Abstract
Reducing the use of chemical inputs (fertilizers, pesticides) in agriculture while maintaining crop productivity is the main challenge facing sub-Saharan African family farming systems. The use of effective microorganisms (EM) is among the various innovative approaches for minimizing chemical inputs and the environmental impact of agricultural production and protecting soil health while enhancing crop yields and improving food security. This study sought to characterize the microbial biodiversity of local beneficial microorganisms (BMs) products from locally fermented forest litter and investigate their ability to enhance tomato plant growth and development. Beneficial microorganisms (BMs) were obtained by anaerobic fermentation of forest litter collected in four agroecological regions of Senegal mixed with sugarcane molasses and various types of carbon sources (groundnut shells, millet stovers, and rice bran in different proportions). The microbial community composition was analyzed using next-generation rDNA sequencing, and their effects on tomato growth traits were tested in greenhouse experiments. Results show that regardless of the litter geographical collection site, the dominant bacterial taxa in the BMs belonged to the phyla Firmicutes (27.75–97.06%) and Proteobacteria (2.93–72.24%). Within these groups, the most prevalent classes were Bacilli (14.41–89.82%), α-proteobacteria (2.83–72.09%), and Clostridia (0.024–13.34%). Key genera included Lactobacillus (13–65.83%), Acetobacter (8.91–72.09%), Sporolactobacillus (1.40–43.35%), and Clostridium (0.08–13.34%). Fungal taxa were dominated by the classes Leotiomycetes and Sordariomycetes, with a prevalence of the acidophilic genus Acidea. Although microbial diversity is relatively uniform across samples, the relative abundance of microbial taxa is influenced by the litter’s origin. This is illustrated by the PCoA analysis, which clusters microbial communities based on their litter source. Greenhouse experiments revealed that five BMs (DK-M, DK-G, DK-GM, NB-R, and NB-M) significantly (p < 0.05) enhanced tomato growth traits, including plant height (+10.75% for DK-G and +9.44% for NB-R), root length (+56.84–62.20%), root volume (+84.32–97.35%), root surface area (+53.16–56.72%), and both fresh and dry shoot biomass when compared to untreated controls. This study revealed that forest-fermented litter products (BMs), produced using litter collected from various regions in Senegal, contain beneficial microorganisms known as plant growth-promoting microorganisms (PGPMs), which enhanced tomato growth. These findings highlight the potential of locally produced BMs as an agroecological alternative to inorganic inputs, particularly within Senegal’s family farming systems.
1. Introduction
In Senegal, family farming plays a crucial role in supplying both local and national markets with fresh and perishable agricultural products. However, the high added value of these crops, combined with their high sensitivity to pests, leads producers to use unsustainable farming methods. These methods heavily rely on imported chemical inputs (fertilizers, pesticides), exposing the environment and consumers to multiple pollutants [1,2]. Therefore, the current challenge for agriculture in sub-Saharan Africa is to design sustainable and resilient low-input farming systems and practices that improve ecosystem services such as primary productivity (yields), nutrient recycling (fertility), and soil health. Many technologies have been developed to increase agricultural production. Nevertheless, some, including the use of mineral fertilizers and agrochemical products, have high costs and harmful effects on the environment.
The use of effective microorganisms (EM) is among the various agroecological techniques that can minimize the environmental impact of agricultural production while enhancing efficiency and profitability. These bioproducts are formed by fungi and bacteria isolated from soil or litter and can coexist in a liquid fermentation medium. Effective microorganism (EM) technology, first described by Teruo Higa in [3,4], is a robust and versatile approach already tested in Latin America and Southeast Asia “https://www.emrojapan.com/what/ (accessed on 22 January 2025)”. Although known by a few in sub-Saharan Africa, its use remains relatively limited. These bioproducts are based on the principle of simple fermentation by farmers using native microorganisms collected locally from forest litter areas.
The beneficial effects of EM on seed germination and the vigor of certain plant species and soil have been well-documented. Studies by [5,6,7] demonstrated EM’s ability to stimulate wheat seed germination and enhance the vigor of maize, sorghum, and sunflower seedlings. Previous studies have shown that applications of EM to the soil and plant ecosystem can improve soil quality, soil health [8,9], and the growth yield and quality of crops [10,11,12,13]. Others studies reported that EM enhanced organic composting [14] and is used in agriculture as bio-fertilizer or bio-pesticide [12,15,16]; it was also found that EM at concentrations of 1% and 2% improved seed germination and seedling vigor in Albizia saman and Acacia auriculiformis. Additionally, Ney et al., 2020 highlighted the impact of EM inoculation on increasing nitrogen availability in soils, thereby boosting legume productivity [17,18]. EMs appeared therefore as an innovation with great potential to support the agro-ecological transition by strengthening the autonomy of producers in terms of inputs by making their practices greener and their products safer. It seems clear that developing farming system practices based on the use of EM can lead to sustainable, productive agriculture in the Sahelian conditions, especially for market gardening.
In Senegal, tomatoes (Solanum lycopersicum) are a widely cultivated vegetable used as fresh fruit, puree, and sauces. Tomato is ranked as the second most important horticultural crop after onions in Senegal, playing a significant socio-economic role in the country’s economy [19]. The production is among the high-gross-margin vegetable crops that could help small farmers modernize family agriculture. The production remained relatively stable over the past five years, reaching 152,950 metric tons in 2023, with an average yield of 1.7 metric tons per hectare [20].
For this study, forest-fermented litter products—referred to as local beneficial microorganisms (BMs)—were produced by anaerobically fermenting local litter collected from various agroecological regions of Senegal. Thus, this study examined the BM products for the presence of beneficial microorganisms with potential plant growth-promoting characteristics to be used as biofertilizers or biostimulants.
This study aims to perform microbial characterization of bioproduct BMs and to evaluate their ability to improve tomato production in Senegal.
2. Materials and Methods
2.1. Litter—Collection and the Local Fermented Forest Litter Preparation
Local fermented forest litters, referred to in this study as local beneficial microorganisms (BMs), are mixed cultures of different components with forest litters collected in four different agroecological regions of Senegal following a climatic gradient (Figure 1). They were collected in the North Groundnut Basin (14°57.562′ N; 16°27.489′ O), Saint-Louis region (16°12.468′ N; 16°23.145′ O), and South Groundnut Basin (14°36.125′ N; 16°26.682′ O), and an urban forest spot in Dakar (14°42.125′ N; 17°25.593′ O).
