Abstract
The rising global demand for medicinal cannabis necessitates the optimization of cultivation, harvesting, and extraction techniques to maximize cannabinoid yield and purity. This study investigates the Foi Thong Phu Pha Yon strain under controlled environmental conditions, evaluating the effects of temperature, humidity, CO2 concentration, and light exposure on plant growth and cannabinoid biosynthesis. A total of 170 seeds were germinated, with an 85% germination success rate, and various growth strategies, including soil composition, nutrient application, and irrigation methods, were tested to determine the most effective approach. The research findings indicate that vegetative growth was optimal at 27 °C, 70% humidity, and 1200 ppm CO2 while flowering required a reduced temperature (22 °C), lower humidity (50%), and elevated CO2 levels (1900 ppm) to enhance cannabinoid production and prevent disease. Furthermore, harvest timing significantly influenced CBD yield, with peak cannabinoid content observed when 80% of trichomes were cloudy white. Over two growing cycles, this study produced 43,200 g of fresh buds, resulting in 7560 g of dried cannabis buds. The extraction process, utilizing dynamic maceration with 95% ethanol, followed by winterization and chromatography, yielded 2343.60 g of cannabis extract, including 589.68 g of CBD, with an average purity of 86.599%. Advanced techniques such as flash chromatography and distillation further refined the CBD isolate, ensuring pharmaceutical-grade quality. These findings highlight the effectiveness of precise environmental control, strategic harvesting, and advanced extraction methodologies in optimizing cannabis production. This research provides valuable insights for agricultural researchers, policymakers, and the pharmaceutical industry, supporting sustainable cultivation practices and improved product quality in the expanding medicinal cannabis market.
1. Introduction
Cannabis sativa is a versatile plant known for its diverse array of secondary metabolites, including cannabinoids, terpenes, and flavonoids, which contribute to its medicinal and pharmacological properties [1,2]. The primary bioactive compounds in cannabis are tetrahydrocannabinol (THC) and cannabidiol (CBD), which are widely recognized for their therapeutic effects [3]. THC is primarily responsible for the psychoactive effects and has been used to alleviate appetite loss, insomnia, muscle spasms, and chronic pain, whereas CBD is non-psychoactive and has shown potential in treating epilepsy, depression, inflammation, migraines, and anxiety disorders [4,5]. The biosynthesis of these cannabinoids predominantly occurs in the glandular trichomes of female cannabis flowers, which serve as the main site for cannabinoid production. While other plant parts, such as leaves and stems, contain secondary metabolites, their concentrations are significantly lower than in flowers. The composition of these metabolites varies depending on genetic factors, environmental conditions, and agronomic practices [6].
Historically, cannabis was classified as a Class 5 substance under Thailand’s Drugs Act of 1979, restricting its use for research and governmental initiatives [7]. However, recent policy reforms have facilitated the controlled cultivation and use of cannabis for medicinal purposes, aligning with global trends in cannabis legalization. Countries such as Canada, the Netherlands, and parts of the United States have established regulatory frameworks that enable medical cannabis cultivation under strict guidelines. In Thailand, government policies promote cannabis cultivation under specific licensing agreements, primarily for medical and research purposes. The Thai cannabis policy emphasizes the localized cultivation of native strains to ensure genetic purity, adapt to regional climatic conditions, and support economic growth [8]. By fostering indigenous cannabis production, Thailand can reduce reliance on imports, enhance local revenue, and establish a competitive position in the global cannabis market [9,10].
Several agronomic and environmental factors play a crucial role in influencing cannabis growth and secondary metabolite production [11]. Genetic strain selection is a key determinant, as different cannabis strains exhibit varying cannabinoid profiles, directly impacting their medicinal and industrial applications [12]. Environmental factors, including temperature, humidity, light intensity, and soil conditions, significantly affect cannabinoid biosynthesis and overall plant yield. Additionally, cultivation practices such as indoor versus outdoor growing conditions, nutrient supplementation, and irrigation techniques influence plant development and metabolic expression [13,14]. Beyond cultivation, harvesting and post-harvest handling play a critical role in maintaining cannabinoid potency, with factors like harvest timing, drying, curing, and storage conditions directly impacting cannabinoid stability and degradation. The extraction methods and metabolomics’ analysis determine the efficiency of cannabinoid isolation, influencing both the purity and concentration, while advanced metabolomics’ techniques help identify bioactive compounds [15].
The “Foi Thong Phu Pha Yon” strain, a native Thai cannabis variety, has been chosen for its high adaptability to local climatic conditions and rich cannabinoid content, making it an ideal candidate for sustainable cultivation. This strain aligns with Thailand’s policy-driven approach to developing high-quality, locally sourced cannabis for pharmaceutical applications, ensuring genetic consistency and economic viability. To contribute to the advancement of standardized cannabis production, this study aims to evaluate cannabis strains and environmental conditions that optimize cannabinoid production, investigate harvesting techniques and post-harvest storage conditions to maximize yield retention, assess different extraction methods for improving cannabinoid purity and efficiency, and analyze the chemical composition of extracts to refine purity enhancement techniques. By integrating strain selection, environmental optimization, and extraction efficiency, this research supports the development of Thailand’s medicinal cannabis industry and strengthens its role in the global cannabis market. Future studies should explore genetic stability, alternative extraction methods, and environmental influences on cannabinoid biosynthesis to further refine cannabis production and processing.
