Abstract
Phaseolus vulgaris L. is one of the most important legumes for human consumption due to its contents of proteins, antioxidants, minerals, and bioactive compounds. In the last decade, there has been a growing research interest in increasing yields while reducing or replacing the use of chemical fertilizers. This has led to a focus on plant growth-promoting Rhizobacteria (PGPR) as biofertilizers in sustainable agricultural practices. The aim of this study was to determine the growth-promoting activity of a culture broth of the Gram-negative soil bacteria Rhizobium sp. (F7), which is conserved in the Collection of Beneficial Bacteria at the Institute of Fundamental Research in Tropical Agriculture (INIFAT), and to identify the main secondary metabolites present in the ethyl acetate crude extract using high-resolution liquid chromatography coupled with mass spectrometry (UHPLC-ESI-MS/MS). The growth-promoting activity of the culture broth on Phaseolus vulgaris L. seeds was evaluated. The ethyl acetate extract was obtained by liquid–liquid extraction with ethyl acetate from the culture broth, and UHPLC-ESI-MS/MS was used to identify secondary metabolites. The results indicated that the culture broth of Rhizobium sp. exhibited an in vitro growth-stimulating effect. Furthermore, ten secondary metabolites were identified in the ethyl acetate extract (p-coumaric acid, indole-3-lactic acid, naringenin, and siderophores B and C, among others). These findings highlight the bioactive metabolites produced by Rhizobium sp., a bacterial strain of the INIFAT collection, which have a positive effect as growth promoters in plants. They reveal the potential of Rhizobium sp. as a promising candidate for inclusion in agricultural management practices.
1. Introduction
In Cuba, the consumption of common beans (Phaseolus vulgaris L.) has a long-standing tradition and they are in high demand, playing a fundamental role in the population’s diet as an alternative source of proteins and minerals. However, the production of these beans remains insufficient [1,2]. Accordingly, one of the priorities of agriculture today is to increase the production of this crop using environmentally friendly technologies, including the use of bioproducts such as biostimulants [3]. Their use is gaining significant attention from scientists and producers as an alternative to reduce the application of chemical fertilizers and mitigate the environmental pollution they cause [1,4]. In this context, plant growth-promoting bacteria (PGPBs) have emerged as an important and promising tool for sustainable agriculture. They activate, in interaction with plants, different mechanisms that include, in addition to biological control of phytopathogenic organisms, biological atmospheric nitrogen fixation, nutrient solubilization, and phytohormone production [5,6]. PGPBs represent about 2 to 5% of the rhizospheric bacteria, including genera such as Agrobacterium, Arthrobacter, Azotobacter, Bacillus, Enterobacter, Flavobacterium, Klebsiella, Pseudomonas, Rhizobium, and Serratia, among others. Rhizobacteria associate symbiotically with leguminous plants, creating root nodules in which biological nitrogen formation takes place. Different factors can affect nodulation, including soil conditions and increased temperature, but some rhizobium strains can establish efficient symbiosis under these conditions. For instance, Rivera et al. discovered two Rhizobium genotypes, identified as Rhizobium hidalgonense and R. redcepovicci, in association with their host legumes in different cultivation areas in Mexico [7]. The first one was found in slightly acidic soil (pH = 6.5) while the latter was found both in acidic and neutral soil. Emisha et al. (2024) studied the effect of the NPS rate and Rhizobium inoculation on the yield and yield components of Phaseolus vulgari L. in Ethiopia and found a poor effect of the Rhizobium strain on most agronomic parameters, which might have resulted from either the low adaptability of the strain or low soil acidity of the experimental locations [8]. Co-inoculation of growth-promoting rhizobacteria in legumes is a common, ecofriendly, and highly relevant practice nowadays. Studies on the application of Pseudomonas sp. with Rhizobium leguminosarum have shown positive effects on Lens culinaris, Cajanus cajan, and Phaseolus vulgaris, with PGPBs enhancing plant growth, nutrient uptake, nodulation, and crop yield in all cases [9]. Horácio et al. (2020) demonstrated that inoculating rhizobia with Azospirillium and cyanobacteria is recommended, as an agricultural practice, to supply the common bean with sufficient nitrogen when cultivated in low-nitrogen soils [10]. Similarly, De Oliveira et al. (2024) found that co-inoculation with Rhizobium, Azospirillum, and microalgae increases the common bean yield and profitability in both sandy and clayey soils [11]. The PGPBs produce metabolites with a broad spectrum of bioactivity [12,13].