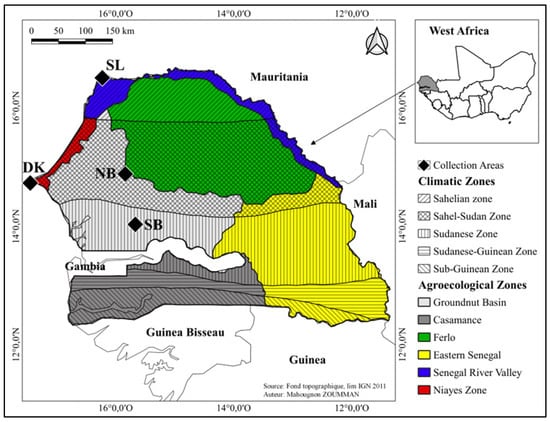
Figure 1.
Map showing climatic zones and the forest litter collection sites along the climatic gradient in Senegal. DK: forest litter collection site in Dakar, located in the Sahel-Sudanese climatic zone, within the agroecological zone of the Niayes, in a peri-urban setting. SL: forest litter collection site in Saint-Louis, situated in the Sahelian climatic zone, within the agroecological zone of the Senegal River Valley. NB: Forest litter collection site in the Sahel-Sudanese climatic zone, within the agroecological zone of the Groundnut Basin. SB: Forest litter collection site located in the Sudanese climatic zone, within the agroecological zone of the Groundnut Basin.
The preparation process of the local fermented forest litters containing beneficial microorganisms (BMs) consists of two successive fermentation steps to produce liquid containing beneficial microorganisms originating from forest litter [16,21,22]. In a 42-L container, 4.8 kg of litter was mixed to 2.1 kg of sugarcane molasses and 1.05 kg of plain yogurt. Then, 9.6 kg of each of the following different residues (groundnut shells, millet stovers, rice bran, and a 50:50 mixture of peanut shells and rice bran) was added as carbon sources to compare their effect on the microbial communities. The mixture was supplemented with water until the optimal humidity was obtained. The containers were then closed hermetically and left to ferment in an anaerobic condition at room temperature until the pH reached 5, usually in one month.
To create an active liquid product, 2.1 kg of the previously fermented material was combined with 42 L of water, 1.05 kg of yogurt, and 2.1 kg of sugarcane molasses in a second fermentation phase. After being put in airtight containers, the mixture was allowed to ferment anaerobically at room temperature for seven to fourteen days, or until the pH fell to four or below. After fermentation was finished, plant remnants were removed from the liquid BMs result by coarsely filtering it through a screen before storing it at room temperature. A total of fifteen local liquid-fermented products were produced, each made with a distinct carbon source and trash from a different origin (Table 1). These products are expected to contain a diverse and substantial number of effective microorganisms. For reference, our study also included a commercial bioproduct obtained from the Songhai Center in Benin (BJ-CCS).

Table 1.
Litters and different carbon resources used for BMs preparations. The various carbon sources are agricultural residues readily available throughout Senegal. These residues are low in nutrients and rich in carbon and serve as a growth support for microorganisms.
2.2. DNA Extraction and Sequencing
The total genomic DNA was extracted from 50 mL of each BMs product using the FastDNA™ SPIN kit for Soil (MP Biomedicals, 29525 Fountain Parkway, Solon, OH-44139 USA), with modification of the manufacturer’s instructions [23]. Agarose gel (1.5% w/v) electrophoresis was used to confirm adequate genomic DNA, which was then quantified using a Thermo Scientific spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific 3411 Silverside Road, Bancroft Building, Suite 100, Wilmington, DE 19810, USA). High-throughput sequencing was performed by the ADNID company (Montferrier, France; http://www.adnid.fr) with the Illumina MiSeq system (Illumina, San Diego, CA, USA) by targeting the 16S rRNA gene with the 515F/806R primers set and the ITS gene with the ITS3F-ITS4R primers. The sequences were deionized, and operational taxonomic units (OTU) were defined by clustering at 3% divergence (97% similarity) followed by the removal of singletons and chimeras. Final OTUs were taxonomically classified using BLASTn https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 25 January 2021) against a curated database derived from GreenGenes and SYLVA. We then produced the final OTU tables containing the number of sequences per sample per OTU matching the designated taxonomic classification. The whole process was conducted by ADNID (Montferrier, France; http://www.adnid.fr).
2.3. Effect of BMs Product on Tomato
The agronomic efficacy of BMs on the tomato plants was conducted for 28 days under natural light in a greenhouse at the ISRA/IRD Bel Air Campus in Dakar (latitude: 14.701778, longitude: −17.426229, altitude: 9 m). The experimentation took place from January to February 2021, with the monthly average midday temperature ranging between 23 °C and 29 °C. The variety AMIRAL F1 hybrid was used in the greenhouse test. This trial was conducted in 600 mL gardening pots with horticultural potting soil as substrate (NFU42001 1 kg/m3; NPK 10/10/20; pH 5.8). Tomato seeds were soaked in 2% of BMs for 1 h before pre-sprouting on agarose water (0.8% agarose) in Petri dishes. After pre-germination, the seedlings were transplanted into pots. Ten days after potting, the seedlings were treated with 10 mL of BMs at 2% concentration (0.2 mL of BMs per pot). Each treatment was replicated 12 times, including the untreated control, which received 10 mL of water. Growth parameters such as plant height, leaf chlorophyll content, and fresh and dry biomass aerial parts were evaluated. Chlorophyll content of leaves was measured with a chlorophyll meter (SPAD-502 Plus) in the middle of the day, between 10 and 11 AM, under maximum light capacity. Plant height (cm) was measured from the ground to the tip of the stem with a tape measure. The plants were uprooted at 28 days post-inoculation (DPI). The roots were carefully washed and scanned, and root traits (root area, root length, root volume, and root diameter) were evaluated using WinRhizo version 2012b.
