Somatic Embryogenesis in Native Peruvian Fine-Flavor Cocoa Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Explant Preparation
2.2. Culture Medium and Growth Conditions
2.3. Data Analyses
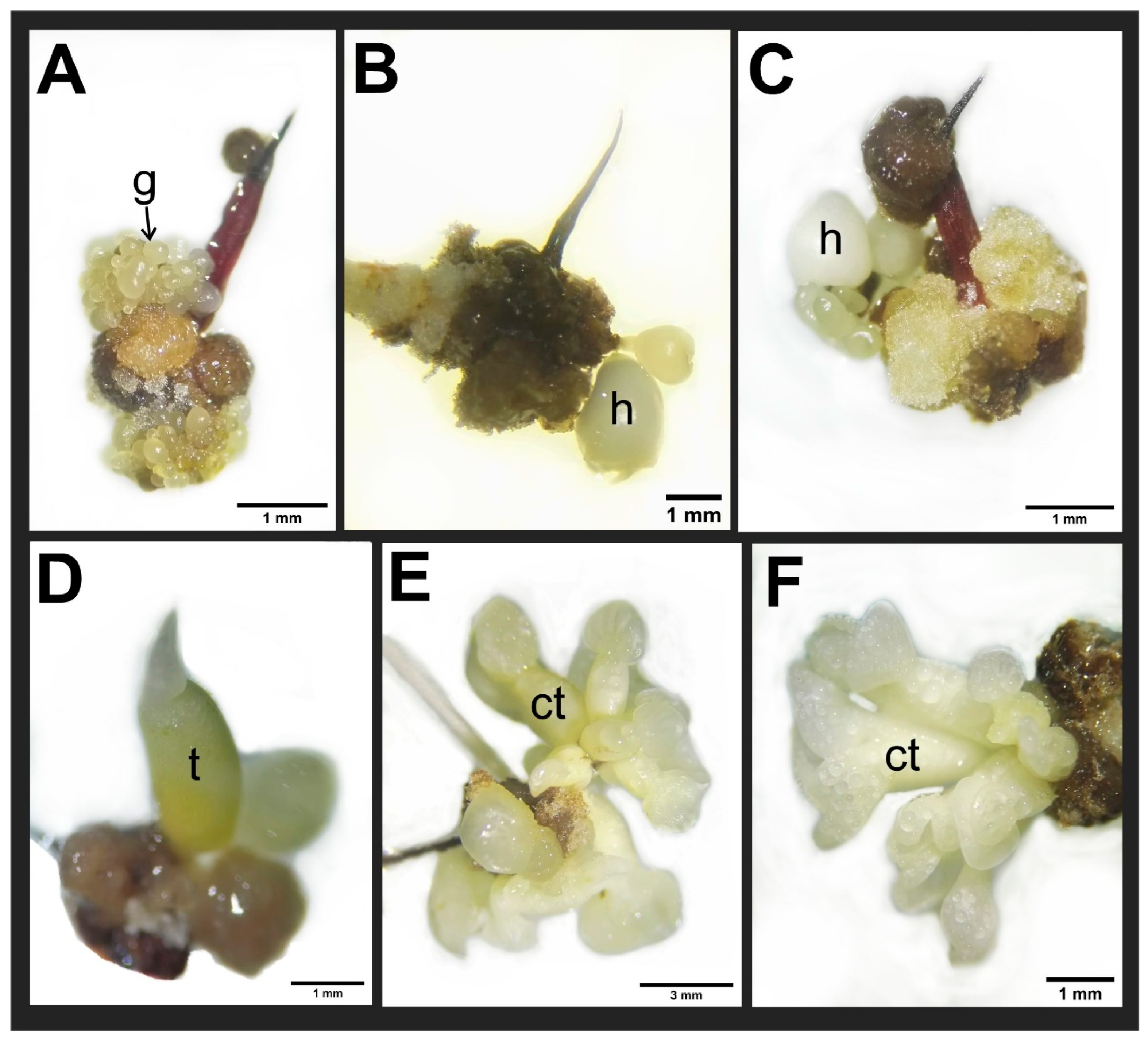
