Abstract
We investigated the genetic and environmental variables determining the glucosinolate (GSL) content of cruciferous vegetables and the implications for cancer prevention. The enzyme myrosinase hydrolyzes GSLs, which are sulfur-containing chemicals found mostly in cruciferous vegetables, producing isothiocyanates (ITCs), which are physiologically active molecules. GSL breakdown products have considerable anti-carcinogenic, antioxidant, and anti-inflammatory capabilities, making them vital to human health. The review dives into genetic heterogeneity among cruciferous species, the importance of individual genes in GSL manufacturing, and breeding techniques for increasing GSL content. It also examines how environmental variables like soil type, pH, plant, nutrient availability, and temperature affect GSL levels. This report also covers the function of GSLs in plant defense, their bioavailability in humans, and their mechanisms in cancer prevention, emphasizing the chemicals’ potential for lowering cancer risk through cruciferous vegetable consumption. The findings highlight the necessity of optimizing both genetic and environmental variables required to increase the nutritional content and medicinal potential of cruciferous vegetables.
1. Introduction
Glucosinolates (GSLs) are sulfur-containing secondary metabolites found mostly in the order Capparales, occurring most abundantly in the Brassicaceae family, including cabbage, broccoli, and Brussels sprouts [1]. Brassicaceae plant species have at least 120 distinct GSLs, each with its own structure consisting of β-thioglucose, sulfonated oxime, and numerous side chains [2]. They are classified as aromatic, indole, aliphatic, and methionine-derived aliphatic compounds [3]. Indole GSLs generated from tryptophan make up 10% of known structures, aliphatic GSLs derived from methionine make up 50%, and aromatic GSLs derived from phenylalanine or tyrosine make up 10% of the known structures of GSLs [2,3]. GSLs comprise β-D-thioglucosides N-hydroximinosulfates and undergo several processes, such as amino acid elongation, core structure formation, and side chain modifications [4].
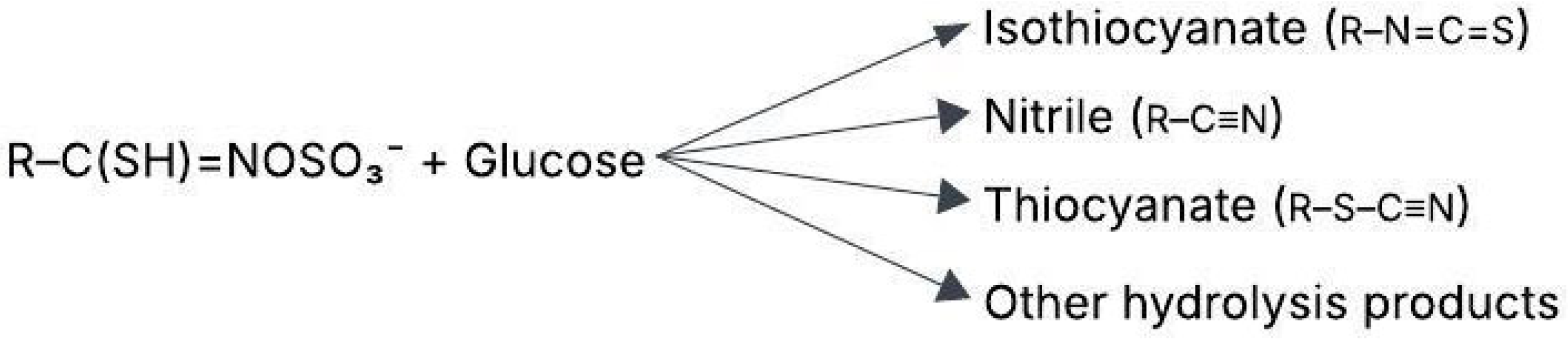
Myrosinase, an enzyme in plant cells, converts GSLs into biologically active compounds such as isothiocyanates (ITCs) as shown in Figure 1. These breakdown products have significant effects on both plant defense and human health. Myrosinase hydrolyzes GSLs, creating ITCs, nitriles, epithionitriles, thiocyanates, and epithioalkanes depending on pH and other factors [1].
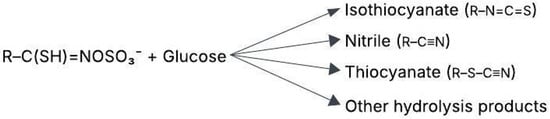
Figure 1.
Hydrolysis of glucosinolates (GSLs) [5].
GSLs have anti-carcinogenic properties that inhibit cell proliferation and induce apoptosis. They also have antioxidant and anti-inflammatory properties that reduce chronic inflammation, a risk factor for cancer, and improve cardiovascular function [6,7]. GSLs, such as glucoraphanin and glucoiberin, may improve cardiovascular health and reduce the risk of heart disease due to their neuroprotective properties and potential to prevent neurodegenerative diseases [6]. Furthermore, GSLs, such as glucoraphanin and sinigrin, exhibit anti-tumor and antibacterial characteristics, highlighting their potential use in biological applications [8]. Epidemiological studies have shown an inverse relationship between cruciferous vegetable consumption and cancer risk, while experimental studies have shown that their compounds, like indole-3-carbinol, can block carcinogenesis in animal models by upregulating phase II detoxification enzymes like glutathione S-transferases and exerting anticarcinogenic effects, indicating their potential cancer-preventive capabilities [9,10].
1.1. GSL Structure, Hydrolysis, and Bioactive Compounds
GSLs are plant secondary metabolites composed of a glucose molecule linked to an amino acid derivative and a sulfate group, with the basic structure RCH(NH2) COS [11]. These compounds are stored in vacuoles within intact plant cells, separate from the enzyme myrosinase. When plant tissue is damaged, myrosinase comes into contact with GSLs, catalyzing their hydrolysis by cleaving the thioglucosidic bond. This reaction releases β-D-glucose and an unstable aglucone intermediate, which rearranges into bioactive compounds such as ITCs, nitriles, thiocyanates, and epithionitriles [12,13]. The specific breakdown products depend on factors such as pH, cofactors like ascorbic acid, and environmental conditions [5,14,15].
ITCs are particularly significant due to their role in plant defense and human health. They exhibit herbicidal, antibacterial, and chemoprotective properties. Nitriles and thiocyanates, while less toxic, contribute to biofumigation and pathogen defense [16]. The production of these bioactive compounds is further influenced by specifier proteins and myrosinase-associated proteins, which guide the enzymatic reaction toward specific products [17]. Atypical myrosinases, such as Arabidopsis thaliana PEN2, may also play unique roles in disease resistance through specialized catalytic mechanisms [18].
In the gastrointestinal tract of humans, GSLs are primarily hydrolyzed by plant-derived myrosinase. The resulting bioactive compounds, especially ITCs, are absorbed and metabolized, where they interact with thiols and proteins to enhance their therapeutic effects [19,20]. This enzymatic process underscores the functional versatility and potential health benefits of GSLs and their hydrolysis products.
The unstable aglycone (non-sugar portion of GSL) produced during GSL hydrolysis undergoes spontaneous rearrangement, known as the Lossen rearrangement, forming various bioactive compounds based on the side chain (R group) as shown in Figure 1 and environmental conditions [13].
Mechanism of GSLs Breakdown Products:
- ITCs are produced at neutral pH levels through the Lossen rearrangement of the aglycone and are highly reactive and biologically active compounds. They play a significant role in activating Phase II detoxification enzymes, inhibiting tumor cell mitosis, and demonstrating antimicrobial properties [12]. ITCs suppress microbial growth by disrupting cell membranes, inhibiting enzymes, and interacting with proteins. Additionally, they generate free radicals, disrupt cellular contents, and bind to intracellular targets, thereby inducing multiple pathways for cell death [5,15].
- Nitriles and epithionitriles are compounds formed under acidic conditions, with their formation influenced by ferrous ions (Fe2+) and epithiospecifier proteins (ESP). Interestingly, these compounds can also develop in low yields in vitro, even in the absence of specifier proteins [12,20].
- Thiocyanates are produced in the presence of specifier proteins, which direct the hydrolysis process [20].
- Oxazolidine-2-thiones are formed by the cyclization of an aglycone with a β-hydroxyl side chain [12].
- Indole-3-Carbinol (I3C) and 3,3′-Diindolylmethane (DIM) are derived from indole GSLs, and these compounds show potential against hormone-responsive cancers such as breast, prostate, and ovarian cancers [11].
1.2. Role of Specific Genes and Alleles in GSL Biosynthesis
A complicated gene network that encodes enzymes involved in amino acid chain elongation, core structure formation, and side-chain modifications controls the manufacture of GSL, as indicated in Table 1. The model species Arabidopsis thaliana has been instrumental in identifying genes responsible for GSL biosynthesis, many of which have homologs in commercially relevant cruciferous crops such as Brassica rapa and Brassica oleracea [21].

Table 1.
Specific genes and their roles in GSL biosynthesis.
