Effect of Plastics (Geotextiles) on Heavy Metal Accumulation by Industrial Hemp Plants Cultivated in Polluted Mediterranean Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodological Framework: Experimental Setup
2.2. Laboratory Analyses
2.3. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Soil Samples
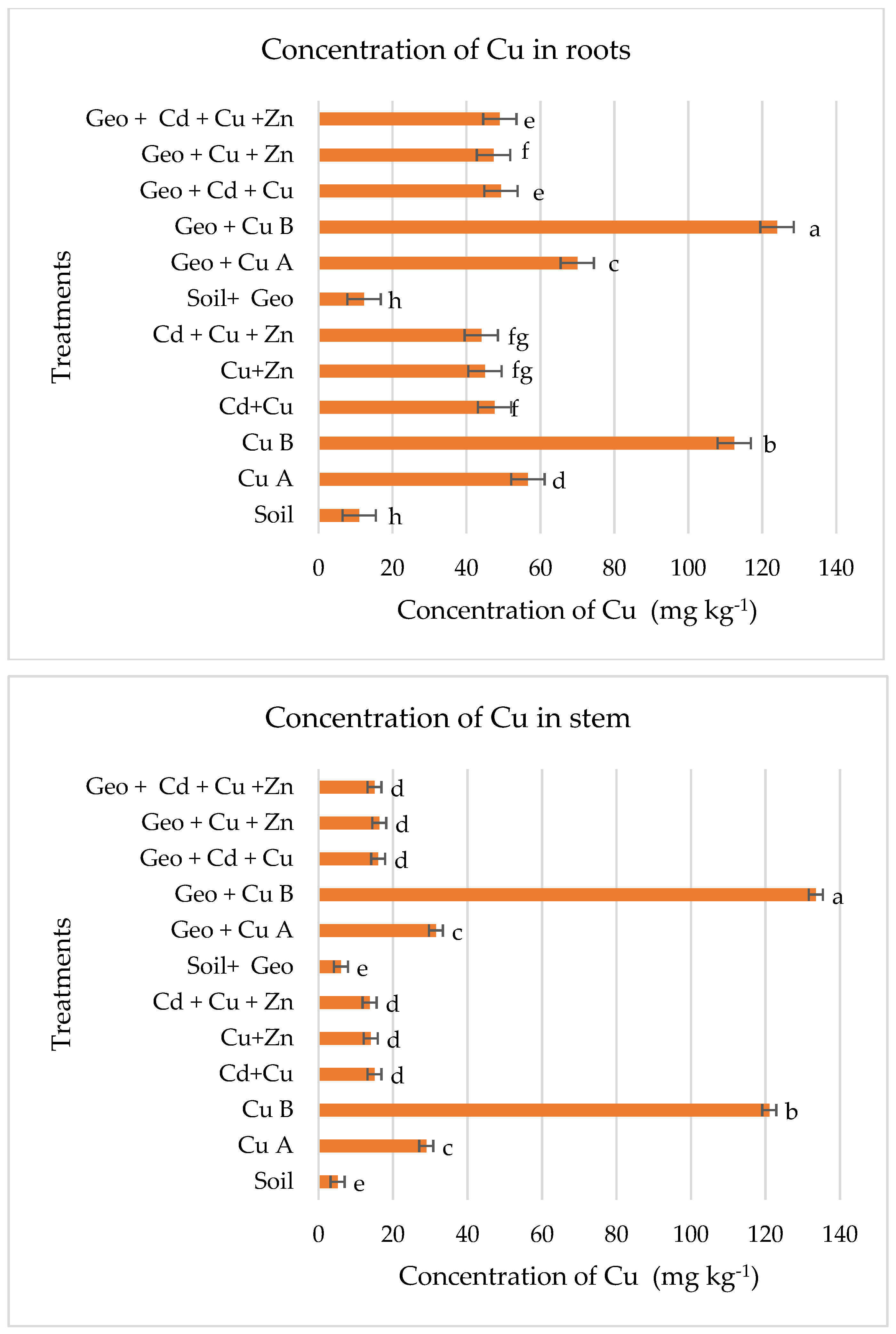
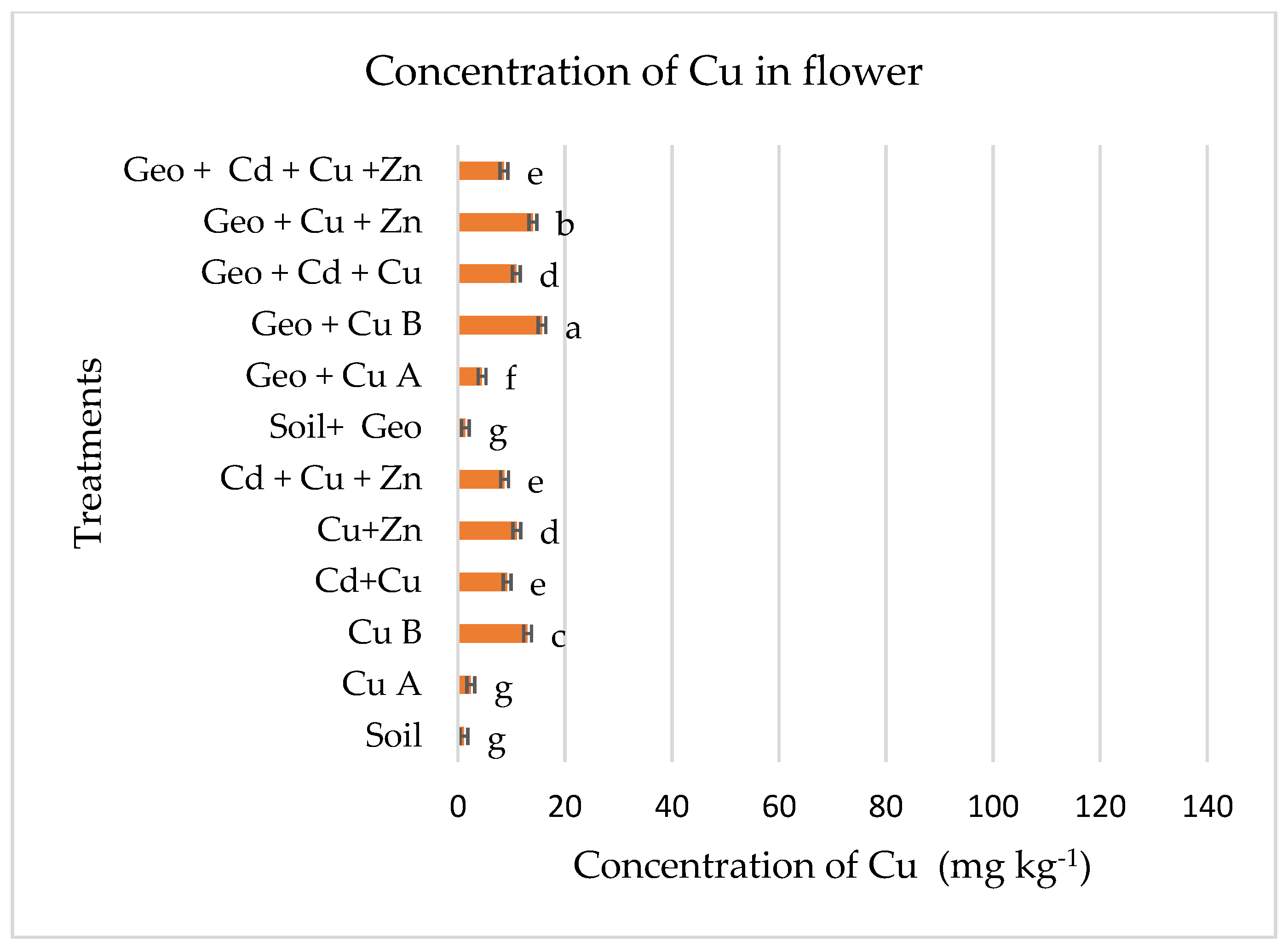
3.2. Levels of Cu in Plant Tissues
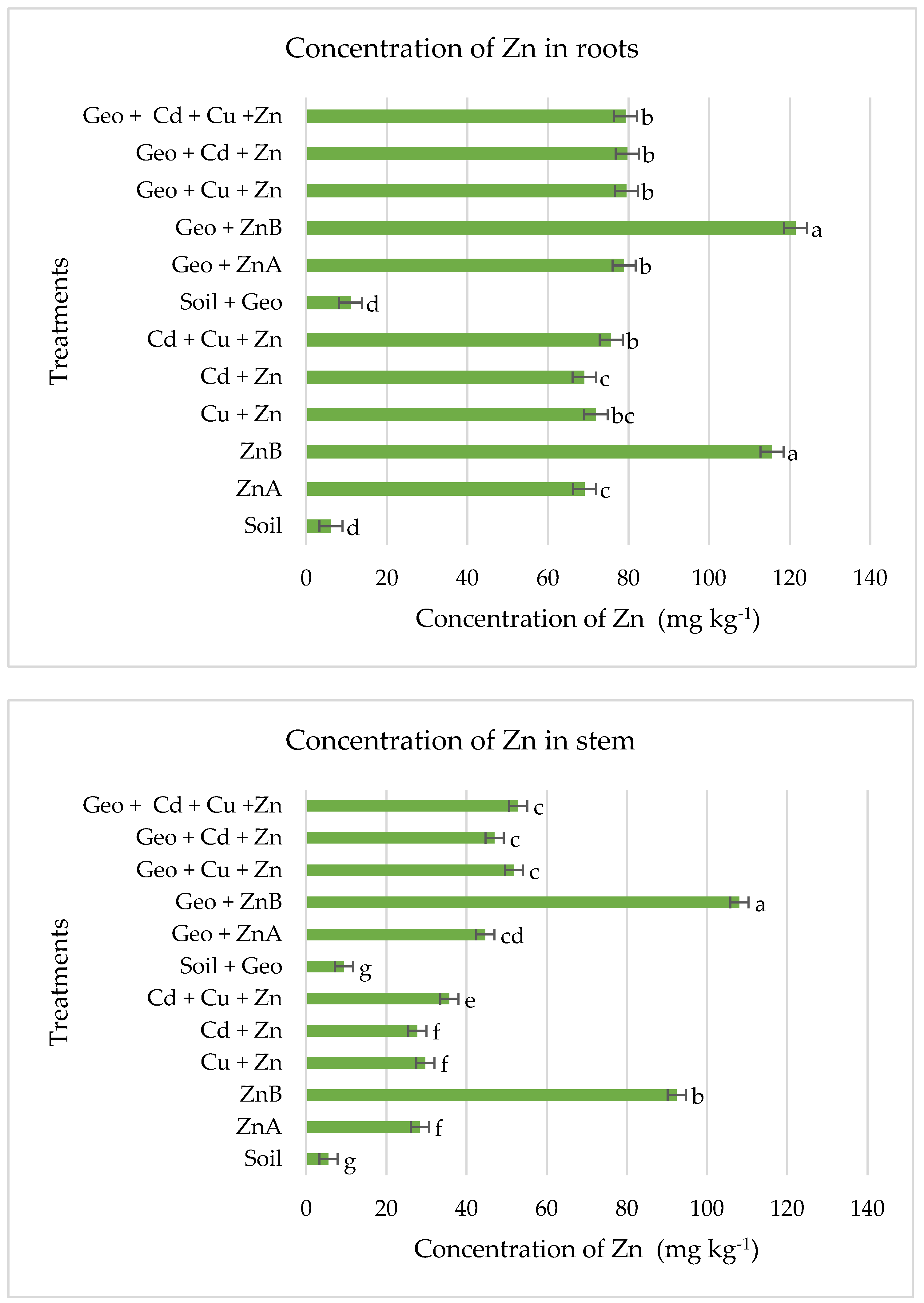
3.3. Levels of Zn in Plant Tissues
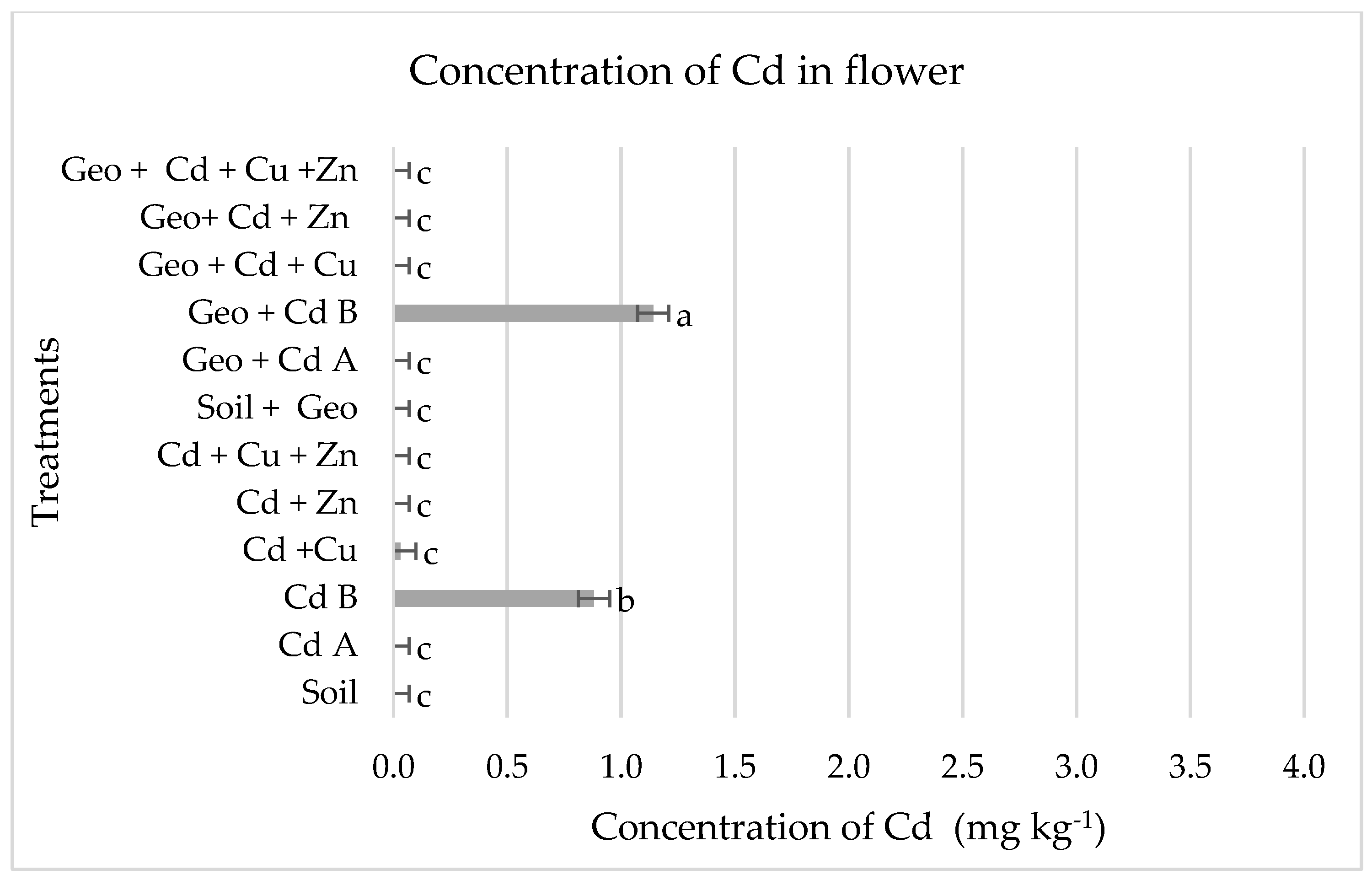
3.4. Levels of Cd in Plant Tissues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanif, S.; Ali, S.; Chaudhry, A.H.; Sial, N.; Marium, A.; Mehmood, T. A Review on Soil Metal Contamination and Its Environmental Implications. Nat. Environ. Pollut. Technol. 2025, 24, D1684. [Google Scholar] [CrossRef]
- El-Sharkawy, M.; Alotaibi, M.O.; Li, J.; Du, D.; Mahmoud, E. Heavy Metal Pollution in Coastal Environments: Ecological Implications and Management Strategies: A Review. Sustainability 2025, 17, 701. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Loan, T.T.H.; Anh, T.T.; Hai, V.H.; Phuong, H.T.; Van Thang, N.; Ba, V.N. Evaluation of Heavy Metal Content in Agricultural Soil Samples in the Mekong Delta Region, VietNam and Human Health Risks. Environ. Geochem. Health 2025, 47, 170. [Google Scholar] [CrossRef]
