [13,33,35].
Regarding hybrid acclimatization, difficulties have been observed in plant survival under high-temperature conditions. Therefore, further research is needed to improve this stage, aiming to increase grapevine survival rates [10], in studying the same hybrids evaluated in the present research, reported challenges during acclimatization and proposed the use of flasks sealed with microporous membranes as an alternative to enhance plant survival, thereby minimizing mortality rates during the transition to the ex vitro environment [31,33].
5. Conclusions
Low sucrose levels and reduced temperatures contribute to the slow growth of interspecific grapevine hybrids from the collection of the Universidade Estadual do Norte Fluminense Darcy Ribeiro. These hybrids can be regenerated and maintained in ½ MS culture medium supplemented with 4.44 µmol L−1 BA and 10 g L−1 sucrose, under BOD conditions at a temperature of 18 ± 1 °C. Additionally, high temperatures during the acclimatization phase may lead to plant mortality. Therefore, further studies are recommended to improve this stage and enhance plant survival rates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijpb16030083/s1, Table S1: Analysis of variance of the minimum in vitro culture at 60 days of cultivation of the CH1.2 and CH1.3 hybrids of Vitis spp.; Table S2: Analysis of variance of the minimum in vitro culture at 120 days of cultivation of the CH1.2 and CH1.3 hybrids of Vitis spp.; Table S3: Analysis of variance of the minimum in vitro culture at 160 days of cultivation of the CH1.2 and CH1.3 hybrids of Vitis spp.; Table S4: Analysis of variance of the in vitro regeneration of interspecific hybrids of Vitis spp. conserved in vitro for 180 days under different sucrose levels and temperatures.
Author Contributions
Conceptualization, L.M.d.S.; methodology, L.M.d.S.; software, L.M.d.S. and D.P.M.; validation, V.S.C. and A.P.V.; formal analysis, L.M.d.S. and V.S.C.; investigation, L.M.d.S., O.D.d.C.J. and K.M.D.; resources, A.P.V. and V.S.C.; data curation, L.M.d.S. and D.P.M.; writing—original draft preparation, L.M.d.S.; writing—review and editing, L.M.d.S., V.S.C. and D.P.M.; visualization, L.M.d.S.; supervision, V.S.C. and A.P.V.; project administration, A.P.V. and V.S.C.; funding acquisition, CAPES and FAPERJ. All authors have read and agreed to the published version of the manuscript.
Funding
To the Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the finantial support and the scholarships granted during this research. Also, to the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Financial Code 001.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hannin, H. Breeding, consumers and market issues: Main evolutions in the vine and wine industry. Acta Hortic. 2019, 1248, 1–6. [Google Scholar] [CrossRef]
- Kumsa, F. Factors affecting in vitro cultivation of grape (Vitis vinifera L.): A review. Int. J. Agric. Res. Innov. Technol. 2020, 10, 1–5. [Google Scholar] [CrossRef]
- Abido, A.I.A.; Aly, M.A.M.; Hassanen, S.A.; Rayan, G.A. In vitro propagation of grapevine (Vitis vinifera L.) Muscat of Alexandria cv. for conservation of endangerment. Middle-East J. Sci. Res. 2013, 13, 328–337. [Google Scholar]
- Amaral, B.D.; Viana, A.P.; Santos, E.A.; Ribeiro, R.M.; Silva, F.A.D.; Ambrósio, M.; Walker, A.M. Prospecting for resistance of interspecific hybrids of Vitis spp. to Plasmopara viticola. Euphytica 2020, 216, 68. [Google Scholar] [CrossRef]
- Payán, A.; Martin, B. Hybridization Methods in Vitis. In Advances in Viticulture; de Tal, F., Ed.; Editora XYZ: Sao Paulo, Brazil, 1975; pp. 45–60. [Google Scholar]
- Withers, L.A. Cryopreservation of Cultured Plant Cells and Protoplasts. In Cryopreservation of Plant Cells and Organs; Kartha, K.K., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 243–267. [Google Scholar]
- AlMousa, R.; Hassan, N. Effect of osmotic regulators on In vitro conservation of grape. J. Genet. Environ. Resour. Conserv. 2022, 10, 60–66. [Google Scholar]
- Hassanen, S.A.; Abido, A.I.A.; Aly, M.A.M.; Rayan, G.A. In vitro preservation of grapevine (Vitis vinifera L.) Muscat of Alexandria and Black Monukka cultivars as genetic resource. Afr. J. Basic Appl. Sci. 2013, 5, 55–63. [Google Scholar]
- Santos, P.R.; Viana, A.P.; Santos, E.A.; Barros, W.F.H.; Riaz, S.; Walker, A.M. Molecular genetic diversity in segregates of Vitis: Implications for the breeding of grapevine aiming at resistance to Pratylenchus brachyurus. Euphytica 2019, 215, 78. [Google Scholar] [CrossRef]
- Silva, L.M.; Carvalho, V.S.; Generoso, A.L.; Miranda, D.P.; da Costa Júnior, O.D.; Simioni, P.F.; Viana, A.P. Micropropagation of interspecific hybrids of Vitis spp. in microenvironments with different gas exchanges. Sci. Hortic. 2022, 305, 111413. [Google Scholar] [CrossRef]
- Butiuc-Keul, A.; Coste, A. Biotechnologies and strategies for grapevine improvement. Horticulturae 2023, 9, 62. [Google Scholar] [CrossRef]