While the beneficial effects of metabolites produced by PGPBs are well-documented in the literature, research on the production of these compounds by rhizobia remains limited. The objective of the present work is to determine the growth-promoting activity of a culture broth of Gram-negative soil bacteria Rhizobium sp., conserved in the Collection of Beneficial Bacteria at the Institute of Fundamental Research in Tropical Agriculture (INIFAT), and to identify, for the first time, the main secondary metabolites present in the ethyl acetate crude extract using ultra-high-resolution liquid chromatography coupled with tandem mass spectrometry (UHPLC-ESI-MS/MS).
2. Materials and Methods
2.1. Bacterial Strain and Growth Media and Conditions
Rhizobium sp. strain was used in this study. The registration number and the origin are shown in Table 1.

Table 1.
Rhizobium sp. (F7) conserved in the INIFAT Collection of Beneficial Bacteria (ICBB).
2.1.1. Revitalization of Bacterial Strains
This work was conducted at the Bacteria Laboratory of INIFAT. Vials preserved in glycerol at −20 °C were used to revitalize the bacterial strain. The microorganism was seeded by depletion in 90 mm Petri dishes with YMA culture media [14]. The pure colonies of the strain were then used as an inoculum for the fermentative process.
2.1.2. Bacterial Strain Fermentation
Rhizobium strains were inoculated in liquid YMA culture media and incubated for 72 h at 28 to 30 °C in a rotating sieve at 150 r.p.m. until microbial growth was complete. The microbial concentration (3 × 1010 UFCmL−1) was measured using the McFarland scale. The pH of the fermented broth (pH = 6.3) was determined with a HANNA potentiometer HI6221-01 (Woonsocket, RI, USA).
2.1.3. In Vitro Growth-Stimulating Effect of Bacterial Strains
The experiments were conducted at the Chemistry Laboratory of INIFAT. For the tests, common bean seeds (Phaseolus vulgaris L.) of the commercial variety ’Caujerí 2170’ (black bean), obtained from INIFAT according to the Catalogue of Varieties 2014 [15] and the Official List of Commercial Varieties 2016, were used.
Sterilized petri dishes of 150 mm diameter were prepared by autoclaving for one hour at 121 °C and 1.5 atm. pressure. The plates were lined with two filter paper discs moistened with sterile distilled water before placing the seeds.
For the application of the microbial broths and solutions, the seeds (previously disinfested with 2% sodium hypochlorite) were soaked for 15 min and then aerated for 30 min, following the methodology described by Rodríguez et al. (2010) [16]. Subsequently, the seeds were placed in sterile Petri dishes (20 seeds per dish), placed uncovered inside transparent nylon bags and subjected to alternating light conditions (16/8 h) at 25 °C [17]. Four replicates per treatment and controls were used.
Seed germination was evaluated from 24 to 168 h after the trials began (NSG). At 96 h (4th day), the number of true leaves was determined. At the end of the experiment (168 h), growth parameters were measured: root length (cm), stem length (cm), number of leaves (u), and plant fresh and dry weights (g) [18]. The evaluation days were determined based on a previous growth dynamics study carried out at the INIFAT Seed Laboratory. Positive control: Dimargón; negative control: yeast mannitol agar.
Data were statistically processed by analysis of variance with a completely randomized design, and the significance of differences was determined by Duncan’s multiple range test (p ≤ 0.05) [19], using the INFOSTAT statistical program. Homogeneity and normality of variances were tested using the Kolmogorov–Smirnov, Cochran C, Hartley, and Bartlett tests.
2.2. Ethyl Acetate Crude Extract
The pH of the supernatants was adjusted to 2.0 using H3PO4 20% (v/v) and then extracted with ethyl acetate (3 × 25 mL). The organic phase was dried under a vacuum to yield 112 mg of the crude metabolite.
2.3. Sample Clean-Up
A 2 mL solution of the extract (1 mg/mL) in MeOH/H2O 8:2 (v/v) was subjected to solid-phase extraction using Chromabond® C18 SPE cartridges (loading 200 mg/3 mL, particle size 54 μm, Macherey-Nagel, Düren, Germany) and eluted with MeOH/H2O 8:2 (v/v). After drying, 1 mg was dissolved in 1 mL of MeOH/H2O 8:2 (v/v) solution (solution A), and an aliquot (10 μL) was diluted with MeOH/H2O 8:2 (v/v) up to a final volume of 1 mL, then filtered through a 0.22 μm nylon membrane filter. The solution (150 μL) was further diluted with MeOH/H2O 8:2 (v/v) up to a final concentration of 1 ppm and submitted to UHPLC-ESI-HRMS/MS.
