Abstract
Strawberry production in tropical and subtropical climates has been adversely affected by rising temperatures and reduced cooling periods, leading to a decrease in flower induction and overall yield. This experiment aimed to investigate the effects of root zone cooling on short-day strawberry plants cultivated under evaporative greenhouse conditions. The cooling methods employed included of four root zone cooling treatments: normal water dripping (NWD), cold-water (10 °C) dripping (CWD), cold-water dripping plus cold-water pipe (CWD + CWP), and normal water dripping plus cold-water pipe (NWD + CWP) embedded within the growing media. The results indicated that the CWD + CWP treatment was particularly effective, reducing RZT by approximately 2 °C compared to other treatments, thereby promoting both vegetative and reproductive growth, particularly in the ‘Akihime’ strawberry. In the absence of root zone cooling, ‘Akihime’ and ‘Pharachatan 88’ were still capable of producing flowers and yield, whereas ‘Pharachatan 80’ was not. In addition, ‘Pharachatan 80’ was affected by CWD + CWP which showed the highest levels of total phenolic compound, total anthocyanin, and total vitamin C among all treatments. It can be concluded that reducing the root zone temperature through the integrated application of cold-water dripping and embedded cold-water pipes in the growing media can enhance the growth and development of short-day strawberry plants cultivated under evaporative greenhouse conditions in a tropical climate.
1. Introduction
Strawberry (Fragaria × ananassa Duch.) is among the most extensively cultivated economic fruits across numerous countries with temperate, tropical, and subtropical regions. As a subtropical fruit, strawberries demonstrate adaptability and robust performance under a variety of climatic conditions. Recently, the value of fresh strawberry production in California, which accounts for 91% of the total strawberry crop, reached USD 14 billion in 2020, reflecting an increase in consumption [1]. Global strawberry production reached 9.2 million tons in 2020 [2]. In 2025, China is the world’s largest producer, yielding 3.39 million tons, followed by the USA with 1.21 million tons, and Turkey with 0.67 million tons [3]. In Thailand, the northern subtropical region is a significant area for strawberry production. Strawberry cultivation in Thailand predominantly occurs in open fields, with ‘Pharachatan 80’ being the major commercial cultivar. In addition to ‘Pharachatan 80’, this study incorporated two additional strawberry cultivars ‘Pharachatan 88’ and ‘Akihime’ to assess varietal responses to root zone cooling under tropical greenhouse conditions. ‘Pharachatan 88’, a recently developed short-day cultivar by the Royal Project Foundation in Thailand, demonstrates enhanced adaptability to highland environments, improved resistance to common diseases, firmer fruit texture, and extended post-harvest shelf life compared to ‘Pharachatan 80’, whose traits make it particularly suitable for commercial production and distribution in subtropical markets [4]. Conversely, ‘Akihime’, a Japanese cultivar widely cultivated across tropical and subtropical regions of Asia, is characterized by its early flowering, high yield potential, and favorable fruit attributes, including large size, glossy red skin, and a high sugar content with low acidity [5,6]. Due to its pronounced sensitivity to elevated temperatures, ‘Akihime’ serves as a critical genotype for evaluating the impact of root zone cooling on reproductive development and yield stability. The inclusion of these three cultivars, ‘Pharachatan 80’, ‘Pharachatan 88’, and ‘Akihime’, offers a comprehensive framework for comparing genetic and physiological responses to temperature regulation strategies in tropical greenhouse cultivation systems. Strawberry planting is typically carried out during September and October. The plants require low temperatures of approximately 10–15 °C and short-day conditions (daylengths of less than 14 h) for flower initiation, and they prefer cool weather for optimal fruit growth and development [7,8]. Vernalization at 2–4 °C can induce off-season flowering by upregulating the expression of the flowering genes VRN5, FT, and SOC1, indicating their involvement in the flowering process of the subtropical strawberry cultivar ‘Pharachatan 80’ [9].
Temperature plays a crucial role in determining flowering, yield, and fruit quality in strawberries. Climate change, characterized by rising temperatures, induces heat stress in strawberry plants, leading to poor flowering, accelerated fruit ripening, and consequently, lower fruit quality, including smaller size, reduced sugar content, diminished aroma, and inferior texture. Additionally, these temperature increases affect the incidence and severity of pests and diseases [10,11]. As a result, climate change has had a significant impact on strawberry production. To mitigate these effects, strategies such as protected cultivation and controlled weather conditions have been considered.