3. Results
3.1. Composition of Bacterial and Fungal Communities of the Liquid BMs Samples
The taxonomic inventory of the bacterial sequences across all the BM samples identified, respectively, three bacterial phyla and five classes. The most dominant bacterial phyla (>1% of total relative bacterial abundance) in terms of relative abundance were Firmicutes (27.75–97.06%) and Proteobacteria (2.93–72.24%), which together accounted for more than 99.99% of the bacterial sequences (Figure 2a). Actinobacteriota was a rare class (<1% of total relative bacterial abundance) found across the BM samples. The most predominant bacteria classes within the BM samples were Bacilli (14.41–89.82%), Alphaproteobacteria (2.83–72.09%), and Clostridia (0.024–13.34%), followed by the rare classes Gammaproteobacteria and Actinobacteria. At the genus level, the BM samples compositions were predominated by Lactobacillus (13–65.83%), Acetobacter (8.91–72.09%), Sporolactobacillus (1.40–43.35%), Clostridium (0.08–13.34%), and Bacillus (0.17–1.79%), followed by the rare genus Brevibacillus, Rummeliibacillus, Brevibacterium, and Anaerocolumna (Figure 2b). The fungal community composition of the BM samples was also subject to variation in the relative abundance of classes dominated by Leotiomycetes (88.72–100%) followed by Sordariomycetes (0.00–11.28%) and Cystobasidiomycetes (0.00–4.27%) (Figure 3a). The most dominant fungal genus was Acidea accounting for 82.72–100% of the fungal abundance in each sample, followed by Gaeumannomyces (0.00–9.77%), Bannoa (0.00–4.27), and Knoxdavieasia (0.00–3.26%) (Figure 3b).
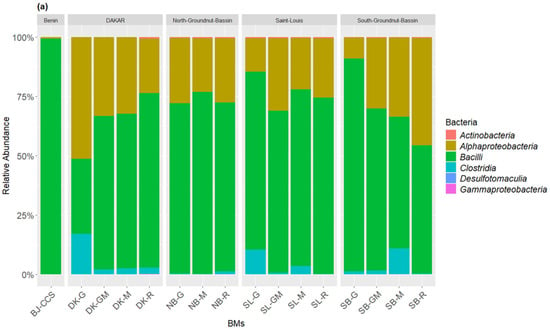
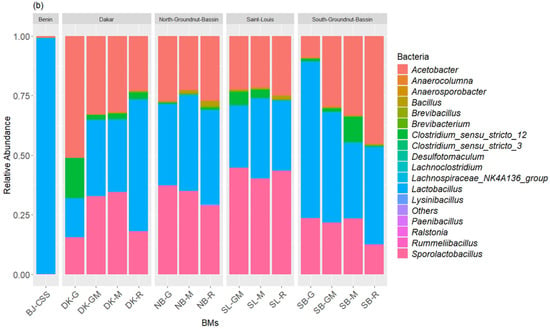
Figure 2.
Taxonomic composition of the bacterial communities at the class (a) and genus (b) levels in the BM samples. The graph illustrates the different taxonomic groups (at the class and genus levels) present in the BMs, with each group represented by a distinct color. The size of each taxonomic group corresponds to its relative abundance in the BMs.
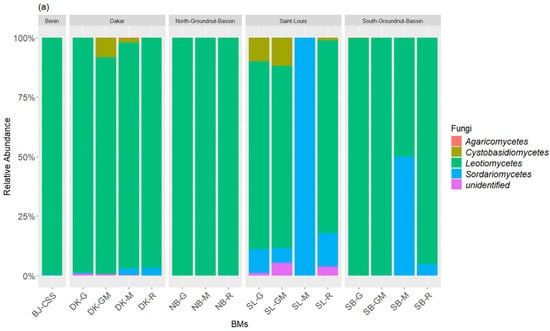
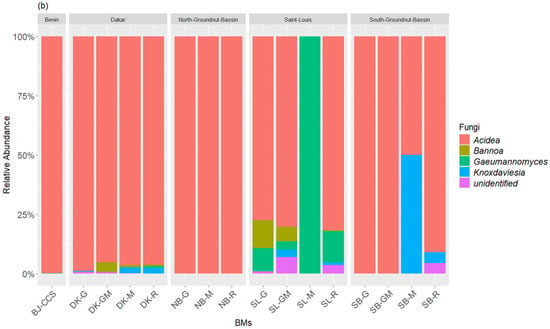
Figure 3.
Taxonomic composition of the fungal communities at the class (a) and genus (b) levels in the BM samples. The graph illustrates the different taxonomic groups (at the class and genus levels) present in the BMs, with each group represented by a distinct color. The size of each taxonomic group corresponds to its relative abundance in the BMs.
A notable diversity of fungi was observed, particularly in the composition of BMs made with ingredients from St-Louis. These compositions showed a higher dominance of the genus Acidea (77% to 82% in SL-G, SL-GM, and SL-R). Additionally, they contained the genera Gaeumannomyces (100% in SL-M, and 3% to 13% in SL-GM, SL-G, and SL-R) and Knoxdavieasia (1% and 3% in SL-GM and SL-R, respectively). Additionally, the genus Bannoa was identified in BMs from the Dakar region, with respective proportions of 0.9%, 0.6%, and 4.2% in DK-M, DK-R, and DK-GM. Similarly, BMs derived from litter collected in the Saint-Louis region (SL-R, SL-G, and SL-GM) showed proportions of 0.5%, 11.8%, and 6.2%, respectively.
The commercial BM (BJ-CCS) studied was predominantly composed of Firmicutes (97.06%) and Proteobacteria (2.93%). Among bacterial classes, Bacilli dominated at 97.04%, followed by Alphaproteobacteria at 2.83%. Regarding fungi, the Leotiomycetes class overwhelmingly dominated, accounting for 99.99% of the fungal composition. At the genus level, BJ-CCS was primarily characterized by Lactobacillus (96.92%), Acetobacter (2.84%), and the fungal genus Acidea (99.99%).
3.2. Bacterial α and β-Diversity
Alpha diversity (OTU richness and Shannon indices) of the bacterial community was higher than that of the fungal community in all samples (Table 2). OTU richness and Shannon indices did not differ significantly between litter collection sites.

Table 2.
Bacterial and fungal community richness and diversity of the BM samples.
The β-diversity analysis of the bacterial community among the different BM samples, assessed using principal coordinates analysis (PCoA) (Figure 4), indicated that the samples clustered according to their litter collection origin. This clustering suggests that spatial factors significantly influenced the β-diversity of the bacterial communities.