2.4. UHPLC-ESI-HRMS/MS Conditions and Data Analysis
The negative-ion high-resolution ESI mass spectra were obtained using an Orbitrap Elite mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with a heated ESI electrospray ion source (spray voltage negative-ion mode 3.5 kV; source heater temperature 150 °C; capillary temperature 325 °C; FTMS resolution 30,000). Nitrogen was used as sheath and auxiliary gas. The MS system was coupled online to an ultra-high-performance liquid chromatography (UHPLC) system (Dionex UltiMate 3000, Thermo Fisher Scientific), equipped with an RP C18 column (1.8 µm; 100 × 1.0 mm; ACQUITY UPLC HSS T3 C18; Waters, column temperature 45 °C) and a photodiode array detector (PDA, Thermo Fisher Scientific). For the UHPLC, a gradient system was used starting from H2O (A; Milli-Q, Merck Millipore):CH3CN (B; Chormasolv LC-MS, Riedel-de-Haen, Honeywell) 95:5 (each of them containing 0.1% (v/v) formic acid (eluent additive for LC-MS, Honeywell Fluka), isocratic for 1 min) raised to 50:50 within 3 min, and in a further 10 min, to 10:90, then finally, to 5:95 within 1 min, followed by holding at 5:95 for a further 3 min, with a flow rate of 150 μL min−1. The wavelength range of the PDA measurements used for detection was 190–400 nm. The CID tandem mass spectra (buffer gas: helium; FTMS resolution: 15,000) were recorded in a data-dependent acquisition mode (DDA) using a normalized collision energy (NCE) of 35%. The instrument was externally calibrated by the Pierce® LTQ Velos ESI negative-ion calibration solution (product number 88324, Thermofisher Scientific, Rockford, IL 61105, USA). The data were evaluated by the Xcalibur software 2.2 SP1 (Thermo Fisher Scientific).
3. Results
3.1. Determination of In Vitro Growth-Stimulating Effect of Bacterial Strains
The effect of Rhizobium sp. (F7) strain culture broth on the growth parameters of cv. Caujerí beans was analyzed. Figure presents the results of the plant growth-stimulating activity after applying the sample. In all cases, the culture broth significantly increased the evaluated parameters compared to the negative control (YMA). Notably, significant differences in root length and the number of leaves were observed compared to the positive control (Dimargón) (Figure 1A,C). Additionally, stem length showed statistically higher values than the negative control.
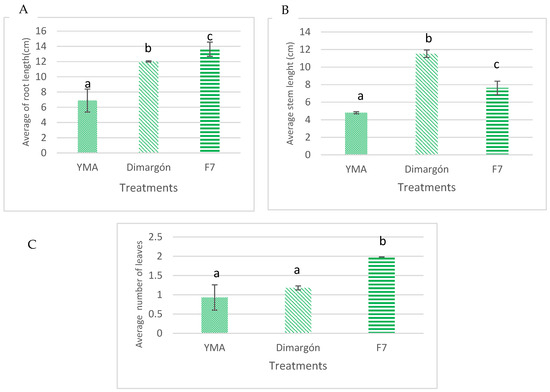
Figure 1.
In vitro plant growth-promoting activity of Rhizobium sp. culture broth. (A) Average root length; (B) average stem length; (C) average number of leaves. Negative control YMA, positive control commercial Dimargón. Different letters indicate significant differences (Duncan’s multiple range test (p ≤ 0.05)).
3.2. UHPLC-ESI-HRMS/MS Analysis
Figure 2 shows the typical total ion chromatogram (TIC) of ethyl acetate extract from Rhizobium sp. in the negative ion mode. The chromatogram reveals several peaks corresponding to compounds eluted under the present chromatographic conditions, reflecting their varying polarities.

Figure 2.
Total ion chromatogram (TIC) of ethyl acetate crude extract of Rhizobium sp. (F7).
The total ion spectrum in the extended m/z 140–400 region is shown in Figure 3. It represents the relative abundance of the different ions as a function of their mass-to-charge ratio (m/z). In the negative ion mode, the metabolites exhibited deprotonated [M−H]− molecular ions, some of which were selected as precursor ions for the MS2 spectra (Figure 3).

Figure 3.
Total ion spectrum of ethyl acetate crude from Rhizobium sp. in the extended region of m/z 140–400.
In this region, the presence of phenolic acids, indolic acid, flavonoids, and siderophores was detected, as shown in Table 2.

Table 2.
Secondary metabolites identified in ethyl acetate crude extract of Rhizobium sp.
4. Discussion
Rhizobium spp. are Gram-negative soil bacteria best known for their symbiotic relationship with legumes. These bacteria are typically found in the soil and participate in nodule formation after infecting the roots of leguminous plants. Thus, they help in the fixation of nitrogen from the atmosphere and play an important role in the growth and development of plants.