Growing strawberry plants in greenhouses has proven highly successful in many temperate-zone countries. Harvesting can occur 3–4 weeks earlier, and yields are often higher compared to open field production, while also mitigating damage from heavy rain and cold temperatures during the autumn and winter seasons [12,13]. In Thailand, evaporative greenhouses optimize growing conditions by regulating temperature, humidity, and light. Compared to air conditioning and refrigeration systems, evaporative cooling systems have lower installation and operating costs. These systems function by reducing temperature through the evaporation of water. However, achieving adequate cooling in tropical and subtropical regions with high ambient temperatures, particularly during the daytime, remains a challenge. The temperature inside an evaporative greenhouse is independent of the outdoor temperature, being primarily influenced by the dew point temperature and maintaining relatively low relative humidity. Consequently, in addition to reducing the ambient temperature, cooling the root zone temperature can significantly enhance the quality and yield of strawberry production. Studying root zone temperature is crucial for understanding the growth and development, floral cluster formation, and chemical composition of various plants [14,15]. The author of [15] demonstrated that a root zone temperature of 30 °C decreases these values, whereas temperatures of 10 °C and 20 °C maintain similar levels that enhance the inflorescences and fruit production in strawberries. The author of [16] found that nutrient uptake was greatest at a root zone temperature of 18 °C. Cooling treatments improve water and nutrient absorption by promoting better root function, preventing water stress, and maintaining optimal hydration levels in the plant. This is particularly important for strawberry plants, as they possess shallow root systems that are susceptible to desiccation in warm conditions. Cooling the root zone can help alleviate heat stress in strawberry plants. Elevated root temperatures can induce early flowering and accelerate fruit ripening, resulting in lower fruit quality and a shorter harvesting period. By cooling the root zone, strawberry plants were better equipped to withstand heat stress, leading to healthier plants and improved yields [17,18]. Cooling root zone techniques, such as drip irrigation, can be employed to regulate soil temperature and maintain moisture levels in the root system. Studies have shown that fruits contained higher levels of fructose, glucose, and total carbohydrates when root zone temperatures were maintained at lower levels (18/12 °C) [19,20]. Techniques such as plastic mulching, air conditioning devices, and underground source cooling with heat pumps are employed to reduce the temperature of the growth medium in greenhouse strawberry cultivation. Monitoring soil temperature and adjusting cooling strategies accordingly is crucial to ensure optimal growing conditions for strawberry plants. In the previous research has revealed a paucity of studies focused on root zone cooling. Therefore, this study aimed to investigate the growth and development of strawberries grown under evaporative greenhouse systems and to assess the impact of root zone cooling on the growth and fruit quality of strawberries in subtropical regions.
2. Materials and Methods
The experiment was conducted at the Agriculture Innovation Research, Integration, Demonstration and Training Center, Faculty of Agriculture, Chiang Mai University, located in Chiang Mai Province, Thailand. The geographical coordinates are latitude 18°44′36″ N, longitude 98°57′50″ E, at an altitude of 304 m above sea level.
2.1. Plant Materials and Experimental Design
Disease-free strawberry plantlets derived from tissue culture were acclimatized in the greenhouse for one day and subsequently transplanted into 3-inch pots containing a mixture of perlite and coconut coir. After 45 days, the strawberry plants produced stolons and daughter plants. The three-week-old daughter plants from each cultivar were then collected, transplanted, and nursed in the greenhouse for the experiment. A factorial 3 × 4 design in a completely randomized design (CRD) was employed for this experiment, with 16 replications, where each experimental unit consisted of one strawberry plant. Factor A comprised three short-day strawberry cultivars: (1) ‘Pharachatan 88’ (‘P88’), (2) ‘Pharachatan 80’ (‘P80’), (3) ‘Akihime’. Factor B consisted of four root zone cooling treatments: (1) normal water dripping (NWD), (2) cold-water (10 °C) dripping (CWD), (3) cold-water dripping plus cold-water pipe (CWD + CWP), (4) normal water dripping plus cold-water pipe (NWD + CWP) (Figure 1).

Figure 1.
Four cooling root zone treatments: (a) normal water dripping (NWD), (b) cold-water dripping (CWD), (c) cold-water dripping + cold-water pipe (CWD + CWP), and (d) normal water dripping + cold-water pipe (NWD + CWP).
2.2. Greenhouse Conditions, Root Zone Cooling System, and Strawberry Planting
The evaporative greenhouse, measuring 9.6 by 40 m, features a circular cover wrapped in 1.5 mm thick transparent plastic, with cooling pads and fans covered on two sides by a 300-micron-thick, single-layer plastic material.