- Das, S.; Sultana, K.W.; Ndhlala, A.R.; Mondal, M.; Chandra, I. Heavy Metal Pollution in the Environment and Its Impact on Health: Exploring Green Technology for Remediation. Environ. Health Insights 2023, 17, 11786302231201260. [Google Scholar] [CrossRef]
- Papadimou, S.G.; Barbayiannis, Ν.; Golia, E.E. Preliminary Investigation of the Use of Silybum marianum (L.) Gaertn. as a Cd Accumulator in Contaminated Mediterranean Soils: The Relationships among Cadmium (Cd) Soil Fractions and Plant Cd Content. EuroMediterr. J. Environ. Integr. 2024, 9, 405–417. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive Mechanisms of Heavy Metal Toxicity in Plants, Detoxification, and Remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef]
- Moustakas, M. Molecular Mechanisms of Metal Toxicity and Plant Tolerance. Int. J. Mol. Sci. 2023, 24, 7810. [Google Scholar] [CrossRef]
- Vasilou, C.; Tsiropoulos, N.G.; Golia, E.E. Phytoremediation & Valorization of Cu-Contaminated Soils Through Cannabis sativa (L.) Cultivation: A Smart Way to Produce Cannabidiol (CBD) in Mediterranean Soils. Waste Biomass Valorization 2024, 15, 1711–1724. [Google Scholar] [CrossRef]
- Monrroy, M.; Solis, H.; Quiel, D.; Araúz, O.; García, J.R. Evaluation of Heavy Metal Uptake by Pigeon Pea (Cajanus cajan) Plants. J. Chem. 2024, 2024, 5047702. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, Plant Selection and Enhancement by Natural and Synthetic Agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Małecka, A.; Konkolewska, A.; Hanć, A.; Ciszewska, L.; Staszak, A.M.; Jarmuszkiewicz, W.; Ratajczak, E. Activation of Antioxidative and Detoxificative Systems in Brassica juncea L. Plants against the Toxicity of Heavy Metals. Sci. Rep. 2021, 11, 22345. [Google Scholar] [CrossRef]
- Alsafran, M.; Saleem, M.H.; Rizwan, M.; Al Jabri, H.; Usman, K.; Fahad, S. An Overview of Heavy Metals Toxicity in Plants, Tolerance Mechanism, and Alleviation through Lysine-Chelation with Micro-Nutrients—A Novel Approach. Plant Growth Regul. 2023, 100, 337–354. [Google Scholar] [CrossRef]
- Bethanis, J.; Golia, E.E. Micro- and Nano-Plastics in Agricultural Soils: A Critical Meta-Analysis of Their Impact on Plant Growth, Nutrition, Metal Accumulation in Plant Tissues and Crop Yield. Appl. Soil Ecol. 2024, 194, 105202. [Google Scholar] [CrossRef]
- Jadhav, B.; Medyńska-Juraszek, A. Microplastic and Nanoplastic in Crops: Possible Adverse Effects to Crop Production and Contaminant Transfer in the Food Chain. Plants 2024, 13, 2526. [Google Scholar] [CrossRef]
- Leitão, I.A.; van Schaik, L.; Ferreira, A.J.D.; Alexandre, N.; Geissen, V. The Spatial Distribution of Microplastics in Topsoils of an Urban Environment—Coimbra City Case-Study. Environ. Res. 2023, 218, 114961. [Google Scholar] [CrossRef]
