Phytotoxicity of Two Bauhinia Species on Four Triticum aestivum Varieties in Laboratory Bioassay
Abstract
1. Introduction
2. Materials and Methods
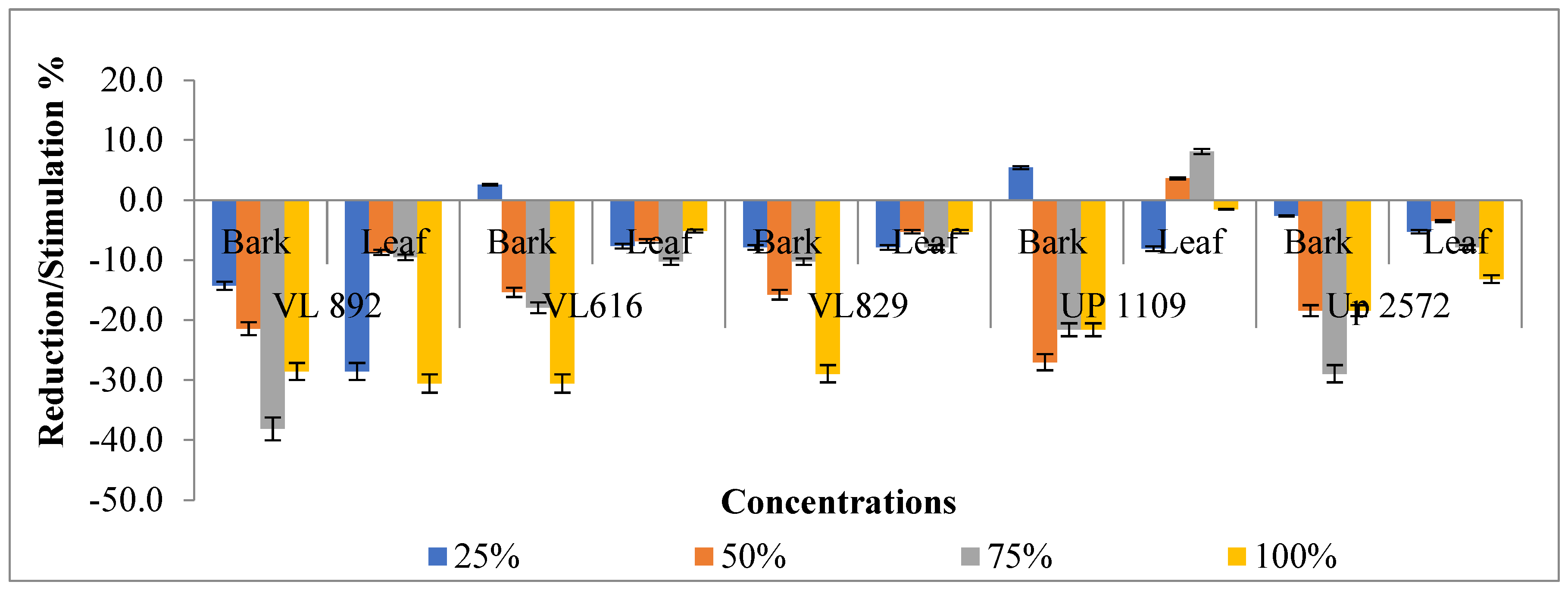
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagar, A.; Khanduri, V.P.; Singh, B.; Riyal, M.K.; Singh, I. Altitudinal variation in morphometric traits of pod, seed, and seedling growth of Bauhinia variegata L. in Garhwal Himalaya. Ann. Silvic. Res. 2022, 47, 84–95. [Google Scholar] [CrossRef]
- Yadav, N.; Singh, B.; Khanduri, V.P. Seasonal variation in nutrient composition in the leaves of two Bauhinia species. Folia For. Polonica. Ser. A For. 2023, 65, 173–178. [Google Scholar] [CrossRef]
- Yadav, N.; Khanduri, V.P.; Singh, B.; Dhanai, C.S.; Riyal, M.K.; Rawat, D.; Ahmad, T.; Kumar, M. Effect of temperature, seed size, sowing depth, and position on seed germination and seedling growth of Bauhinia retusa Roxb. and Bauhinia variegata L. Forest 2023, 14, 1664. [Google Scholar] [CrossRef]
- Coder, K.D. Allelopathy in Trees and Forests: A Selected Bibliography; University of Georgia: Athens, GA, USA, 1999; p. 7. [Google Scholar]
- Putnam, A.R.; Tang, C.S. The Science of Allelopathy; John Wiley: NewYork, NY, USA, 1986; pp. 1–22. [Google Scholar]
- Al-Snafi, A.E. The pharmaceutical importance of Althaea officinalis and Althaea rosea: A review. Int. J. Pharm. Tech. Res. 2013, 5, 1378–1385. [Google Scholar]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and Allelochemicals of Leucaena leucocephala as an Invasive Plant Species. Plants 2022, 11, 1672. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.R.; Khnaduri, V.P.; Singh, B.; Riyal, M.K.; Kumar, S.; Kumar, P.; Rawat, D. Allelopathic potential of Ficus auriculata and Ficus semicordata on the growth of four traditional food crops of Garhwal Himalaya. J. Agric. Food Res. 2022, 9, 100352. [Google Scholar]
- Nakano, H.; Fujii, Y.; Yamada, K.; Kosemura, S.; Yamamura, S.; Hasegawa, K.; Suzuki, T. Isolation and identification of plant growth inhibitors as candidate(s) for allelopathic substance(s), from aqueous leachate from mesquite (Prosopis juliflora (Sw.) DC.) leaves. Plant Growth Regul. 2002, 37, 113–117. [Google Scholar]
- Nakano, H.; Nakajima, E.; Fujii, Y.; Yamada, K.; Shigemori, H.; Hasegawa, K. Leaching of the allelopathic substance,-tryptophan from the foliage of mesquite (Prosopis juliflora (Sw.) DC.) plants by water spraying. Plant Growth Regul. 2003, 40, 49–52. [Google Scholar] [CrossRef]