The strawberry root zone cooling system comprises a cooling water tank (a one-cubic-meter insulated plastic container), an electric pump, pipelines embedded in the growing media, and a control unit. The tank is filled with nutrient-rich water proportional to the anticipated daily water consumption. A cooling coil from a 12,000 BTU modified air-conditioning unit (Smart IoT Co., Ltd., Chiang Mai, Thailand) is submerged in the tank, utilizing a type-K thermocouple thermostat to maintain the tank water temperature at approximately 10 °C. The cooled water circulates continuously through the pipe and returns to the tank. The system includes a 220 V, 0.5 horsepower centrifugal water pump.
In late September 2022, 192 strawberry daughter plants were grown with a spacing of 25 cm in a planting trough measuring 16 m in length. The growing medium consists of a mixture of perlite and peat moss at a 30:70 ratio by volume, subjected to four different root zone cooling treatments (Figure 1 and Figure 2). Sensors for measuring temperature, relative humidity, carbon dioxide levels, and light intensity were installed. The IoT system controlled the daily water drip schedule from a 10 °C cooling water tank, administering water five times a day between 13:00 and 17:00, with each drip delivering 20 mL over one minute. As a result, each strawberry plant received a total of 100 mL of water per day.
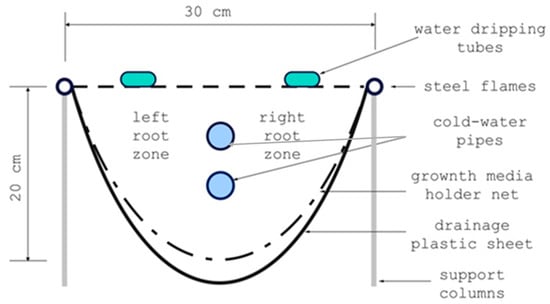
Figure 2.
Water dripping tubes and double cold-water pipe positions in strawberry-growing trough.
2.3. Greenhouse Monitoring System
Climate conditions in the evaporative greenhouse, including temperature, relative humidity, light intensity, and CO2 content, as well as media temperature and moisture, were monitored by installed sensors and an IoT system that collects the data. A temperature monitor embedded in the growing media controls the operation of the pump. Cold water was returned circulated through insulated PVC pipelines and double steel pipes inserted into the growing media, then to the storage tank. The use of double pipelines maximizes the surface area for heat transfer between the cold water inside the pipes and the growing media. The control box performs multiple functions: it regulates the pump operation based on readings from the soil temperature probe, manages the refrigeration systems according to the water temperature probe in the tank, and collects data from all sensors in the growth channel to relay to the IoT junction box (Figure 3).
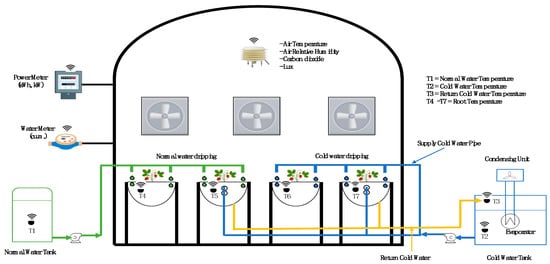
Figure 3.
Diagram of Greenhouse Monitoring System.
2.4. Physiology Measurement
After planting using 16 replications, plant growth parameters were recorded, including plant height, canopy width, leaf width, and leaf length, all measured using a standard measuring ruler, and crown diameter was determined using a Vernier caliper (Model 1108-150, INSIZE, Suzhou, China). Additional observations included the percentage of flowering. Chlorophyll content was estimated using a SPAD-502 chlorophyll meter (Minolta Corp., Spectrum Technologies, Osaka, Japan).
Post-harvest evaluations were conducted when fruits reached approximately 80% ripeness, corresponding to 45–50 days after flowering. At this stage, fruit weight, size, and firmness were recorded. Chemical analyses of the fruit included measurements of total soluble solids (TSS), titratable acidity (TA), pH, total vitamin C content, total phenolic compounds, and total anthocyanin content.
2.5. Chemical Analysis
2.5.1. Total Soluble Solid (TSS), Titratable Acidity (TA), and pH
The TSS content was measured using a digital refractometer with a wide measurement range of 0.0–53.0% Brix (ATAGO Pal-1, ATAGO Co., Tokyo, Japan). The titratable acidity (TA) was determined following AOAC methods [21]. For this, 1 g of homogenized fresh sample was added to 100 mL of distilled water and titrated with 0.1 N NaOH until the endpoint was reached, indicated by a pH of 8.2, measured using a pH meter (FE20-1, Mettler-Toledo, Greifensee, Switzerland). The results are expressed as a percentage of citric acid.