- Deng, Y.; Zeng, Z.; Feng, W.; Liu, J.; Yang, F. Characteristics and Migration Dynamics of Microplastics in Agricultural Soils. Agriculture 2024, 14, 157. [Google Scholar] [CrossRef]
- Kajal, S.; Thakur, S. Coexistence of Microplastics and Heavy Metals in Soil: Occurrence, Transport, Key Interactions and Effect on Plants. Environ. Res. 2024, 262, 119960. [Google Scholar] [CrossRef]
- Pinto-Poblete, A.; Retamal-Salgado, J.; López, M.D.; Zapata, N.; Sierra-Almeida, A.; Schoebitz, M. Combined Effect of Microplastics and Cd Alters the Enzymatic Activity of Soil and the Productivity of Strawberry Plants. Plants 2022, 11, 536. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Ma, Q.; Lai, J.; Luo, X.; Ji, X. Cytotoxic and Genotoxic Evaluation and the Toxicological Mechanism of Uranium in Vicia Faba Root. Environ. Exp. Bot. 2020, 179, 104227. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Qiu, T.; Duan, C.; Chen, L.; Zhao, S.; Zhang, X.; Fang, L. Microplastics Addition Reduced the Toxicity and Uptake of Cadmium to Brassica chinensis L. Sci. Total. Environ. 2022, 852, 158353. [Google Scholar] [CrossRef] [PubMed]
- Bethanis, J.; Golia, E.E. Revealing the Combined Effects of Microplastics, Zn, and Cd on Soil Properties and Metal Accumulation by Leafy Vegetables: A Preliminary Investigation by a Laboratory Experiment. Soil Syst. 2023, 7, 65. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A Critical Review on the Interactions of Microplastics with Heavy Metals: Mechanism and Their Combined Effect on Organisms and Humans. Sci. Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef]
- Kumar, R.; Ivy, N.; Bhattacharya, S.; Dey, A.; Sharma, P. Coupled Effects of Microplastics and Heavy Metals on Plants: Uptake, Bioaccumulation, and Environmental Health Perspectives. Sci. Total Environ. 2022, 836, 155619. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, L.; Liu, C.; Dong, M.; Luo, Y.; Tan, S.; uz Zaman, Q.; Hayat, Z.; El-Kahtany, K.; Fahad, S.; et al. Macronutrients Dynamics in Copper-Contaminated Soils: Implications for Hemp Growth and Its Phytoremediation Potential. J. Agric. Food Res. 2024, 18, 101299. [Google Scholar] [CrossRef]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative Stress and Antioxidant Defense Mechanisms in Plants Under Salt Stress. In Plant Abiotic Stress Tolerance; Springer International Publishing: Cham, Switzerland, 2019; pp. 191–205. [Google Scholar]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy Metal Stress and Responses in Plants. Int J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Ningombam, L.; Hazarika, B.N.; Yumkhaibam, T.; Heisnam, P.; Singh, Y.D. Heavy Metal Priming Plant Stress Tolerance Deciphering through Physiological, Biochemical, Molecular and Omics Mechanism. South Afr. J. Bot. 2024, 168, 16–25. [Google Scholar] [CrossRef]