- Inderjit; Seastedt, T.R.; Callaway, R.M.; Pollock, J.L.; Kaur, J. Allelopathy and plant invasions: Traditional, congeneric, and bio-geographical approaches. Biol. Invasions 2008, 10, 875–890. [Google Scholar] [CrossRef]
- Hemada, M.; Youssef, R.; El-Darier, S. Allelopathic potential of Eucalyptus litter on some ecophysiological parameters of Vicia faba L. seedlings. El-Minia Sci. Bull. 2004, 15, 411–427. [Google Scholar]
- Abou-Zeid, H.M.; EL-Darier, S.M. Allelotoxic activity of Eucalyptus rostrata Schlecht. On seed germination and seedling growth of Chenopodium album L. and Portulaca oleracea L. Int. J. Agron. Agric. Res. 2014, 4, 39–50. [Google Scholar]
- Padu, K.; Khanduri, V.P.; Singh, B.; Rawat, D.; Riyal, M.K.; Kumar, K.S. Phytotoxicity of common weeds on germination, seedling growth, NPK uptake and chlorophyll content of four hill crops of Garhwal Himalaya. J. Agric. Food Res. 2023, 12, 100539. [Google Scholar] [CrossRef]
- Amoo, I.A.; Adebayo, O.T.; Oyeleye, A.O. Chemical evaluation of winged beans (Psophocarpus tetragonolobus), Pitanga Cherries (Eugenia uniflora) and orchid fruit (Orchid fruit myristica). Afr. J. Food Agric. Nutr. Dev. 2006, 6, 2. [Google Scholar] [CrossRef]
- Šourková, M.; Adamcová, D.; Zloch, J.; Skutnik, Z.; Vaverková, M.D. Evaluation of the phytotoxicity of leachate from a Municipal solid waste Landfill: The case study of Bukov Landfill. Environments 2020, 7, 111. [Google Scholar] [CrossRef]
- Nesrine, S.; El-Darier, S.M.; Taher, H.M. The allelochemicals effect of Zygophyllum album on control of Bromus tectorum. J. Life Sci. 2012, 6, 182–186. [Google Scholar]
- Singh, B.; Uniyal, A.K.; Todaria, N.P. Studies on the allelopathic influence of Zanthoxylum armatum D.C. on important field crops seeking its sustainable domestication in existing agroforestry systems of Garhwal Himalaya, India. J. Sustain. Agric. 2007, 30, 87–95. [Google Scholar] [CrossRef]
- Thapaliyal, A.; Bali, R.S.; Singh, B.; Todarai, N.P. Allelopathic effects of trees of economic importance on germination and growth of food crops. J. Herbs Spices Med. Plants 2007, 13, 11–23. [Google Scholar] [CrossRef]
- Tukey, H.B. The leaching of substance from plants. Annu. Rev. Plant Physiol. 1970, 21, 305–324. [Google Scholar] [CrossRef]
- Tanveer, A.; Rehman, A.; Javaid, M.M.; Abbas, R.N.; Sibtain, M.; Ahmad, A.; Ibin-I-Zamir, M.S.; Chaudhary, K.M.; Aziz, A. Allelopathic potential of Euphorbia helioscopia L. against wheat (Triticum aestivum L.), chickpea (Cicer arietinum L.) and lentil (Lens culinaris Medic.). Turk. J. Agric. For. 2010, 34, 75–81. [Google Scholar]