The most specific interaction known in the world is that of beans and other legume species with bacteria of the genus Rhizobium and other related genera. A mutualistic relationship develops between these plants and the bacteria. As a consequence of this, the growth and yield increase in beans [22]. In addition, it is possible to reduce the application of mineral fertilizers by up to 30% during the crop cycle. The plant growth-stimulating activity observed in this research aligns with findings by Valero et al. in 2021 [23], who reported an increased root length in Vigna unguiculata L. inoculated with Rhizobium sp. These results also corroborate the study by Castillo et al. (2016) [24], which showed a positive effect on the number of leaves in bean plants (var. butter) using a Rhizobium miluonense strain under controlled and field conditions. Similar outcomes were recently reported by Ortiz et al. (2021), who evaluated the in vitro growth-stimulating effect of 10 bacterial strains, including Rhizobium spp. (F7) on common bean (Phaseolus vulgaris L. cv. ‘Lewa’) seeds. Among the Rhizobium strains, F7 showed the highest stem and root lengths [25].
The plants form nodules, structures where the bacteria spend most of their life cycle. They also provide root exudates, which serve as an energy source for these microorganisms. In turn, the bacteria fix and supply up to 90% of the nitrogen the plants consume, in the form of ammonium compounds and a wide range of physiologically active substances and phytohormones involved in all phenological phases of the plants [26].
To identify the secondary metabolites produced by the Cuban Rhizobium strain that could enhance plant growth, such as antioxidants, as well as molecules that contribute to the fitness of the plant against pathogens and abiotic stress, ethyl acetate solvent was selected. This research detected the presence of phenolic acids, an indole acid, flavonoids, and siderophores.
Phenolic acids play a crucial role as signaling molecules in plant–microbe interactions, acting as node gene inducers of legume–rhizobia symbioses [27]. The antioxidant activity of phenolic acids and flavonoids is well-documented in the scientific literature. Oxidative stress is a major factor affecting the proper development of crops. Plants that invest energy in defense mechanisms face issues with nutrient absorption and metabolism, significantly reducing their growth potential [28]. In the present investigation, different phenolic acids were identified using the UHPLC-ESI-MS/MS analytical technique. Pseudomolecular ions [M−H]− at m/z 147.0358, 163.0330, 165.0609, and 181.0508 were assigned to cinnamic, p-coumaric, dihydro p-coumaric, and dihydrocaffeic acids, respectively. Zhao et al. (2018) showed that cinnamic acid inhibited the growth of faba beans and increased the incidence of Fusarium Wilt [29]. On the other hand, Wang et al. (2019) demonstrated that this phenolic acid has the potential to be used as an alternative to commercial fungicides in treating Sclerotinia sclerotiorum. The authors showed that this secondary metabolite inhibited the mycelial growth and development of the highly destructive phytopathogen, which affects soybean, among other plants [30].
Auxin production, particularly that of 3-indolacetic acid (IAA), is the most extensively analyzed mechanism in PGPBs. Other indole compounds and related metabolites, such as indole pyruvic acid, indole lactic acid, indole acetamide acid, indole acetaldehyde acid, and other unidentified non-indole compounds, have been identified but are less studied [31]. Scientific research shows that anabolic pathways in the presence of tryptophan significantly increase AIA production [32,33]. In this investigation, the absence of AIA was noted, which can be attributed to the lack of an inducer in the fermentation medium. Several scientific studies have demonstrated the importance of L-tryptophan in promoting the production of this auxin [34].
The presence of indole-3-lactic acid was detected in this research and corroborated by the pseudomolecular ion at m/z 204.0669, corresponding to [M−H]−. Second-order mass spectra analysis revealed fragment ions at m/z 186, 158, 130, and 116 corresponding to H2O, HCOO−, and side chain losses, respectively. The fragmentation scheme (Scheme 1) shows the structures proposed to identify the fragment ions that respond to the data found in the spectrum. This analysis takes into account the fragments of negative-mode 3-substituted indole compounds proposed by Kyriaki et al. in 2019 for indole acids and other derivatives [35].

Scheme 1.
Proposed fragmentation pattern for indole-3-lactic acid in the negative ionization mode.
Furthermore, Imada et al. [30] identified this auxin through the analysis of indolic compounds produced by R. tropici CIAT 899 using the UPLC-MS technique. The authors detected this bioactive metabolite in the supernatant across all growth conditions studied.
The ion at m/z 253.0509 was identified as the deprotonated molecule of flavone chrysin. This compound was characterized based on the fragmentation pattern of the second-order spectrum. The structure was confirmed by the losses of CO and CO2. The absence of hydroxyl groups on the B-ring suggests that CO2 loss can only occur on ring A (Scheme 2). Furthermore, the fragmentation pattern was compared with the data reported in the literature [36]. Recently, Yuce et al. (2024) studied the exogenous application of chrysin in pepper under copper stress conditions. They evaluated its effects on the morphological, biochemical, and enzymatic responses during the early stage of plant growth. Their findings demonstrated that chrysin exhibits strong antioxidant and chelating properties under heavy metal stress conditions by regulating antioxidant enzymes’ activity, plant nutrients, and phytohormones’ content in pepper [37].