2. Materials and Methods
2.1. Growth Materials
This cannabis cultivation study was conducted on the rooftop of the Faculty of Science and Technology Building, Rajamangala University of Technology Phra Nakhon, Bangkok, focusing on the “Foi Thong Phu Pha Yon” strain to develop a high CBD-to-THC ratio (≥1:1) for medicinal use. To mitigate the risk of contamination, five distinct varieties of growing medium were sourced from reputable suppliers, as follows: elephant manure castings, peat moss, fine coconut coir, bat guano, and vermiculite. These materials were combined in specific ratios as shown in Table 1 and labeled as the formula (F1, F2, and F3) and subjected to a fermentation process lasting 72 h before planting.

Table 1.
Types and percentages of cannabis planting materials.
2.2. Experimental Setup
A total of 170 seeds were selected based on heart-shaped morphology (indicative of female plants), with only those achieving a ≥85% germination rate proceeding to cultivation. Seeds were soaked for 12–18 h, wrapped in moist paper, and stored at 25 °C and 85% humidity until sprouting. A total of 15 plants were utilized to evaluate each formula (Table 1) in triplicates. Seedlings were cultivated in two phases. Phase 1 involved 5–7 days of indoor growth in 3-inch pots under 90% humidity, 25 °C, and 18 h of LED light (300–400 µmol PPFD) with liquid nutrients. Phase 2 lasted 7–10 days, with seedlings transplanted into 8-inch coconut coir-filled pots until they developed seven pairs of leaves. Plants were then propagated in peat moss pots under 90% humidity and 24 h lighting for 10–15 days, followed by an 8 h light cycle for 15–30 days to encourage sex differentiation. Once identified, female plants were cultivated indoors (48 plants/cycle) or outdoors (24 plants/cycle), while male, hermaphrodite, and immature female plants were removed. To enhance flower quality, plants were transferred to Smart Pots (60 × 40 cm2 for outdoors, 40 × 35 cm2 for indoors).
2.3. Flower Quality Improvement
Cannabis cultivation was conducted in a 40 m2 fully controlled environment to ensure optimal growth. Conditions were maintained at 70% relative humidity, 20–25 °C temperature, and LED lighting (PPFD 500–800 µmol) for 8 h daily, with CO2 enrichment [16]. The 5 × 8 m2 cultivation room, built with ISOWALL material, provided a moisture-proof and climate-controlled setting. An air conditioning system with filtration regulated temperature, humidity, and airflow, while humidity sensors and automated fans ensured optimal air circulation. A drip irrigation system supplied water and nutrients, and fans simulated natural airflow to enhance plant strength and flower quality. The setup included 48 QR-coded planting containers (1–48), each reinforced with a two-tiered wire mesh to support branch expansion and maximize light exposure. During pre-flowering (up to 8 weeks/60 days), conditions were strictly regulated with an 8 h light/16 h dark cycle and CO2 enrichment to accelerate flower formation. Pruning was discontinued as plants transitioned to flowering, and watering was reduced to prevent waterlogging. By week 10, full flower development was reached, marked by resin exudation on leaves and flower clusters. Trichome coloration analysis determined the optimal harvest time, ensuring premium medical-grade cannabis flowers.
2.4. Harvesting and Pre-Processing
To optimize flower development, light exposure was reduced before harvest [17]. In outdoor cultivation, cannabis plants were covered with black shade cloth for two days, while in indoor cultivation, grow lights were turned off for 48 h under controlled conditions (25–27 °C, 50% humidity). After harvesting flowers were hung upside down on racks in a well-ventilated, dark curing room at 18–20 °C, with 50–60% humidity, ensuring gradual moisture loss over 10–14 days while preventing mold formation. Fans maintained airflow stability, and once dried, flowers were trimmed, removing excess leaves and reducing stems to ≤0.5 inches. The trimmed flowers were then cured in a humidity-controlled room (≤60%) to regulate moisture release. Fully dried buds were stored in airtight glass jars to prevent odor emission and kept in the dark at 27 °C.
2.5. Extraction and Purification
The dried flowers were finely ground (200–300 μ) and analyzed for CBD and THC content before undergoing decarboxylation at 150 °C for 90 min in a vacuum dry oven, converting CBDA, CBGA, and THCA into their active forms (CBD, CBG, and THC). Terpene emissions were captured at −80 °C during this process. For ethanol heat extraction, 7000 g of ground cannabis was processed in a 50 L jacketed glass reactor with >95% ethyl alcohol (35 L) as the solvent. The water circulator heater was set at 60 °C, and the DL-10-2000 condenser maintained 5 °C under 500 mbar air pressure, with a blade speed of 150 rpm for a 3 h extraction cycle. The extract was then filtered (15 min) and residual material was extracted using a compression machine (30 min) before the reactor was cleaned (15 min), totaling 4 h per extraction cycle. The thick, dark-colored extract was mixed with 5–6 parts ethanol, gently heated for dissolution, and then cooled at −20 °C for 24 h followed by vacuum filtration of the mixture. To eliminate chlorophyll, activated charcoal (5%) was added, with careful control to minimize CBD loss. HPLC analysis was conducted before and after treatment to monitor cannabinoid concentration. The solution was stirred for 15 min with a magnetic stirrer, followed by vacuum filtration, yielding yellow-to-orange CBD oil, depending on the strain. The filtered oil was subjected to solvent evaporation via a rotary evaporator, removing ethanol and producing a concentrated cannabinoid extract by vacuum distillation. An external condenser has collected terpenes, while cannabinoid vapor condenses internally and flows into a collection flask. Higher boiling point impurities adhered to chamber walls and were separately collected.
