Abstract
Juniperus communis L. can be used for essential oils and ornamental purposes, but currently the population is declining in its natural habitats as the seeds are very slow to germinate, with many seeds also empty. These findings could support both the conservation of J. communis and its adoption in sustainable agricultural systems. This study investigated the potential of vegetative propagation evaluating the effects of rooting medium, gender, and Indole 3-Butyric Acid (IBA) treatment on the rooting success of J. communis cuttings. Two types of rooting medium (RM_I vs. RM_II), gender (male vs. female) and two IBA concentrations (0 vs. 4000 ppm) were used. Rooting medium I (RM_I) consists of substrate and perlite (50% + 50%), and rooting medium II (RM II) contains unfertilized blonde peat (65%), substrate (25%), and perlite (10%). The results show the influence of rooting medium, IBA treatment, and gender on the rooting percentage and the number of the primary roots. Female cuttings are more likely to induce rooting than male cuttings (29.69% vs. 19.90%), and the RM_II produces a higher percentage of rooting than RM_I (28.89% vs. 20.70%). In relation to the number of roots per cutting, RM_II was higher than RM_I (7.46 vs. 6.04). Interaction between rooting medium and IBA treatment showed significant differences (p ≤ 0.05) in the rooting percentage. Results showed that cuttings treated with 4000 ppm IBA in RM_II achieved the highest rooting percentage (45.57%), with female cuttings outperforming male cuttings.
1. Introduction
Juniper species are resistant to different climates, poor soils, and harsh environments. Juniperus communis L. (common juniper) can grow in acid, neutral, and alkaline soil and can survive in very acid and very alkaline soil [1,2]. It grows at low and high altitudes on pastureland, in abandoned fields, clearcuts, as well as on mountains and in scrub [3]. In Spain, it is found most commonly in the Northeast, more frequently between 400 and 1700 m above sea level [4]. Its adaptability to poor soils and harsh climates makes it an ideal candidate for cultivation in marginal lands with low productivity potential as a medicinal and aromatic plant.
Common juniper has been used for centuries in traditional medicine [5], for ornamental purposes [6,7], and in various industrial applications [8]. Nowadays, the essential oils are valued for their antimicrobial, antioxidant, anti-inflammatory [9], and anticancer activities [10,11,12].
Common juniper is a dioecious species of the Cupressaceae family. The seeds are in female specimens and annually produce fleshy, spherical, berry-like cones of approximately 6.5 mm in diameter that take 2 or 3 years to ripen and contain between one to four seeds per cone [13]. The natural regeneration of this plant using seeds is very slow and difficult in field conditions [14,15]. It has certain problems, such as producing a large number of empty seeds and seeds that are not viable for different reasons each year [16,17], along with the difficulty of breaking seed dormancy [9,18,19]. In particular, under natural conditions, one study found that only one seedling survives the first summer out of every 1572 seeds dispersed by birds [18]. Therefore, the spontaneous regeneration of the species is limited [20], leading to strong population decline [6] in many regions [4,17], emphasizing the urgent need for alternative propagation methods, such as vegetative propagation, to ensure its availability for conservation and commercial purposes.
Clonal propagation from stem cuttings enables the multiplication of select superior trees with the same characteristics as that of selected parents and the production of genetically homogenous plant material, resulting in improved efficiency in management and finished product [21], which can be applied to large-scale cultivation and reduce costs for the farmers. Although some studies have explored in vitro propagation, whole plantlet development from callus as indirect organogenesis could not be achieved [22,23].
Difficulties in rooting of cutting in coniferous species have been reported [7,21,24] and their vegetative propagation has not been sufficiently developed [21,24,25] despite this being the method normally used for the commercial propagation of Juniperus species [24,26]. In relation to J. communis studies have been carried out [14,27,28,29,30], but further investigation is necessary.
The auxin concentrations and cutting collection dates have been evaluated in most rooting studies, but there are few studies on the effect of the rooting medium composition on the rooting of different Juniperus species in vegetative propagation. The rooting medium is a factor influencing the rooting potential of conifers cuttings [31].
The substrate used should provide adequate drainage to prevent waterlogging during root initiation [24], but it should also have sufficient water-holding capacity to prevent rapid drying out [32,33]. Therefore, the most suitable substrates to facilitate rooting are mixtures of different materials, so that they have characteristics of water retention, aeration, porosity, bulk density, and ideal particle size. Besides, they should adhere to the roots formed after rooting is complete. In this way, the cuttings are transplanted with the root ball, and the success of the operation is guaranteed [34].
In the literature, the vegetative propagation of J. communis cuttings was performed using a mixture of equal parts of peat, bark and perlite [19], sand and peat [35], pine bark and compost mixes [16], or perlite [36]. There must be adequate amounts of sterile, highly draining media such as sand, vermiculite, or perlite [9].
In relation to the application of auxins, Indole 3-Butyric Acid (IBA) has been used in the rooting of cuttings since the 1930s [37] to stimulate adventitious root formation in stem cuttings because it is probably the best material for general use since it is non- toxic over a wide range of concentrations. It is also quite effective in promoting the rooting of a large number of plant species [24,29,38] and moves little into the plant, so it remains longer at the application site [34]. In common juniper, the levels of IBA ranged from 2000 to 12,000 ppm [25,28,29].
Concerning the propagation procedures, according to the consulted literature, the following aspects must be taken into account:
- Bed conditions: Cuttings should be planted with background heat on the tables to generate basal heating to accelerate root formation. For most species, the most suitable temperature to induce rooting was between 15–25 °C [16,38,39,40].