2.5.2. Total Vitamin C
The total vitamin C content was determined according to AOAC methods [21]. A 1 g fresh sample was added to 0.4% oxalic acid, and the volumetric flask was filled to 100 mL. Subsequently, 10 mL of this solution was pipetted into each 50 mL Erlenmeyer flask and titrated with 0.04% 2,6-dichloroindophenol (DPIP) until a light but distinct rose-pink color persisted. The obtained values were recorded and compared to the standard curve.
2.5.3. Total Phenolic Compound Extraction
The total phenolic content was determined using a modified Folin–Ciocalteu method [22] with reagent spectrophotometry adapted from Sellappan’s method [23]. The sample was ground using a mortar grinder mixer, and 1 g of the sample was mixed with 100% methanol to a total volume of 20 mL. The mixture was then covered with parafilm and shaken for 2 h in the dark. The extract was filtered using Whatman No.1 paper, and 5 mL of the supernatant was adjusted to 25 mL with 100% methanol. The extract was then filtered again using a 0.45 μM nylon syringe filter and stored in an amber vial in the dark at 4 °C.
For the analysis, 50 μL of the extract was mixed with 50 μL of 100% methanol, 2000 μL of distilled water, 375 μL of Na2CO3, and 125 μL of Folin–Ciocalteu reagent. The mixture was incubated in the dark for 2 h and then measured using a UV-Spectrophotometer (Genesys 20 Spectrophotometer, Thermo Scientific, Waltham, MA, USA) at a wavelength of 765 nm. The obtained optical density (OD) was used to calculate the phenolic compounds using the standard curve formula y = 0.0186x − 0.0176, R2 = 0.9893, where the concentration range is 0–100 micrograms, expressed as micrograms of gallic acid equivalents per gram of fresh weight.
2.5.4. Total Anthocyanin Content
The total anthocyanin content was determined using a modified method by Ranganna [24]. The sample was ground, and 1 g was added to 20 mL of ethanolic solvent (ethanol/hydrochloric acid ratio 85:15). This mixture was shaken in the dark for 2 h and then filtered using Whatman No.1 filter paper. The filtrate was adjusted to a volume of 100 mL with the ethanolic solvent in a volumetric flask. A spectrophotometer (Genesys 20 Spectrophotometer, Thermo Scientific, Waltham, MA, USA) was used to measure the absorbance at a wavelength of 535 nm, with ethanolic HCl serving as the blank. The optical density (OD) obtained was used to calculate the total anthocyanin content, expressed as milligrams per 100 g of fresh weight, using the following equations.
2.6. Statistical Analysis
Statistically comparative data were analyzed using an analysis of variance and the mean difference was compared using LSD at a 95% confidence level by using Statistical 9 software (analytical software SX, version 9, Tallahassee, FL, USA).
3. Results
The data collected from the IoT sensor system indicated that, from October 2022 to January 2023, the maximum and minimum temperatures, as well as the relative humidity (RH) within the evaporative greenhouse, were recorded as 29.6 °C, 15.9 °C, 90%, and 56%, respectively. During this period, the average carbon dioxide (CO2) concentration was measured at 495 ppm, with photosynthetic photon flux density averaging 976.07 μmol m−2 s−1. In contrast, outdoor conditions saw maximum and minimum temperatures of 33.8 °C and 14.3 °C, with RH values ranging from 98% to 34%. Notably, the maximum temperature inside the greenhouse was approximately 4 °C lower than the outdoor maximum, while the minimum temperature was about 2 °C higher than the outdoor minimum.
In the first month following the application of cooling treatments (October), root zone temperatures (RZTs) of strawberry plants showed no significant differences across various treatments, with averages of 23.8 °C, 24.3 °C, 22.9 °C, and 24.4 °C for NWD, CWD, CWD + CWP, and NWD + CWP treatments, respectively. By January, RZTs decreased to 19.2 °C, 19.7 °C, 17.7 °C, and 19.5 °C, respectively (Figure 4), with the CWD + CWP treatment being particularly effective, reducing RZT by approximately 2 °C compared to other treatments.