3. Results
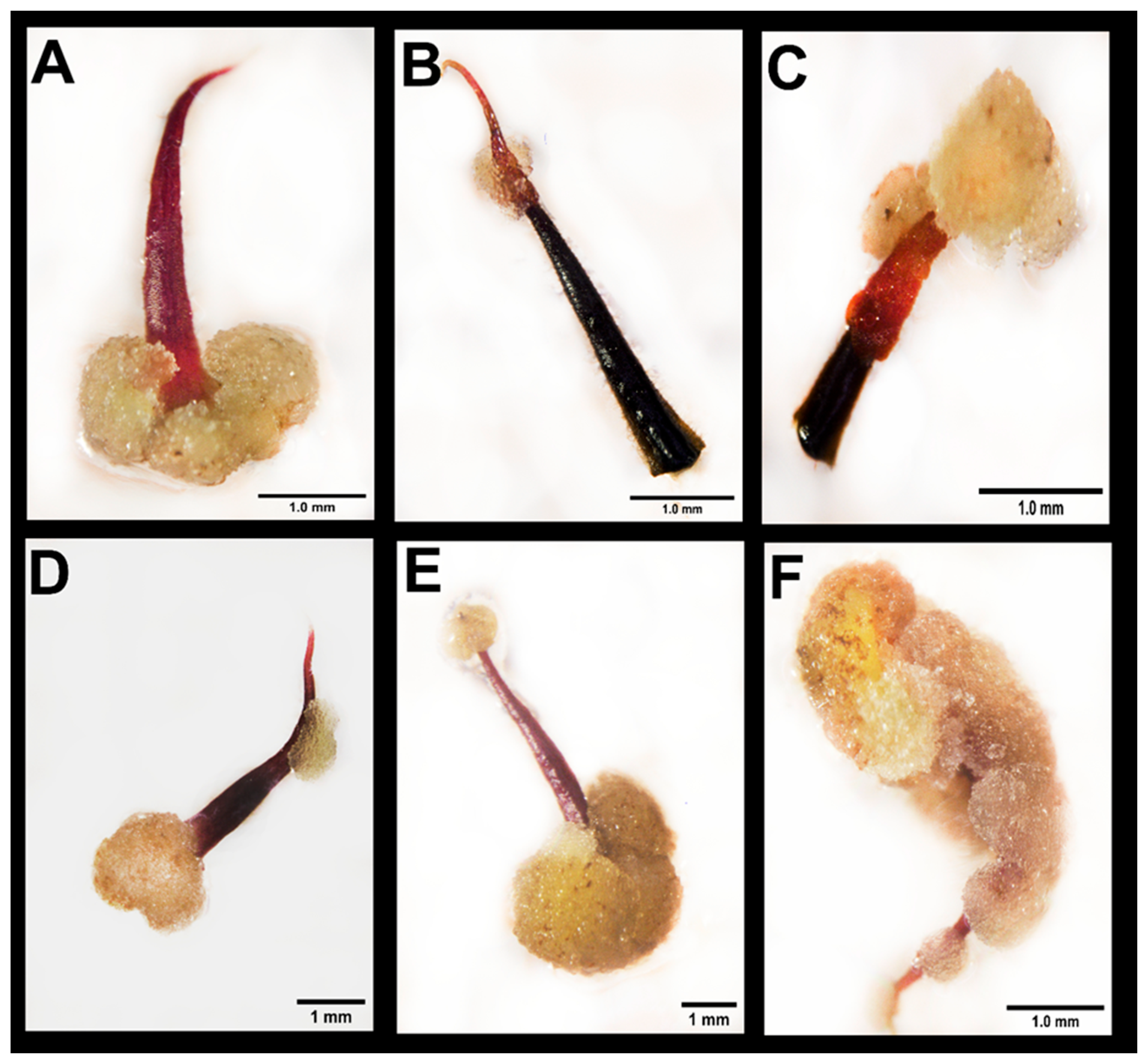
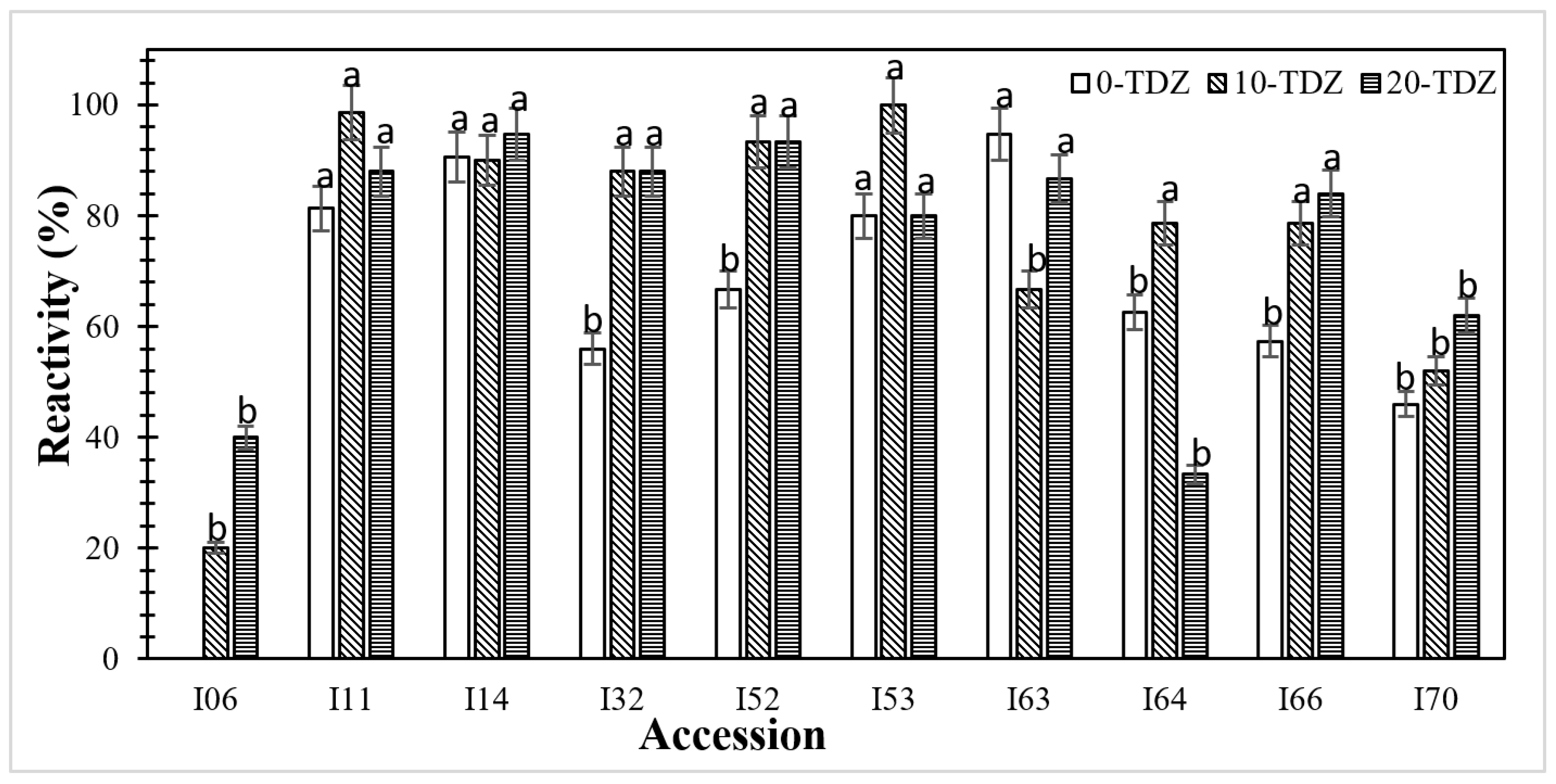
3.1. Effect of Thidiazuron on Callogenesis in Staminodes
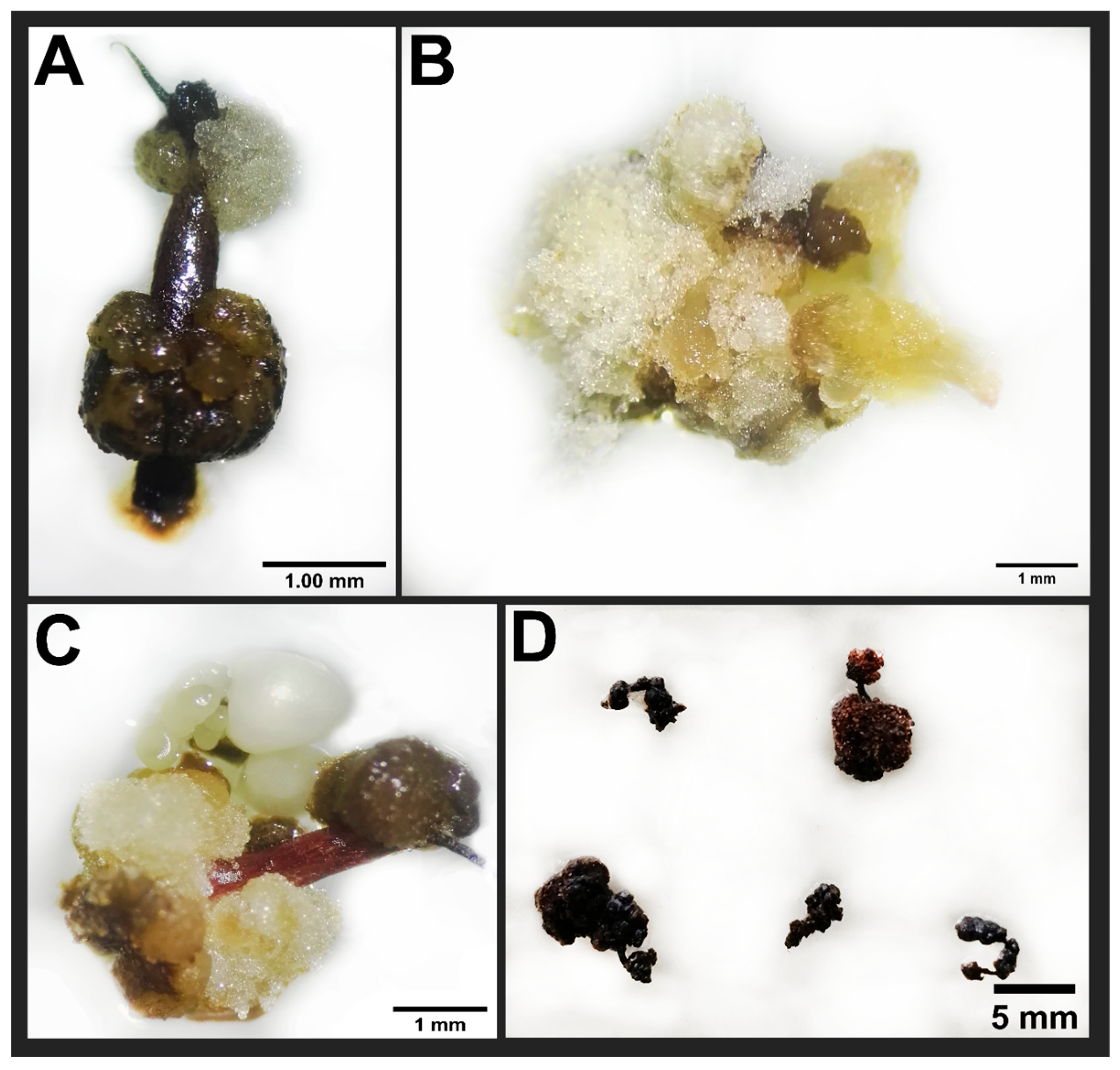