- Ambient: The juniper cuttings have leaves, so they continue to transpire from the moment they are cut, even though they have no roots—and it takes several months to a year for them to root—so a humidifier needs to be installed. If relative air humidity (RH) is not maintained, spaces can form inside the cuttings, which prevent circulation and therefore cause the death of the cuttings [41]. The use of vaporizers [42] or frequent misting [43] reduces evapotranspiration because a uniform film of water forms on the surface of the foliage, allowing rooting even in full sunlight.
- Cuttings preparation: The removal of part of the leaves from cuttings reduces the evapotranspiration process. If there is too much foliage, too much water is lost, causing the cuttings dehydrate before roots can form. Therefore, it is recommended to leave only one third of the leaves on each cutting.
The aim of this study was to determine the effects of the rooting medium on adventive root formation in J. communis cuttings in relation to gender and IBA treatment. The cuttings were collected from wild specimens in Soria province (Spain) in March 2022. This research will improve our knowledge of cutting propagation of common juniper with the aim of encouraging planting and afforestation of this species, which is currently declining in many regions, while it is of increasing interest for its valuable bioproducts.
2. Materials and Methods
2.1. Study Site and Collection of Stem Cuttings
Small branches of J. communis were collected from its natural habitat in Fuentelcarro (Soria), as shown in Figure 1. The site is situated at an altitude of 1024 m. The local climate has an average annual temperature of 9.7 °C and annual rainfall of 440 mm, based on 20-year averages (CEDER meteorological station). The soil texture is sandy loam with acidic pH.
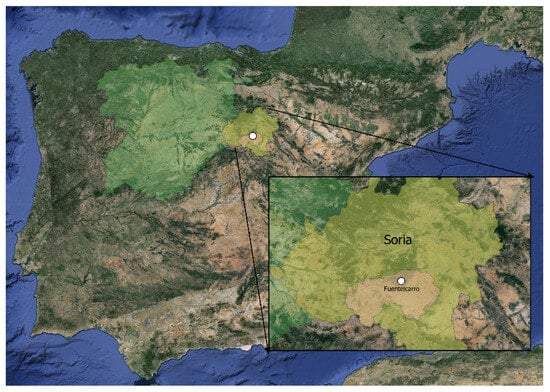
Figure 1.
Map of the Juniperus communis L. cuttings collection area.
These branches were collected between from 1 to 31 March 2022 in the early morning, from different individual male and female wild plants in a radius of less than 50 m, between the geographical coordinates 41°31′27″ N and 2°32′35″ W, as shown in Figure 1. According to Broome [19] and Edson et al. [44], cuttings of J. communis collected in February and March showed moderate to good success. Common juniper females of the sampled populations always carry berries at different stages of maturity throughout the year. The berries take 2–3 years to fully mature, so these find a mix of immature green berries, partially ripened berries, and ripe purple-black berries. In the absence of berries, the plants are considered male. The branches are preserved in pre-labelled bags separated by gender.
2.2. Preparation of Rooting Medium
The rooting medium was prepared by the homogeneous manual mixing of the previously weighed components. Two types of rooting media were established: (RM_I), a combination of professional substrate (50%) and perlite (50%); (RM_II), a combination of unfertilized blonde peat (65%), professional substrate (25%) and perlite (10%). The components were unfertilized blonde peat “Projar OPM525W Madrid, Spain”, professional substrate “Fertiberia, Madrid, Spain” and perlite “Projar, Madrid, Spain”.
2.3. Cutting Preparation and Vegetative Propagation Experiment
Each day, cuttings of branches were collected and planted in a greenhouse at CEDER. This is a polycarbonate greenhouse with a surface of 252 m2 with automatic climate control systems (temperature and moisture), shadow screen, boilers fueled with biomass pellets, heated table, cooling system, and micro-sprinklers.
These cuttings were treated immediately after collection and planted in the rooting medium (RM), as shown in Figure 2. Cuttings that could not be planted on the day of collection were eliminated to prevent the risk of drying out or deterioration.
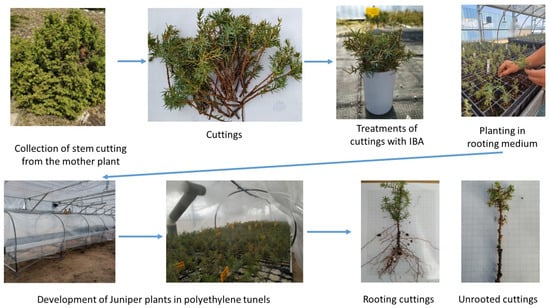
Figure 2.
Stages of propagation of J. communis using stem cuttings.
Cuttings from the tips of lateral branches were recut to a length of 12 to 15 cm and a diameter of 0.3–1.0 cm after collection, with two- thirds of basal leaves removed to prevent rotting. Several notches were made on the bark, to induce adventitious rooting. Any berries were removed from the branches, and male and female plants were marked and separated, as shown in Figure 2.
Two IBA treatments were applied to the basal part of the cuttings: 0 parts per million (0 ppm; control) and 4000 ppm. As IBA hormone is available in powder form, 4 g of IBA hormone was dissolved in 300 mL of 96% ethyl alcohol, and then distilled water was added until it reached 1000 mL of volume. In the present study, IBA at 4000 ppm was applied, corroborating the results reported by Al-Kinany [29], while the hormone-free treatment aligns with Broom’s [19] conviction that rooting hormones are not necessary.
The prepared cuttings were dipped into hormone solutions for 10 s, and a treatment control without IBA was established as well. They were planted in pre-moistened substrate.
After treatment, each cutting was planted in 45 × 45 × 140 mm cells in polyethylene forest trays, with 48 cells per pack, for propagation in the greenhouse. In total, 3456 cuttings were planted for rooting (Table 1), equivalent to 72 forest trays, which were arranged in a table according to the “randomized complete block design”. The cuttings were then watered immediately.