- Zzet, K.L.; Yusuf, Y. Allelopathic effects of plants extracts against seed germination of some weeds. Asian J. Plant Sci. 2004, 3, 472–475. [Google Scholar]
- Fujii, Y.; Furukawa, M.; Hayakawa, Y.; Sugahara, K.; Shibuya, T. Survey of Japanese medicinal plants for the detection of allelopathic properties. Weed Res. Tokyo 1991, 36, 36–42. [Google Scholar]
- Alam, S.M.; Islam, E.U. Effect of aqueous extract of leaf, stem and root of nettleleaf goosefoot and NaCl on germination and seedling growth of rice. Pak. J. Biol. Sci. 2002, 1, 47–52. [Google Scholar]
- Khailov, K.M. The Biochemical Trophodynamics in Coastal Sea Ecosystems; Naukova Dumka: Kiev, USSR, 1974. (In Russian) [Google Scholar]
- Wickens, G.E. Economic Botany: Principles and Practices; Springer Kluwer Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Kamara, A.K.; Akobunda, I.O.; Sanginga, N.; Jutzi, S.C. Effect of mulch from 14 multipurpose tree species (MPTs) on early growth and nodulation of cowpea (Vigna unguiculata L.). J. Agron. Crop Sci. 1999, 182, 127–138. [Google Scholar] [CrossRef]

| Variety | Plant Parts | Tree Crops | B. retusa | B. variegata | B. retusa | B. variegata | B. retusa | B. variegata | B. retusa | B. variegata | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Extract Concentrations | |||||||||||
| 0% | 25% | 25% | 50% | 50% | 75% | 75% | 100% | 100% | |||
| VL 892 | Bark | R | 4.1 ± 0.40 | 4.3 ± 0.39 (+6.39) | 4.2 ± 1.06 (+1.97) | 3.5 ± 0.62 (−13.02) | 6.5 ± 0.50 (+59.71) | 2.9 ± 0.6 (−29.48) | 3.5 ± 0.1 (−14.74) | 3.2 ± 0.64 (−20.24) | 4.5 ± 0.0 (11.30) |
| P | 3.7 ± 0.22 | 3.4 ± 0.45 (−6.28) | 3.6 ± 0.36 (−1.91) | 2.8 ± 0.58 (−24.04) | 5.4 ± 1.03 (+47.54) | 2.6 ± 0.5 (−29.78) | 4.7 ± 0.2 (+27.60) | 3.6 ± 0.21 (−2.46) | 4.0 ± 0.7 (+9.56) | ||
| Leaves | R | 4.1 ± 0.40 | 4.2 ± 1.04 (+1.97) | 4.9 ± 0.49 (+23.83) | 4.3 ± 0.60 (+4.67) | 4.9 ± 0.58 (+22.60) | 5.4 ± 0.2 (+33.17) | 4.9 ± 0.8 (+20.39) | 2.5 ± 2.46 (−39.31) | 4.1 ± 1.7 (+5.16) | |
| P | 3.7 ± 0.22 | 4.3 ± 0.63 (+16.94) | 5.4 ± 1.68 (+48.91) | 4.8 ± 0.85 (+31.15) | 4.5 ± 1.05 (+23.22) | 6.2 ± 1.0 (+69.95) | 3.7 ± 0.8 (+2.73) | 4.9 ± 1.57 (+36.34) | 3.1 ± 0.1 (−14.21) | ||
| VL 616 | Bark | R | 5.7 ± 0.44 | 4.5 ± 0.51 (−20.94) | 4.6 ± 0.63 (−19.90) | 2.4 ± 0.17 (−58.81) | 7.1 ± 0.84 (+23.56) | 3.5 ± 0.9 (−39.44) | 5.2 ± 1.6 (−8.73) | 2.1 ± 0.60 (−64.05) | 5.2 ± 0.9 (−9.42) |