2.6. Bioactive Compounds Analysis
Flash chromatography was used to separate THC, producing broad-spectrum crude oil. This method isolates CBD, CBN, and THC using a stationary phase column and an organic solvent pump as the mobile phase. A UV detector identifies separated compounds, which are collected by a fraction collector and then concentrated via rotary evaporation, yielding cannabinoids with >90% purity (CBD isolate). A dense water-based CBD solution was prepared by mixing 1 part CBD isolate with 5 parts NanoStabilizer-LT. The mixture was then blended with purified water in a mixing tank using a high-intensity ultrasonic machine, by controlling nanoemulsion droplet size. The emulsion was pumped into a reactor chamber at a controlled flow rate, then filtered through a 220 nm filter into tinted bottles and stored at 4 °C. Physical and chemical properties were assessed by measuring moisture content, total ash, and acid-insoluble ash to determine purity and composition. After extraction, unused cannabis plant parts were stored in a designated waste storage room. Components were categorized and stored under controlled conditions: roots, stems, branches, leaves, and residual biomass were sealed in bags to ensure preservation and safety. Unusable waste was incinerated in a specialized furnace at 1000–1400 °C.
2.7. Statistical Analysis
Data were analyzed using descriptive statistics. Independent t-tests compared indoor vs. outdoor yields, while one-way ANOVA assessed cannabinoid variations across extraction methods. HPLC results were validated using relative standard deviation (RSD) and confidence intervals. Regression analysis evaluated the relationship between drying conditions, extraction efficiency, and yield. Statistical tests were performed using SPSS 30 with significance set at p < 0.05 to ensure reliability.
3. Results
3.1. Germination Rate
Out of 200 seeds planted, 177 seeds germinated successfully, resulting in 131 vigorous plants. Out of these, 87 were female plants (66.41%), 24 were male plants (18.32%), and 20 were hermaphrodite plants (15.27%). The consistent germination performance between the two greenhouses, with only a slight variation, indicates that the cultivation conditions in both areas were relatively uniform and effective. The elevated germination rate and the advantageous ratio of female to male plants suggest that the “Foi Thong Phu Pha Yon” strain is highly conducive for cultivation, assuming that suitable selection and care practices are implemented.
3.2. Media Optimization
Five unique growing media were evaluated. The average weights of the dehydrated cannabis flowers obtained from the indoor system were as follows: F1 generated 90.23 g, F2 produced 115.63 g, and F3 resulted in 95.15 g (Table 2). The control group, maintained under standard conditions, achieved an average weight of 92.82 g. This notable variation highlights the importance of selecting an appropriate growing medium composition to maximize cannabis yield [18]. The superior performance of the F2 indicates that a well-balanced mixture of organic and inorganic components may provide the essential nutrients and support required for optimal cannabis cultivation. Notably, the outdoor cultivation failed to produce any dried cannabis flowers, yielding only leaves, which were excluded from the weight assessments.

Table 2.
Dry flower yield from indoor cultivation.
3.3. Growth Parameters
The process of harvesting cannabis flowers to optimize CBD levels requires a systematic approach [19]. The findings indicated that the optimal harvest time for plants was when approximately 80% of the trichomes appeared cloudy white. To achieve this, a 48 h dark period was implemented within the cultivation environment, maintaining a temperature of 25 °C and a humidity level of 50%. As the plants near the end of their growth cycle, essential compounds become more concentrated in the flowers, while the leaves begin to yellow, indicating maturity. The initial harvesting phase began when 60% of the trichomes on the parent plants exhibited a cloudy white appearance. During this phase, all environmental parameters were carefully controlled in the same manner as the final harvest. After drying and curing, the flowers from both cultivation cycles were analyzed to determine their CBD content. The results confirmed that peak CBD levels are achieved when 80% of the trichomes are hazy white, reinforcing the importance of precise timing in the harvesting process. This study revealed that when trichomes were only 60% opaque, significant cannabinoid levels were still present, although they remained notably lower than those in fully matured plants. This underscores the critical role of environmental regulation and strategic harvest timing in maximizing cannabinoid concentration. These findings demonstrated that precise environmental control during the final growth phase directed the plant’s energy toward producing cannabinoid-rich flowers, ensuring optimal CBD yield.