1.3. Genetic Variation and GSL Content
Natural variation in these genes leads to differences in GSL profiles among cruciferous vegetables. Polymorphisms in AOP2, for instance, determine whether plants accumulate higher levels of beneficial ITC precursors or less bioactive nitriles [23]. Additionally, variation in FMOGS-OX influences sulforaphane content in broccoli, affecting its potential anticancer properties [24]. Some Brassica rapa genotypes are naturally enriched in glucoraphanin, a precursor to sulforaphane, while others contain higher levels of progoitrin, a less desirable GSL form [21].
However, the environment modulates these genotype-determined profiles in complex ways. Abiotic factors such as sulfur and nitrogen availability, light intensity, and temperature can differentially influence individual GSL classes. For instance, sulfur limitation can sharply reduce aliphatic GSL levels in MAM1-dependent pathways, whereas indole GSLs derived via CYP79B2/B3 are more responsive to high-intensity light and cooler temperatures [26,27]. Biotic stresses such as pathogen attack, insect herbivory, or even root-colonizing microbes, often trigger selective up-regulation of indole GSLs (via enhanced CYP81F2 expression) over aliphatic forms, reflecting their roles in localized defense [28].
Furthermore, gene expression levels are influenced by environmental conditions such as sulfur availability, nitrogen levels, and temperature. Variants in MAM1 and BCAT4 impact the efficiency of methionine elongation, leading to genotypic differences in aliphatic GSLs [22]. In Brassica oleracea, differences in TGG1 and TGG2 expression modulate the enzymatic activity of myrosinase, affecting ITC release upon tissue damage [20].
These genotype, environment interactions must also be viewed through an evolutionary lens: GSLs and their hydrolysis products (ITC and nitriles) function as herbivore feeding deterrents. The classic “mustard-oil bomb” mechanism, where tissue damage by chewing insects triggers rapid myrosinase-mediated GSL hydrolysis, illustrates how selective pressures from herbivory have shaped both GSL diversity and inducible expression of TGG1/TGG2 myrosinases across Brassica species [28,29].
Advances in genome editing and marker-assisted breeding provide opportunities to enhance GSL content in cruciferous vegetables. Potential strategies include:
- CRISPR-Cas9-based knockout of ESP: Reduces nitrile formation, ensuring higher ITC production [25].
- Overexpression of FMOGS-OX: Increases the conversion of methylthioalkyl GSLs to sulforaphane precursors, enhancing cancer-preventive properties [24].
- Selection of high-glucoraphanin genotypes: Targeted breeding of Brassica rapa and Brassica oleracea lines to enhance glucoraphanin accumulation to improve dietary benefits [21].
Understanding the genetic basis of GSL biosynthesis enables the development of functional foods with optimized phytochemical content. This knowledge can support breeding programs aimed at increasing the health benefits of cruciferous vegetables while maintaining desirable agronomic traits [23].
1.4. Impact of Soil Type, pH, and Nutrient Availability
Soil type, pH, and nutrient availability all have a significant influence on GSL levels in Brassica plants, impacting microbial activity and nutrient availability for biosynthesis. Soil management measures that increase GSL content and release ITC can aid in the control of soil-borne illnesses due to ITC’s biofumigant qualities [30]. Different soil types can have a significant impact on GSL levels in plants. In Brassica napus, GSL content varied among soil types in England, despite receiving the same amount of sulfur fertilizer [31]. Swede roots from loam and sandy soils exhibited similar GSL profiles, with sandy soil having lower amounts of GSL than peat soil [32]. Cabbages planted in sludge-amended soil had much lower GSL levels than cabbages grown in unamended soil [33]. High zinc (Zn) accumulation in soil is associated with lower GSL levels in leaves but with higher levels in roots of Noccaea caerulescens (formerly Thlaspi caerulescens), a hyperaccumulator of Zn, Cd, and Ni, whose response may not represent that of non-accumulating food crops, whereas high copper (Cu) levels in soil are associated with higher total GSL content, particularly indolic GSLs, in Chinese cabbage roots [34].
Soil pH fluctuations also impact the availability of minerals needed for GSL synthesis, which may affect overall GSL concentration [35]. Chinese cabbage cultivated in soil with a pH of 7.6 had higher GSL, gluconasturtiin, and gluconapin than cabbage grown in soil with a pH of 6.2 [33]. The pH of the soil and plant environment has a considerable impact on GSL hydrolysis and subsequent product formation. Acidic pH (pH 4) promotes the synthesis of ITCs, which are beneficial to human health, but neutral pH (pH 6) inhibits ITC formation while increasing the creation of nitriles and epithionitriles, which are less beneficial. Basic pH (pH 8) promotes ITC synthesis in the same manner as acidic conditions [36].
GSL synthesis requires nutrients, especially sulfur (S), and changes in soil fertility and nutrient levels can directly affect its production, leading to varying quantities in plants [32]. Total GSL concentrations in broccoli rose with low nitrogen (N) delivery, regardless of sulfur level, and dropped with a low sulfur supply paired with an adequate N supply [33]. Sulfur (S) fertilizer has been shown to increase GSL levels in several plant species, emphasizing the importance of nutrient availability [31]. High S and low N applications increased total GSL and glucobrassicin concentrations in turnip cultivars, while increased selenium fertilization caused a dose-dependent decrease in all GSL classes [37]. S is a necessary component of the GSL structure, and its availability directly affects GSL synthesis, with sulfur fertilization increasing GSL levels [38]. In broccoli plants, optimal S and N supply improved GSL concentrations, particularly alkyl GSL such as glucoraphanin and glucoiberin [39]. Phosphorus (P) availability and light intensity affect GSL levels in pakchoi (Brassica rapa), with low P supply increasing total GSL by 164% under normal light intensity [40].
1.5. Influence of Climate and Weather Conditions (Water Availability, Temperature, Light)
Climate and climatic circumstances have a significant influence on plant GSL levels, influencing nutritional value and health benefits [37]. Water shortage appears to be a crucial factor in increasing GSL content, as vegetables grown under water-stressed conditions exhibited higher GSL levels, most likely due to improved synthesis of amino acids and sugars, which are precursors in GSL biosynthesis [38]. Excessive water, on the other hand, might cause a decrease in GSL levels, depending on the unique plant species and environmental conditions [41]. Water availability is important since decreased water supply can increase GSL concentration, as seen in turnip roots grown under water stress conditions (25% accessible soil water) vs. plentiful water supply [37]. Turnips grown with three water-supply treatments (25%, 50%, and 75% of available soil water) showed that the 25% available soil water treatment increased total GSLs concentrations more than the 50% and 75% [37]. Similarly, drought stress has a significant influence on GSL content, with drought-stressed plants having higher GSL levels in their leaves than in their roots, particularly in broccoli and cauliflower [42].
Temperature also plays an important role, with higher growth temperatures (21/15 °C) producing greater GSL concentrations than lower temperatures (15/9 °C). Extremely high temperatures, on the other hand, may promote growth and reduce functional components in leaves due to increased transpiration [43,44]. Temperature fluctuations affect vegetable output and quality, demonstrating a balance between higher temperatures at the start of the development phases and lower temperatures near the end of the development phases [45]. Low temperatures can enhance the concentration of some GSLs, such as glucoraphanin, in broccoli, and freezing increases the content of sulforaphane [34]. In Chinese cabbage, a temperature of around 28 °C during early developmental stages maximizes GSL accumulation, whereas sustained high temperatures above 22 °C during later growth stages impair overall yield and quality [45]. Ref. [38] discovered that vegetables grown in a year with less rainfall and higher temperatures had a significantly higher GSL content than those grown in a year with more favorable conditions (more rainfall and moderate temperatures).
Light exposure, including intensity and photoperiod, is also a significant concern. Crops grown in spring with high light intensity and longer days often have higher GSL content [34]. Excessive sun exposure combined with high temperatures can reduce various GSLs, including glucoiberin [44]. Light quality, such as the red-to-far-red ratio, affects GSL concentrations by affecting biosynthetic pathways [41]. Seasonal variations influence GSL profiles due to changes in temperature, light, and other environmental factors. Crops grown in spring, characterized by moderate temperatures, high light intensity, and less rainfall, have higher GSL levels compared to autumn/winter crops exposed to lower temperatures, shorter days, and increased rainfall [34]. According to [46], GSL levels peak in the spring because of moderate temperatures, low humidity, and longer photoperiods. Cold acclimatization may alter GSL levels, with an overall decrease observed, although specific compounds like sinigrin can increase [44]. Elevated CO2 levels (1300–1600 ppm) positively affect GSL content by enhancing growth, particularly in nitrogen-deficient environments [43].
1.6. Effects of Agricultural Practices on GSL Formation
1.6.1. Pesticides
GSL and their breakdown products, particularly ITC, provide a sustainable alternative to synthetic pesticides by efficiently suppressing soil-borne illnesses and pests using their antimicrobial capabilities [47]. Incorporating Brassica residues into the soil has shown potential in controlling soil-borne diseases, with outcomes depending on factors like species, GSL content, and timing of incorporation [47].