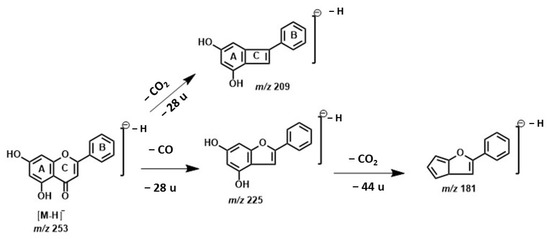
Scheme 2.
Proposed fragmentation of the pseudomolecular ion of chrysin at m/z 253.0509 [M−H]−.
Additionally, the pseudomolecular ion at m/z 269.0459 [M−H]− was identified as the isoflavone genistein. This compound is the precursor to the phytoalexin kievitone produced by Phaseolus vulgaris [38] and it is also involved in the pathway leading to the glyceollin response of soybean cells [39,40]. Genistein plays a crucial role in specialized plant physiological and metabolic functions, as well as plant responses to various stresses, such as changes in light or temperature, competition, herbivore pressure, and pathogenic attacks. It also participates in the initiation of symbiosis between legumes and mycorrhizal fungi or nitrogen-fixing bacteria as a signaling molecule [41,42]. In the study conducted by Rivera et al., the antifungal activity of genistein was demonstrated, showing inhibitory effects at concentrations of 60–120 μM and a fungicidal effect at 240 μM [43].
The peak at m/z 271.1666 [M−H]− revealed the presence of flavanone naringenin, which Singh et al. (2018) identified among the secondary metabolites of Rhizobium leguminusarum bv. viciae [44]. Yildiztugay et al., in 2020, demonstrated that the exogenous application of naringenin protected chloroplasts of Phaseolus vulgaris by minimizing disturbances caused by NaCl or PEG exposure via the AsA or GSH redox-based systems and POX activity [45]. Furthermore, naringenin from soybean exhibits antifungal effects against Phytophthora sojae [43].
Peaks at m/z 749.3759 [M−H]− and 773.3841 [M−H]− were tentatively assigned as siderophore B and C, respectively. Several investigations have shown that the production of siderophores by PGPB bacteria constitutes one of the growth-promoting characteristics of this group, due to their capacity to stimulate plant development through different mechanisms [46]. In this study, siderophores B and C were identified with the findings of Wright et al. (2013) in Rhizobium leguminusarum ATCC 14479 bv. trifolii [20]. Siderophores play a crucial role as iron-chelating agents, facilitating essential plant functions such as DNA synthesis and photosynthesis. Bacterial siderophores can improve the plant nutritional status by releasing ferric ions (Fe3+) from the siderophore complex, which plants can then utilize for growth in iron-deficient environments. Additionally, siderophores indirectly promote plant growth by mitigating damage from phytopathogenic microorganisms. They achieve this by either decreasing the amount of iron available for their development or by triggering plant defense mechanisms [47].
In Figure 4, the structures of the flavonoids and lipopeptides identified are shown, with pseudomolecular ions [M−H]− detected at m/z 269, 271, 749, and 773, respectively.

Figure 4.
Structures of (A) genistein, (B) naringenin, (C) siderophore B, and (D) siderophore C.
5. Conclusions
Different studies have demonstrated the symbiotic relationship between bacteria of the genus Rhizobium and the host plants, as well as the secondary metabolites that these bacteria activate in the plant when they interact with the crop’s roots. However, there is limited research on the secondary metabolites produced by Rhizobium species when fermented in vitro. This study provides evidence of the growth-promoting activity of Rhizobium sp. (F7) on Phaseolus vulgaris L seeds and, to the best of our knowledge, constitutes the first report identifying secondary metabolites produced by the bacterial strain F7 of the INIFAT bacterial collection using UHPLC-ESI-MS/MS.