3.4. Analysis of Compounds
Indoors, 48 female plants were grown in a 40 m2 space (0.833 m2/plant), producing an average yield of 400 g per plant, totaling 19,200 g of fresh buds. Outdoors, 24 female plants occupied 30 m2 (1.25 m2/plant), yielding 100 g per plant, for a total of 2400 g of fresh buds. The combined indoor and outdoor yield was 21,600 g of fresh buds from 72 plants. After drying, bud weight shrank by ~65%, reducing the yield to 105 g per plant, resulting in 7560 g of dried cannabis buds. This analysis highlights yield efficiency across cultivation environments. Annual cannabis cultivation across two cycles yielded 43,200 g of fresh buds (21,600 g/cycle). After drying (~65% reduction), this produced 7560 g of dried buds per cycle and 15,120 g annually. The extraction process achieves 70–90% purity and can process up to 7.5 kg of oil per hour. Following distillation, the CBD extract reaches 70–90% concentration, often exceeding the 0.2% THC. From each cycle, 2343.6 g of cannabis extract was obtained (31% yield), which when diluted fivefold, produced 11,718 mL of cannabis oil, filling approximately 2343 bottles (5 mL). Additionally, CBD extraction from dried buds yielded 589.68 g per cycle (7.8% yield), totaling 1179.36 g annually. This analysis highlights the efficiency and scalability of cannabis cultivation and extraction across indoor and outdoor cycles.
A comparative analysis of compound concentrations was conducted using HPLC, focusing on the differences between the mother plants and the initial planting round. Notable differences in cannabis CBD concentrations were identified between the mother plant round and the initial planting round, as demonstrated by the high-performance liquid chromatography (HPLC) analysis as shown in Table 3. The average CBD concentration in the mother plant round was 0.424%, while no CBD was detected in the initial cultivation cycle. Conversely, the average concentration of d9-THC rose from 1.176% in the mother plant round to 3.160% in the initial planting round. Similarly, the average THCA-A content experienced a significant increase from 5.473% to 48.214% across these two phases. This fluctuation in THC levels underscores the influence of differing environmental conditions and developmental stages on the chemical profile of cannabis plants. The marked rise in THCA-A concentration during the initial planting round indicates that targeted agricultural practices can effectively enhance the levels of specific CBDs.

Table 3.
The concentration of bioactive compounds in cannabis plants grown in an indoor greenhouse system during the mother plant cycle and the first cultivation round.
3.5. Purification of CBD
The process of extracting and purifying cannabidiol (CBD) from dried cannabis flowers involves several intricate steps that are crucial for obtaining a high-quality product [20]. They are subjected to extraction methods, including solvent extraction, CO2 extraction, or steam distillation. Quantitative data on the yield of CBD obtained represent the purity levels achieved, and possibly comparisons with other extraction methods; the meticulous approach to extracting and purifying CBD not only enhances the efficacy of the final product but also contributes to the growing body of research and understanding surrounding the therapeutic potential of CBD. Three cannabinoid compounds CBDV (cannabidivarin), CBDA (cannabidiolic acid), and CBD (cannabidiol) in two different samples were obtained in the analysis as displayed in Figure 1. The results indicate that CBD is the most abundant compound, with an average concentration of 86.599%, followed by CBDA at 0.407%, and CBDV at 0.456%. The values for each compound are consistent across the graph demonstrating minimal variation. This suggests a high level of uniformity and reliability in the cannabinoid content of the analyzed samples. The significantly higher percentage of CBD compared to CBDA and CBDV indicates that the samples are rich in cannabidiol, which is a key factor in determining their therapeutic potential.
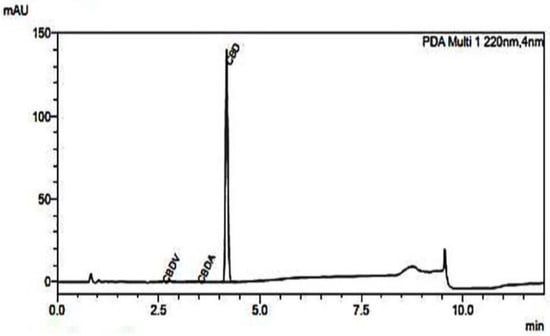
Figure 1.
Graph representing the types and quantities of key substances and the purity of CBD.
The chromatogram (Figure 1) has a dominant peak at approximately 5 min, corresponding to CBD (cannabidiol), indicating it is the primary component in the sample. The intensity of this peak, reaching nearly 150 mAU, highlights the high concentration of CBD, confirming its significant presence. Additionally, smaller peaks at earlier retention times (~2–3 min) are labeled as CBGV (cannabigerovarin) and CBDA (cannabidiolic acid), suggesting trace amounts of these minor cannabinoids. The absence of significant peaks beyond the CBD peak indicates a high level of purity in the sample, with minimal impurities or other cannabinoids detected. This result confirms that the sample predominantly contains CBD, making it suitable for applications requiring a high-purity cannabinoid profile.
The overlaid chromatograms from Figure 2 and the data provided in Table 4 confirmed that the CBD isolate is highly purified, with a dominant peak at approximately 10 min corresponding to CBD (red peaks), as confirmed by alignment with the standard mix (black peaks). The intensity of this peak in the isolate indicates that CBD is the primary component, showcasing its high concentration. In contrast to the standard mix, which contains multiple peaks representing a variety of cannabinoids, the CBD isolate displays significantly fewer peaks, highlighting its purity. Minor trace peaks observed at earlier retention times (~2–5 min) suggest the presence of minimal residual cannabinoids or impurities. Overall, the results confirm the successful isolation of CBD, with a clean profile suitable for applications requiring high-purity cannabinoid content.