Table 1.
Number of cuttings per pretreatment.
The forest trays were placed on a 12 m2 heated table under a polyethylene tunnel fitted with a mist generator (Figure 2). The mist was applied automatically to maintain 50% relative humidity (RH) in order to replenish losses through evapotranspiration. Basal heat was maintained between 20 and 24 °C using a heating blanket. High RH reduces evapotranspiration losses and improves foliage health in a misting bed covered by a polyethylene tent. Additionally, an automatic drip irrigation system was activated for 3 min per day.
In addition, all the cuttings were treated with amino acids during the assay. The amino acid treatments were Kenogard Enraigar, at doses of 3 mL/l 1 month after establishment; N-biol Radix, N-biol 5 stars and N-biol 1 star every 15 days at a dose of 3 mL/l until the cuttings were transplanted. These amino acids contain copper (fungicidal action), manganese, and zinc.
2.4. Percentage of Rooting, Number of Roots, Length, and Diameter of the Main Root
To determine the rooting percentage, the roots were examined 50 days after the establishment of the cuttings. The biometric measurements performed after extracting the cuttings included the number of the primary roots, as well as the length and diameter of the longest root.
Subsequently, the cuttings were transplanted from 1 August to 27 September of the same year in 110 × 110 × 200 mm cells, with six cells x pack, as shown in Figure 3. Unrooted stem cuttings, which still had their green leaves, were left in their respective cells and returned to previous conditions to continue rooting. This process was carried out very meticulously to avoid damaging the cuttings removed from the rooting medium. Brown cuttings were eliminated.

Figure 3.
Transferring rooted cuttings to six cells per pack. (a) Cuttings growing in RM in polyethylene forest trays with 48 cells per pack. (b) Rooted cuttings. (c) Cuttings growing in professional substrate in polyethylene forest trays with six cells per pack.
The cuttings were transferred into a professional substrate, ‘Fertiberia’, and were fertilized. The fertilizer consisted of 0.75 g per plant of slow-release fertilizer (9% N, 13% P2O5, 18% K2O), Osmocote Scotts, 12–14-month release rate at 21 °C.
2.5. Statistical Analysis
Statistical analysis of the obtained dataset was performed using IBM SPSS Statistics software package, version 29.0 (IBM, Armonk, NY, USA). We used multifactor analysis of variance (three-way ANOVA) at a probability level of 5% (p value < 0.05) to compare the effects of three factors (gender, treatment, rooting medium) on the rooting percentage (%) of J. communis.
Fisher’s least significant difference (LSD) test (p value < 0.05) was used to determine significant differences between treatments. To meet the assumption of normality, the rooting percentage data underwent cube root transformation prior to the ANOVA testing.
3. Results
Common juniper stem cuttings were successfully rooted in controlled RH at 50% and 20–24 °C basal heat using a heating blanket.
Observations on rooting percentage were recorded for each treatment and are shown in Table 2 and Figure 4 in relation to plant gender, IBA treatment, and rooting medium. In all experiments, IBA treatment, gender, and composition of rooting medium significantly affected the rooting.

Table 2.
Mean rooting percentage in RM_I and RM_II in relation to IBA treatment and gender. The values followed by the same lower-case letters in each column are not significantly different at the 5% level of significance (p value < 0.05), according to the LSD test.
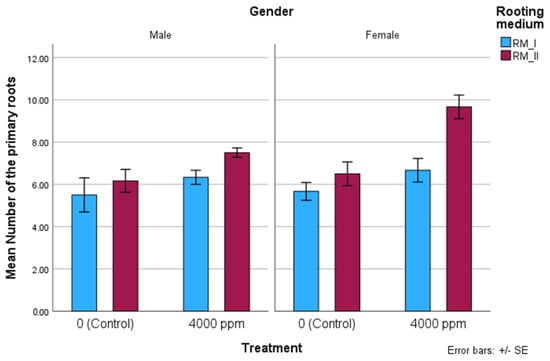
Figure 4.
Number of the primary roots in RM_I and RM_II in relation to IBA concentrations. The treatment method followed by the same lower-case letters in each column are not significantly different from one another at the 5% level of significance (p value < 0.05), according to the LSD test.
In relation to gender, the average rooting percentages were higher in female plants than in male plants in both rooting mediums (29.69% vs. 19.90%) (Table 2).
Regarding the effect of the hormone, cuttings treated with 4000 ppm IBA generally showed better rooting than untreated ones (Table 2). Rooting percentages among cuttings treated with IBA ranged from 21.31% to 45.57%, while for untreated cuttings, the rooting percentage was between 11.79% and 23.48%. IBA applied exogenously significantly increased the rooting of cuttings. Thus, this species could be reliably propagated using 4000 ppm IBA. The trend of higher rooting success in female compared to male plants was maintained. The rooting percentage of male plants was very low (Table 2).
With respect to the rooting medium (RM), the composition was found to affect the rooting. The highest rooting percentage was found in RM_II. The average rooting percentage was 20.70% in RM_I and 28.89% in RM_II. Female plants showed higher average rooting percentages than male plants in both RMs, as shown in Table 2.
The number of primary roots, the length and diameter of the longest root in RM_I and RM_II in relation to IBA treatment and gender are shown in Figure 4 and Figure 5. In all measures, RM_II performed better than RM_I. The average number of roots, an important quality indicator of the cuttings, was positively influenced by IBA treatment and the rooting medium. The average number of roots was 5.92 root units/cutting in male and 7.13 root units/cutting in female, with the highest value being 9.67 in females in RM_II. The average roots length ranged from 7.17 cm to 8.83 cm, and root thickness was between 2.50 mm and 2.83 mm.

Figure 5.