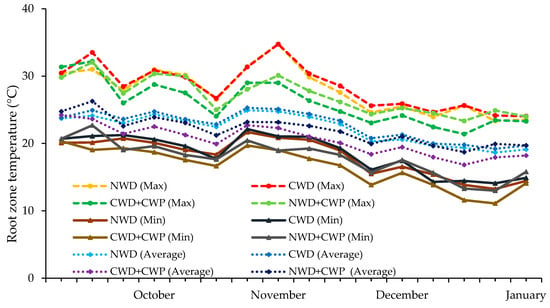
Figure 4.
The root zone temperatures in different cooling treatments.
Three months after the treatments, there was an interaction between two factors, as in Table 1. Each strawberry cultivar responded to the root zone cooling treatments differently. ‘Akihime’ and ‘P80’ strawberries in all root zone cooling treatments showed significantly higher performances in canopy width and leaf width than ‘P88’. On the other hand, ‘Akihime’ and ‘P88’ strawberries together with CWD + CWP treatment produced bigger crowns than ‘P80’, which showed no difference in crown diameter. Interestingly, the cooling treatments resulted in a higher percentage of flowering in each cultivar compared to the NWD treatment. However, the ‘P80’ strawberry did not produce any flowers under either the NWD or CWD treatments. Interactions between two factors were not found in plant height, leaf length, and chlorophyll content. ‘Akihime’ gave the highest plant height and leaf width as compared to ‘P80’ and ‘P88’, whereas ‘P80’ had the lowest chlorophyll content (Table 2). The root zone cooling treatments had no effect on plant height and chlorophyll content. Anyhow, NWD + CWP showed less leaf length than other cooling treatments (Table 3).

Table 1.
Canopy width, leaf width, crown diameter, and percentage of flowering in different treatments.

Table 2.
Plant height, leaf length, and chlorophyll content of three cultivars of strawberry.

Table 3.
Plant height, leaf length, and chlorophyll content in different root zone cooling treatments.
There was an interaction between two factors in terms of fruit quality and yield. The root zone cooling treatments tended to have better fruit qualities, particularly in ‘Akihime’, in terms of fruit weight, fruit length, TSS, and fruit color. In any case, ‘P88’ gave higher fruit firmness values than the other cultivars (Table 4 and Table 5; Figure 5 and Figure 6).

Table 4.
Strawberry fruit qualities and yield in different treatments.

Table 5.
pH, TA, and TSS/TA of strawberry fruits after treatments.
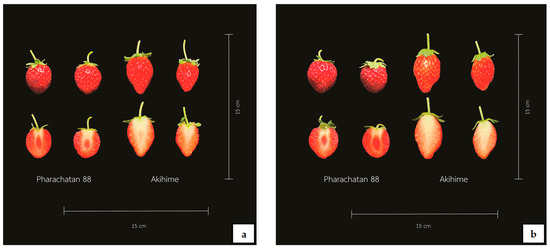
Figure 5.
‘P88’ and ‘Akihime’ strawberry fruits after NWD (a) and CWD (b) treatments.
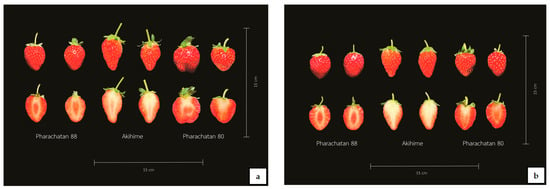
Figure 6.
‘P88’, ‘Akihime’, and ‘P80’ strawberry fruit color after CWD + CWP (a) and NWD + CWP (b) treatments.
The total acidity content (TA) and TSS/TA in strawberry fruits were different in each cultivar after cooling treatments. It seemed that the cooling treatments reduced the TA then led to higher TSS/TA (Table 5). In addition, the vitamin C content was increased after the cooling treatments. Phenolic compounds and anthocyanin contents in ‘P80’ and ‘Akihime’ seemed higher after cooling treatments as compared to ‘P88’ (Table 6).

Table 6.
Total phenolic compound, anthocyanin, and vitamin C contents of strawberry fruits after treatments.
4. Discussion
In Thailand, situated in tropical and subtropical regions, strawberry production in greenhouses encounters high temperatures during the summer, resulting in poor flowering and a shortened harvesting period. This study aimed to manipulate the root zone temperature (RZT) of three short-day strawberry cultivars grown in an evaporative greenhouse by automatically dripping cold water (10 °C) and embedding a cold-water pipe into the growing media. The results indicated that dripping cold water directly onto the surface of the growing media was ineffective in reducing RZT during the period from October 2022 to January 2023. The growing media, composed of a 70:30 mixture of peat moss and perlite, demonstrated insulating properties, which maintained consistent temperatures. The practice of dripping cold water (from a 10 °C cooling water tank) for 1 min, five times in the afternoon (20 mL/time), was insufficient to lower the temperature of the growing media. This may be attributed to an increase in water temperature to 13.4 °C after passing through the drip tube and the growing substrate.