| P | 4.4 ± 0.82 | 4.2 ± 0.94 (−5.18) | 3.9 ± 1.20 (−11.26) | 2.5 ± 0.70 (−43.92) | 6.9 ± 0.68 (+55.86) | 3.3 ± 0.0 (−25.00) | 5.6 ± 0.4 (+25.23) | 2.4 ± 1 (−45.95) | 4.0 ± 0.7 (−9.68) | ||
| Leaves | R | 5.7 ± 0.44 | 4.1 ± 0.11 (−27.92) | 4.6 ± 0.50 (−19.37) | 6.8 ± 0.61 (+19.02) | 6.9 ± 0.43 (+22.16) | 7.5 ± 0.7 (+30.89) | 4.5 ± 0.8 (−21.82) | 5.6 ± 1.25 (−1.40) | 5.3 ± 0.3 (−6.98) | |
| P | 4.4 ± 0.82 | 5.4 ± 0.74 (+22.30) | 4.6 ± 1.18 (+4.50) | 6.4 ± 0.64 (+44.37) | 7.9 ± 0.66 (+79.95) | 7.2 ± 0.4 (+61.71) | 3.6 ± 0.7 (−18.92) | 4.7 ± 0.45 (+6.98) | 5.1 ± 0.0 (+15.32) | ||
| VL 829 | Bark | R | 4.1 ± 0.40 | 5.3 ± 0.78 (+54.79) | 5.5 ± 1.30 (+34.89) | 4.9 ± 0.54 (−19.90) | 6.7 ± 0.52 (+64.13) | 4.5 ± 1.8 (−41.77) | 3.9 ± 0.5 (−2.70) | 2.2 ± 0.57 (-45.21) | 4.9 ± 0.4 (21.62) |
| P | 3.7 ± 0.22 | 6.5 ± 1.12 (+77.60) | 5.1 ± 0.54 (+39.07) | 2.9 ± 0.32 (-19.95) | 5.9 ± 1.21 (+61.48) | 3.1 ± 1.5 (−15.85) | 5.4 ± 0.6 (+48.36) | 2.3 ± 0.67 (−36.07) | 3.7 ± 0.8 (+2.19) | ||
| Leaves | R | 4.1 ± 0.40 | 5.7 ± 0.48 (+39.56) | 4.9 ± 0.32 (+20.15) | 7 ± 0.51 (+71.99) | 5.4 ± 0.91 (+31.45) | 8.0 ± 0.0 (+97.79) | 5.8 ± 0.0 (+43.98) | 4.6 ± 1.43 (+13.76) | 4.7 ± 0.9 (+17.69) | |
| P | 3.7 ± 0.22 | 6.2 ± 0.91 (+68.85) | 5.7 ± 054 (+55.74) | 6.8 ± 0.72 (+87.43) | 5.3 ± 0.97 (+43.72) | 6.8 ± 0.1 (+84.97) | 4.7 ± 0.2 (+29.23) | 6.2 ± 1.87 (+68.58) | 3.7 ± 0.2 (+3.55) | ||
| UP 1109 | Bark | R | 4.6 ± 0.63 | 4.9 ± 0.40 (+8.28) | 5.3 ± 1.06 (+14.38) | 2.9 ± 0.34 (−35.73) | 5.3 ± 1.01 (+15.25) | 3.2 ± 0.7 (−31.37) | 5.0 ± 0.3 (+9.80) | 3.5 ± 0.10 (−23.75) | 4.2 ± 0.6 (−8.28) |
| P | 4.3 ± 0.75 | 6.7 ± 0.52 (+56.88) | 3.7 ± 0.73 (−12.82) | 3.1 ± 0.84 (−23.81) | 4.2 ± 0.59 (−3.03) | 2.6 ± 0.2 (−38.23) | 4.2 ± 0.6 (−3.03) | 4.3 ± 0.40 (+0.23) | 3.3 ± 0.8 (−22.14) | ||
| Leaves | R | 4.6 ± 0.63 | 4.3 ± 0.32 (−6.32) | 5.1 ± 0.95 (+11.33) | 5.7 ± 2.28 (+23.53) | 5.4 ± 0.89 (+17.43) | 6.2 ± 1.5 (+34.64) | 4.9 ± 0.4 (−4.58) | 5.3 ± 0.86 (+15.90) | 4.9 ± 0.9 (+7.19) | |
| P | 4.3 ± 0.75 | 5.1 ± 1.46 (+19.35) | 5.9 ± 1.01 (+38.69) | 5.6 ± 1.03 (+30.77) | 6.4 ± 0.98 (+48.48) | 7.5 ± 1 (+75.76) | 3.8 ± 0.2 (−10.96) | 4.1 ± 0.21 (−3.73) | 3.8 ± 0.7 (−10.26) | ||
| UP 2572 | Bark | R | 4.7 ± 0.44 | 5.1 ± 0.14 (+7.91) | 4.3 ± 0.83 (+7.48) | 3.7 ± 0.19 (−20.09) | 5.8 ± 1.50 (+25.00) | 2.9 ± 0.5 (−38.03) | 4.5 ± 1.4 (−3.42) | 2.6 ± 0.57 (−43.80) | 4.5 ± 0.59 (−4.27) |