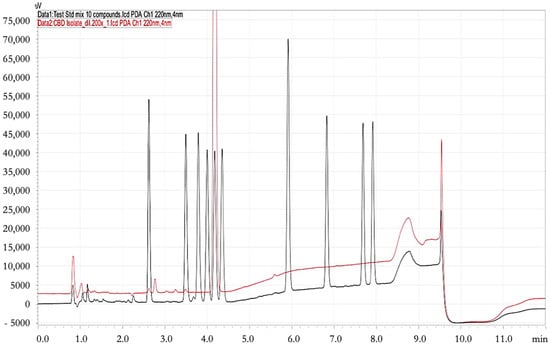
Figure 2.
Comparative analysis between the standard mix (black peaks) and the Foi Thong Phu Pha Yon strain of cannabis (red peaks).

Table 4.
Types and quantities of key substances of CBD from the Foi Thong Phu Pha Yon strain of cannabis.
4. Discussion
The findings of this study align with the existing literature emphasizing the significance of environmental conditions in optimizing cannabis yield and cannabinoid production. Temperature plays a crucial role in cannabis growth, influencing photosynthesis, respiration, and secondary metabolite biosynthesis. The research indicates that optimal temperatures for cannabis cultivation range between 24 and 30 °C during the light period and 18 and 22 °C during the dark period, ensuring efficient growth and cannabinoid accumulation [21]. High temperatures can lead to heat stress, reduced stomatal conductance, and increased transpiration rates, negatively affecting biomass production and cannabinoid synthesis [22]. Similarly, relative humidity (RH) affects stomatal function and water use efficiency, with an optimal RH range of 50–60% recommended to prevent excessive water loss and mold formation [23]. Another critical factor is CO2 concentration, which directly impacts photosynthesis and biomass accumulation. Studies have shown that CO2 enrichment up to 1200–1500 ppm enhances photosynthetic efficiency, resulting in higher yields and increased cannabinoid content, particularly THC and CBD [24]. Light exposure is equally important, as light spectrum and intensity influence cannabinoid biosynthesis. Blue and red wavelengths are most effective in promoting photosynthetic activity and secondary metabolite production, with high-intensity LED lighting proving beneficial for maximizing THC and CBD concentrations [25]. The results of this study, particularly on the Foi Thong Phu Pha Yon strain, reinforce these findings by demonstrating the direct impact of controlled environmental conditions on cannabis flower maturation, cannabinoid accumulation, and overall yield. By optimizing these factors, growers can significantly enhance the quality and quantity of bioactive compounds, making controlled cultivation strategies essential for both medicinal and industrial cannabis production.
This study effectively demonstrates how optimizing environmental conditions during the vegetative and flowering phases enhances cannabis growth and cannabinoid biosynthesis. During the vegetative phase, maintaining a temperature of 27 °C, relative humidity of 70%, and CO2 concentration of 1200 ppm fosters enhanced photosynthesis, nutrient uptake, and metabolic efficiency, which is in agreement with previous studies highlighting the role of CO2 enrichment in increasing biomass production [26]. High humidity during this stage is beneficial for leaf expansion and stomatal function, reducing water loss and ensuring steady growth [27]. As plants transition to the flowering phase, adjusting environmental parameters becomes essential to promote cannabinoid biosynthesis while preventing microbial contamination. Lowering the temperature to 22 °C and reducing humidity to 50% minimizes the risk of mold formation, particularly in the dense floral structures of cannabis [28]. Moreover, increasing CO2 concentration to 1900 ppm enhances carbon assimilation, providing additional energy for terpene and cannabinoid biosynthesis, a trend observed in previous research that demonstrated increased THC and CBD levels under CO2-enriched conditions [29]. The reduction in the photoperiod from 18 h to 12 h is a well-documented requirement for inducing the flowering phase, as cannabis is a short-day plant that requires extended darkness to activate flowering genes [30]. Harvest timing is another key determinant of cannabinoid potency, as CBD and THC concentrations peak when trichomes appear cloudy white. This study confirms that harvesting at 80% cloudy trichomes results in the highest CBD levels, which aligns with findings that cannabinoid biosynthesis reaches peak activity in the late flowering stages [31]. Conversely, early harvesting at 60% opacity leads to lower cannabinoid levels, reinforcing the understanding that cannabinoid accumulation continues late into the flowering phase, and slight delays in harvest can improve potency without degrading quality [32]. These findings highlight the importance of precise environmental control and optimized harvest timing for maximizing yield and cannabinoid content in cannabis cultivation.