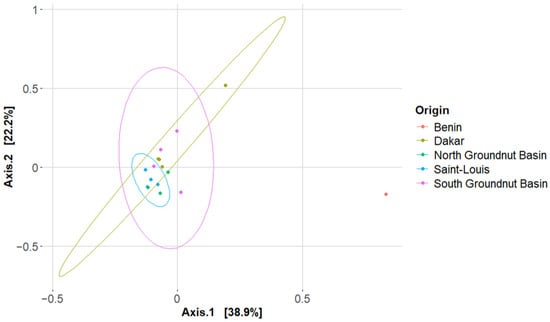
Figure 4.
β-diversity of microbial communities in BMs according to litter origin. The first two axes explain nearly 61% of the total variance. Each point represents the observed OTU count of BMs from a specific litter origin, with grouping patterns reflecting geographic variation in microbial composition.
The PCoA clustering was statistically supported by PERMANOVA analysis, (F-value of 4.2700, adonis R2 of 0.59, and p-value < 0.001; Table 3). These results confirm the presence of significant dissimilarities in the bacterial community compositions among the different collection origins. However, no significant dissimilarities were observed in the fungal community, regardless of the collection zone or the carbon sources used in the BM fabrication.

Table 3.
Effect of litter origin and carbon sources on BM microbial β-diversity.
3.3. Effects of BMs on Aerial Growth of Tomato
Data on the effects of BM treatment on tomato plants after 28 days indicate that 5 of 15 products had a significantly positive effect on the chlorophyll content of leaves and plant growth (Figure 5 and Table 4). Results indicate that the products DK-G, DK-GM, and DK-M significantly enhanced (p < 0.001) the chlorophyll content in tomato leaves compared to control plants. The leaves’ chlorophyll content increased by 17.94% for DK-G, 18.21% for DK-GM, and by 15.37% for DK-M compared to the controls and the reference BJ-CCS. Moreover, plant height significantly increased by 10.75%, 10.98%, 13.10%, and 9.44% for DK-G, DK-GM, DK-M, and NB-R, respectively, compared to the control.
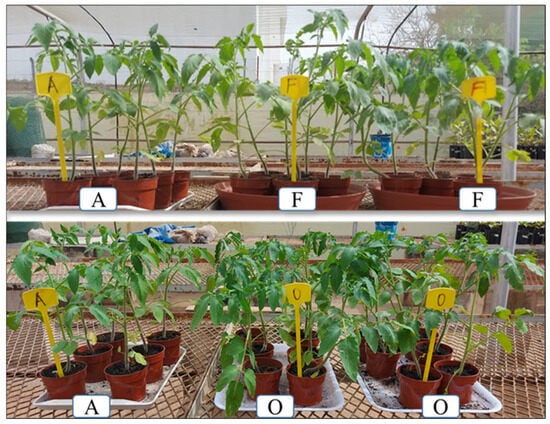
Figure 5.
Effect of BMs on the growth of tomato at 28 days after treatment. A: Untreated control plants; F: plants treated with NB-R; and O: plants treated with DK-G. An increase in the height of the treated plants compared with the untreated control showed that some bioproducts have a better effect on chlorophyll content than others (fewer green plants).

Table 4.
Effect of BMs on tomato growth properties, BMs that induced a significant increase in chlorophyll content and plant height are highlighted with asterisks indicating the level of statistical significance. The letters indicate the bioproducts grouped in the same statistical group by the comparison test.
3.4. Effect of BMs on Tomato Root Traits
At 28 DAT, treatments with NB-R, NB-M, DK-M, DK-G, and DK-GM significantly (p < 0.001) increased root length, root volume, and root surface area in tomato plants compared to the control. Notably, root length increased by 56.84%, 75.81%, and 62.20% for DK-G, NB-M, and NB-R, respectively. Interestingly, root volume increased by 59.21% for DK-M, 84.32% for DK-G, 85.44% for DK-GM, and 97.35% for NB-R. Root surface area also saw significant increases: 53.16% for DK-G, 51.61% for DK-GM, and 56.72% for NB-R. However, there was no statistical difference in root diameter (Table 5). Tomato plants treated with BJ-CCS showed no significant difference compared to the control.

Table 5.
Effect of BMs on tomato root traits. BMs with significant variation for each root trait.
3.5. Effects of BMs on the Aerial and Root Biomass of Tomato Plants
Figure 6 illustrates the effects of BM products on tomato plant aerial and root biomass, focusing on fresh shoot biomass, dry shoot biomass, fresh root biomass, and dry root biomass. Analysis of biomass data indicates that the fresh and dry shoot biomass weight of plants treated with DK-G, DK-M, and NB-R significantly increased (p < 0.0001), ranging from 8% to 52% compared with the non-inoculated control (C-NI) for dry above-ground biomass (Figure 6a,c). The best performances are obtained with beneficial microorganisms (BMs) from the Dakar region. For root fresh biomass, plants treated with NB-R and SL-GM showed a significant increase (p < 0.0001) compared to the control. In contrast, no significant difference was obtained in root dry biomass, although there was an increase in the mean of about 29% in NB-R compared with the control (Figure 6b,d).
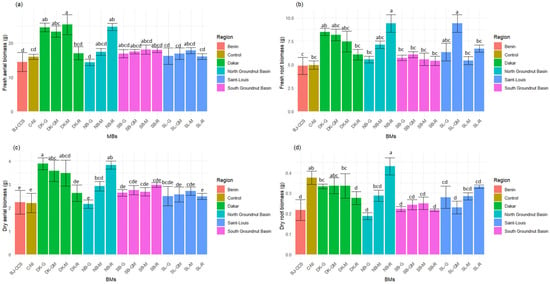
Figure 6.
Effect of BMs on tomato plant shoot and root biomass; (a) fresh aerial biomass; (b) fresh root biomass; (c) dry aerial biomass; and (d) dry root biomass. Different colors represent forest litter collection areas. Overall, BMs DK-G, DK-GM, DK-M, NB-M, and SL-GM positively impacted tomato plant biomass compared to non-inoculated plants. The letters indicate the bioproducts grouped in the same statistical group by the comparison test.