Pesticide application influences GSL levels depending on the type, timing, and frequency of use. Some pesticides induce stress responses in plants, leading to increased GSL synthesis as a defensive mechanism. However, excessive pesticide use can disrupt natural balances, potentially reducing GSL levels by harming beneficial organisms that support plant health [48,49]. Conventional synthetic pesticides often result in higher GSL concentrations compared to organic methods due to the stress reactions they elicit in plants [50]. Biofumigant crops, such as mustard (‘Caliente 199’, ‘Pacific Gold’) and broccoli, significantly influence soil organic matter (SOM), pH, and GSL levels. These crops release ITCs from GSLs during tissue breakdown, providing effective treatment for soilborne diseases when released into the soil [51].
1.6.2. Crop Rotation
Crop rotation helps mitigate soil-borne diseases and pests that can negatively impact GSL production. Rotations incorporating Brassica species improve soil health, nutrient availability, and overall stability, promoting increased GSL levels [48,49]. Additionally, rotating with legumes such as soybeans, peas, and beans enhances nitrogen fixation and soil fertility, creating conditions conducive to higher GSL production [52]. These practices collectively support soil structure and fertility, fostering an optimal environment for GSL-producing plants.
1.7. Post-Harvest Factors Influencing GSL Content
Post-harvest factors have a considerable impact on broccoli’s GSL content, influencing its nutritional value and health benefits [53]. Harvesting methods, post-harvest treatments, packaging, storage conditions, light exposure, storage time, mechanical processing, relative humidity, controlled atmosphere storage, and other specific treatments all have an impact on glucosinolate levels.
- Harvesting Methods: Delayed or improper harvesting can physically damage broccoli, triggering myrosinase activation and reducing GSL levels but increasing downstream hydrolysis products [53].
- Post-Harvest Treatments: Processes like blanching (with controlled temperature and duration), chemical treatments (e.g., calcium chloride to reduce enzyme activity), and irradiation (to lower microbial burden) help maintain GSL levels [53,54].
- Packaging: Modified Atmosphere Packaging (MAP), vacuum packaging, and emerging smart packaging technologies (e.g., intelligent indicators that monitor gas levels and temperature) help retain GSL levels by reducing oxidative stress and precisely managing the internal atmosphere [53,55].
- Storage Conditions: Cold storage preserves GSLs, but freezing and thawing can activate myrosinase, causing degradation. Maintaining proper humidity prevents microbial growth and desiccation, both of which reduce GSL levels [53,56].
- Light Exposure: Continuous light exposure enhances GSL levels through metabolic activity but can lead to quality issues like floret yellowing [53].
- Storage Time: GSL levels vary with storage duration. Broccoli refrigerated for seven days retains higher GSL content compared to four days [57].
- Pre-Storage and Storage Temperature: Storage at 0 °C or 4 °C stabilizes GSL levels. Pre-storage duration and subsequent storage at moderate temperatures (10 °C) can help maintain higher GSL levels compared to higher temperatures (18 °C) [57].
- Mechanical Processing (Chopping): Chopping activates myrosinase, increasing the conversion of GSLs into bioactive compounds [58].
- Relative Humidity (RH): High RH (98–100%) is critical for GSL retention, as lower humidity and higher temperatures accelerate GSL degradation [56].
- Controlled Atmosphere (CA) Storage: Low oxygen (1–2%) and high carbon dioxide (5–10%) levels extend broccoli’s post-harvest life and preserve GSL content [56].
1.8. Bioavailability and Absorption of GSLs and Their Metabolites in Humans
When GSLs are consumed, some are absorbed by the stomach, while most are absorbed in the small intestine, with up to 5% excreted via urine. Cooking vegetables deactivates the enzyme myrosinase, which hydrolyzes GSLs. Any remaining plant myrosinase in the proximal intestine can hydrolyze GSLs, while gut bacteria in humans convert GSLs into ITCs. Strains like Bifidobacterium may convert GSLs to nitriles. Bacterial myrosinase in the colon hydrolyzes non-hydrolyzed GSLs, with ITCs either absorbed or expelled. ITC absorption peaks about 3 h after ingestion. Once absorbed, ITCs can be transported back to the gut, enter plasma, or undergo processing. Studies in rats showed that ITCs accumulate mainly in the intestinal mucosa, liver, kidneys, and bladder, with lesser amounts in the brain and heart. Mercapturic acid in urine is a biomarker for ITC elimination, which is higher after raw cruciferous vegetable consumption, indicating improved bioavailability from raw vegetables [12,59].
Quantifying ITC metabolites like dithiocarbamates in urine or plasma aids pharmacokinetic studies [12,58]. Studying GSL absorption and metabolism provides insights into their potential health benefits, including anticancer properties [60,61]. Gut bacteria’s role in GSL hydrolysis increases bioactive ITC exposure [61]. Research on kale showed GSL breakdown products like allyl nitrile and sulforaphane increase after digestion, while intact GSLs decrease [62]. Similar studies on broccoli found faster and higher sulforaphane absorption from raw compared to cooked broccoli [63]. Studies in mice and humans confirmed that some intact GSLs are absorbed into the bloodstream, though gut bacteria play a limited role in GSL bioactivation [64]. Further, benzyl isothiocyanate (BITC) metabolites like BITC-N-acetyl-L-cysteine were detected in human plasma and urine after consuming Indian cress [65]. Sulforaphane and other metabolites excreted in urine confirmed bioavailability following the consumption of broccoli sprouts [66]. Overall, to maximize ITC bioavailability and harness the full spectrum of their potential health benefits, consumption of raw or minimally cooked Brassica vegetables is recommended.
1.9. Quantification of GSLs in Cruciferous Vegetables
Quantification of GSLs in cruciferous vegetables involves precise preparation and extraction. Initially, samples are immersed in boiling methanol to deactivate endogenous myrosinase, which would release compounds such as ITC and glucose after GSL breakdown [67]. After cooling, the samples are mixed and vacuum-filtered with a Buchner funnel and Whatman filter paper No. 1, and the residue is washed and reprocessed to create a pure aqueous extract [30]. To eliminate methanol, the extract is concentrated using a rotating vacuum evaporator at 40 °C [14]. Centrifugation and filtering through celite provide a homogeneous extract from which GSLs are extracted using DEAE-Sephadex A-25 ion-exchange resin [30,67].
Glucose extracted from Brassica plants via hot water extraction is purified and quantified using colorimetric or enzymatic techniques [14]. For precise GSL quantification, advanced methods like high-performance liquid chromatography (HPLC) coupled with mass spectrometry (MS) and Ultra-High-Performance Liquid Chromatography coupled with Mass Spectrometry (UHPLC-MS) are used. These methods accurately assess GSL types and content, identifying significant variations among Brassica genotypes and leaf [68,69,70,71]. A comprehensive understanding of GSL quantification is required to advance scientific research and enhance agricultural and nutritional practices [69].
2. Mechanisms of Cancer Prevention by GSL and Their Metabolites
Brassicaceae plants exhibit anticancer properties due to bioactive compounds generated during myrosinase-mediated GSL hydrolysis. These compounds, primarily ITCs and indoles, reduce systemic oxidative stress, inhibit angiogenesis, and promote cancer cell apoptosis. Sulforaphane, a potent ITC, exhibits strong antioxidant and anti-tumor activity, while phenethyl isothiocyanate (PEITC) affects gene expression associated with cancer progression. Indole-derived GSL metabolites, such as indole-3-carbinol (I3C) and 3,3′-diindolylmethane (DIM), have shown promise as preventive and therapeutic agents for colorectal, prostate, and breast cancer [1].