| P | 3.9 ± 0.53 | 4.5 ± 0.54 (+13.35) | 4.0 ± 1.05 (+1.26) | 2.8 ± 0.35 (−28.21) | 5.9 ± 0.47 (+50.88) | 2.8 ± 0.1 (−29.22) | 4.4 ± 1.5 (+10.08) | 2.9 ± 0.17 (−29.95) | 3.7 ± 0.48 (−5.29) | ||
| Leaves | R | 4.7 ± 0.44 | 5.5 ± 0.96 (−4.56) | 4.9 ± 0.86 (+4.91) | 4.6 ± 0.15 (−2.56) | 6.8 ± 0.47 (+45.73) | 5.5 ± 0.5 (+17.74) | 4.9 ± 0.2 (+5.56) | 4.2 ± 0.24 (−9.62) | 5.2 ± 0.93 (+11.11) | |
| P | 3.9 ± 0.53 | 5.3 ± 1.03 (+32.75) | 4.9 ± 1.83 (+24.18) | 4.9 ± 0.43 (+25.69) | 5.9 ± 0.58 (+50.88) | 7.5 ± 0.3 (+89.42) | 3.8 ± 0.4 (−3.78) | 3.5 ± 0.04 (−12.34) | 4.1 ± 0.54 (+2.02) | ||
| Source of Variation | Degree of Freedom | F-Value | |||||
|---|---|---|---|---|---|---|---|
| B. retusa | B. variegata | ||||||
| Germination | Radical | Plumule | Germination | Radical | Plumule | ||
| Treatments | 1 | 38.64 ** | 110.25 ** | 195.81 ** | 1.41 NS | 2.47 NS | 1.79 NS |
| Concentration | 4 | 12.41 ** | 11.26 ** | 13.28 ** | 26.05 ** | 19.71 ** | 29.28 ** |
| Varieties | 4 | 0.33 NS | 8.20 ** | 10.10 ** | 1.36 NS | 9.65 ** | 7.80** |
| Treatment × Con. | 4 | 21.81 ** | 30.88 ** | 39.62 ** | 0.75 NS | 2.02 NS | 7.48 ** |
| Treatment × varieties | 4 | 0.24 NS | 6.79 ** | 3.16 * | 0.04 NS | 1.59 NS | 1.71 NS |
| Con. × Varieties | 16 | 1.36 NS | 2.41 * | 2.13 * | 0.72 NS | 2.08 * | 2.84 ** |
| Treatment × con. × varieties | 16 | 0.59 NS | 2.90 * | 2.48 * | 0.97 NS | 2.47 ** | 1.49 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, N.; Khanduri, V.P.; Singh, B.; Rawat, D.; Riyal, M.K. Phytotoxicity of Two Bauhinia Species on Four Triticum aestivum Varieties in Laboratory Bioassay. Int. J. Plant Biol. 2024, 15, 599-606. https://doi.org/10.3390/ijpb15030045
Yadav N, Khanduri VP, Singh B, Rawat D, Riyal MK. Phytotoxicity of Two Bauhinia Species on Four Triticum aestivum Varieties in Laboratory Bioassay. International Journal of Plant Biology. 2024; 15(3):599-606. https://doi.org/10.3390/ijpb15030045
Chicago/Turabian StyleYadav, Neeraj, Vinod Prasad Khanduri, Bhupendra Singh, Deepa Rawat, and Manoj Kumar Riyal. 2024. "Phytotoxicity of Two Bauhinia Species on Four Triticum aestivum Varieties in Laboratory Bioassay" International Journal of Plant Biology 15, no. 3: 599-606. https://doi.org/10.3390/ijpb15030045
APA StyleYadav, N., Khanduri, V. P., Singh, B., Rawat, D., & Riyal, M. K. (2024). Phytotoxicity of Two Bauhinia Species on Four Triticum aestivum Varieties in Laboratory Bioassay. International Journal of Plant Biology, 15(3), 599-606. https://doi.org/10.3390/ijpb15030045