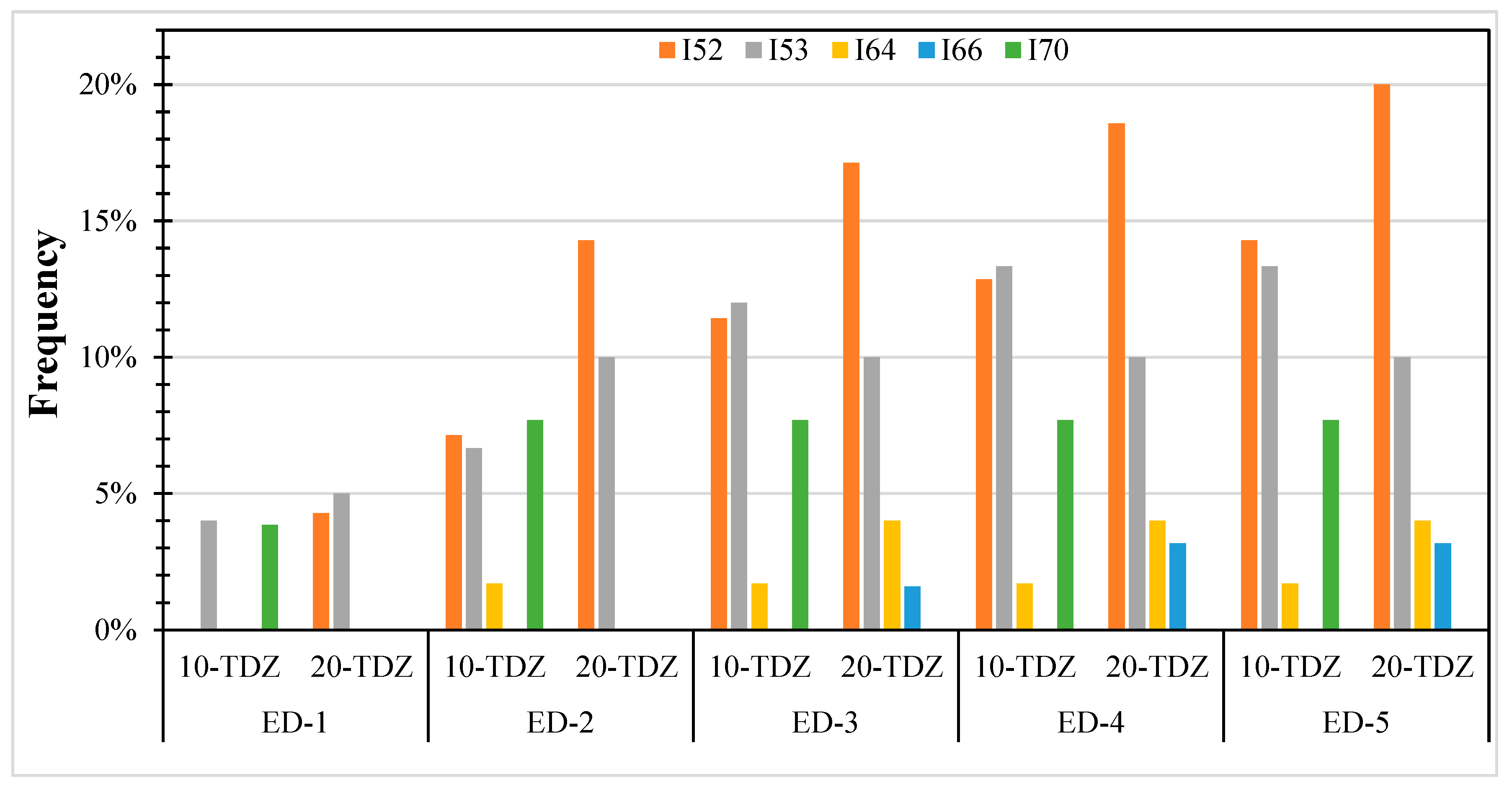
3.2. Effect of Thidiazuron on Somatic Embryogenesis Expression
4. Discussion
4.1. TDZ Treatment Promotes Explant Reactivity
4.2. Embryo Formation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zarrillo, S.; Gaikwad, N.; Lanaud, C.; Powis, T.; Viot, C.; Lesur, I.; Fouet, O.; Argout, X.; Guichoux, E.; Salin, F.; et al. The Use and Domestication of Theobroma cacao during the Mid-Holocene in the Upper Amazon. Nat. Ecol. Evol. 2018, 2, 1879–1888. [Google Scholar] [CrossRef]
- SEIA. Estadística Agropecuaria. Available online: https://siea.midagri.gob.pe/portal/siea_bi/index.html (accessed on 24 September 2022).