- Fengqin, Z.; Fangmei, L.; Dabin, G. Studies on germplasm resources of wild grape species (Vitis spp.) in China. Vitis 1990, 1, 50–57. [Google Scholar]
- Santos, P.R.; Viana, A.P.; Gomes, V.M.; Preisigke, S.C.; Santos, E.A.; Cavalcante, N.R.; Walker, M.A. Clonal selection in interspecific Vitis spp. hybrids resistant to the root-lesion nematode Pratylenchus brachyurus by REML/BLUP. Fruits 2018, 73, 191–197. [Google Scholar] [CrossRef]
- Garcia, R.; Pacheco, G.; Falcão, E.; Borges, G.; Mansur, E. Influence of type of explant, plant growth regulators, salt composition of basal medium, and light on callogenesis and regeneration in Passiflora suberosa L. (Passifloraceae). Plant Cell Tissue Organ Cult. 2011, 106, 47–54. [Google Scholar] [CrossRef]
- Pacheco, G.; Garcia, R.; Lugato, D.; Vianna, M.; Mansur, E. Plant regeneration, callus induction and establishment of cell suspension cultures of Passiflora alata Curtis. Sci. Hortic. 2012, 144, 42–47. [Google Scholar] [CrossRef]
- Pence, V.C. The possibilities and challenges of in vitro methods for plant conservation. Kew Bull. 2010, 65, 539–547. [Google Scholar] [CrossRef]
- Vettorazzi, R.G.; Carvalho, V.S.; Sudré, C.P.; Rodrigues, R. Developing an in vitro optimized protocol to sweet potato landraces conservation. Acta Sci. Agron. 2017, 39, 359–367. [Google Scholar] [CrossRef]
- Pavlova, I.A.; Klimenko, V.P.; Zlenko, V.A.; Luschay, E.A.; Abdurashitova, A.S.; Grigorenko, M.I. Conservation of grape genetic resources in the system in vitro. BIO Web Conf. 2023, 78, 02005. [Google Scholar] [CrossRef]
- Benelli, C.; Tarraf, W.; Izgu, T.; De Carlo, A. In vitro conservation through slow growth storage technique of fruit species: An overview of the last 10 years. Plants 2022, 11, 3188. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudzadeh, H. In vitro regeneration of virus-free grapevine (Vitis vinifera L.) in some commercial cultivars. Int. J. Agric. Nat. Sci. 2018, 1, 69–74. [Google Scholar]
- Trejgell, A.; Kamińska, M.; Tretyn, A. In vitro slow growth storage of Senecio macrophyllus shoots. Acta Physiol. Plant. 2015, 37, 1–9. [Google Scholar] [CrossRef]
- Pan, X.J.; Zhang, W.E.; Li, X. In vitro conservation of native Chinese wild grape (Vitis heyneana Roem. And Schult) by slow growth culture. Vitis 2014, 53, 207–214. [Google Scholar]
- Sharma, S.; Shahzad, A.; Akhtar, R.; Upadhyay, A. Micropropagation: A Boon for Conservation of Valuable Vines and Lianas. In Biotechnological Strategies for the Conservation of Medicinal and Ornamental Climbers; Springer: Cham, Switzerland, 2016; pp. 163–193. [Google Scholar]
- Shibli, R.D.; Shatnawi, M.A.; Subaih, W.S.; Viera, R.F.; Ajlouni, M.M. In vitro conservation and cryopreservation of plant genetic resources: A review. World J. Agric. Sci. 2006, 2, 372–382. [Google Scholar]
- Normah, M.N.; Chin, H.F.; Reed, B.M. Conservation of Tropical Plant Species; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Trevisan, F.; Mendes, B.M.J. Optimization of in vitro organogenesis in passion fruit (Passiflora edulis f. flavicarpa). Sci. Agric. 2005, 62, 346–350. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 3 February 2025).
- Bettoni, J.C.; Dalla Costa, M.; Gardin, J.P.P.; Kretzschmar, A.A.; Pathirana, R. Cryotherapy: A new technique to obtain grapevine plants free of viruses. Rev. Bras. Frutic. 2016, 38, e-833. [Google Scholar] [CrossRef]
- Lima, J.; Silva, M.S. Produção de uvas no Brasil em 2023: Análise regional e perspectivas. Rev. Bras. Frutic. 2024, 45, 123–135. [Google Scholar]
- Generoso, A.L.; Carvalho, V.S.; Sales, R.A.; Brito, N.L.; Viana, A.P.; Pereira, T.N.S. New evidence for in vitro conservation of nodal segments of the passion fruit ‘UENF Rio Dourado’ (Passiflora edulis Sims). Acta Sci. Agron. 2023, 45, e59498. [Google Scholar] [CrossRef]
- Luz, R.F.M.; Román, C.M.C.; Jorge, C.I.; del Mar, R.P.L.; Marcos, S.H.R.; Lourdes, A.G.; Israel, C.J. In vitro shoot regeneration and callogenesis of Sechium compositum (Donn. Sm.) C. Jeffrey for plant conservation and secondary metabolites product. Horticulturae 2024, 10, 537. [Google Scholar] [CrossRef]
- Gammoudi, N.; San Pedro-Galán, T.; Ferchichi, A.; Gisbert Domenech, M.C. Improvement of regeneration in pepper: A recalcitrant species. Vitr. Cell Dev. Biol. Plant. 2018, 54, 145–153. [Google Scholar] [CrossRef]
- Yaacob, J.S.; Mahmad, N.; Taha, R.M.; Mohamed, N.; Yussof, A.I.M.; Saleh, A. Optimization of culture conditions (sucrose, pH, and photoperiod) for in vitro regeneration and early detection of somaclonal variation in ginger lime (Citrus assamensis). Sci. World J. 2014, 2014, 262710. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Puzirnova, V.; Doroshenko, N. Preserving grapevine variety Fioletoviy Ranniy in the collection in vitro. E3S Web Conf. 2021, 273, 01007. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).