Author Contributions
Conceptualization, G.H., Y.R., T.H.G., Y.L., I.S. and Y.O.; methodology, G.H., Y.R., I.S. and Y.O.; software, Y.R. and G.H.; validation, Y.R., T.H.G., Y.L., I.S. and Y.O.; formal analysis, G.H., Y.R., T.H.G., Y.L., I.S. and Y.O.; investigation, G.H., Y.R., T.H.G., Y.L., I.S. and Y.O.; resources, Y.O.; data curation, G.H., Y.R., T.H.G., Y.L., I.S. and Y.O.; writing—original draft preparation, G.H.; writing—review and editing, Y.R., T.H.G., I.S. and Y.O.; supervision, Y.O. and I.S.; project administration, Y.O.; funding acquisition, Y.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the research project “Prospección de microorganismos estimuladores del crecimiento vegetal para aumentar la producción de Phaseolus vulgaris L.”, financed by the Cuban Ministry of Agriculture, under research grant number PN131LH001.40, and International Project “Mejoramiento de la resiliencia y adaptación al cambio climático en Guantánamo, Cuba”, under research grant number 01.20.98.20.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank the Cuban Program: Food Production and Agroindustry for its administrative support, the Italian Agency for Development Cooperation (AICS) for its financial support to carry out this research, and Nadia Bergamini, an Alliance of Bioversity International and CIAT expert, for her English correction.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Calero, A.H.; Quintero, E.R.; Olivera, D.V.; Pérez, Y.D.; Castro, I.L.; Jiménez, J.; López, E.D. Respuesta de dos cultivares de frijol común a la aplicación foliar de microorganismos eficientes. Cultiv. Trop. 2018, 39, 5–10. [Google Scholar]
- Oficina Nacional de Estadística. Anuario Estadístico de Guantánamo 2020. Edición 2021. 265p. Available online: www.onei.gob.cu (accessed on 15 December 2024).
- Quintero, R.E.; Calero, H.A.; Pérez, D.Y.; Enríquez, G.L. Efecto de diferentes bioestimulantes en el rendimiento del frijol común. Rev. Cent. Agrícola 2018, 45, 73–80. [Google Scholar]
- Chen, S.; Chen, D.; Cai, R.; Cui, H.; Long, Y.; Lu, Y.; Li, C.; She, Z. Cytotoxic and Antibacterial Preussomerins from the Mangrove Endophytic Fungus Lasiodiplodia theobromae. ZJ-HQ1. J. Nat. Prod. 2016, 79, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Palacio, R.R.; Ramos, B.P.; Coria-Arellano, J.L.; Nava, R.B.; Sáenz-Mata, J. Mecanismos de las PGPR para mitigar el estrés abiótico de plantas. Árido-Ciencia 2016, 1, 4–11. [Google Scholar]
- Rojas-Badía, M.M.; Bello-González, M.A.; Ríos-Rocajull, Y.; Lugo-Moya, D.; Rodríguez-Sánchez, J. Utilización de cepas de Bacillus como promotores de crecimiento en hortalizas comerciales. Acta Agronómica 2020, 69, 54–60. [Google Scholar] [CrossRef]
- Rivera Ortuña, F.N.; Guevara-Luna, J.; Yan, J.; Lopez Amezcua, E.; Arroyo-Herrera, I.; Li, Y.; Vásquez-Murrieta, M.S.; Rojas Arellano, D.; Wang, E.T. Rhizobium hidalgonense and Rhizobium redzepovicii as faba bean (Vicia faba L.) microsymbionts in Mexican soils. Arch. Microbiol. 2024, 206, 281. [Google Scholar] [CrossRef]
- Emisha, L.; Abera, D.; Fayisa, H. Effect of NPS Rate and Rhizobium Inoculation on Yield and Yield Components of Common Bean (Phaseolus vulgari L.) at Kellem Wollega Zone, Western Oromia, Ethiopia. Adv. Biosci. Bioeng. 2024, 12, 81–92. [Google Scholar] [CrossRef]
- Granda-Mora, K.; Correa-Ullauri, C.; Collahuazo-Reinoso, Y.; Robles-Carrión, Á. Inoculantes microbianos comerciales con PGPR sobre variables productivas y económicas de fríjol común (Phaseolus vulgaris L.). Agron. Mesoam. 2024, 35, 55654. [Google Scholar] [CrossRef]
- Horácio, E.H.; Zucareli, C.; Gavilanes, F.Z.; Yunes, J.S.; Sanzovo, A.W.; Andrade, D.S. Co-inoculation of rhizobia, azospirilla and cyanobacteria for increasing common bean production. Semin.-Cienc. Agrar. 2020, 41, 2015–2028. [Google Scholar] [CrossRef]
- De Oliveira, K.S.; Telles, T.S.; Andrade, D.S.; Yunes, J.S.; Mendes, A.D.R.; Volsi, B. Co-inoculation with Rhizobium, Azospirillum, and microalgae increases common bean yield and profitability. Agron. J. 2024, 117, e21719. [Google Scholar] [CrossRef]