4. Discussion
Effective microorganisms are a robust technology for improving soil quality, plant growth, and yield. The main microorganisms found in EMs are photosynthetic bacteria (Rhodopseudomonas palustris and Rhodobacter sphaeroides), lactic acid bacteria (Lactobacillus plantarum, L. casei, and Streptococcus lactis), yeasts (Saccharomyces spp.), actinomycetes (Streptococcus spp.), and fermenting fungi such as yeasts and actinomyces (Streptomyces spp.) [24,25,26]. While several studies highlight their abundance and potential benefits, the existing research remains incomplete, leaving their effectiveness insufficiently demonstrated. This gap often leads to legitimate questions regarding their real usefulness [27].
The metabarcoding analysis conducted in our study confirms the presence of these microorganisms in BMs produced with local ingredients collected from four different agroecological regions in Senegal. The microbial content revealed the predominance of the bacterial genera Lactobacillus, Acetobacter, and Sporolactobacillus, as well as an extremely acidophilic fungal genus, Acidea. This finding is consistent with the few studies that describe the composition of microbial community groups that constitute effective microorganisms [12,28,29,30]. Lactobacillus are widely recognized for their holistic and ecological contributions to both agriculture and human health. Their remarkable capabilities include preserving and enhancing nutritional quality, serving as biocontrol agents, improving soil conditions, and stimulating plant growth [31,32,33].
Our results show some variations in the structure of the BMs’ microbial communities due to the litter’s origin. These results align with those of Marois et al. [16], who observed the relative importance of the impact of the origin of litter from Mediterranean and temperate climates in fermented forest litter (LFF) on the structure of microbial communities. Previous studies revealed that most of the microbes belonging to those genera are well known as plant growth and health-promoting microorganisms [34,35,36,37]. The presence of those microbial communities is linked to the decrease in the pH of the medium, which can reach 4 during the fermentation process [25]. The locally derived Senegal BMs demonstrated greater microbial community diversity compared to commercial BMs. Among their shared microbial communities are genera such as Lactobacillus and Acetobacter. These effective microorganisms play diverse roles in crop enhancement, including boosting seed germination, fostering seedling growth, increasing chlorophyll content in plant leaves, and promoting overall plant development [25,38,39,40]. In the present work, the application of 15 indigenous beneficial microorganisms (BMs) to tomato plants revealed that 3 of them significantly contributed to the improvement of plant growth and chlorophyll content in tomato leaves 28 days after treatment. Indeed, the BMs DK-M, DK-G, and DK-GM made with forest litter collected in the Dakar region, mixed with millet stover stalks, groundnut shells, or a mixture of both, were shown to be very effective in optimizing growth and chlorophyll synthesis in tomato plants. These results are in line with those of Kalaji et al. [41], who showed that the inoculation of 10 mL of EM to Arabidopsis plants significantly improves the leaves’ chlorophyll content. A similar study carried out on Kalanchoe daigremontiana [42] showed that the microorganisms effectively contribute to increased root development and germination of K. daigremontiana.
All tomato plants treated with the three most effective BMs in this study showed a significant increase in plant height, number of leaves, root weight, and chlorophyll content. This improvement can be attributed to their ability to degrade organic matter in the substrate, making essential nutrients such as iron and magnesium available, which are necessary for plant growth [43,44]. Furthermore, these microorganisms have been identified to enhance the absorption capacity of minerals, resulting in increased chlorophyll content and enhanced photosynthetic abilities [37,45]. The production of biologically active substances, such as phytohormones and growth factors, by these microorganisms, can also contribute to the observed improvements in phenotypic traits [46,47]. Indeed, the effect of several phytohormones, such as AIA, allows root growth, thus increasing the plant’s ability to explore the rhizosphere and mobilize more nutrients [48,49,50].
The studies of Youssef et al. [51] show an improvement in the size and fresh and dry aboveground biomass of Stevia rebaudiana inoculated with BMs compared to non-inoculated plants. Our results corroborate their conclusions, showing a significant increase in plant height and fresh and dry aerial biomass for plants inoculated with BMs NB-R, NB-M, DK-G, DK-M, and DK-GM. The performance of these BMs observed in 28 days could be maintained by a double or triple inoculation during the plant cycle. Indeed, studies conducted by Javaid and Bajwa [24] showed that multiple inoculations of EM at 0.2% at 15-day intervals to wheat increased aboveground dry biomass by nearly 272%.
The Dakar region of Senegal has been recognized as the source of the most effective locally beneficial microorganisms (BMs). Molecular studies revealed a spatial effect on the beta diversity of these microorganisms, suggesting that the highly diverse BM compositions for DK-G, DK-GM, and DK-M may contribute to their beneficial properties through complementary functional interactions [52]. This hypothesis is supported by observations that microorganisms with different ecological and functional characteristics can collaborate to enhance both root and aerial plant traits [53,54].
A similar study on Kalanchoe daigremontiana by Domenico [42] showed that the use of EMs led to a significant increase in plant height, number of leaves, vegetative and radical weight, number and weight of new shoots, and leaf area. Limanska et al. [55] reported that inoculation of tomato seeds and seedlings with Lactobacillus plantarum strains resulted in stimulated plant growth, with an increase in shoot, main root, and lateral root length, as well as the development of root hairs. Additionally, their studies showed that applying a mixture of effective microorganisms resulted in an acceleration of the decomposition of organic materials in soil, which in its turn led to the quicker and better release of nutrients for plant growth. A recent study showed a yield increase of 0.23 t/ha following the inoculation of EM-based fertilizers [56]. These findings agree with our results that show an increase in root length, root area, and root diameter for plants inoculated with DK-M, DK-G, and DK-GM at 28 DAT.
The significant positive effects of BMs from the Dakar region might be due to the limited microbial diversity in this area, which consists of only three or four microbial genera, all belonging to the Bacilli class. This suggests that they share similar functions, such as promoting growth, protecting against pathogens, and enhancing plant health. In other regions, in addition to Bacilli, there are bacteria from the Alphaproteobacteria class, primarily involved in nitrogen fixation. These bacteria also aid in promoting plant growth. However, due to their different mechanisms of action, they may disrupt the synergy with beneficial bacteria from other classes. Moreover, as the diversity of microbial groups increases, competition for resources can arise, potentially hindering their ability to benefit the plant.
The uniqueness of this study lies in the use of local resources (forest litters, rice bran, millet stover, and peanut shells available throughout Senegal) to produce local BM. It allowed us to confirm the presence of beneficial microbial communities, regardless of the type of carbon source used or the region where forest litter was collected. These results will enable farmers to produce BM using any available resources.