Notably, a combination of cold-water dripping and embedding a cold-water pipe into the growing media successfully reduced RZT by approximately 2 °C. Under this treatment, the maximum RZT was recorded at 23.4 °C, and the minimum at 11.1 °C, with the average air temperature and relative humidity being 21.5 °C and 84%, respectively. The average CO2 concentration and light intensity in the evaporative greenhouse were recorded at 495 ppm and 976.07 μmol m−2 s−1, respectively. These conditions were conducive to promoting both vegetative and reproductive growth of strawberry plants in our experiment. Previous research has demonstrated that increasing photosynthetic photon flux density from 600 to 1200 μmol m−2 s−1 at temperatures between 15 and 21 °C enhances CO2 uptake and photosynthesis in June-bearing strawberries [25]. Optimal root zone temperature (RZT) is crucial as it enhances root respiration, oxygen consumption, and cell viability, leading to increased nutrient uptake and biomass accumulation. Furthermore, RZT can significantly influence the vegetative and reproductive growth of hydroponically grown strawberry plants [15]. The author of [25] also found that large fluctuations in RZT negatively impacted strawberry plant growth, leaf area, and the uptake of certain nutrients. Similarly, the author of [26] reported that groundwater cooling reduced the rhizosphere temperature from 26.9 °C to 24.9 °C, effectively improving the growth and productivity of strawberries under high-temperature conditions.
Furthermore, cooling root zone temperatures (RZTs) to around 20 °C, combined with short-day (8 h daylength) treatment for 22 days, has been shown to accelerate and stabilize flower bud formation in the ‘Nyoho’ and ‘Tochiotome’ cultivars [17]. Conversely, maintaining root zone temperatures at 20–23 °C through heating was found to be effective in increasing the productivity and quality of strawberries during winter cultivation in greenhouses [27]. In our experiment, the ‘Akihime’ and ‘P88’ cultivars exhibited better flowering and yield following root zone cooling (RZC) treatments compared to the ‘P80’ cultivar. The RZC treatments did not affect chlorophyll content, although ‘P80’ demonstrated lower chlorophyll content than the other cultivars. Additionally, RZC treatments appeared to enhance fruit weight, TSS, and overall yield compared to the non-cooling treatment. Different strawberry cultivars responded variably to environmental conditions. For example, when six strawberry cultivars (‘Maehyang’, ‘Seolhyang’, and ‘Keumhyang’—Korean short-day cultivars; ‘Akihime’, ‘Red Pearl’, and ‘Sachinoka’—Japanese short-day cultivars) were exposed to short-day (10 h photoperiod) conditions at an elevation of 800 m for 35 days starting in late June, they exhibited different numbers of flowers and fruits [28]. The treatment of seven 9 h short days at 21/21 °C (day/night) followed by seven 9 h short days at 21/12 °C (day/night) was identified as the most effective conditioning method for off-season ‘Sweet Charlie’ strawberry production in greenhouses [29]. The author of [30] reported that strawberries harvested at a root zone temperature (RZT) of 15 °C had greater soluble solid content (SSC) compared to those harvested at 22 °C. Higher RZTs negatively impacted the of phosphorus (P), potassium (K), and magnesium (Mg), reducing their uptake by approximately 35%. Potassium is known to play a crucial role in sugar translocation from source to sink. The author of [31] indicated that the K nutrient is positively correlated with sugar content (°Brix) in ‘Fortuna’ strawberries. Similar studies on other plants, such as tomatoes, have confirmed that cooling RZTs promotes growth and nutrient uptake, as well as affects sugar accumulation in the fruit. Two tomato cultivars exhibited differential responses to root cooling treatments. Specifically, root cooling enhanced the accumulation of vitamin C and lycopene in the ‘Delioso’ cultivar, likely due to the activation of protection mechanisms against low-temperature-induced oxidative stress [32]. This study concludes that root zone cooling treatments can improve the growth, flowering, fruit quality, and yield of short-day strawberries under evaporative greenhouse conditions. Nevertheless, the effectiveness of these cooling treatments is influenced by additional climatic conditions and cultivar-specific responses. The findings offer practical implications for enhancing the sustainability of strawberry production systems in tropical areas, potentially enabling farmers to achieve higher yields and greater economic returns.