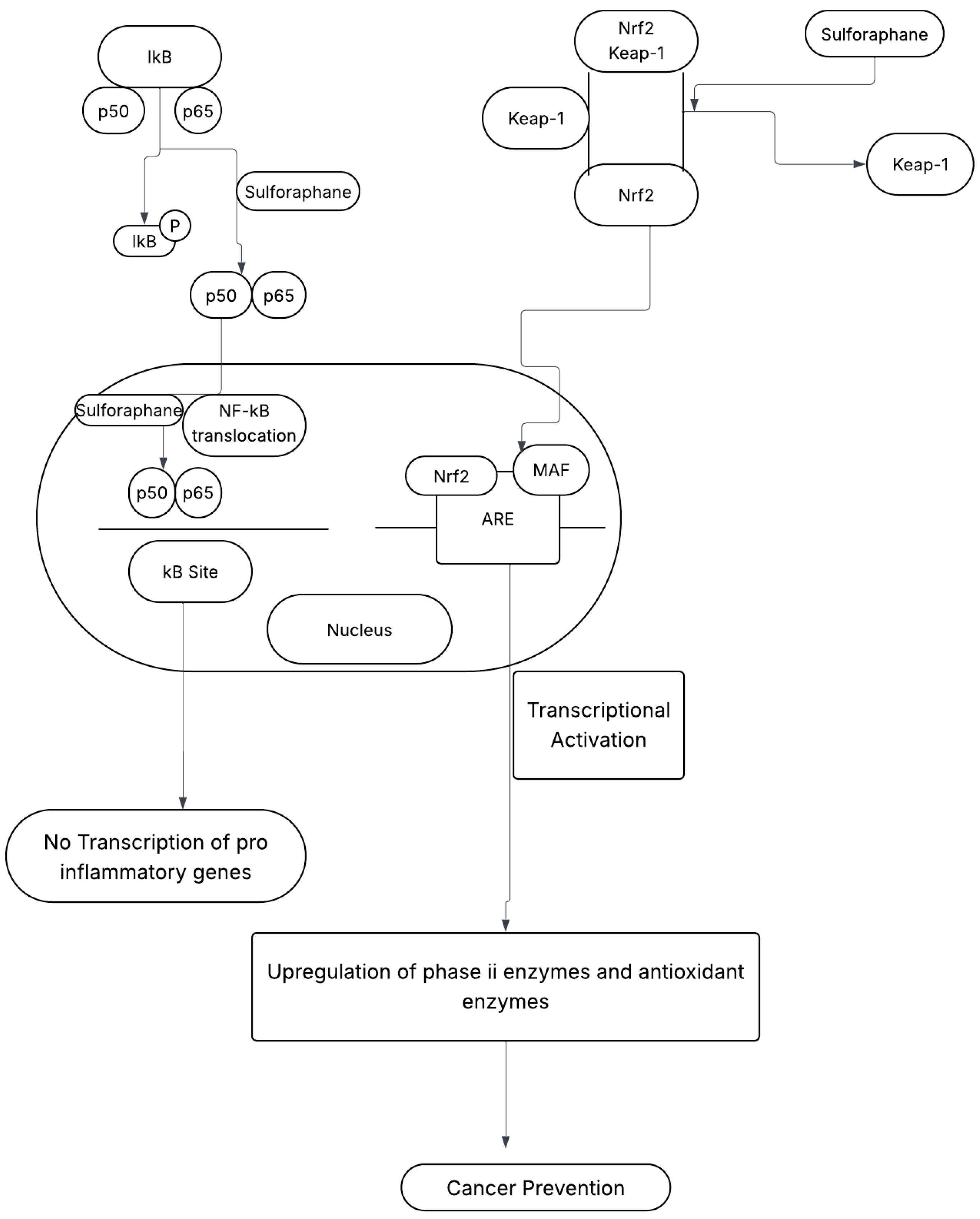
Sulforaphane prevents cancer development through its interaction with two critical pathways: the nuclear factor-kappa B (NF-κB) pathway and the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway as shown in Figure 2 [4].
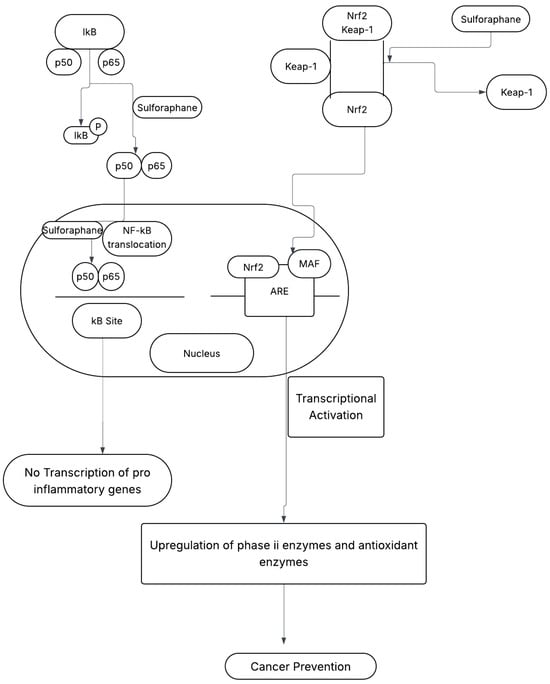
Figure 2.
Sulforaphane’s (SFN)—Mediated Cancer-Preventive Mechanisms [1]. SFN = Sulforaphane, IkB = inhibitor of kB, NF-kB = nuclear factor kB (p65/p50), Keap-1 = Kelch-like ECH-associated protein 1, Nrf2 = nuclear factor erythroid 2-related factor 2, MAF = musculoaponeurotic fibrosarcoma oncogene homolog, ARE = antioxidant response element.
2.1. Mechanism A: NF-κB Pathway
The NF-κB pathway plays a crucial role in regulating immune responses, inflammation, and cell survival. Hyperactivation of NF-κB contributes to chronic inflammation, tumor proliferation, and survival. SFN inhibits this pathway by:
- Preventing IκB Phosphorylation: SFN inhibits the phosphorylation of IκB, thereby blocking the nuclear translocation of the NF-κB subunit, p65. This suppression reduces the transcription of pro-inflammatory genes, lowering inflammation and cancer risk [1,4].
- Suppressing Tumor-Promoting Signals: SFN reduces the production of cytokines and growth factors associated with tumor growth, creating an unfavorable environment for cancer development [1].
2.2. Mechanism B: Nrf2 Pathway
The Nrf2 pathway is central to cellular defense against oxidative damage and carcinogenesis. Under normal conditions, Nrf2 is sequestered by Kelch-like ECH-associated protein 1 (Keap1) and degraded. SFN activates the Nrf2 pathway through:
- Disrupting Nrf2–Keap1 Interaction: SFN releases Nrf2 from Keap1, enabling its translocation to the nucleus.
- Enhancing Antioxidant Response: Once in the nucleus, Nrf2 binds to antioxidant response elements (AREs) in gene promoters, increasing the production of phase II detoxification enzymes and antioxidant proteins. This enhances detoxification processes and reduces oxidative stress, protecting cells from carcinogenesis [1,4].
SFN targets both the NF-κB and Nrf2 pathways, providing a comprehensive cancer prevention strategy. The NF-κB pathway reduces inflammation and suppresses tumor-promoting signals, while the Nrf2 pathway improves antioxidant defenses and detoxification [1]. This dual mechanism highlights sulforaphane’s potential as a potent dietary chemopreventive agent that addresses multiple aspects of cancer development and progression [4].
3. Molecular Mechanism of ITCs in Cancer Prevention
This section examines the effects of Benzyl Isothiocyanate (BITC), Phenethyl Isothiocyanate (PEITC), and SFN on several cancer cell lines, focusing on their potential targets in cell signaling for cancer prevention. Cruciferous vegetables are rich dietary sources of these compounds, as shown in Table 2.

Table 2.
Dietary Sources of Isothiocyanates and Their Precursors [4].
- Benzyl Isothiocyanate (BITC)
BITC, found in cruciferous vegetables such as cabbage, garden cress, and Indian cress, possesses antioxidant, anticancer, and antimetastatic properties. Its mechanisms of action against various cancer types are summarized in Table 3.

Table 3.
Mechanisms of BITC in Cancer Prevention [4].
- b.
- Phenethyl Isothiocyanate (PEITC)
PEITC, primarily found in watercress (Nasturtium officinale), a green leafy vegetable, has been shown to induce oxidative stress and generate Reactive Oxygen Species (ROS) in cancer cells, leading to oxidative damage and apoptosis. Its mechanisms of action in various cancer types are summarized in Table 4.

Table 4.
Mechanisms of PEITC in Cancer Prevention [4].
- c.
- Sulforaphane (SFN)
SFN is a strong Nrf2 inducer found in broccoli, Brussels sprouts, and cabbage. It plays a critical role in cancer prevention by modulating cell signaling pathways, enhancing antioxidant responses, and inducing apoptosis. The mechanisms of action of SFN in various cancer types are summarized in Table 5.

Table 5.
Mechanisms of SFN in Cancer Prevention [4].
4. Epidemiological Evidence Linking Cruciferous Vegetables Consumption to Cancer Risk Reduction
Epidemiological studies investigating the relationship between a cruciferous vegetable diet and cancer risk have yielded varying results across cancer types.
Lung Cancer: Several case–control studies have found that people with lung cancer consume fewer cruciferous vegetables than cancer-free controls. However, prospective studies have been mixed; while some found an inverse association between higher cruciferous vegetable intake and lower lung cancer risk (e.g., in Dutch men and women, American women, and Finnish men), other studies failed to replicate this finding [72]. Individuals in the highest category of cruciferous vegetable intake experienced a 43% lower risk of lung cancer compared with those in the lowest category (relative risk: 0.57; 95% CI: 0.36–0.89) [73].
Colorectal Cancer: Early case–control studies suggested that lower cruciferous vegetable consumption increased colorectal cancer risk. However, most prospective cohort studies found no significant association, except for a Dutch study that linked higher intake to decreased colon cancer risk but increased rectal cancer risk in women [72]. The Netherlands Cohort study observed no overall significant association between total vegetable intake and colorectal cancer risk. However, among women, those in the highest quintile of Brassica vegetable consumption had a lower colon cancer risk (rate ratio: 0.66; 95% CI: 0.44–1.01), with no clear association for rectal cancer [74].