5. Conclusions
This study aimed to determine the composition and abundance of effective microorganisms present in forest litter in Senegal and to evaluate their agronomic efficacy on tomatoes. A total of 18 bacterial genera were identified in forest litter. Among these, Lactobacillus was prominent, along with Acetobacter, Sporolactobacillus, Clostridium, and Bacillus. The composition of beneficial microorganisms (BMs) in fungi was very low, with the dominant fungal genus being Acidea, a non-pathogenic genus. Inoculating tomato plants with the 15 Senegalese BMs from our study revealed that three of them were highly effective compared to uninoculated plants. They significantly improved plant growth, chlorophyll content, and both above-ground and root biomass, among other parameters. These results highlight the value of local beneficial microorganisms as a low-cost, agroecological solution to boost crop resilience and productivity in Senegal. To consolidate these findings, further studies are needed. The metabarcoding analysis will be complemented by total genomic DNA sequencing to enable precise species-level identification of beneficial microbes within the BMs. Additionally, field trials under real farming conditions will be conducted using a dual inoculation strategy in order to validate the effects of the best-performing BMs on tomato yield. This next phase is crucial for assessing the scalability of the approach and its relevance for widespread adoption by smallholder farmers.
Author Contributions
Conceptualization, A.M.A.Z., K.A. and P.F.; methodology, A.M.A.Z., K.A., P.F., C.C., A.K. and L.C.; validation, K.A., P.F. and L.C.; formal analysis, A.M.A.Z.; investigation, A.M.A.Z. and M.G.; resources, P.F., K.A. and M.G.; data curation, A.M.A.Z.; writing—original draft preparation, A.M.A.Z.; writing—review and editing, K.A., P.F. and A.M.A.Z.; visualization, A.M.A.Z.; supervision, L.C. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was granted by ACEPT MAB and VALIMAB projects (respectively funded by Fondation de France and Fondation Olga Triballat).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
Thanks to DAAD and CERAAS for the PhD fellowship granted to the first author. The authors are grateful for the technical assistance of the members of the Laboratoire Mixte International Intensification Ecologique des Sols Cultivés en Afrique de l’Ouest, (IESOL) Dakar, Sénégal. Authors acknowledge the affiliated institutions.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Diédhiou, S.O.; Ndiaye, T.M.N. Caractérisation des catégories d’espaces et contribution du maraîchage à la sécurité alimentaire dans la ville de Ziguinchor au Sénégal. Rev. Afr. D’environ. D’agric. 2021, 4, 10–22. [Google Scholar]
- Faye, C.; Sané, B.; Cissokho, D.; Diédhiou, S.O. Structure of peri-urban market gardening and degradation of resources (soils and water) in the zone of Boutoute in Ziguinchor (Senegal). J. D’econ. Manag. D’environ. Droit 2019, 2, 49–60. [Google Scholar]
- Higa, T.; Wididana, G.N. The Concept and Theories of Effective Microorganisms. In Proceedings of the First International Conference on Kyusei Nature Farming, Khon Kaen, Thailand, 17–21 October 1989; US Department of Agriculture: Washington, DC, USA, 1991; pp. 118–124. [Google Scholar]
- Higa, T. Effective Microorganisms: A New Dimension for Nature Farming. In Proceedings of the Second International Conference on Kyusei Nature Farming, Piracicaba, Brazil, 7–11 October 1991; US Department of Agriculture: Washington, DC, USA, 1994; pp. 20–22. [Google Scholar]
- Faltyn, U.; Miszkielo, T. The influence of effective microorganisms on germinability of dressed spring wheat seeds. Zesz. Nauk. Uniw. Przyr. We Wroclawiu Rolnictwo. Pol. 2008, 92, No. 568. 31–35 ref. 9. [Google Scholar]
- Ertekin, M. Effects of Microorganisms, Hormone Treatment and Stratification on Seed Germination of Goldenrain Tree (Koelreuteria paniculata). Int. J. Agric. Biol. 2011, 13, 38–42. [Google Scholar]
- Van Tonder, N.C.P.; Van der Westhuizen, C.; Van der Westhuizen, R.J. Interaction Effects of Effective Microorganisms and Prolonged Storage on Germination and Seedling Vigour of Maize, Sorghum and Sunflower. J. New Gener. Sci. 2014, 12, 147–161. Available online: https://journals.co.za/doi/abs/10.10520/EJC166483 (accessed on 5 May 2022).
- Hu, C.; Qi, Y. Long-Term Effective Microorganisms Application Promote Growth and Increase Yields and Nutrition of Wheat in China. Eur. J. Agron. 2013, 46, 63–67. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Gyushi, M.A.H.; Hemida, K.A.; El-Saadony, M.T.; Abd El-Mageed, S.A.; Abdalla, H.; AbuQamar, S.F.; El-Tarabily, K.A.; Abdelkhalik, A. Coapplication of Effective Microorganisms and Nanomagnesium Boosts the Agronomic, Physio-Biochemical, Osmolytes, and Antioxidants Defenses Against Salt Stress in Ipomoea Batatas. Front. Plant Sci. 2022, 13, 883274. [Google Scholar] [CrossRef]
- Mbouobda, H.; Fotso; Astride Carole, D.; Kilovis, F.; Ndoumou, D. Impact of Effective and Indigenous Microorganisms Manures on Colocassia Esculenta and Enzymes Activities. Afr. J. Agric. Res. 2013, 8, 1086–1092. [Google Scholar] [CrossRef]
- Desfontaines, L.; Rotin, P.; Ozier-Lafontaine, H. Les Biostimulants: Qu’en savons-nous? Quelles alternatives pour l’agriculture Guyanaise? Innov. Agron. 2018, 64, 31–46. [Google Scholar] [CrossRef]
- Joshi, H.; Somduttand, C.P.; Mundra, S.L. Role of Effective Microorganisms (EM) in Sustainable Agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 172–181. [Google Scholar] [CrossRef]
- Ahirwar, N.K.; Singh, R.; Chaurasia, S.; Chandra, R.; Prajapati, S.; Ramana, S. Effective Role of Beneficial Microbes in Achieving the Sustainable Agriculture and Eco-Friendly Environment Development Goals: A Review. Front. Environ. Microbiol. 2020, 5, 111–123. [Google Scholar] [CrossRef]
- Ezeagu, G.; Ijah, U.; Abioye, O.; Dauda, B. Efficacy of Organic Fertilizers Produced Using Locally Formulated Effective Microorganisms on the Growth and Yield Responses of Maize. Asian J. Biotechnol. Bioresour. Technol. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Khan, B.M.; Hossain, M.K.; Mridha, M.A.U. Effect of Microbial Inoculants on Albizia Saman Germination and Seedling Growth. J. For. Res. 2006, 17, 99–102. [Google Scholar] [CrossRef]
- Marois, J.; Lerch, T.Z.; Dunant, U.; Silva, A.-M.F.D.; Christen, P. Chemical and Microbial Characterization of Fermented Forest Litters Used as Biofertilizers. Microorganisms 2023, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q.; Dahal, S. Impact of Inoculation with Local Effective Microorganisms on Soil Nitrogen Cycling and Legume Productivity Using Composted Broiler Litter. Appl. Soil Ecol. 2020, 154, 103567. [Google Scholar] [CrossRef]
- Yamada, K.; Xu, H.-L. Properties and Applications of an Organic Fertilizer Inoculated with Effective Microorganisms. J. Crop Prod. 2001, 3, 255–268. [Google Scholar] [CrossRef]
- FAOSTAT FAOSTAT. Available online: https://www.fao.org/faostat/fr/#data/QCL (accessed on 5 May 2022).