Length and diameter of the longest root in RM_I and RM_II in relation to IBA concentrations. The treatment method followed by the same lower-case letters in each column are not significantly different from one another at the 5% level of significance (p value < 0.05) according to the LSD test.
According to the results obtained with ANOVA, significant differences were found among RMs, gender, IBA treatment, and number of the primary roots. The RM composition xIBA pretreatment interaction significantly affected the rooting percentage. These results confirmed a strong relationship between the variables studied and the rooting capacity of the stem cuttings. Nevertheless, there were no significant differences in rooting percentages between RM xgender, and gender xIBA treatment (Table 3).

Table 3.
Results of the multifactor ANOVA on the effects of the main factors on the rooting performance of cuttings. p-values for three main factors (substrate, gender, and pretreatment with IBA) are shown. Effects were considered significant when p value < 0.05. (x) Effects between main factors. A total of 3456 stem cuttings were planted.
4. Discussion
Our findings demonstrate that vegetative propagation using 4000 ppm IBA and RM_II provides a viable solution for large-scale cultivation of J. communis. The higher rooting success observed in female cuttings emphasizes the importance of selecting suitable plant material for propagation programs, particularly in contexts where rapid multiplication is required for commercial or conservation purposes. In the case of cuttings treated with rooting hormone, the rooting percentage was higher, compared to those untreated in both genders.
The cuttings were collected at the tips of the branches from selected wild plants in their natural habitat in order to obtain young material. Hernández and Leal [45] and Houle and Babeux [28] showed a better rooting when the cuttings were selected from young or new material than from older branches in J. communis. Young tissues have a higher auxin content, where Indole-3-acetic acid (IAA) is synthesized, and also contain the enzymes necessary for the conversion of tryptophan into IAA [39,46].
J. excels showed higher adventitious root induction in autumn and winter [47]. In the present study, the cuttings were collected in March, similarly to Roshca [27], who studied J. communis ‘Meyer’ cuttings collected on two dates—in January and March—and the rooting percentages were higher in March. Therefore, hardwood cuttings have moderate to good success when the dormancy phase ends and the shrub begins to grow actively.
In relation to the vegetative propagation environment, in our experiment, we applied 50% RH using a humidifier in a polyethylene tunnel, reducing the levels proposed by López and Mateo [42] of 80% RH during the day and increasing the RH to 90%, as we had observed fungal infestation problems with these values.
In the present study, the temperatures applied by basal heat on the tables using an electric blanket and a thermostat were between 20 °C and 24 °C, as for most species, the suitable temperature for rooting cuttings is between 20 °C and 25 °C [40].
The rooting media were a mixture of unfertilized blonde peat, substrate, and perlite. Perlite and blonde peat are readily available and have been used in the propagation of other juniper species [44,48]. The mixture of these substrates in the right proportions has the most desirable attributes for good plant growth. In our studies, RM_II achieved a higher rooting success than RM_I. This may be due to the fact that RM_I has a much higher proportion of perlite (50%), which is beneficial for root aeration but dries out quickly. RM_II, on the other hand, contains more unfertilized blonde peat (65%) and substrate (25%) resulting in better water-retention capacity. The water-retention capacity of perlite is lower than that of peat [49]. According to the properties described by the manufacturer, blonde peat has a high porosity with a high drainage capacity and an average absorption capacity of 10–15 times its weight in water. In addition, the smaller proportion of perlite led to the development of more compact root balls, which helped minimize root damage during transplanting, as was instead observed with RM_I.
However, in other cases where intermittent watering is used, the substrate must drain well, so the materials need to have a coarse or medium grain size, and the aeration capacity must be very high. Therefore, RM_I could give good results. Runkle [40] suggests using at least 50% perlite during propagation to prevent the medium from becoming saturated.
The application of auxins to the base of stem cuttings enhances root development in most species during vegetative propagation, and IBA is the most effective across species [50,51]. IBA hormone is a growth regulator that promotes and accelerates adventitious root formation in plants, promotes nutrient uptake, accelerates growth, and optimizes metabolic functions [38]. The exogenous application of IBA may increase the speed of translocation and movement of carbohydrates from the leaf to the cutting area of the stem and promote root growth and development of seedlings [52].
In common juniper, the IBA concentrations were between 2000 and 12,000 ppm [25,28,29]. With IBA at 12,000 ppm, rooting percentages were very low in male plants, between 0% and 8%. Therefore, we need to explore whether the IBA hormone applied to J. communis stimulates root formation or whether it depends on the species, since some studies show negative effects of high concentrations of IBA on root formation [28] and no effect with low concentrations.
In our results, we observed that both 0 ppm and 4000 ppm treatments induced rooting in juniper cuttings; however, the rooting success varied according to the treatment. Cuttings untreated with IBA showed a lower rooting capacity than treated ones, proving that the application of IBA was very effective. In alignment with prior studies [29], our results confirm the efficacy of 4000 ppm IBA in enhancing rooting percentage for J. communis cuttings. The same results were found by Al-Kinany [29], who studied rooting with treatments of 0, 2000 and 4000 ppm in cuttings of J. communis collected in April. However, this study achieved percentages as high as 45.57%, higher than in their trial, likely due to the optimized substrate composition and controlled nursery conditions employed. The rooting percentages were higher in comparison to other studies [29].