Breast Cancer: The link between cruciferous vegetables and breast cancer risk is unclear. Some evidence suggests these vegetables may reduce risk by regulating estrogen metabolism, but larger studies have found no significant associations. Genetic differences in estrogen metabolism may influence these results [72]. In a pooled analysis of prospective cohorts, women consuming more than 5.5 servings/day of cruciferous and other vegetables had a modestly reduced breast cancer risk compared to those consuming ≤ 2.5 servings/day (hazard ratio = 0.89; 95% CI: 0.83–0.96) [75].
Prostate Cancer: Studies on prostate cancer have also shown mixed results. Some case–control studies found lower cruciferous vegetable consumption in men with prostate cancer, but most prospective studies found no significant link. However, one long-term study reported a significant inverse association among men who had prostate-specific antigen (PSA) testing, suggesting a possible protective effect [72].
Stomach Cancer: Epidemiological studies have found an inverse relationship between cruciferous vegetable (CV) intake and cancer risk, particularly stomach cancer, with a high cruciferous vegetable intake showing a relative risk of 0.81 (95% CI: 0.75–0.88) [76].
5. Review of Cohort and Case–Control Studies
Research from Italy and Switzerland supports this, showing CV consumption protects against various malignancies, including cancers of the oral cavity/pharynx, esophagus, stomach, liver, pancreas, and more [77]. Verhoeven et al. found 71% of prospective and 70% of case–control studies reported an inverse association between Brassica vegetables like cabbage, broccoli, cauliflower, and Brussels sprouts and cancer risk [9]. Studies on breast cancer indicate a strong link between high CV intake and reduced risk (RR = 0.85, 95% CI = 0.77–0.94), particularly postmenopausal breast cancer [78].
6. Meta-Analyses and Systematic Reviews on Cruciferous Vegetables Intake and Cancer Incidence
Several meta-analyses and systematic reviews support the preventative role of CV against various cancers, particularly stomach, colorectal, breast, and lung cancers [76,77]. A meta-analysis of 22 studies found a pooled risk ratio of 0.81 (95% confidence interval: 0.75–0.88) for stomach cancer, while another analysis revealed that consuming 10 g of CVs per day was associated with an 8% reduction in colorectal cancer risk [9]. Similarly, increased CV consumption was linked to a 15% decreased risk of breast cancer, with subgroup analyses showing consistent inverse relationships across different demographic and research variables [78]. A meta-analysis of 11 independent studies (4306 cases and 375,562 controls) revealed that CV consumption is linked to a reduced risk of ovarian cancer, with case–control studies showing a significant association (RR = 0.84; 95% CI, 0.75–0.94), though cohort studies did not [79]. The anticancer effects of CVs are attributed to glucosinolates and their breakdown products, such as isothiocyanates (ITCs) and indoles, which influence biotransformation enzyme systems [77,80].
7. Potential Confounding Factors and Limitations of Existing Studies
Research on gastric cancer risk factors is limited due to confounding variables and restrictions. Adjusting for significant confounders such as smoking status, alcohol use, and socioeconomic status helps, but residual confounding cannot be entirely removed. Case–control studies may overstate relationships due to recall or selection bias, while prospective studies-cohort investigations that enroll disease-free participants and record their diet before any diagnosis, may still fail to account for changes in dietary habits over time. Only one study addressed Helicobacter pylori infection, a major risk factor for stomach cancer. Dietary assessments using food frequency questionnaires may misclassify CV consumption, and heterogeneity in reporting and categorization may contribute to disparities [76].
The correlation between total vegetable consumption and CV intake makes it difficult to differentiate their effects. Food frequency surveys may miss unique CV consumption patterns, leading to inaccurate estimates [77]. Despite the potential for recall bias in case–control studies, evidence consistently shows no association between CVs and increased breast cancer risk [78].
Soil quality, rainfall, solar exposure, and cultivar or crop variety impact the levels of chemopreventive compounds in CVs, and bioavailability may be influenced by food preparation methods. Dietary and lifestyle factors are often overlooked, affecting observed relationships. The synergistic effects of compounds in CVs remain unclear, requiring further research [9].
Both cohort and case–control studies have limitations due to their observational nature. Genetic variations in glutathione S-transferase enzyme activity can yield inconsistent results, while random errors in dietary assessments may weaken observed associations, complicating efforts to link vegetable consumption and cancer risk [81]. Even after controlling for confounders like age and socioeconomic status, residual confounding from unmeasured covariates remains an issue.
Inaccurate dietary measurements and fluctuations in glucosinolate levels further impair study validity. Case–control studies may suffer from recall and selection bias, but cohort studies have yielded similar risk estimates. Cooking methods can affect CV bioavailability, particularly when high heat deactivates myrosinase, reducing ITC bioavailability. Genetic polymorphisms such as GSTM1 and GSTT1 also affect CV efficacy, complicating the interpretation of epidemiological data [82,83].
In conclusion, increased consumption of cruciferous vegetables is associated with a reduced risk of stomach cancer, especially non-cardia cancer [76]. While epidemiological evidence suggests CVs protect against various cancers, these findings should be interpreted cautiously due to study limitations and potential confounding factors [77]. More prospective studies with detailed dietary assessments and stratified analyses are needed to better understand the protective mechanisms of CVs [76]. Despite these limitations, further research into the cancer-preventive properties of CV phytochemicals, especially through prospective cohort studies and randomized controlled trials, remains essential [84].
8. Future Directions and Research Gaps
Numerous studies have examined the effects of genetic and environmental factors on the amount of glucosinolate (GSL) in cruciferous vegetables; nevertheless, many important research gaps still need to be filled. For instance, although multi-environment trials in broccoli and oilseed rape have quantified genotype–environment interactions for GSL accumulation, these efforts seldom integrate genomic selection models to predict performance across diverse agroecological zones. Standardized analytical protocols for GSL quantification exist, but inter-laboratory variability still hinders reliable cross-study comparisons [85]. The influence of the soil and gut microbiomes on plant GSL profiles and the subsequent human microbiota-mediated conversion of those GSLs into bioactive ITCs remains poorly characterized [86]. Furthermore, the contribution of specific promoter variants of key GSL biosynthetic genes to environmental inducibility in field-grown crops has yet to be explored [87]. Longitudinal field studies assessing the stability of GSL traits in commercial cultivars under projected climate change scenarios are scarce, limiting predictive breeding strategies [33]. Human clinical intervention trials evaluating the bioavailability and chemopreventive efficacy of GSL-derived isothiocyanates beyond sulforaphane are limited in scale and inconsistent in design, impeding the translation of preclinical findings into clear dietary guidelines [88]. Moreover, most human studies focus on acute consumption effects, with few assessing long-term intake and health outcomes [89]. Finally, while several studies have characterized G×E interactions in various species, the lack of high-resolution phenotypic and environmental data hampers the development of robust predictive models for GSL content across growing regions.
Second, despite extensive research on environmental modulation of GSL biosynthesis, reported outcomes remain highly variable and sometimes contradictory. Soil properties, such as pH, can modify leaf GSL concentrations in kale [46], temperature gradients influence total and individual GSL profiles under both greenhouse and field conditions [90], and nutrient availability (notably nitrogen and sulfur fertilization) alters aliphatic and indolic GSL levels in Brassica napus and B. oleracea. Moreover, biotic stresses, particularly insect herbivory, consistently induce increases in specific GSL classes as part of the glucosinolate–myrosinase defense response [91]. However, no integrative synthesis currently identifies which abiotic and biotic parameters reliably modulate GSL levels across species, cultivars, and growth conditions. Clarification of these broadly relevant environmental and biotic factors is critical to inform practical agricultural recommendations for optimizing GSL content in cruciferous crops.
Further clinical trials are also necessary to confirm the anti-carcinogenic properties of GSLs and develop clear dietary recommendations. These studies will also explore the possible risks and long-term health benefits of GSL ingestion. According to [92]. Further study is needed to fully comprehend the health consequences of GSLs, specifically regarding their bioavailability and metabolism in humans.
Addressing these gaps with controlled, long-term human intervention studies would significantly improve the use of glucosinolates in sustainable agriculture and dietary interventions.
9. Conclusions
Cruciferous vegetables are rich sources of glucosinolates (GSLs) and phytochemicals with multiple health advantages, particularly in cancer prevention. Genetic variation and environmental factors (e.g., soil quality, climate, and farming practices) influence GSL concentration in these crops. Optimizing these characteristics can increase the chemoprotective potential of cruciferous vegetables.