- Centro de Comercio Internacional. Cacao: Guía de Prácticas Comerciales; UNCTAD/OMC: Geneva, Switzerland, 2001. [Google Scholar]
- Paredes, J.; Canals, M.; González, A.; Ventura, M. Evaluación de Sustratos en el Enraizamiento de Estacas de Cacao (Theobroma cacao L.). In Proceedings of the 14th International Cocoa Research Conference, Accra, Ghana, 13–18 October 2003; pp. 400–497. [Google Scholar]
- Li, Z.; Traore, A.; Maximova, S.; Guiltinan, M.J. Somatic Embriogenesis and Plant Rengeneration from Floral Explants of Cacao (Theobroma cacao L.) Using Thidiazuron. Vitr. Cell. Dev. Biol.-Plant 1998, 34, 293–299. [Google Scholar] [CrossRef]
- Henao Ramírez, A.M.; de la Hoz Vasquez, T.; Ospina Osorio, T.M.; Atehortúa Garcés, L.; Urrea Trujillo, A.I. Evaluation of the Potential of Regeneration of Different Colombian and Commercial Genotypes of Cocoa (Theobroma cacao L.) via Somatic Embryogenesis. Sci. Hortic. 2018, 229, 148–156. [Google Scholar] [CrossRef]
- Chanatásig, C.I. Inducción de la Embriogénesis Somática en Clones Superiores de Cacao (Theobroma cacao L.), Con Resistencia a Enfermedades Fungosas. Master’s Thesis, Centro Agronómico Tropical de Investigación y Enseñanza, Turrialba, Costa Rica, 2004. [Google Scholar]
- Maximova, S.N.; Young, A.; Pishak, S.; Guiltinan, M.J. Field Performance of Theobroma cacao L. Plants Propagated via Somatic Embryogenesis. Vitr. Cell. Dev. Biol.-Plant 2008, 44, 487–493. [Google Scholar] [CrossRef]
- Maximova, S.N.; Alemanno, L.L.; Young, A.; Ferriere, N.; Traore, A.; Guiltinan, M.J. Efficiency, Genotypic Variability, and Cellular Origin of Primary and Secondary Somatic Embryogenesis of Theobroma cacao L. Vitr. Cell. Dev. Biol.-Plant 2002, 38, 252–259. [Google Scholar] [CrossRef]
- Solano, W. Embriogénesis Somática en Clones Superiores de Cacao (Theobroma cacao L.) Obtenidos en el Programa de Mejoramiento Genético Del CATIE. Master’s Thesis, Centro Agronómico Tropical de Investigación y Enseñanza, Turrialba, Costa Rica, 2008. [Google Scholar]
- Urrea, A.I.; Atehortúa, L.; Gallego, A.M. Regeneración vía Embriogénesis Somática de Una Variedad Colombiana Élite de Theobroma cacao L. Rev. Colomb. Biotecnol. 2011, 13, 39–50. [Google Scholar]
- Driver, J.; Kuniyuki, A. In Vitro Propagation of Paradox Walnut Rootstock. HortScience 1984, 19, 507–509. [Google Scholar] [CrossRef]
- Tulecke, W.; McGranahan, G. Somatic Embryogenesis and Plant Regeneration from Cotyledons of Walnut, Juglans regia L. Plant Sci. 1985, 40, 57–63. [Google Scholar] [CrossRef]
- Fontanel, A.; Gire-Bobin, S.; Labbé, G.; Favereau, P.; Álvarez, M.; Rutte, S.; Pétiard, V. In Vitro Multiplication and Plant Regeneration of Theobroma cacao L. via Stable Embryogenic Calli. 10 IAPTC Congress. Plant Biotechnol. 2002, 1, 23–28. [Google Scholar]
- Garcia, C.; Marelli, J.P.; Motamayor, J.C.; Villela, C. Somatic Embryogenesis in Theobroma cacao L. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1815, pp. 227–245. [Google Scholar]
- Kan Kouassi, M.; Kahia, J.; Kouame, C.N.; Tahi, M.G.; Kouablan, K. Comparing the Effect of Plant Growth Regulators on Callus and Somatic Embryogenesis Induction in Four Elite. HortScience 2017, 52, 142–145. [Google Scholar] [CrossRef]
- Bustamante, D.E.; Motilal, L.A.; Calderon, M.S.; Mahabir, A.; Oliva, M. Genetic Diversity and Population Structure of Fine Aroma Cacao (Theobroma cacao L.) from North Peru Revealed by Single Nucleotide Polymorphism (SNP) Markers. Front. Ecol. Evol. 2022, 10, 895056. [Google Scholar] [CrossRef]
- Penn State University. Integrated System for Vegetative Propagation of Cacao: Protocol Book; Guiltinan, M.J., Maximova, S.N., Eds.; Penn State University: University Park, PA, USA, 2010. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, version 2020; Universidad Nacional de Córdoba: Córdoba, Argentina, 2020.