IBA treatments have also been reported to be more effective for root induction in stem cuttings of J. excelsa, J. procera, J. osteosperma, J. virginiana, J. scopuluru, and J. sabina using plant growth regulators than untreated cuttings [26,33,47,53,54,55]. J. excelsa (Bieb) cuttings collected in autumn and winter, treated with 4000 ppm of IBA, showed better rooting [47], and the same IBA concentration was found to be optimal for J. procera [53] cuttings taken from more mature stock plants. Greater IBA concentrations were applied in treating J. osteosperma with IBA in talc at 8000 ppm, achieving a rooting percentage of 69% [26]; 5000 ppm IBA for J. virginiana resulted in a maximum rooting percentage of 87% [33]; and, for J. scopuluru, at least 9000 ppm of indole-3-butyric acid potassium salt (K-IBA) was necessary [54]. Lower IBA concentrations of 1000 ppm used for J. sabina cuttings collected in spring induced rooting percentages of 60–62% [55]. The differences observed in these studies were due to variations in species and cultivars. Auxins enhance rooting, with easy-to-root species (J. horizontalis) treated at lower concentrations and more hard-to-root species (J. virginiana) treated with higher auxin levels [41].
The cuttings from female plants produce significantly more roots than those from male plants. Similar observations were recorded in this same species by Houle and Babeux [28] and Sarmast et al. [56]. These differences were associated with genetic differences between male and female J. communis, as observed in DNA extracted from leaves [28]. The female shrub rooting percentages ranged between 6 and 58% against 0–8% in male shrubs rooted using cuttings treated with both 8000 and 12,000 ppm of IBA [28]. The rooting percentages in September and December in female J. communis were 1.5 times those of male plants [56].
Root initiation in stem cuttings occurred 50–60 days after the establishment of the cuttings. This is in accordance with the literature, where stem cuttings are found to take 60–70 days to root [55], and it is in line with the findings of López and Mateo [42], with root formation in conifers occurring between 30 and 90 days, although it depends on the species, as some species take up to 1 year. According to McKeon [9], root initiation generally occurs within 84–112 days, and well-rooted systems are established after 24 weeks. After root formation, a change usually occurs in the appearance of the foliage of the cuttings.
RM_II induced a higher number of roots than RM_I, which resulted in a higher rooting percentage. There were no significant differences between genders in RM_I and treatments. However, in the RM_II and 4000 ppm treatment, there were significant differences in female plants. In relation to the rest of the biometric measures, such as length and diameter of the longest root, there were no significant differences between treatments. The results were similar to those obtained by Wani [14] and Roshca [27].
Irrigation can cause the nutrients within the substrate to leach out, which can lead to chlorosis in the cuttings and delayed root development. Fertilization ensures a constant supply of nutrients to the plants and prevents nutritional deficiencies. Therefore, fertilization treatment is necessary to improve root formation in the cuttings.
Fertirrigation refers to the supply of fertilizers directly through the irrigation water. In the present study, liquid fertilizers were used. These nutrients were initially absorbed through the leaves and later by the roots developed by the cuttings. The biofertilizers used contained amino acids and essential elements such as nitrogen, phosphorus, potassium, manganese, copper, molybdenum, iron, zinc and boron. Zinc is a precursor of tryptophan, which in turn is a precursor of auxins plant hormone that improve root formation. Boron, when combined with IBA, has also been recommended as a root promoter, as it increases the rooting percentage, the number and length of roots, and the speed of rooting [57].
Another option is mineral fertilization. Shukla et al. [57] applied an NPK 20-20-20 fertilizer at a dose of 2 g/L every 15 days, along with copper-based fungicides (Captan) every 8–15 days to prevent the appearance of pathogenic fungi or used weekly fertirrigation with 300–400 ppm of nitrogen [58]. Runkle [40] suggests two treatments: one involving the application of a low concentration of fertilizer (e.g., 40–50 ppm of nitrogen) in the misting water to deliver nutrients to cuttings; the other involved drenching the cuttings with fertilizer (200–300 ppm) once roots have developed, typically 10 days after sticking the cuttings for many crops. McKeon [9] recommended applying an NPK 9-45-15 fertilizer to the cuttings twice a week starting at the bud break.
5. Conclusions
This investigation demonstrated that the propagation of common juniper through stem cuttings is a reliable and rapid method of multiplication with IBA treatment. By utilizing 4000 ppm IBA and RM_II rooting medium, plantable seedlings can be produced within 1 year, with a 45.57% rooting success in female cuttings. Cuttings offer a significantly reduced production time compared to those grown from seeds, which take several years to be established in the field.
Moreover, the plants produced through vegetative propagation retain the traits of the mother plant, making them a viable option for the cultivation and conservation of the species. This consistency is crucial for the large-scale cultivation of common juniper, addressing the gap between the increasing demand and limited supply of essential oils and extracts. The findings suggest a pathway for integrating J. communis into sustainable agricultural systems, particularly in marginal lands, where its resilience and economic value can support biodiversity and enhance rural livelihoods.
These findings provide practical insights for large-scale propagation of J. communis in marginal lands offering a scalable solution for the conservation and cultivation of this economically and ecologically valuable species, supporting its sustainable use for essential oils and other uses.
Author Contributions
Conceptualization, M.S.G. and L.S.E.P.; Methodology, M.S.G., L.S.E.P. and M.T.G.; Software, M.S.G.; Validation, M.S.G.; Formal analysis, M.S.G.; Investigation, M.S.G. and L.S.E.P.; Resources, L.S.E.P.; Data curation, M.S.G. and M.T.G.; Writing—original draft preparation, M.S.G.; Writing—review and editing, M.S.G. and L.S.E.P.; Visualization, L.S.E.P.; Supervision, L.S.E.P.; Project administration, L.S.E.P.; Funding acquisition, L.S.E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried within the BeonNAT and BIOVALOR projects. BeonNAT has received funding from the Bio Based Industries Join Undertaking (JU) under grant agreement No 887917 BeonNAT. The JU receives support from the European Union Horizon 2020 research and innovation program and the Bio Based Industries Consortium. The BIOVALOR Project is supported by Fundación Biodiversidad from the Spanish Ministry of Ecological Transition and Demographic Challenge (MITERD) and it is funded by the European Union-NextGenerationEU.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oostermeijer, J.G.B.; de Knegt, B. Genetic Population Structure of the Wind-Pollinated, Dioecious Shrub Juniperus communis in Fragmented Dutch Heathlands. Plant Species Biol. 2004, 19, 175–184. [Google Scholar] [CrossRef]
- El-Arabi, T.; Hindaqy, S.; Tsianou, E.; Vasilakos, I. Catalogue of Selected Plants: Description, Availability and Product Development Options; HYDROUSA Project: 2020; p. 354. Available online: https://www.hydrousa.org/wp-content/uploads/2020/12/HYDROUSA-Catalogue-of-selected-plants-description-availability-and-product-development-options.pdf (accessed on 17 December 2023).