This integrated study combines findings from molecular genetics, highlighting the regulation of key biosynthetic transcription factors (MYB28, AOP2) and CRISPR/Cas9-driven enhancements in Brassica spp with agronomic evidence demonstrating how tailored sulfur and nitrogen fertilization regimes modulate GSL accumulation in field trials of broccoli and oilseed rape. Post-harvest biology studies show that controlled storage conditions, including modified atmosphere packaging and optimized temperature management, preserve myrosinase activity and sustained ITC release upon consumption, while epidemiological investigations correlate higher plasma biomarker levels of ITCs with reduced lung and colorectal cancer incidence in high-GSL diet cohorts. This multifaceted framework underscores the necessity of iterative feedback loops between breeding programs, agronomic practice optimization, post-harvest treatment standardization, and large-scale population trials to translate GSL enrichment into effective cancer-preventive strategies.
Despite the progress already achieved, our present understanding has significant limits. Inconsistent GSL measuring techniques and research designs impede the cross-comparison of findings. Most human evidence is observational, with few long-term studies, making causality uncertain. Furthermore, food preparation and genetic variances complicate the GSL-cancer relationship; for example, high-heat cooking deactivates myrosinase (limiting isothiocyanate bioavailability), and enzyme polymorphisms (GSTM1/T1) affect individual responses. Such intricacies introduce a need for caution when interpreting data and formulating dietary suggestions.
Given these shortcomings, future research should take numerous approaches to improve our understanding and application of glucosinolates (GSLs) in human health. Functional genomics and breeding efforts should be improved to create cultivars with higher GSL concentrations using contemporary genomic methods and marker-assisted selection. Simultaneously, agronomic studies under controlled settings are required to investigate how soil composition, nutrient availability, and climatic factors affect GSL accumulation. Furthermore, well-designed, long-term human clinical trials must show conclusive causal links between GSL consumption and cancer risk reduction. Finally, the investigation of individualized nutrition methods through nutrigenetic research is promising, as genetic heterogeneity among individuals substantially impacts GSL metabolism and bioavailability, altering their efficacy in disease prevention.
As highlighted in this analysis, integrating genetic insights and environmental control will be critical to realizing the full potential of GSLs in cancer prevention.
Author Contributions
Conceptualization, Methodology, Investigation, Writing-original draft preparation: S.K.; writing-review and editing, Funding acquisition: G.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the United States Department of Agriculture, National Institute of Food and Agriculture (USDA/NIFA) to Kentucky State University under the agreement # KYX-10-23-80P Accession 7005611 to Kentucky State University.
Data Availability Statement
Not applicable.
Conflicts of Interest
There are no competing interests to declare.
References
- Melim, C.; Lauro, M.R.; Pires, I.M.; Oliveira, P.J.; Cabral, C. The Role of Glucosinolates from Cruciferous Vegetables (Brassicaceae) in Gastrointestinal Cancers: From Prevention to Therapeutics. Pharmaceutics 2022, 14, 190. [Google Scholar] [CrossRef]
- Hopkins, R.J.; Van Dam, N.M.; Van Loon, J.J.A. Role of Glucosinolates in Insect-Plant Relationships and Multitrophic Interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef]
- Bekaert, M.; Edger, P.P.; Hudson, C.M.; Pires, J.C.; Conant, G.C. Metabolic and Evolutionary Costs of Herbivory Defense: Systems Biology of Glucosinolate Synthesis. New Phytol. 2012, 196, 596–605. [Google Scholar] [CrossRef]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [PubMed]
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolates and Biofumigation: Fate of Glucosinolates and Their Hydrolysis Products in Soil. Phytochem. Rev. 2009, 8, 299–310. [Google Scholar] [CrossRef]
- Buxdorf, K.; Yaffe, H.; Barda, O.; Levy, M. The Effects of Glucosinolates and Their Breakdown Products on Necrotrophic Fungi. PLoS ONE 2013, 8, e70771. [Google Scholar] [CrossRef]
- Yi, G.-E.; Robin, A.; Yang, K.; Park, J.-I.; Kang, J.-G.; Yang, T.-J.; Nou, I.-S. Identification and Expression Analysis of Glucosinolate Biosynthetic Genes and Estimation of Glucosinolate Contents in Edible Organs of Brassica oleracea Subspecies. Molecules 2015, 20, 13089–13111. [Google Scholar] [CrossRef]
- Melrose, J. The Glucosinolates: A Sulphur Glucoside Family of Mustard Anti-Tumour and Antimicrobial Phytochemicals of Potential Therapeutic Application. Biomedicines 2019, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Murillo, G.; Mehta, R.G. Cruciferous Vegetables and Cancer Prevention. Nutr. Cancer 2001, 41, 17–28. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Koutsidis, G.; Mavroudis, N.; Trafalis, D.T.; Botaitis, S.; Franco, R.; Zoumpourlis, V.; Amery, T.; Galanis, A.; Pappa, A.; et al. The Role of Isothiocyanates as Cancer Chemo-Preventive, Chemo-Therapeutic and Anti-Melanoma Agents. Antioxidants 2019, 8, 106. [Google Scholar] [CrossRef]
- Becker, T.; Juvik, J. The Role of Glucosinolate Hydrolysis Products from Brassica Vegetable Consumption in Inducing Antioxidant Activity and Reducing Cancer Incidence. Diseases 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, S.F.; Berhow, M.A. Glucosinolate Hydrolysis Products from Various Plant Sources: pH Effects, Isolation, and Purification. Ind. Crops Prod. 2005, 21, 193–202. [Google Scholar] [CrossRef]
- Antonious, G.F.; Bomford, M.; Vincelli, P. Screening Brassica Species for Glucosinolate Content. J. Environ. Sci. Health Part B 2009, 44, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Abreu, A.C.; Ferreira, C.; Saavedra, M.J.; Simões, L.C.; Simões, M. Antibacterial Activity and Mode of Action of Selected Glucosinolate Hydrolysis Products against Bacterial Pathogens. J. Food Sci. Technol. 2015, 52, 4737–4748. [Google Scholar] [CrossRef]
- Antonious, G.F. Glucosinolates in Collard Greens Grown under Three Soil Management Practices. J. Environ. Sci. Health Part B 2015, 50, 368–373. [Google Scholar] [CrossRef]
- Wittstock, U.; Kurzbach, E.; Herfurth, A.-M.; Stauber, E.J. Glucosinolate Breakdown. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 80, pp. 125–169. ISBN 978-0-08-100327-5. [Google Scholar]
- Sugiyama, R.; Hirai, M.Y. Atypical Myrosinase as a Mediator of Glucosinolate Functions in Plants. Front. Plant Sci. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and Stability of Glucosinolates and Their Breakdown Products in Foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450. [Google Scholar] [CrossRef]
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and Biological Activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef]
- He, H.; Ping, L.; Bonnema, G.; Dekker, M.; Verkerk, R. Genetic variation in glucosinolate content within Brassica rapa vegetables. Acta Hortic. 2012, 944, 129–140. [Google Scholar]
- Kang, J.Y.; Ibrahim, K.E.; Juvik, J.A.; Kim, D.H.; Kang, W.J. Genetic and environmental variation of glucosinolate content in Chinese cabbage. HortScience 2006, 41, 1382–1385. [Google Scholar] [CrossRef]
- Bellostas, N.; Sørensen, A.D.; Sørensen, J.C.; Sørensen, H. Genetic Variation and Metabolism of Glucosinolates. Adv. Bot. Res. 2007, 45, 369–415. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Okamura, Y.; Dort, H.; Reichelt, M.; Tunström, K.; Wheat, C.W.; Vogel, H. Testing hypotheses of a coevolutionary key innovation reveals a complex suite of traits involved in defusing the mustard oil bomb. Proc. Natl. Acad. Sci. USA 2022, 119, e2208447119. [Google Scholar] [CrossRef] [PubMed]