- Lopez-Báez, O.; Moreno, J.; Pacheco, S. Avanzes en Propagación de Cacao Theobroma cacao Por Embriogénesis Somática en México. In Proceedings of the International Workshop on New Technologies and Cocoa Breeding, Kota Kinabalu, Malaysia, 16–17 October 2000; Ingenic: Montpellier, France, 2001; pp. 163–176. [Google Scholar]
- Ajijah, N.; Rubiyo; Sudarsono, D. Callogenesis and Somatic Embryogenesis of Cacao Using Thidiazuron through One Step of Callus Induction. J. Penelit. Tanam. Ind. 2014, 20, 179–186. [Google Scholar] [CrossRef]
- Ajijah, N.; Hartati, R.S.; Rubiyo; Sukma, D.; Sudarsono, S. Effective Cacao Somatic Embryo Regeneration on Kinetin Supplemented DKW Medium and Somaclonal Variation Assessment Using SSRs Markers. Agrivita 2016, 38, 80–92. [Google Scholar] [CrossRef]
- Paisic-Ramirez, R.; Hernández-Amasifuen, A.D.; Sánchez-Aguilar, W.D.; Corazon-Guivin, M.A.; Bobadilla, L.G.; Mansilla-Córdova, P.J.; Caetano, A.C.; Silva-Zuta, Z.M.; Guerrero-Abad, J.C. Effect of Osmoregulatory on the Secondary Somatic Embryogenesis of Cocoa (Theobroma cacao L.). J. Appl. Biol. Biotechnol. 2024, 12, 177–183. [Google Scholar] [CrossRef]
- Gárate-navarro, M.A.; Arévalo-gardini, E. Induction of Somatic Embryogenesis from Cocoa Farmer Field Collection of ICT-Peru. Int. Ann. Sci. 2017, 2, 6–11. [Google Scholar] [CrossRef]
- Alemanno, L.; Berthouly, M.; Michaux-Ferrière, N. Histology of Somatic Embryogenesis from Floral Tissues Cocoa. Plant Cell Tissue Organ Cult. 1996, 46, 187–194. [Google Scholar] [CrossRef]
- Ndoumou, D.O.; Ndzomo, G.T.; Niemenak, N. Phenol Content, Acidic Peroxidase and IAA-Oxidase during Somatic Embryogenesis in Theobroma cacao L. Biol. Plant 1997, 39, 337–347. [Google Scholar] [CrossRef]
- Quiroz-Figueroa, F.; Mendez-Zeel, M.; Larque-Saavedra, A.; Layola-Vargas, V. Picomolar Concentrations of Salicycates Induce Cellular Growth and Enhance Somatic Embriogénesis in Coffea arabica Tissue Culture. Plant Cell Rep. 2001, 20, 679–684. [Google Scholar] [CrossRef]
- Alemanno, L. Localization and Identification of Phenolic Compounds in Theobroma cacao L. Somatic Embryogenesis. Ann. Bot. 2003, 92, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Gallego, A.; Henao, A.M.; Urrea, A.; Atehortúa, L. Análisis de Distribución de Polifenoles y Sustancias de Reserva en Embriogénesis Somática de Cacao. Rev. Acta Biol. Colomb. 2016, 21, 335–345. [Google Scholar]
- Tan, C.L.; Furtek, D.B. Development of an in Vitro Regeneration System for Theobroma cacao from Mature Tissues. Plant Sci. 2003, 164, 407–412. [Google Scholar] [CrossRef]
- Fernández, R.; Villarroel, A.; Cuamo, L.; Storaci, V. Evaluation of a Somatic Embryogenesis Regenetation System for Neem (Azadirachta indica). Acta Biol. Colomb. 2016, 21, 581–592. [Google Scholar] [CrossRef]
- Fisichella, M.; Silvi, E.; Morini, S. Regeneration of Somatic Embryos and Roots from Quince Leaves Cultured on Media with Different Macroelement Composition. Plant Cell Tissue Organ Cult. 2000, 63, 101–107. [Google Scholar] [CrossRef]