- García, D.; Zamora, R.; Gómez, J.M.; Jordano, P.; Hódar, J.A. Geographical Variation in Seed Production, Predation, and Abortion in Juniperus communis throughout Its Range in Europe. J. Ecol. 2000, 88, 436–446. Available online: http://www.jstor.org/stable/2648450 (accessed on 5 August 2024). [CrossRef]
- Felicísimo, A.M.; Muñoz, J.; Villalba, C.J. Ficha Juniperus communis communis. In Impactos, Vulnerabilidad y Adaptación al Cambio Climático de la Biodiversidad Española; Flora y Vegetación; Ministerio para la Transición Ecológica y el Reto Demográfico: Madrid, Spain, 2011; pp. 124–125. Available online: https://www.miteco.gob.es/content/dam/miteco/es/biodiversidad/temas/inventarios-nacionales/Juniperus_communis_tcm30-200426.pdf (accessed on 2 February 2024).
- Bais, S.; Gill, N.S.; Rana, N.; Shandil, S.A. Phytopharmacological Review on a Medicinal Plant: Juniperus communis. Int. Sch. Res. Not. 2014, 2014, 634723. [Google Scholar] [CrossRef]
- Sarmast, M.K. Genetic Transformation and Somaclonal Variation in Conifers—A Review. Plant Biotechnol. Rep. 2016, 10, 309–325. [Google Scholar] [CrossRef]
- Tektas, I.; Türkoglu, N.; Causoglu, S. Effects of Auxin Doses on Rooting of Juniperus L. Prog. Nutr. 2017, 19, 130–136. Available online: https://www.mattioli1885journals.com/index.php/progressinnutrition/article/view/5786 (accessed on 17 December 2023).
- McCune, L.M.; Johns, T. Antioxidant Activity in Medicinal Plants Associated with the Symptoms of Diabetes Mellitus Used by the Indigenous Peoples of the North American Boreal Forest. J. Ethnopharmacol. 2002, 82, 197–205. [Google Scholar] [CrossRef]
- McKeon, C. Juniperus communis: Revisiting Use of Common Juniper for Modern Culinary Uses and Producing Drought-Resistant Cultivars for Evolving Markets. Master’s Thesis, University of Minnesota, Minneapolis, MN, USA, 2015. Available online: https://hdl.handle.net/11299/175834 (accessed on 14 February 2025).
- Čmiková, N.; Vukic, M.D.; Vukovic, N.L.; Verešová, A.; Bianchi, A.; Garzoli, S.; Ben Saad, R.; Ben Hsouna, A.; Ban, Z.; Kačániová, M. Phytochemical Investigation, Evaluation of the Biological Activities and Preservative Effect of the Essential Oil of Juniperus communis L. Dried Berries on the Vacuum-Packed Carrot after the Application of Salmonella enterica. Sci. Hortic. 2024, 336, 113442. [Google Scholar] [CrossRef]
- Esteban, L.S.; Mediavilla, I.; Xavier, V.; Amaral, J.S.; Pires, T.C.S.P.; Calhelha, R.C.; López, C.; Barros, L. Yield, Chemical Composition, and Bioactivity of Essential Oils from Common Juniper (Juniperus communis L.) from Different Spanish Origins. Molecules 2023, 28, 4448. [Google Scholar] [CrossRef]
- Xavier, V.; Finimundy, T.C.; Heleno, S.A.; Amaral, J.S.; Calhelha, R.C.; Vaz, J.; Pires, T.C.S.P.; Mediavilla, I.; Esteban, L.S.; Ferreira, I.C.F.R.; et al. Chemical and Bioactive Characterization of the Essential Oils Obtained from Three Mediterranean Plants. Molecules 2021, 26, 7472. [Google Scholar] [CrossRef]
- García, D.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Annual Variability in Reproduction of Juniperus communis L. in a Mediterranean Mountain: Relationship to Seed Predation and Weather. Écoscience 2002, 9, 251–255. [Google Scholar] [CrossRef]
- Wani, M.A. Rooting Responses of Hardwood Stem Cutting of Juniper to Exogenous Hormone Treatment. Indian For. 2018, 144, 1179–1187. [Google Scholar]
- Pack, D.A. After-Ripening and Germination of Juniperus Seeds. Bot. Gaz. 1921, 71, 32–60. Available online: https://www.jstor.org/stable/2560383 (accessed on 5 August 2024). [CrossRef]
- Benito Matías, L.; Villar Salvador, P.; García Viñas, J.I.; Gastón González, A.; Prada Sáez, M.A. Juniperus communis L. In Producción y Manejo de Semillas y Plantas Forestales; Pemán, J., Navarro-Cerrillo, R.M., Nicolás, J.L., Prada, M.A., Serrada, R., Eds.; Organismo Autónomo Parques Nacionales: Madrid, Spain, 2012; Volume I, pp. 632–646. [Google Scholar]
- Verheyen, K.; Adriaenssens, S.; Gruwez, R.; Michalczyk, I.M.; Ward, L.K.; Rosseel, Y.; Van den Broeck, A.; García, D. Juniperus communis: Victim of the Combined Action of Climate Warming and Nitrogen Deposition? Plant Biol. 2009, 11, 49–59. [Google Scholar] [CrossRef]
- Zamora, R.; Barea-Azcón, J.M.; Pérez-Luque, A.J.; García, D.; Aspízua, R.; Cano-Manuel, F.J. Los Enebrales de la Alta Montaña de Sierra Nevada: Conservación y Restauración; Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible (Junta de Andalucía), Universidad de Granada: Granada, Spain, 2022. [Google Scholar]
- Broome, A. Growing Juniper: Propagation and Establishment Practices. Forestry Commission Information Note. 50.2003. Available online: https://cdn.forestresearch.gov.uk/2003/01/fcin050.pdf (accessed on 25 July 2024).