- Ji, J.; Zhong, Y.; Xiao, M.; Wang, X.; Hu, Z.; Zhan, M.; Ding, J.; Zhu, Z.; Ge, T. Synergistic Effect of Microplastics and Cadmium on Microbial Community and Functional Taxa in Wheat Rhizosphere Soil. Soil Ecol. Lett. 2025, 7, 240260. [Google Scholar] [CrossRef]
- Papadimou, S.G.; Golia, E.E.; Barbayiannis, N.; Tsiropoulos, N.G. Dual Role of the Hyperaccumulator Silybum marianum (L.) Gaertn. in Circular Economy: Production of Silymarin, a Valuable Secondary Metabolite, and Remediation of Heavy Metal Contaminated Soils. Sustain. Chem. Pharm. 2024, 38, 101454. [Google Scholar] [CrossRef]
- Golia, E.E.; Liava, V. The Use of Geotextiles in Agricultural Soils and Their Effects on Soil Properties and Nutrients Availability. Are Wastes Plastics Likely to Become Useful Materials in Agriculture? Sustain. Chem. Pharm 2024, 39, 101544. [Google Scholar] [CrossRef]
- Page, A.L. Methods of Soil Analysis-Part 2: Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; Volume 9, pp. 421–422. [Google Scholar]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Allison, L.E.; Moodie, C.D. Carbonate. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 2016; pp. 1379–1396. [Google Scholar]
- ISO/DIS 11466; Environment Soil Quality. ISO Standards Compendium: Geneva, Switzerland, 1994.