- Jha, C.K.; Saraf, M. Plant growth promoting Rhizobacteria (PGPR): A review. J. Agric. Res. Dev. 2015, 5, 108–119. [Google Scholar]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Martínez, V.R.; López, M.; Brossard, F.M.; Tejeda, G.G.; Pereira, A.H.; Parra, Z.C.; Rodríguez, S.J.; Alba, A. Procedimientos Para el Estudio y Fabricación de Biofertilizantes Bacterianos; INIA: Maracay, Venezuela, 2006. [Google Scholar]
- Fernández, G.L.; Shagarodsky, S.T.; Cristóbal, S.R.; Muñoz de Con, L.; Gil, V.J.F.; Sánchez, R.Y.; González, C.D.M.; Moreno, F.V.; Fundora, M.Z.M.; Castiñeira, A.L.; et al. Catálogo de Variedades; INIFAT: Havana, Cuba, 2014; 165p. [Google Scholar]
- Rodríguez, S.J. Formulación de un Bioproducto Mixto a Partir de Azotobacter chroococcum y Bacillus subtilis Para el Tratamiento de Semillas de Tomate (Solanum lycopersicum L.). Doctoral Dissertation, University of Havana, Havana, Cuba, 2010. [Google Scholar]
- International Seed Testing Association (ISTA). International Rules for Seed Testing. Seed Sci. Technol. 1999, 27, 25–30. [Google Scholar]
- Moeinzadeh, A.; Sharif-Zahed, F.; Ahmadzadeh, M.; Heidari, F. Biopriming of sunflower (Helianthus annuus L.) seed with Pseudomonas fluorescens for improvement of seed invigoration and seedling growth. Aust. J. Crop Sci. 2010, 4, 564–570. [Google Scholar]
- Lerch, G. La Experimentación en las Ciencias Biológicas y Agrícolas; Editorial Científico-Técnico: Havana, Cuba, 1987. [Google Scholar]
- Wright, W.; Little, J.; Liu, F.; Chakraborty, R. Isolation and structural identification of the trihydroxamate siderophore vicibactin and its degradative products from Rhizobium leguminosarum ATCC 14479 bv. trifolii. Biometals 2013, 26, 271–283. [Google Scholar] [CrossRef]
- Pluhácek, T.; Lemr, K.; Ghosh, D.; David Milde, D.; Novák, J.; Havlícek, V. Characterization of microbial siderophores by mass spectrometry. Mass Spectrom. Rev. 2016, 35, 35–47. [Google Scholar] [CrossRef]
- Sendi, Y.; Pfeiffer, T.; Koch, E.; Mhadhbi, H.; Mrabet, M. Potential of common bean (Phaseolus vulgaris L.) root microbiome in the biocontrol of root rot disease and traits of performance. J. Plant Dis. Prot. 2020, 4, 453–462. [Google Scholar] [CrossRef]
- Valero-Valero, N.O.; Vergel-Castro, C.M.; Ustate, Y.; Gómez-Gómez, L.C. Bioestimulación de frijol guajiro y su simbiosis con Rhizobium por ácidos húmicos y Bacillus mycoides. Rev. Biotecnol. Sect. Agropecu. Agroindustrial 2021, 19, 119–134. [Google Scholar]
- Castillo, A.C. Evaluación de Rizobacterias Promotoras del Crecimiento Vegetal en Frijol (Phaseolus vulgaris L.) var. Mantequilla. Bachelor’s Thesis, Universidad Nacional de Loja, Loja, Ecuador, 2016. [Google Scholar]
- Ortiz, Y.; Ríos, Y.; Aguado, Y.; Rodríguez, L.C.; Lorenzo, Y.; Deliz, L.; Álvarez, M.E.; Rodríguez, J.; Zulueta, I.; Fresneda, J.A. Selección de cepas bacterianas con potencial estimulador del crecimiento vegetal en Phaseolus vulgaris L. (CV. ‘LEWA’). Agrotec. Cuba 2021, 45, 42–58. [Google Scholar]
- Vega-Celedón, P.; Canchignia Martínez, H.; González, M.; Seeger, M. Biosynthesis of indole-3-acetic acid and plant growth promoting by bacteria. Cultiv. Trop. 2016, 37, 33–39. [Google Scholar]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Vicent, A.; Sancho Ortega, J.; Baigorri, R.; San-Francisco, S. El efecto de los antioxidantes sobre el estrés oxidativo en los cultivos. Phytoma España 2019, 314, 76–79. [Google Scholar]
- Zhao, Q.; Chen, L.; Dong, K.; Dong, Y.; Xiao, J. Cinnamic Acid Inhibited Growth of Faba Bean and Promoted the Incidence of Fusarium Wilt. Plants 2018, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, L.-Y.; Cao, J.; Li, Y.-L.; Ding, L.-N.; Zhu, K.-M.; Yang, Y.-H.; Tan, X.-L. Recent Advances in Mechanisms of Plant Defense to Sclerotinia sclerotiorum. Front. Plant Sci. 2019, 10, 1314. [Google Scholar] [CrossRef]