- García, D.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Age Structure of Juniperus communis L. in the Iberian Peninsula: Conservation of Remnant Populations in Mediterranean Mountains. Biol. Conserv. 1999, 87, 215–220. [Google Scholar] [CrossRef]
- Sutton, B. Commercial Delivery of Genetic Improvement to Conifer Plantations Using Somatic Embryogenesis. Ann. For. Sci. 2002, 59, 657–661. [Google Scholar] [CrossRef]
- Koçer, Z.; Gözen, A.; Onde, S.; Kaya, Z. Indirect Organogenesis from Bud Explants of Juniperus communis L.: Effects of Genotype, Gender, Sampling Time, and Growth Regulator Combinations. Dendrobiology 2011, 66, 33–40. [Google Scholar]
- Koçer, Z.A. In Vitro Induction of Growth and Development of Common Juniper (Juniperus communis L.) from Shoot and Bud Explants. Master’s Thesis, Middle East Technical University, Ankara, Türkiye, 2005. [Google Scholar]
- Ragonezi, C.; Klimaszewska, K.; Castro, M.R.; Lima, M.; Oliveira, P.; Zavattieri, M.A. Adventitious Rooting of Conifers: Influence of Physical and Chemical Factors. Trees 2010, 24, 975–992. [Google Scholar] [CrossRef]
- Sarmast, M.K. In Vitro Establishment of Conifers by Mature Shoots. J. For. Res. 2018, 29, 565–574. [Google Scholar] [CrossRef]
- Cope, K.R.; Rupp, L.A. Cutting Propagation of Juniperus osteosperma (Utah Juniper). Acta Hortic. 2013, 1014, 157–159. [Google Scholar] [CrossRef]
- Roshca, I. Consideration on Vegetative Propagation from Cuttings in Plant Trays of the Cultivar Juniperus communis ‘Meyer’. Mediu. Ambiant. 2009, 5, 24–26. Available online: https://ibn.idsi.md/sites/default/files/imag_file/Consideration%20on%20vegetative%20propagation%20from.pdf (accessed on 5 July 2024).
- Houle, G.; Babeux, P. Variations in Rooting Ability of Cuttings and in Seed Characteristics of Five Populations of Juniperus communis var. depressa from Subarctic Quebec. Can. J. Bot. 1994, 72, 493–498. [Google Scholar] [CrossRef]
- Al-Kinany, A. Effect of Auxins on Root Formation in the Vegetative Propagation of Populus alba, Populus tremula, Picea abies and Juniperus communis. Pak. J. For. 1980, 30, 84–97. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19820677668 (accessed on 23 December 2023).
- Güney, D.; Hossein, S.; Bayraktar, A.; Fahrettin, A. The Effects of Temperature and Exogenous Auxin on Cutting Propagation of Some Junipers. Dendrobiology 2021, 86, 26–38. [Google Scholar] [CrossRef]
- Ivanoova, Z. Rapid vegetative propagation of conifers. Sci. Hortic. 1981, 14, 347–356. [Google Scholar] [CrossRef]
- Zorg, P.G. The Propagation of Junipers from Cuttings. Proc. Int. Plant Propag. Soc. 1953, 3, 81–85. [Google Scholar]
- Henry, P.H.; Blazich, F.A.; Hinesley, L.E. Vegetative Propagation of Eastern Redcedar by Stem Cuttings. HortScience 1992, 27, 1272–1274. [Google Scholar] [CrossRef]
- Martínez, I.; Farré, F.X.; Aguila, I.; Sancho, J.F. Horticultura: Revista de Industria, Distribución y Socioeconomía Hortícola: Frutas, Hortalizas, Flores, Plantas, Árboles Orna-mentales y Viveros 1989, 50, 9–42. Available online: https://www.mapa.gob.es/ministerio/pags/biblioteca/revistas/pdf_hortint%5CHortint_2009_67Red1_completa.pdf (accessed on 20 February 2024).
- Torchik, V. Effect of Donor Plant Phenological Phase on Root Formation of Stem Cuttings of Ornamental Juniperus L. cultivars. Propag. Ornam. Plants 2005, 5, 51–55. [Google Scholar]
- Moore, G. Perlite: Start to Finish. Comb. Proc. Int. Plant Propag. Soc. 1987, 37, 48–52. [Google Scholar]
- Kroin, J. Advances Using Indole-3-Butyric Acid (IBA) Dissolved in Water for Rooting Cuttings, Transplanting and Grafting; Hortus USA Corp.: New York, NY, USA, 1956. [Google Scholar]
- Hartmann, H.T.; Kester, D.E. Propagación de Plantas: Principios y Prácticas, Octava Reimpresión; Editorial Continental: Mexico City, México, 2001. [Google Scholar]
- Acosta, M.; Sánchez, J.; Bañón, M. Auxinas. In Fundamentos de Fisiología Vegetal; Azcón-Bieto, J., Talón, M., Eds.; McGraw-Hill Education/Interamericana: Barcelona, Spain, 2008; pp. 377–398. Available online: https://exa.unne.edu.ar/biologia/fisiologia.vegetal/FundamentosdeFisiologiaVegetal2008Azcon..pdf (accessed on 24 July 2024).