5. Conclusions
Research indicates that cold-water dripping (10 °C) combined with embedding a cold-water pipe into the growing media can reduce the root zone temperature by approximately 2 °C, thereby enhancing the vegetative and reproductive growth of short-day strawberry cultivars, as well as improving fruit quality and yield. Without the root zone cooling treatment, the ‘Akihime’ and ‘Pharachatan 88’ cultivars were still capable of producing flowers and yield, whereas the ‘Pharachatan 80’ cultivar was not. Therefore, the ‘Pharachatan 80’ cultivar appears to be more dependent on root zone cooling for optimal growth and productivity.
Author Contributions
Conceptualization, D.N.; data curation, W.P. and A.P.; formal analysis, D.N.; resources, S.B.; software, A.P.; supervision, D.N.; validation, C.S. and A.P.; writing—original draft, D.N. and W.P.; writing—review and editing, D.N. All authors have read and agreed to the published version of the manuscript.
Funding
Fundamental Fund, 2022 was supported by (i) Chiang Mai University (CMU) which funded by (ii) Thailand Science Research and Innovation (TSRI): 2377069, and (iii) the National Science, Research, and Innovation Fund (NSRF), Thailand Science, Research, and Innovation (TSRI).
Data Availability Statement
D.N. and A.P. are responsible for data keeping, and data are available upon request.
Acknowledgments
We wish to thank the Central Laboratory, Faculty of Agriculture, and Faculty of Engineering, Chiang Mai University, for the supporting materials, scientific equipment, and places to conduct research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hernández-Martínez, N.R.; Blanchard, C.; Wells, D.; Salazar-Gutiérrez, M.R. Current state and future perspectives of commercial strawberry production: A review. Sci. Hortic. 2023, 312, 111893. [Google Scholar] [CrossRef]
- Strawberry Production in 2021. In Crops/Regions/World List/Production Quantity (Pick Lists); UN: San Francisco, CA, USA, 2021.
- Indian Defence Review. 2025. “They Control France’s Favorite Fruit”: China Cultivates 200,000 Hectares (20 Times the Size of Paris) Dominating the Global Market. Available online: https://indiandefencereview.com/they-control-frances-favorite-fruit-china-cultivates-200000-hectares-dominating-the-global-market/ (accessed on 15 May 2025).
- Highland Research and Development Institute (HRDI). Pharachatan Strawberry Cultivars: Characteristics and Recommendations; HRDI: Chiang Mai, Thailand, 2020. [Google Scholar]
- Hirakawa, M.; Nishizawa, T.; Yamamoto, T. Development and Characteristics of ‘Akihime’ Strawberry. Jpn. Agric. Res. Q. 2010, 44, 123–130. [Google Scholar]
- Mori, T.; Honda, T. Strawberry Cultivation and Breeding in Japan. Acta Hortic. 2009, 842, 89–94. [Google Scholar]
- Phiphatthanawong, N. Strawberry New Economic Crops; Kasetsart University: Bangkok, Thailand, 2007; p. 158. [Google Scholar]
- Phiphatthanawong, N. Strawberry production and the royal project foundation, Thailand. J. Dev. Sustain. Agric. 2015, 10, 15–18. [Google Scholar]
- Thammasophon, T.; Pusadee, T.; Bundithya, W.; Naphrom, D. Effects of Vernalization on Off–Season Flowering and Gene Expression in Sub-Tropical Strawberry cv. Pharachatan 80. Horticulturae 2023, 9, 87. [Google Scholar] [CrossRef]
- Wang, S.Y.; Camp, M.J. Temperatures after bloom affect plant growth and fruit quality of strawberry. Sci. Hortic. 2000, 85, 183–199. [Google Scholar] [CrossRef]
- Palencia, P.; Martinez, F.; Medina, J.J.; Vázquez, E.; Flores, F.; López-Medina, J. Effects of climate change on strawberry production. Acta Hortic. 2009, 838, 51–54. [Google Scholar] [CrossRef]
- Khammayom, N.; Maruyama, N.; Chaichana, C. The effect of climatic parameters on strawberry production in a small walk-in greenhouse. AgriEngineering 2022, 4, 104–121. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Rafiee, S.; Mousazadeh, H. Environmental impact assessment of open field and greenhouse strawberry production. Eur. J. Agron. 2013, 50, 29–37. [Google Scholar] [CrossRef]