Beyond cultivation, optimizing extraction and purification techniques is critical for obtaining high-purity CBD suitable for pharmaceutical and medicinal applications. This study successfully employed dynamic maceration with 95% ethanol, followed by winterization and flash chromatography, to extract CBD with an average purity of 86.599%. Ethanol-based extraction is widely recognized for its efficiency in solubilizing cannabinoids while maintaining their structural integrity [33]. The subsequent winterization process effectively removed unwanted lipids and waxes, a necessary step to improve extract clarity and purity [34]. Flash chromatography played a crucial role in isolating CBD from other cannabinoids and impurities by leveraging differential solubility and polarity to separate compounds efficiently. Similar studies have demonstrated that chromatographic separation techniques improve cannabinoid isolation, particularly in refining CBD while minimizing THC contamination [34]. Further purification was achieved through distillation, which utilizes boiling point differences to selectively isolate cannabinoids. This approach aligns with existing research confirming that distillation enhances cannabinoid purity by eliminating residual solvents and undesired compounds [35]. The results of this study reinforce the efficacy of modern solvent-based extraction combined with chromatographic separation in maximizing CBD yield while maintaining high purity levels. These methods are essential for producing medically viable cannabis isolates, ensuring consistency and compliance with pharmaceutical standards. The high purity achieved in this study underscores the potential of advanced extraction technologies in meeting the increasing demand for high-quality cannabinoid products in therapeutic and industrial applications.
This study provides significant contributions to sustainable cannabis production by evaluating the genetic stability, growth patterns, and optimal cultivation conditions of the Foi Thong Phu Pha Yon strain. The integration of controlled environmental conditions, precision harvesting techniques, and advanced extraction methodologies highlights the potential for scalable CBD production while ensuring product integrity. These findings align with the existing research emphasizing precision agriculture as a key factor in maximizing cannabinoid yield and maintaining consistency in medicinal cannabis production [27]. By optimizing environmental variables such as temperature, humidity, CO2 concentration, and photoperiod, this study reinforces the importance of precision-controlled cultivation in enhancing biomass accumulation and secondary metabolite production [36]. Additionally, the application of advanced post-harvest processing techniques, including precision harvesting and chromatographic purification, further supports the pharmaceutical industry’s demand for high-purity cannabinoid isolates [37]. The emphasis on genetic stability is particularly crucial for sustainable cannabis production, as genetic variations can lead to fluctuations in cannabinoid profiles and yield efficiency. While this study provides a baseline for strain optimization, future research should focus on evaluating genetic stability under varying environmental conditions to ensure resilience and consistency in large-scale cultivation [38]. Furthermore, optimizing extraction techniques to refine cannabinoid isolation and purity enhancement will be critical for maintaining pharmaceutical-grade CBD products and minimizing THC contamination [39]. These advancements will be instrumental in bridging the gap between cannabis cultivation and pharmaceutical applications, ultimately fostering the development of standardized cannabis-based therapeutics.
5. Conclusions
This study successfully demonstrated the importance of optimizing environmental parameters, precision harvesting, and advanced extraction techniques for maximizing cannabis yield and cannabinoid purity, particularly in the Foi Thong Phu Pha Yon strain. By maintaining controlled conditions during the vegetative and flowering stages, the research identified optimal temperature, humidity, CO2 concentration, and light exposure that significantly influenced plant growth, cannabinoid biosynthesis, and overall crop quality. The findings confirmed that vegetative growth was best supported by a temperature of 27 °C, 70% humidity, and 1200 ppm CO2, facilitating strong leaf and stem development. In contrast, the flowering phase required lower temperatures (22 °C), reduced humidity (50%), and elevated CO2 levels (1900 ppm) to enhance cannabinoid production and minimize disease risks. The importance of harvest timing was also established, with the highest CBD concentrations observed when 80% of trichomes appeared cloudy white, reinforcing that late-stage cannabinoid accumulation is essential for optimizing potency. In addition, this study demonstrated the effectiveness of dynamic maceration with ethanol, winterization, and chromatography in achieving high-purity CBD extraction (86.599%), ensuring that isolated cannabinoids met pharmaceutical-grade standards. Techniques such as flash chromatography and distillation played crucial roles in removing unwanted compounds, leading to high purity extracts suitable for medical applications. These findings provide valuable insights for cannabis cultivators, researchers, and the pharmaceutical industry, emphasizing the need for precision agriculture, sustainable cultivation practices, and advanced extraction methods. Future research should explore genetic stability, alternative extraction techniques, and environmental influences on cannabinoid profiles to further optimize cannabis production and medicinal applications. By integrating scientific advances with sustainable practices, this research contributes to the growing body of knowledge on high-quality cannabis cultivation and extraction, supporting the expansion of medicinal cannabis industries worldwide.