- Karpouzas, D.G.; Pantelelis, I.; Menkissoglu-Spiroudi, U.; Golia, E.; Tsiropoulos, N.G. Leaching of the Organophosphorus Nematicide Fosthiazate. Chemosphere 2007, 68, 1359–1364. [Google Scholar] [CrossRef]
- En-Nejmy, K.; EL Hayany, B.; Al-Alawi, M.; Jemo, M.; Hafidi, M.; El Fels, L. Microplastics in Soil: A Comprehensive Review of Occurrence, Sources, Fate, Analytical Techniques and Potential Impacts. Ecotoxicol. Environ. Saf. 2024, 288, 117332. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Zhang, Z.; Cui, M. The Positive Effects of Polypropylene and Polyvinyl Chloride Microplastics on Agricultural Soil Quality. J. Soils Sediments 2023, 23, 1304–1314. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, H.; Yang, Y.; Chen, H.; Chen, C.; Cheng, W. Metabonomics Reveals the Mechanism of Stress Resistance in Vetiveria Zizanioides Inoculated with AMF under Copper Stress. Sci. Rep. 2025, 15, 6005. [Google Scholar] [CrossRef]
- Sabir, M.; Baltrėnaitė-Gedienė, E.; Ditta, A.; Ullah, H.; Kanwal, A.; Ullah, S.; Faraj, T.K. Bioaccumulation of Heavy Metals in a Soil–Plant System from an Open Dumpsite and the Associated Health Risks through Multiple Routes. Sustainability 2022, 14, 13223. [Google Scholar] [CrossRef]
- Anisimov, V.S.; Anisimova, L.N.; Sanzharov, A.I. Zinc Plant Uptake as Result of Edaphic Factors Acting. Plants 2021, 10, 2496. [Google Scholar] [CrossRef]
- Asare, M.O.; Száková, J.; Tlustoš, P.; Kumar, M. Zinc Contamination in Soils and Its Implications on Plant Phytoalexins. Int. J. Environ. Sci. Technol. 2025, 22, 8581–8600. [Google Scholar] [CrossRef]
- Xu, L.; Yu, C.; Xie, W.; Liang, X.; Zhan, J.; Dai, H.; Skuza, L.; Xu, J.; Jing, Y.; Zhang, Q.; et al. Effects of Polyethylene Microplastics on Cadmium Accumulation in Solanum nigrum L.: A Study Involving Microbial Communities and Metabolomics Profiles. J. Hazard. Mater. 2025, 489, 137621. [Google Scholar] [CrossRef]
- An, Q.; Zheng, N.; Chen, C.; Li, X.; Ji, Y.; Peng, L.; Xiu, Z.; Lin, Q. Regulation Strategies of Microplastics with Different Particle Sizes on Cadmium Migration Processes and Toxicity in Soil-Pakchoi System. J. Hazard. Mater. 2025, 488, 137505. [Google Scholar] [CrossRef]
- Liu, F.; Hu, J.; Zhang, Y.; Li, X.; Yang, Y.; Du, G.; Tang, K. Hemp (Cannabis sativa L.) Tolerates Chelator Stress Showing Varietal Differences and Concentration Dependence. Agronomy 2023, 13, 2325. [Google Scholar] [CrossRef]


| pH (1:2.5) | EC (μS cm−1) | OM (%) | CaCO3 (%) | Clay (%) | Texture | |
|---|---|---|---|---|---|---|
| MinimumValue | 7.48 | 457 | 2.62 | 12.2 | 39 | Clayey |
| MaximumValue | 7.77 | 553 | 3.18 | 14.3 | 44 | |
| Mean Value | 7.61 | 498 | 2.91 | 13.3 | 41 | |
| Relative Standard Deviation (%) | 2.2 | 10.7 | 1.1 | 1.7 | 0.9 |
| pH (1:2.5) | EC (μS cm−1) | OM (%) | CaCO3 (%) | Clay (%) | Texture | |
|---|---|---|---|---|---|---|
| MinimumValue | 6.61 | 344 | 3.1 | 12.2 | 39 | Clayey |
| MaximumValue | 7.83 | 905 | 3.7 | 14.3 | 44 | |