- FAOSTAT FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 11 October 2024).
- Manuel de la Litière Forestière Fermentée Une Préparation Simple et Économique Pour Des Cultures Vigoureuses-Broché–Terre Et Humanisme, Marc Dufumier—Achat Livre|Fnac. Available online: https://www.fnac.com/a15514025/Terre-Et-Humanisme-Manuel-de-la-litiere-forestiere-fermentee (accessed on 27 October 2024).
- Christen, P.; Marois, J.; Ammar, I.; Abecassis, V.; Lerch, T.; Criquet, S.; Greff, S.; Davidson, S.; Gries, A.; Martinez, M.; et al. In Proceedings of the Taller Científico Internacional Biochar en la Agricultura Sostenible, Matanzas, Cuba, 14–16 March 2023.
- Tournier, E.; Amenc, L.; Pablo, A.L.; Legname, E.; Blanchart, E.; Plassard, C.; Robin, A.; Bernard, L. Modification of a Commercial DNA Extraction Kit for Safe and Rapid Recovery of DNA and RNA Simultaneously from Soil, without the Use of Harmful Solvents. MethodsX 2015, 2, 182–191. [Google Scholar] [CrossRef]
- Javaid, A.; Bajwa, R. Field Evaluation of Effective Microorganisms (EM) Application for Growth, Nodulation, and Nutrition of Mung Bean. Turk. J. Agric. For. 2011, 35, 443–452. [Google Scholar] [CrossRef]
- Olle, M.; Williams, I.H. Effective Microorganisms and Their Influence on Vegetable Production—A Review. J. Hortic. Sci. Biotechnol. 2013, 88, 380–386. [Google Scholar] [CrossRef]
- Maheswari, N.U.; Abirami, R. A Review on: Effective Microorganisms and It Applications. Asian J. Multidimens. Res. 2019, 8, 121. [Google Scholar] [CrossRef]
- Denis, C. Effets de la Litière Forestière Fermentée sur la Croissance Végétative, la Santé et le Rendement de Lactuca sativa (Laitue) et Solanum lycopersicum (Tomate) en Conditions Expérimentales; Terre & Humanisme: Lablachère, France, 2021; p. 58+annexes. [Google Scholar]
- Higa, T. What Is EM Technology. EM World J. 2000, 1, 1–6. [Google Scholar]
- Ezeagu, G.G.; Omotosho, A.O.; Suleiman, K.O. Effective Microorganisms: A Review of Their Products and Uses. Nile J. Eng. Appl. Sci. 2023, 1. [Google Scholar] [CrossRef]
- Miché, L.; Dries, A.; Ammar, I.B.; Davidson, S.; Cagnacci, L.; Combet-Blanc, Y.; Abecassis, V.; Penton Fernandez, G.; Christen, P. Changes in Chemical Properties and Microbial Communities’ Composition of a Forest Litter–Based Biofertilizer Produced through Aerated Solid-State Culture under Different Oxygen Conditions. Environ. Sci. Pollut. Res. 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Hou, Q.; Kwok, L.; Yu, Z.; Zheng, Y.; Sun, Z.; Menghe, B.; Zhang, H. Bacterial Microbiota Compositions of Naturally Fermented Milk Are Shaped by Both Geographic Origin and Sample Type. J. Dairy Sci. 2016, 99, 7832–7841. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.; Kim, J.-S.; Choi, K.R.; Eun, H.; Yang, D.; Ko, Y.-J.; Kim, S.-J. Application of Lactic Acid Bacteria (LAB) in Sustainable Agriculture: Advantages and Limitations. Int. J. Mol. Sci. 2022, 23, 7784. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Garcia, Y.L.; Nery-Flores, S.D.; Campos-Muzquiz, L.G.; Flores-Gallegos, A.C.; Palomo-Ligas, L.; Ascacio-Valdés, J.A.; Sepúlveda-Torres, L.; Rodríguez-Herrera, R. Lactic Acid Fermentation in the Food Industry and Bio-Preservation of Food. Fermentation 2024, 10, 168. [Google Scholar] [CrossRef]
- Gashash, E.A.; Osman, N.A.; Alsahli, A.A.; Hewait, H.M.; Ashmawi, A.E.; Alshallash, K.S.; El-Taher, A.M.; Azab, E.S.; Abd El-Raouf, H.S.; Ibrahim, M.F. Effects of Plant-Growth-Promoting Rhizobacteria (PGPR) and Cyanobacteria on Botanical Characteristics of Tomato (Solanum lycopersicon L.) Plants. Plants 2022, 11, 2732. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Mukherjee, A.; Ganguli, S.; Chakraborti, A.; Roy, S.; Choudhury, S.S.; Subramaniyan, V.; Kumarasamy, V.; Sayed, A.A.; El-Demerdash, F.M. Marvels of Bacilli in Soil Amendment for Plant-Growth Promotion toward Sustainable Development Having Futuristic Socio-Economic Implications. Front. Microbiol. 2023, 14, 1293302. [Google Scholar] [CrossRef]
- Samain, E.; Duclercq, J.; Ait Barka, E.; Eickermann, M.; Ernenwein, C.; Mazoyon, C.; Sarazin, V.; Dubois, F.; Aussenac, T.; Selim, S. PGPR-Soil Microbial Communities’ Interactions and Their Influence on Wheat Growth Promotion and Resistance Induction against Mycosphaerella graminicola. Biology 2023, 12, 1416. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Chen, Y.; Xie, Z. Developing Plant-Growth-Promoting Rhizobacteria: A Crucial Approach for Achieving Sustainable Agriculture. Agronomy 2023, 13, 1835. [Google Scholar] [CrossRef]