- Litz, R. In Vitro Somatic Embriogénesis from Nucellar Callus of Monoembryonic Mango. HortScience 1984, 19, 715–717. [Google Scholar] [CrossRef]
- Issali, A.E.; Traoré, A.; Konan, J.L.; Mpika, J.; Andi, J.; Ngoran, K.; Sangaré, A. Relationship between Five Climatic Parameters and Somatic Embryogenesis from Sporophytic Floral Explants of Theobroma cacao L. Afr. J. Biotechnol. 2010, 9, 6614–6625. [Google Scholar]
- Minyaka, E.; Nimeenak, N.; Fotso; Sangara, A.; Ndoumuo, D. Effect of MgSO4 and K2SO4 on Somatic Embryo Differentiation in Theobroma cacao L. Plant Cell Tissue Organ Cult. 2008, 94, 149–160. [Google Scholar] [CrossRef]
| Accession Code | Acronym | UTM | Sector | Altitude (m.a.s.l.) |
|---|---|---|---|---|
| INDES-06 | I06 | 9369168 17M 787894 | El Chalán | 754 |
| INDES-11 | I11 | 9369112 17M 787792 | El Chalán | 736 |
| INDES-14 | I14 | 9366961 17M 793728 | El Limoncito | 817 |
| INDES-32 | I32 | 9367833 17M 779564 | Quebrada Seca | 421 |
| INDES-52 | I52 | 9366649 17M 794441 | Diamante Bajo | 725 |
| INDES-53 | I53 | 9366665 17M 794453 | Diamante Bajo | 757 |
| INDES-63 | I63 | 9365734 17M 793806 | Naranjos Alto | 727 |
| INDES-64 | I64 | 9364133 17M 792251 | Naranjos Alto | 665 |
| INDES-66 | I66 | 9364181 17M 792346 | Naranjos Alto | 667 |
| INDES-70 | I70 | 9371938 17M 787756 | Lluhuana | 970 |
| Genotypes | [TDZ] | Treatments | ED5 (70 Days) | |||
|---|---|---|---|---|---|---|
| E | EE | ME | TE | |||
| INDES-52 | 10-TDZ | 14 | 10 | 17.6 | 44 | 176 |
| INDES-52 | 20-TDZ | 15 | 14 | 16.93 | 39 | 237 |
| INDES-53 | 10-TDZ | 17 | 10 | 5.1 | 10 | 51 |
| INDES-53 | 20-TDZ | 18 | 4 | 15.5 | 38 | 62 |
| INDES-64 | 10-TDZ | 23 | 1 | 1 | 1 | 1 |
| INDES-64 | 20-TDZ | 24 | 1 | 22 | 22 | 22 |
| INDES-66 | 20-TDZ | 27 | 2 | 3.5 | 4 | 7 |
| INDES-70 | 10-TDZ | 29 | 2 | 28 | 32 | 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio, K.; Leiva, S.; Oliva, M.; Diaz-Valderrama, J.R.; Guerrero-Abad, J.C. Somatic Embryogenesis in Native Peruvian Fine-Flavor Cocoa Genotypes. Int. J. Plant Biol. 2025, 16, 84. https://doi.org/10.3390/ijpb16030084
Rubio K, Leiva S, Oliva M, Diaz-Valderrama JR, Guerrero-Abad JC. Somatic Embryogenesis in Native Peruvian Fine-Flavor Cocoa Genotypes. International Journal of Plant Biology. 2025; 16(3):84. https://doi.org/10.3390/ijpb16030084
Chicago/Turabian StyleRubio, Karol, Santos Leiva, Manuel Oliva, Jorge R. Diaz-Valderrama, and Juan Carlos Guerrero-Abad. 2025. "Somatic Embryogenesis in Native Peruvian Fine-Flavor Cocoa Genotypes" International Journal of Plant Biology 16, no. 3: 84. https://doi.org/10.3390/ijpb16030084
APA StyleRubio, K., Leiva, S., Oliva, M., Diaz-Valderrama, J. R., & Guerrero-Abad, J. C. (2025). Somatic Embryogenesis in Native Peruvian Fine-Flavor Cocoa Genotypes. International Journal of Plant Biology, 16(3), 84. https://doi.org/10.3390/ijpb16030084