| Mean Value | 7.37 | 512 | 3.2 | 13.3 | 41 | |
| Relative Standard Deviation % | 1.9 | 8.9 | 1.5 | 1.6 | 1.1 |
| Treatment | Root Concentration Ratio | Stem Concentration Ratio | Flower Concentration Ratio | Above-Ground Concentration Ratio |
|---|---|---|---|---|
| Soil | 1.00 | 1.00 | 1.00 | 1.00 |
| ZnA | 5.16 | 5.66 | 2.18 | 5.22 |
| ZnB | 10.3 | 23.7 | 11.8 | 22.2 |
| Cu + Zn | 4.34 | 2.94 | 8.36 | 3.62 |
| Cd + Zn | 4.10 | 2.74 | 10.0 | 3.65 |
| Cd + Cu + Zn | 4.01 | 2.68 | 7.91 | 3.34 |
| Soil + Geo | 1.00 | 1.00 | 1.00 | 1.00 |
| Geo + ZnA | 5.69 | 5.25 | 3.24 | 4.98 |
| Geo + ZnB | 10.1 | 22.3 | 11.3 | 20.8 |
| Geo + Cu + Zn | 4.01 | 2.67 | 7.84 | 3.36 |
| Geo + Cd + Ζn | 3.85 | 2.72 | 10.1 | 3.70 |
| Geo + Cd + Cu +Zn | 3.98 | 2.50 | 6.19 | 2.99 |
| Treatment | Root Concentration Ratio | Stem Concentration Ratio | Flower Concentration Ratio | Above-Ground Concentration Ratio |
|---|---|---|---|---|
| Soil | 1.00 | 1.00 | 1.00 | 1.00 |
| ZnA | 11.3 | 5.14 | 3.21 | 4.31 |
| ZnB | 18.8 | 16.8 | 9.32 | 13.58 |
| Cu + Zn | 11.7 | 5.39 | 3.53 | 4.60 |
| Cd + Zn | 1.24 | 5.03 | 3.05 | 4.18 |
| Cd + Cu + Zn | 12.3 | 6.48 | 4.85 | 5.78 |
| Soil + Geo | 1.00 | 1.00 | 1.00 | 1.00 |
| Geo + ZnA | 7.17 | 4.77 | 4.06 | 4.52 |
| Geo + ZnB | 11.0 | 11.5 | 9.23 | 10.7 |
| Geo + Cu + Zn | 7.23 | 5.53 | 5.13 | 5.39 |
| Geo + Cd + Ζn | 7.24 | 5.01 | 5.03 | 5.02 |
| Geo + Cd + Cu +Zn | 7.21 | 5.65 | 5.12 | 5.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexiadis, D.; Bethanis, J.; Papadimou, S.G.; Barbieri, E.; Vogia, R.; Tatsi, E.; Tziourrou, P.; Tsaliki, E.; Golia, E.E. Effect of Plastics (Geotextiles) on Heavy Metal Accumulation by Industrial Hemp Plants Cultivated in Polluted Mediterranean Soils. Int. J. Plant Biol. 2025, 16, 53. https://doi.org/10.3390/ijpb16020053
Alexiadis D, Bethanis J, Papadimou SG, Barbieri E, Vogia R, Tatsi E, Tziourrou P, Tsaliki E, Golia EE. Effect of Plastics (Geotextiles) on Heavy Metal Accumulation by Industrial Hemp Plants Cultivated in Polluted Mediterranean Soils. International Journal of Plant Biology. 2025; 16(2):53. https://doi.org/10.3390/ijpb16020053
Chicago/Turabian StyleAlexiadis, Dimitrios, John Bethanis, Sotiria G. Papadimou, Edoardo Barbieri, Rafaella Vogia, Eftihia Tatsi, Pavlos Tziourrou, Eleni Tsaliki, and Evangelia E. Golia. 2025. "Effect of Plastics (Geotextiles) on Heavy Metal Accumulation by Industrial Hemp Plants Cultivated in Polluted Mediterranean Soils" International Journal of Plant Biology 16, no. 2: 53. https://doi.org/10.3390/ijpb16020053
APA StyleAlexiadis, D., Bethanis, J., Papadimou, S. G., Barbieri, E., Vogia, R., Tatsi, E., Tziourrou, P., Tsaliki, E., & Golia, E. E. (2025). Effect of Plastics (Geotextiles) on Heavy Metal Accumulation by Industrial Hemp Plants Cultivated in Polluted Mediterranean Soils. International Journal of Plant Biology, 16(2), 53. https://doi.org/10.3390/ijpb16020053