- Huang, W.; Hu, H.; Hu, T.; Chen, H.; Wang, Q.; Chen, G.; Tu, L. Impact of Aqueous Extracts of Cinnamomum septentrionale Leaf Litter on the Growth and Photosynthetic Characteristics of Eucalyptus Grandis Seedlings. New For. 2015, 46, 561–576. [Google Scholar] [CrossRef]
- Huo, X.; Wang, D.; Bing, D.; Li, Y.; Kang, H.; Yang, H.; Wei, G.; Chao, Z. Appropriate Removal of Forest Litter Is Beneficial to Pinus tabuliformis Carr. Regeneration in a Pine and Oak Mixed Forest in the Qinling Mountains, China. Forests 2019, 10, 735. [Google Scholar] [CrossRef]
- Jarboui, R.; Dhouib, B.; Ammar, E. Effect of Food Waste Compost (FWC) and Its Non-Aerated Fermented Extract (NFCE) on Seeds Germination and Plant Growth. Open J. Soil Sci. 2021, 11, 122–138. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Cetner, M.D.; Samborska, I.A.; Lukasik, I.; Oukarroum, A.; Rusinowki, S.; Pietkiewicz, S.; Swiatek, M.; Dabrowski, P. Effective Microorganisms Impact on Photosynthetic Activity of Arabidopsis Plant Grown under Salinity Stress Conditions. Ann. Wars. Univ. Life Sci. SGGW. Land Reclam. 2016, 48, 153–163. [Google Scholar] [CrossRef]
- Domenico, P. Effective Microorganisms for Germination and Root Growth in Kalanchoe daigremontiana. World J. Adv. Res. Rev. 2019, 3, 47–53. [Google Scholar] [CrossRef]
- Cheng, H.; Yuan, M.; Tang, L.; Shen, Y.; Yu, Q.; Li, S. Integrated Microbiology and Metabolomics Analysis Reveal Responses of Soil Microorganisms and Metabolic Functions to Phosphorus Fertilizer on Semiarid Farm. Sci. Total Environ. 2022, 817, 152878. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, H.; Zhao, X.; Liu, P.; Wang, L.; Wang, W. A Functional Metagenomics Study of Soil Carbon and Nitrogen Degradation Networks and Limiting Factors on the Tibetan Plateau. Front. Microbiol. 2023, 14, 170806. [Google Scholar] [CrossRef]
- Morgan, J.A.W.; Bending, G.D.; White, P.J. Biological Costs and Benefits to Plant–Microbe Interactions in the Rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The Role of Microbial Signals in Plant Growth and Development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Tian, H.; Lv, B.; Ding, T.; Bai, M.; Ding, Z. Auxin-BR Interaction Regulates Plant Growth and Development. Front. Plant Sci. 2018, 8, 2256. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Smith, S.M.; Li, J. Genetic Regulation of Shoot Architecture. Annu. Rev. Plant Biol. 2018, 69, 437–468. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yu, J.; Sun, J.; Wang, C.; Chen, S.; Yang, C.; Yang, Y. Exogenous Inoculation of Microorganisms Effect on Root Exudates and Rhizosphere Microorganism of Tobaccos. Adv. Microbiol. 2021, 11, 510–528. [Google Scholar] [CrossRef]
- Youssef, M.A.; Yousef, A.F.; Ali, M.M.; Ahmed, A.I.; Lamlom, S.F.; Strobel, W.R.; Kalaji, H.M. Exogenously Applied Nitrogenous Fertilizers and Effective Microorganisms Improve Plant Growth of Stevia (Stevia rebaudiana Bertoni) and Soil Fertility. AMB Express 2021, 11, 133. [Google Scholar] [CrossRef]
- Pattnaik, S.; Mohapatra, B.; Kumar, U.; Pattnaik, M.; Samantaray, D. Microbe-Mediated Plant Growth Promotion: A Mechanistic Overview on Cultivable Plant Growth-Promoting Members. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.-S., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 435–463. ISBN 978-3-030-18933-4. [Google Scholar]
- Rêgo, M.C.F.; Ilkiu-Borges, F.; de Filippi, M.C.C.; Gonçalves, L.A.; Silva, G.B. da Morphoanatomical and Biochemical Changes in the Roots of Rice Plants Induced by Plant Growth-Promoting Microorganisms. J. Bot. 2014, 2014, 818797. [Google Scholar] [CrossRef]
- Niang, N.; Demaneche, S.; Ndoye, I.; Navarro, E. Genetic Diversity of Rhizobia and Plant Growth Promoting Rhizobacteria of Soil under the Influence of Piliostigma reticulatum (DC.) Hochst and Their Impact on Shrub Growth. Afr. J. Agric. Res. 2018, 13, 2668–2679. [Google Scholar] [CrossRef]
- Limanska, N.; Ivanytsia, T.; Basiul, O.; Krylova, K.; Biscola, V.; Chobert, J.-M.; Ivanytsia, V.; Haertlé, T. Effect of Lactobacillus plantarum on Germination and Growth of Tomato Seedlings. Acta Physiol. Plant. 2013, 35, 1587–1595. [Google Scholar] [CrossRef]
- Ngueuleu, D.A.; Muyang, R.F.; Tchiaze, I.A.V.; Wamba, F.O.S.; Dongho, D.F.F.; Tassong, D.S.; Tefouet, V.D.; Asseng, C.C.; Fotso, M.; Taffouo, V.D. Effects of Indigenous Microorganism Fertilizers (IMO), Effective Fertilizers (EM) and Mineral Fertilizers (NPK) on the Yield and Nutritional Value of Two Varieties of Arachis hypogaea Grown Locally in West Cameroon. Am. J. Plant Sci. 2025, 16, 216–231. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).