- Runkle, E.S. Successfully Propagating Cuttings Takes Planning. GMPRO Grow. Trends 2006, 92, 92–93. Available online: https://www.canr.msu.edu/uploads/resources/pdfs/successfullypropagatingcuttingstakesplanning.pdf (accessed on 5 July 2024).
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Hartmann and Kester’s Plant Propagation: Principles and Practices, 8th ed.; Pearson: Boston, MA, USA, 2011. [Google Scholar]
- López, G.A.; Mateo, J.J. Manual para la Clonación de Coníferas Ornamentales; Universidad Autónoma del Estado de Hidalgo; Instituto de Ciencias Agropecuarias: Hidalgo, México, 2008; Available online: https://www.uaeh.edu.mx/investigacion/icap/LI_IntGenAmb/Juana_Fons/2.pdf (accessed on 5 February 2024).
- Luna, T.; Evans, J.; Hosokawa, J. Propagation Protocol for Production of Container (Plug) Juniperus communis L. Plants, 800 mL Containers. USDI NPS—Glacier National Park West Glacier, Montana. In Native Plant Network. US Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources, 2008. Available online: https://NativePlantNetwork.org (accessed on 31 December 2023).
- Edson, J.L.; Wenny, D.L.; Dumroese, R.K.; Leege-Brusven, A.D. Mass Propagation of Rocky Mountain Juniper from Shoot Tip Cuttings. Tree Plant. Notes 1996, 47, 94–99. [Google Scholar]
- Hernández, S.; Leal, F. Enraizamiento de Estacas de Cacao. Rev. Unellez Cienc. Tecnol. 1997, 15, 1–12. [Google Scholar]
- Salisbury, F.; Ross, C. Fisiología de las Plantas; Editorial Paraninfo Thomson Learning: Madrid, Spain, 2000. [Google Scholar]
- Rifaki, N.; Economou, A.; Hatzilazarou, S. Factors Affecting Vegetative Propagation of Juniperus excelsa Bieb. by Stem Cuttings. Propag. Ornam. Plants 2002, 2, 9–13. [Google Scholar]
- Rein, W.H.; Wright, R.D.; Seiler, J.R. Propagation Medium Moisture Level Influences Adventitious Rooting of Woody Stem Cuttings. J. Am. Soc. Hortic. Sci. 1991, 116, 632–636. [Google Scholar] [CrossRef]
- Markoska, V.; Spalevic, V.; Lisichkov, K.; Atkovska, K.; Gulaboski, R. Determination of Water Retention Characteristics of Perlite and Peat. Agric. For. 2018, 64, 113–126. [Google Scholar] [CrossRef]
- Zaerr, J.B.; Mapes, M.O. Action of Growth Regulators. In Tissue Culture in Forestry; Bonga, J.M., Durzan, D.J., Eds.; Martinus Nijhoff/Dr. W. Junk Publishers: The Hague, The Netherlands, 1982; pp. 231–255. [Google Scholar] [CrossRef]
- Pandey, A.; Tamta, S.; Giri, D. Role of Auxin on Adventitious Root Formation and Subsequent Growth of Cutting-Raised Plantlets of Ginkgo biloba L. Int. J. Biodivers. Conserv. 2011, 3, 142–146. [Google Scholar]
- Shekhawat, M.; Manokari, M. Impact of Auxins on Vegetative Propagation through Stem Cuttings of Couroupita guianensis Aub.: A Conservation Approach. Scientifica 2016, 2016, 6587571. [Google Scholar] [CrossRef]
- Negash, L. Successful Vegetative Propagation Techniques for the Threatened African Pencil Cedar (Juniperus procera Hoechst. Ex Endl.). For. Ecol. Manag. 2002, 161, 53–64. [Google Scholar] [CrossRef]
- Bielenin, M. Rooting and Gas Exchange of Conifer Cuttings Treated with Indole Butyric Acid. J. Fruit Ornam. Plant Res. 2003, 11, 99–105. Available online: https://www.inhort.pl/files/journal_pdf/journal_2003/Full_2003_11.pdf (accessed on 5 July 2024).
- Abshahi, M.; García-Morote, F.A.; Zarei, H.; Zahedi, B.; Nejad, A.R. Improvement of Rooting Performance in Stem Cuttings of Savin Juniper (Juniperus sabina L.) as a Function of IBA Pretreatment, Substrate, and Season. Forests 2022, 13, 1705. [Google Scholar] [CrossRef]
- Sarmast, M.K.; Kordkatoli, R.; Rezaei, Z.; Ghasemnezhad, A. Effect of Seasons, Gender, and Agrobacterium rhizogenes Strains on Adventitious Root Induction of Male and Female Juniperus communis L. J. Ornam. Plants 2019, 9, 23–31. [Google Scholar]
- Shukla, H.S.; Tripathi, V.K.; Awasthi, R.D.; Tripathi, A.K. Effect of IBA, PHB, and Boron on Rooting and Shoot Growth of Hardwood Stem Cuttings of Peach. Int. J. Appl. Agric. Res. 2010, 5, 467–473. Available online: https://www.ripublication.com/IJAER/ijaarv5n4_5.pdf (accessed on 2 August 2024).
- Argo, B.; Hack, K.; Biernbaum, J.; Weesies, A.; Weesies, B. Direct Stick Mist Propagation: Part 1. Greenh. Grow. 1995, 13, 40–44. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).