- Imada, E.L.; de Paiva Rolla dos Santos, A.A.; De Oliveira, A.L.M.; Hungria, M.; Rodrigues, E.P. Indole-3-acetic acid production via the indole-3-pyruvate pathway by plant growth promoter Rhizobium tropici CIAT 899 is strongly inhibited by ammonium. Res. Microbiol. 2017, 168, 283–292. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Zhang, L.; Mu, J.; Jiang, Y.; Fu, H.; Zhang, Y.; Cui, H.; Yu, X.; Ye, Z. Biosynthetic Pathways and Functions of Indole-3-Acetic Acid in Microorganisms. Microorganisms 2023, 11, 2077. [Google Scholar] [CrossRef]
- Gilbert, S.; Xu, J.; Acosta, K.; Poulev, A.; Lebeis, S.; Lam, E. Bacterial Production of Indole Related Compounds Reveals Their Role in Association Between Duckweeds and Endophytes. Front. Chem. 2018, 6, 265. [Google Scholar] [CrossRef]
- Goud, M.S.; Sharma, S.K.; Kharbikar, L.L.; Prasanna, R.; Sangwan, S.; Dahuja, A.; Dixit, A. Bacillus species consortium with tryptophan-dependent and independent pathways mediated production of IAA and its derivatives modulates soil biological properties, growth and yield of wheat. Plant Soil 2024, 1–27. [Google Scholar] [CrossRef]
- Revelou, P.K.; Kokotou, M.; Constantinou-Kokotou, V. Identification of Auxin Metabolites in Brassicaceae by Ultra-Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry. Molecules 2019, 24, 2615. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of Flavone, Flavonol, and Flavanone Aglycones by Negative Ion Liquid Chromatography Electrospray Ion Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Yuce, M.; Ekinci, M.; Turan, M.; Agar, G.; Aydin, M.; Ilhan, E.; Yildirim, E. Chrysin mitigates copper stress by regulating antioxidant enzymes activity, plant nutrient and phytohormones content in pepper. Sci. Hortic. 2024, 328, 112887. [Google Scholar] [CrossRef]
- Garcia-Arenal, F.; Fraile, A.; Sagasta, E.M. The multiple phytoalexin response of bean (Phaseolus vulgaris) to infection by Botrytis cinerea. Physiol. Plant Path. 1978, 13, 151–156. [Google Scholar] [CrossRef]
- Graham, T.L. Flavonoid and flavonol glycoside metabolism in Arabidopsis. Plant Physiol. Biochem. 1998, 31, 135–144. [Google Scholar] [CrossRef]
- Graham, T.L.; Graham, M.Y.; Stacey, G.; Keen, N. Defense potentiation and elicitation competency redox conditioning. Plant Microbe Interact. 2000, 5, 181–220. [Google Scholar]
- Berhow, M.A. Flavonoid accumulation in tissue and cell culture: Studies in citrus and other plant species. In Flavonoids in the Living System; Manthey, J.A., Buslig, B.S., Eds.; Springer: Boston, MA, USA, 1998; pp. 67–84. [Google Scholar]
- Morkunas, I.; Ratajczak, L. Biosynthesis and physiological activity of genistein in plants. In Recent Progress in Medicinal Plants. Flavonoids and Antioxidants Vol 43—Phytotherapeutics; Pathak, M., Govil, J.N., Eds.; Studium Press LLC: Houston, TX, USA, 2016. [Google Scholar]
- Rivera-Vargas, L.I.; Schmitthenner, A.F.; Graham, T.L. Soybean flavonoid effects on and metabolism by Phytophthora sojae. Phytochemistry 1993, 32, 851–857. [Google Scholar]
- Singh, H.B.; Keswani, C.; Reddy, M.S.; Sansinenea, E.; García-Estrada, C. Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms: Discovery and Applications; Springer Nature: Singapore, 2019. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M.; Turkan, I. Flavonoid Naringenin Alleviates Short-Term Osmotic and Salinity Stresses Through Regulating Photosynthetic Machinery and Chloroplastic Antioxidant Metabolism in Phaseolus vulgaris. Front. Plant Sci. 2020, 11, 682. [Google Scholar] [CrossRef]
- Khader, B.; Agsar, D.; Mahadevaswamy, D.; Reshma, R. Isolation, characterization and screening of Rhizobium from leguminous plant. Pharma Innov. J. 2023, 12, 1581–1591. Available online: https://www.thepharmajournal.com/archives/2023/vol12issue11/PartS/12-10-167-110.pdf (accessed on 1 February 2025).
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Bacterial Siderophores: Classification, Biosynthesis, Perspectives of Use in Agriculture. Plants 2022, 11, 3065. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).