Author Contributions
Conceptualization, T.T. and N.I.; methodology, T.T.; software, T.T.; validation, T.T. and N.I.; formal analysis, T.T., R.D. and P.B.; investigation, T.T.; resources, T.T.; data curation, T.T.; writing—original draft preparation, T.T.; writing—review and editing, T.T., N.I., R.D. and P.B.; visualization, T.T.; supervision, R.D., N.I. and P.B.; project administration, T.T.; funding acquisition, T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge Maejo University International College and the Faculty of Science and Technology, Rajamangala University of Technology Phra Nakhon, for providing the space for cannabis cultivation and their support in carrying out this research project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and its secondary metabolites: Their use as therapeutic drugs, toxicological aspects, and analytical determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.M.; Injamum-Ul-Hoque, M.; Shaffique, S.; Ayoobi, A.; Rahman, M.A.; Rahman, M.M.; Choi, H.W. Illuminating Cannabis sativa L.: The power of light in enhancing C. sativa growth and secondary metabolite production. Plants 2024, 13, 2774. [Google Scholar] [CrossRef] [PubMed]
- Al Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A comprehensive review on the techniques for extraction of bioactive compounds from medicinal cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Cannabis Healing: A Guide to the Therapeutic Use of CBD, THC, and Other Cannabinoids; Simon and Schuster: New York, NY, USA, 2020. [Google Scholar]
- Stasiłowicz-Krzemień, A.; Nogalska, W.; Maszewska, Z.; Maleszka, M.; Dobroń, M.; Szary, A.; Kępa, A.; Żarowski, M.; Hojan, K.; Lukowicz, M.; et al. The Use of Compounds Derived from Cannabis sativa in the Treatment of Epilepsy, Painful Conditions, and Neuropsychiatric and Neurodegenerative Disorders. Int. J. Mol. Sci. 2024, 25, 5749. [Google Scholar] [CrossRef]
- Punja, Z.K.; Sutton, D.B.; Kim, T. Glandular trichome development, morphology, and maturation are influenced by plant age and genotype in high THC-containing cannabis (Cannabis sativa L.) inflorescences. J. Cannabis Res. 2023, 5, 12. [Google Scholar] [CrossRef]
- Charoenratana, S.; Anukul, C.; Aramrattana, A. Attitudes towards Kratom use, decriminalization and the development of a community-based Kratom control mechanism in Southern Thailand. Int. J. Drug Policy 2021, 95, 103197. [Google Scholar] [CrossRef]
- Orenstein, D.G.; Glantz, S.A. Cannabis legalization in state legislatures: Public health opportunity and risk. Marquette Law Rev. 2019, 103, 1313. [Google Scholar]
- Avery, G.C. (Ed.) Sufficiency Thinking: Thailand’s Gift to an Unsustainable World; Routledge: London, UK, 2020. [Google Scholar]
- Astutik, S.; Pretzsch, J.; Ndzifon Kimengsi, J. Asian medicinal plants’ production and utilization potentials: A review. Sustainability 2019, 11, 5483. [Google Scholar] [CrossRef]
- Morello, V.; Brousseau, V.D.; Wu, N.; Wu, B.S.; MacPherson, S.; Lefsrud, M. Light quality impacts vertical growth rate, phytochemical yield and cannabinoid production efficiency in Cannabis sativa. Plants 2022, 11, 2982. [Google Scholar] [CrossRef]
- Ndlovu, S.B.; Naidoo, D.; van Staden, J.; Gebashe, F.C. A systematic review of Cannabis sativa L. cultivation techniques: A comprehensive overview of tissue culture innovations and growth optimization. Ind. Crops Prod. 2024, 222, 119539. [Google Scholar]
- Chaachouay, N.; Qureshi, R.; Benkhnigue, O.; Azeroual, A.; Zidane, L. Marijuana (Cannabis sativa L. Cannabaceae). In Comprehensive Guide to Hallucinogenic Plants; CRC Press: Boca Raton, FL, USA, 2024; pp. 57–76. [Google Scholar]
- Ali, A.; Niu, G.; Masabni, J.; Ferrante, A.; Cocetta, G. Integrated nutrient management of fruits, vegetables, and crops through the use of biostimulants, soilless cultivation, and traditional and modern approaches—A mini review. Agriculture 2024, 14, 1330. [Google Scholar] [CrossRef]
- Walker, L.A.; Koturbash, I.; Kingston, R.; ElSohly, M.A.; Yates, C.R.; Gurley, B.J.; Khan, I. Cannabidiol (CBD) in dietary supplements: Perspectives on science, safety, and potential regulatory approaches. J. Diet. Suppl. 2020, 17, 493–502. [Google Scholar]
- Knight, G.; Hansen, S.; Connor, M.; Poulsen, H.; McGovern, C.; Stacey, J. The results of an experimental indoor hydroponic Cannabis growing study, using the ‘Screen of Green’(ScrOG) method—Yield, tetrahydrocannabinol (THC) and DNA analysis. Forensic Sci. Int. 2010, 202, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Moher, M. Lighting Strategies for Indoor Cannabis Propagation, Vegetative Growth, and Flower Initiation. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, December 2020. [Google Scholar]