- Arora, R. Glucosinolates and Their Hydrolytic Products—A Love Story of Environmental, Biological, and Chemical Conditions. J. AOAC Int. 2024, 107, 867–875. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhu, Z.J.; Ni, X.L.; Qian, Q.Q. Effect of nitrogen and sulfur supply on glucosinolates in Brassica campestris ssp. chinensis. Agric. Sci. China 2006, 5, 603–608. [Google Scholar] [CrossRef]
- Malik, M.A.; Poveda, J.; Zuluaga, D.L.; Boccaccio, L.; Hassan, Z.; Ali, J. Defence of Brassicaceae plants against generalist and specialised insect pests through the development of myrosinase mutants: A review. Ind. Crops Prod. 2025, 228, 120945. [Google Scholar] [CrossRef]
- Unger, K.; Raza, S.A.K.; Mayer, T.; Reichelt, M.; Stuttmann, J.; Hielscher, A.; Wittstock, U.; Gershenzon, J.; Agler, M.T. Glucosinolate structural diversity shapes recruitment of a metabolic network of leaf-associated bacteria. Nat. Commun. 2024, 15, 8496. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.; Antonious, A.; Trivette, T. Emerging Technology for Increasing Glucosinolates in Arugula and Mustard Greens. J. Environ. Sci. Health Part B 2017, 52, 466–469. [Google Scholar] [CrossRef]
- Falk, K.L.; Tokuhisa, J.G.; Gershenzon, J. The Effect of Sulfur Nutrition on Plant Glucosinolate Content: Physiology and Molecular Mechanisms. Plant Biol. 2007, 9, 573–581. [Google Scholar] [CrossRef]
- Thomsen, M.; Riley, H.; Borge, G.I.A.; Lea, P.; Rødbotten, M.; Bengtsson, G. Effects of soil type and fertilization on yield, chemical parameters, sensory quality and consumer preference of swede (Brassica napus L. ssp. rapifera). Eur. J. Hortic. Sci. 2018, 82, 294–305. [Google Scholar] [CrossRef]
- Wu, X.; Huang, H.; Childs, H.; Wu, Y.; Yu, L.; Pehrsson, P.R. Glucosinolates in Brassica Vegetables: Characterization and Factors That Influence Distribution, Content, and Intake. Annu. Rev. Food Sci. Technol. 2021, 12, 485–511. [Google Scholar] [CrossRef]
- Biondi, F.; Balducci, F.; Capocasa, F.; Visciglio, M.; Mei, E.; Vagnoni, M.; Mezzetti, B.; Mazzoni, L. Environmental Conditions and Agronomical Factors Influencing the Levels of Phytochemicals in Brassica Vegetables Responsible for Nutritional and Sensorial Properties. Appl. Sci. 2021, 11, 1927. [Google Scholar] [CrossRef]
- Velasco, P.; Cartea, M.E.; González, C.; Vilar, M.; Ordás, A. Factors affecting the glucosinolate content of kale (Brassica oleracea acephala group). J. Agric. Food Chem. 2007, 55, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Klopsch, R.; Oliviero, T.; Schreiner, M.; Verkerk, R.; Dekker, M. Optimizing Isothiocyanate Formation during Enzymatic Glucosinolate Breakdown by Adjusting pH Value, Temperature and Dilution in Brassica Vegetables and Arabidopsis Thaliana. Sci. Rep. 2017, 7, 40807. [Google Scholar] [CrossRef]
- Zhang, H.; Schonhof, I.; Krumbein, A.; Gutezeit, B.; Li, L.; Stützel, H.; Schreiner, M. Water Supply and Growing Season Influence Glucosinolate Concentration and Composition in Turnip Root (Brassica rapa ssp. rapifera L.). J. Plant Nutr. Soil Sci. 2008, 171, 255–265. [Google Scholar] [CrossRef]
- Ciska, E.; Martyniak-Przybyszewska, B.; Kozlowska, H. Content of Glucosinolates in Cruciferous Vegetables Grown at the Same Site for Two Years under Different Climatic Conditions. J. Agric. Food Chem. 2000, 48, 2862–2867. [Google Scholar] [CrossRef]
- Schonhof, I.; Blankenburg, D.; Müller, S.; Krumbein, A. Sulfur and Nitrogen Supply Influence Growth, Product Appearance, and Glucosinolate Concentration of Broccoli. J. Plant Nutr. Soil Sci. 2007, 170, 65–72. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Z.; Gerendás, J. Interactive Effects of Phosphorus Supply and Light Intensity on Glucosinolates in Pakchoi (Brassica campestris L. ssp. chinensis var. communis). Plant Soil 2009, 323, 323–333. [Google Scholar] [CrossRef]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in Plant Protection and Human Health—Influences of Climate, Environment and Agronomic Practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Ben Ammar, H.; Arena, D.; Treccarichi, S.; Di Bella, M.C.; Marghali, S.; Ficcadenti, N.; Lo Scalzo, R.; Branca, F. The Effect of Water Stress on the Glucosinolate Content and Profile: A Comparative Study on Roots and Leaves of Brassica oleracea L. Crops. Agronomy 2023, 13, 579. [Google Scholar] [CrossRef]
- Chowdhury, M.; Kiraga, S.; Islam, M.N.; Ali, M.; Reza, M.N.; Lee, W.-H.; Chung, S.-O. Effects of Temperature, Relative Humidity, and Carbon Dioxide Concentration on Growth and Glucosinolate Content of Kale Grown in a Plant Factory. Foods 2021, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Steindal, A.L.H.; Rødven, R.; Hansen, E.; Mølmann, J. Effects of Photoperiod, Growth Temperature and Cold Acclimatisation on Glucosinolates, Sugars and Fatty Acids in Kale. Food Chem. 2015, 174, 44–51. [Google Scholar] [CrossRef]
- Shim, J.-Y.; Kim, H.-Y.; Kim, D.-G.; Lee, Y.-S.; Chung, S.-O.; Lee, W.-H. Optimizing Growth Conditions for Glucosinolate Production in Chinese Cabbage. Hortic. Environ. Biotechnol. 2018, 59, 649–657. [Google Scholar] [CrossRef]
- Del Carmen Martínez-Ballesta, M.; Moreno, D.; Carvajal, M. The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.A.S.; Rodrigues, P.M.F. Towards a More Sustainable Agriculture System: The Effect of Glucosinolates on the Control of Soil-Borne Diseases. J. Hortic. Sci. Biotechnol. 1999, 74, 667–674. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Monti, A. Energy Crops in Rotation. A Review. Biomass Bioenergy 2011, 35, 12–25. [Google Scholar] [CrossRef]
- Yfantopoulos, D.; Ntatsi, G.; Karkanis, A.; Savvas, D. Evaluation of the Role of Legumes in Crop Rotation Schemes of Organic or Conventionally Cultivated Cabbage. Agronomy 2024, 14, 297. [Google Scholar] [CrossRef]
- Rempelos, L.; Barański, M.; Sufar, E.K.; Gilroy, J.; Shotton, P.; Leifert, H.; Średnicka-Tober, D.; Hasanaliyeva, G.; Rosa, E.A.S.; Hajslova, J.; et al. Effect of Climatic Conditions, and Agronomic Practices Used in Organic and Conventional Crop Production on Yield and Nutritional Composition Parameters in Potato, Cabbage, Lettuce and Onion; Results from the Long-Term NFSC-Trials. Agronomy 2023, 13, 1225. [Google Scholar] [CrossRef]
- Rudolph, R.E.; Sams, C.; Steiner, R.; Thomas, S.H.; Walker, S.; Uchanski, M.E. Biofumigation Performance of Four Brassica Crops in a Green Chile Pepper (Capsicum Annuum) Rotation System in Southern New Mexico. HortScience 2015, 50, 247–253. [Google Scholar] [CrossRef]
- Bak, G.; Lee, G.; Lee, J.; Jee, S. Crop Rotation Affects Biological Properties of Rhizosphere Soil and Productivity of Kimchi Cabbage (Brassica rapa ssp. pekinensis) Compared to Monoculture. Hortic. Environ. Biotechnol. 2022, 63, 613–625. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre- and Post-Harvest Factors Affecting Glucosinolate Content in Broccoli. Front. Nutr. 2020, 7, 147. [Google Scholar] [CrossRef]
- Banerjee, A.; Variyar, P.S.; Chatterjee, S.; Sharma, A. Effect of Post-Harvest Radiation Processing and Storage on the Volatile Oil Composition and Glucosinolate Profile of Cabbage. Food Chem. 2014, 151, 22–30. [Google Scholar] [CrossRef]
- Du, L.; Huang, X.; Li, Z.; Qin, Z.; Zhang, N.; Zhai, X.; Shi, J.; Zhang, J.; Shen, T.; Zhang, R.; et al. Application of Smart Packaging in Fruit and Vegetable Preservation: A Review. Foods 2025, 14, 447. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Faragher, J.D.; Winkler, S. A Review of the Influence of Postharvest Treatments on Quality and Glucosinolate Content in Broccoli (Brassica oleracea var. italica) Heads. Postharvest Biol. Technol. 2006, 41, 1–8. [Google Scholar] [CrossRef]
- Rybarczyk-Plonska, A.; Hagen, S.F.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.K.; Wold, A.-B. Glucosinolates in Broccoli (Brassica oleracea L. var. italica) as Affected by Postharvest Temperature and Radiation Treatments. Postharvest Biol. Technol. 2016, 116, 16–25. [Google Scholar] [CrossRef]