- Greer, D.H.; Wünsche, J.N.; Norling, C.L.; Wiggins, H.N. Root-zone temperatures affect phenology of bud break, flower cluster development, shoot extension growth and gas exchange of ‘Braeburn’ (Malus domestica) apple trees. Tree Physiol. 2006, 26, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Uenishi, M.; Miyamoto, K.; Suzuki, T. Effect of root-zone temperature on the growth and fruit quality of hydroponically grown strawberry plants. J. Agric. Sci. 2016, 8, 122–131. [Google Scholar]
- Udagawa, Y.; Ito, T.; Gomi, K. Effects of root temperature on the absorption of water and mineral nutrients by strawberry plants ‘Reiko’ grown hydroponically. J. Jpn. Soc. Hortic. Sci. 1991, 59, 711–717. [Google Scholar] [CrossRef]
- Mizuno, S.; Muramatsu, Y.; Tateishi, A.; Watanabe, K.; Shinmachi, F.; Koshioka, M.; Kubota, S. Effects of Root-zone Cooling with Short-day Treatment in Pot-grown Strawberry (Fragaria × ananassa Duch.) Nurseries on Flowering and Fruit Production. Hortic. J. 2022, 91, 1–7. [Google Scholar]
- Deschamps, S.S.; Whitaker, V.M.; Agehara, S. White-striped plastic mulch reduces root-zone temperatures during establishment and increases early season yields of annual winter strawberry. Sci. Hortic. 2019, 243, 602–608. [Google Scholar] [CrossRef]
- Kumar, S.; Dey, P. Effects of different mulches and irrigation methods on root growth, nutrient uptake, water-use efficiency and yield of strawberry. Sci. Hortic. 2011, 127, 318–324. [Google Scholar] [CrossRef]
- Moritani, S.; Nanjo, H.; Itou, A.; Imai, T. Root-zone cooling evaluation using heat pump for greenhouse strawberry production. HortTechnology 2018, 28, 570–577. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Folin, O. Tyrosine and tryptophan determination in proteins. J. Biol. Chem. 1927, 3, 649–673. [Google Scholar]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products, 2nd ed.; Tata McGraw-Hill: New Delhi, India, 2004. [Google Scholar]
- Rivero, R.; Sønsteby, A.; Solhaug, K.A.; Heide, O.M.; Remberg, S.F. Effects of temperature and photoperiod on photosynthesis in everbearing strawberry. Acta Hortic. 2021, 1309, 379–386. [Google Scholar] [CrossRef]
- Lee, G.B.; Lee, J.E.; Choe, Y.U.; Park, Y.H.; Choi, Y.W.; Kang, N.J.; Kang, J.S. Effects of Groundwater Cooling Treatment on Growth, Yield, and Quality of Strawberries under High Temperature Conditions. J. Environ. Sci. Int. 2018, 27, 631–639. [Google Scholar] [CrossRef]
- Jo, W.J.; Shin, J.H. Effect of root-zone heating using positive temperature coefficient film on growth and quality of strawberry (Fragaria × ananassa) in greenhouses. Hortic. Environ. Biotechnol. 2022, 63, 89–100. [Google Scholar] [CrossRef]
- Ruan, J.; Lee, Y.H.; Yeoung, Y.R.; Larson, K. Influence of short-day treatment on autumn fruit production of June-bearing strawberry cultivars. Hortic. Environ. Biotechnol. 2011, 52, 259–264. [Google Scholar] [CrossRef]
- Durner, E.F. Photoperiod and temperature conditioning of ‘Sweet Charlie’ strawberry (Fragaria × ananassa Duch.) plugs enhances off-season production. Sci. Hortic. 2016, 201, 184–189. [Google Scholar] [CrossRef]
- MacKenzie, S.J.; Chandler, C.K. The late season decline in strawberry fruit soluble solid content observed in Florida is caused by rising temperatures. Acta Hortic. 2008, 842, 843–846. [Google Scholar] [CrossRef]
- Nakro, A.; Bamouh, A.; Bouslama, H.; San Bautista, A.; Ghaouti, L. The effect of potassium–nitrogen balance on the yield and quality of strawberries grown under soilless conditions. Horticulturae 2023, 9, 304. [Google Scholar] [CrossRef]
- He, F.; Thiele, B.; Watt, M.; Kraska, T.; Ulbrich, A.; Kuhn, A.J. Effects of root cooling on plant growth and fruit quality of cocktail tomato during two consecutive seasons. J. Food Qual. 2019, 2019, 3598172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).