- Pagnani, G.; Pellegrini, M.; Galieni, A.; D’egidio, S.; Matteucci, F.; Ricci, A.; Stagnari, F.; Sergi, M.; Sterzo, C.L.; Pisante, M.; et al. Plant growth-promoting rhizobacteria (PGPR) in Cannabis sativa ‘Finola’ cultivation: An alternative fertilization strategy to improve plant growth and quality characteristics. Ind. Crops Prod. 2018, 123, 75–83. [Google Scholar]
- Crispim Massuela, D.; Hartung, J.; Munz, S.; Erpenbach, F.; Graeff-Hönninger, S. Impact of harvest time and pruning technique on total CBD concentration and yield of medicinal cannabis. Plants 2022, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and extraction methods of medicinal cannabis: A narrative review. J. Cannabis Res. 2021, 3, 32. [Google Scholar] [PubMed]
- Chandra, S.; Lata, H.; Khan, I.A.; ElSohly, M.A. Temperature response of photosynthesis in different drug and fiber varieties of Cannabis sativa L. Physiol. Plant. 2015, 155, 297–306. [Google Scholar]
- Potter, D.J. A review of the cultivation and processing of cannabis (Cannabis sativa L.) for production of prescription medicines in the UK. Drug Test. Anal. 2014, 6, 31–38. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Bianchetti, R.E.; Furlan, C.M.; Ono, E.O. Influence of relative humidity on physiological and biochemical responses in Cannabis sativa L. Ind. Crops Prod. 2021, 171, 113921. [Google Scholar]
- Vanhove, W.; Van Damme, P.; Meert, N. Factors determining yield and quality of illicit indoor cannabis (Cannabis spp.) production. Forensic Sci. Int. 2012, 220, 158–163. [Google Scholar]
- Magagnini, G.; Grassi, G.; Kotiranta, S. The effect of light spectrum on the morphogenesis and cannabinoid content of Cannabis sativa L. Med. Cannabis Cannabinoids 2018, 1, 19–27. [Google Scholar] [PubMed]
- Denton, T.M.; Schmidt, S.; Critchley, C.; Stewart, G.R. Natural abundance of stable carbon and nitrogen isotopes in Cannabis sativa reflects growth conditions. Funct. Plant Biol. 2001, 28, 1005–1012. [Google Scholar]
- Danziger, N.; Bernstein, N. Light matters: Effect of light spectra on cannabinoid profile and plant development of medical cannabis (Cannabis sativa L.). Ind. Crop. Prod. 2021, 164, 113351. [Google Scholar]
- Buirs, L.; Punja, Z.K. Integrated management of pathogens and microbes in Cannabis sativa L. (Cannabis) under greenhouse conditions. Plants 2024, 13, 786. [Google Scholar] [CrossRef]
- Hawley, D.; Graham, T.; Stasiak, M.; Dixon, M. Improving cannabis bud quality and yield with subcanopy lighting. HortScience 2018, 53, 1593–1599. [Google Scholar]
- Spitzer-Rimon, B.; Shafran-Tomer, H.; Gottlieb, G.H.; Doron-Faigenboim, A.; Zemach, H.; Kamenetsky-Goldstein, R.; Flaishman, M. Non-photoperiodic transition of female cannabis seedlings from juvenile to adult reproductive stage. Plant Reprod. 2022, 35, 265–277. [Google Scholar]
- Reichel, P.; Munz, S.; Hartung, J.; Präger, A.; Kotiranta, S.; Burgel, L.; Schober, T.; Graeff-Hönninger, S. Impact of three different light spectra on the yield, morphology and growth trajectory of three different Cannabis sativa L. strains. Plants 2021, 10, 1866. [Google Scholar] [CrossRef]
- Stack, G.M.; Toth, J.A.; Carlson, C.H.; Cala, A.R.; Marrero-González, M.I.; Wilk, R.L.; Gentner, D.R.; Crawford, J.L.; Philippe, G.; Rose, J.K.C.; et al. Season-long characterization of high-cannabinoid hemp (Cannabis sativa L.) reveals variation in cannabinoid accumulation, flowering time, and disease resistance. GCB Bioenergy 2021, 13, 546–561. [Google Scholar] [CrossRef]
- Malabadi, R.B.; Kolkar, K.P.; Chalannavar, R.K.; Baijnath, H. Cannabis sativa: Extraction Methods for Phytocannabinoids: An Update. World J. Biol. Pharm. Health Sci. 2024, 20, 018–058. [Google Scholar] [CrossRef]
- Song, Y.X.; Furtos, A.; Fuoco, D.; Boumghar, Y.; Patience, G.S. Meta-analysis and review of cannabinoids extraction and purification techniques. Can. J. Chem. Eng. 2023, 101, 3108–3131. [Google Scholar]
- Martinez, A.S.; Lanaridi, O.; Stagel, K.; Halbwirth, H.; Schnürch, M.; Bica-Schröder, K. Extraction techniques for bioactive compounds of cannabis. Nat. Prod. Rep. 2023, 40, 676–717. [Google Scholar]
- Backer, R.; Schwinghamer, T.; Rosenbaum, P.; McCarty, V.; Eichhorn Bilodeau, S.; Lyu, D.; Ahmed, B.; Robinson, G.; Lefsrud, M.; Smith, D. Closing the yield gap for cannabis: A meta-analysis of factors determining cannabinoid content. Front. Plant Sci. 2019, 10, 495. [Google Scholar]
- Richins, R.D.; Rodriguez-Uribe, L.; Lowe, K.; Ferral, R.; O’Connell, M.A. Accumulation of bioactive metabolites in cultivated medicinal cannabis. Plant Physiol. Biochem. 2018, 132, 198–207. [Google Scholar]
- Lange, B.M.; Zager, J.J. Comprehensive inventory of cannabinoids in Cannabis sativa L.: Can we connect genotype and chemotype? Phytochem. Rev. 2022, 21, 1273–1313. [Google Scholar]
- Al Bakain, R.Z.; Al-Degs, Y.S.; Cizdziel, J.V.; Elsohly, M.A. Comprehensive chromatographic profiling of cannabis from 23 USA States marketed for medical purposes. Acta Chromatogr. 2020, 33, 78–90. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).