- Verkerk, R.; Dekker, M.; Jongen, W.M.F. Post-harvest Increase of Indolyl Glucosinolates in Response to Chopping and Storage of Brassica Vegetables. J. Sci. Food Agric. 2001, 81, 953–958. [Google Scholar] [CrossRef]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular Structure, Breakdown, Genetic, Bioavailability, Properties and Healthy and Adverse Effects. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 90, pp. 305–350. ISBN 978-0-12-816567-6. [Google Scholar]
- Sørensen, J.C.; Frandsen, H.B.; Jensen, S.K.; Kristensen, N.B.; Sørensen, S.; Sørensen, H. Bioavailability and in Vivo Metabolism of Intact Glucosinolates. J. Funct. Foods 2016, 24, 450–460. [Google Scholar] [CrossRef]
- Shakour, Z.T.; Shehab, N.G.; Gomaa, A.S.; Wessjohann, L.A.; Farag, M.A. Metabolic and Biotransformation Effects on Dietary Glucosinolates, Their Bioavailability, Catabolism and Biological Effects in Different Organisms. Biotechnol. Adv. 2022, 54, 107784. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Bornhorst, G.M.; Oteiza, P.I.; Mitchell, A.E. Assessing the Fate and Bioavailability of Glucosinolates in Kale (Brassica oleracea) Using Simulated Human Digestion and Caco-2 Cell Uptake Models. J. Agric. Food Chem. 2019, 67, 9492–9500. [Google Scholar] [CrossRef]
- Charron, C.S.; Vinyard, B.T.; Ross, S.A.; Seifried, H.E.; Jeffery, E.H.; Novotny, J.A. Absorption and Metabolism of Isothiocyanates Formed from Broccoli Glucosinolates: Effects of BMI and Daily Consumption in a Randomised Clinical Trial. Br. J. Nutr. 2018, 120, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Budnowski, J.; Hanske, L.; Schumacher, F.; Glatt, H.; Platz, S.; Rohn, S.; Blaut, M. Glucosinolates Are Mainly Absorbed Intact in Germfree and Human Microbiota-Associated Mice. J. Agric. Food Chem. 2015, 63, 8418–8428. [Google Scholar] [CrossRef] [PubMed]
- Platz, S.; Kühn, C.; Schiess, S.; Schreiner, M.; Kemper, M.; Pivovarova, O.; Pfeiffer, A.F.H.; Rohn, S. Bioavailability and Metabolism of Benzyl Glucosinolate in Humans Consuming Indian Cress (Tropaeolum majus L.). Mol. Nutr. Food Res. 2016, 60, 652–660. [Google Scholar] [CrossRef]
- Baenas, N.; Suárez-Martínez, C.; García-Viguera, C.; Moreno, D.A. Bioavailability and New Biomarkers of Cruciferous Sprouts Consumption. Food Res. Int. 2017, 100, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The Chemical Diversity and Distribution of Glucosinolates and Isothiocyanates among Plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Kim, D.-G.; Park, K.; Park, J.-T.; Lee, W.-H. Quantitative Analysis of Glucosinolate Content in Chinese Cabbages Under Different Storage Conditions. J. Biosyst. Eng. 2020, 45, 57–64. [Google Scholar] [CrossRef]
- Kim, H.W.; Ko, H.C.; Baek, H.J.; Cho, S.M.; Jang, H.H.; Lee, Y.M.; Kim, J.B. Identification and Quantification of Glucosinolates in Korean Leaf Mustard Germplasm (Brassica juncea var. integrifolia) by Liquid Chromatography–Electrospray Ionization/Tandem Mass Spectrometry. Eur. Food Res. Technol. 2016, 242, 1479–1484. [Google Scholar] [CrossRef]
- Rhee, J.-H.; Choi, S.; Lee, J.-E.; Hur, O.-S.; Ro, N.-Y.; Hwang, A.-J.; Ko, H.-C.; Chung, Y.-J.; Noh, J.-J.; Assefa, A.D. Glucosinolate Content in Brassica Genetic Resources and Their Distribution Pattern within and between Inner, Middle, and Outer Leaves. Plants 2020, 9, 1421. [Google Scholar] [CrossRef]
- Yu, X.; He, H.; Zhao, X.; Liu, G.; Hu, L.; Cheng, B.; Wang, Y. Determination of 18 Intact Glucosinolates in Brassicaceae Vegetables by UHPLC-MS/MS: Comparing Tissue Disruption Methods for Sample Preparation. Molecules 2021, 27, 231. [Google Scholar] [CrossRef]
- Higdon, J.; Delage, B.; Williams, D.; Dashwood, R. Cruciferous Vegetables and Human Cancer Risk: Epidemiologic Evidence and Mechanistic Basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef]
- Lam, T.K.; Ruczinski, I.; Helzlsouer, K.J.; Shugart, Y.Y.; Caulfield, L.E.; Alberg, A.J. Cruciferous Vegetable Intake and Lung Cancer Risk: A Nested Case-Control Study Matched on Cigarette Smoking. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, L.E.; Goldbohm, R.A.; van Poppel, G.A.F.C.; Sturmans, F.; Hermus, R.J.J.; van den Brandt, P.A. Vegetable and Fruit Consumption and Risks of Colon and Rectal Cancer in a Prospective Cohort Study: The Netherlands Cohort Study on Diet and Cancer. Am. J. Epidemiol. 2000, 152, 1081–1092. [Google Scholar] [CrossRef]
- Farvid, M.S.; Chen, W.Y.; Rosner, B.A.; Tamimi, R.M.; Willett, W.C.; Eliassen, A.H. Fruit and Vegetable Consumption and Breast Cancer Incidence: Repeated Measures over 30 Years of Follow-Up. Int. J. Cancer 2019, 144, 1496–1510. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, Y.; Wang, J.; Han, L.; Xiang, Y. Cruciferous Vegetable Consumption and Gastric Cancer Risk: A Meta-analysis of Epidemiological Studies. Cancer Sci. 2013, 104, 1067–1073. [Google Scholar] [CrossRef]
- Bosetti, C.; Filomeno, M.; Riso, P.; Polesel, J.; Levi, F.; Talamini, R.; Montella, M.; Negri, E.; Franceschi, S.; La Vecchia, C. Cruciferous Vegetables and Cancer Risk in a Network of Case–Control Studies. Ann. Oncol. 2012, 23, 2198–2203. [Google Scholar] [CrossRef]
- Liu, X.; Lv, K. Cruciferous Vegetables Intake Is Inversely Associated with Risk of Breast Cancer: A Meta-Analysis. Breast 2013, 22, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Li, X.; Yu, T. Cruciferous vegetables consumption and the risk of ovarian cancer: A meta-analysis of observational studies. Diagn. Pathol. 2014, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W.; Peterson, S. Brassica, Biotransformation and Cancer Risk: Genetic Polymorphisms Alter the Preventive Effects of Cruciferous Vegetables. J. Nutr. 2002, 132, 2991–2994. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, X.-O.; Xiang, Y.-B.; Yang, G.; Li, H.; Gao, J.; Cai, H.; Gao, Y.-T.; Zheng, W. Cruciferous Vegetable Consumption Is Associated with a Reduced Risk of Total and Cardiovascular Disease Mortality. Am. J. Clin. Nutr. 2011, 94, 240–246. [Google Scholar] [CrossRef]
- Johnson, I.T. Cruciferous Vegetables and Risk of Cancers of the Gastrointestinal Tract. Mol. Nutr. Food Res. 2018, 62, 1701000. [Google Scholar] [CrossRef]
- Tse, G.; Eslick, G.D. Cruciferous Vegetables and Risk of Colorectal Neoplasms: A Systematic Review and Meta-Analysis. Nutr. Cancer 2014, 66, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, C.B.; Tang, L. Cruciferous Vegetable Intake and Cancer Prevention: Role of Nutrigenetics. Cancer Prev. Res. 2009, 2, 298–300. [Google Scholar] [CrossRef]
- Grosser, K.; van Dam, N. A straightforward method for glucosinolate extraction and analysis with high-pressure liquid chromatography (HPLC). J. Vis. Exp. JoVE 2017, 121, 55425. [Google Scholar] [CrossRef]
- Bouranis, J.A.; Beaver, L.M.; Jiang, D.; Choi, J.; Wong, C.P.; Davis, E.W.; Williams, D.E.; Sharpton, T.J.; Stevens, J.F.; Ho, E. Interplay between cruciferous vegetables and the gut microbiome: A multi-omic approach. Nutrients 2023, 15, 42. [Google Scholar] [CrossRef]
- Watanabe, H.; Usami, R.; Kishino, S.; Osada, K.; Aoki, Y.; Morisaka, H.; Takahashi, M.; Izumi, Y.; Bamba, T.; Aoki, W.; et al. Enzyme systems involved in glucosinolate metabolism in Companilactobacillus farciminis KB1089. Sci. Rep. 2021, 11, 23715. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Martini, D.; Venturi, S.; Tucci, M.; Porrini, M.; Riso, P.; Del Bo’, C. An overview of registered clinical trials on glucosinolates and human health: The current situation. Front. Nutr. 2021, 8, 730906. [Google Scholar] [CrossRef]
- Sikorska-Zimny, K.; Beneduce, L. The metabolism of glucosinolates by gut microbiota. Nutrients 2021, 13, 2750. [Google Scholar] [CrossRef]
- Muthusamy, M.; Lee, S.I. Abiotic stress-induced secondary metabolite production in Brassica: Opportunities and challenges. Front. Plant Sci. 2024, 14, 1323085. [Google Scholar] [CrossRef]
- Textor, S.; Gershenzon, J. Herbivore induction of the glucosinolate–myrosinase defense system: Major trends, biochemical bases and ecological significance. Phytochem. Rev. 2009, 8, 149–170. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frígola, A. Bioactive Components from Leaf Vegetable Products. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 41, pp. 321–346. ISBN 978-0-444-63294-4. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).