Review of Invasive Plant Functional Traits and Management Using Remote Sensing in Sub-Saharan Africa
Abstract
1. Introduction
2. Literature Review Method
3. Invasive Plants of Sub-Saharan Africa and Their Deleterious Impacts
4. Implications of IAPs for Sustainable Development Goals in Sub-Saharan Africa
5. IAP Functional Traits and Their Ecological Importance
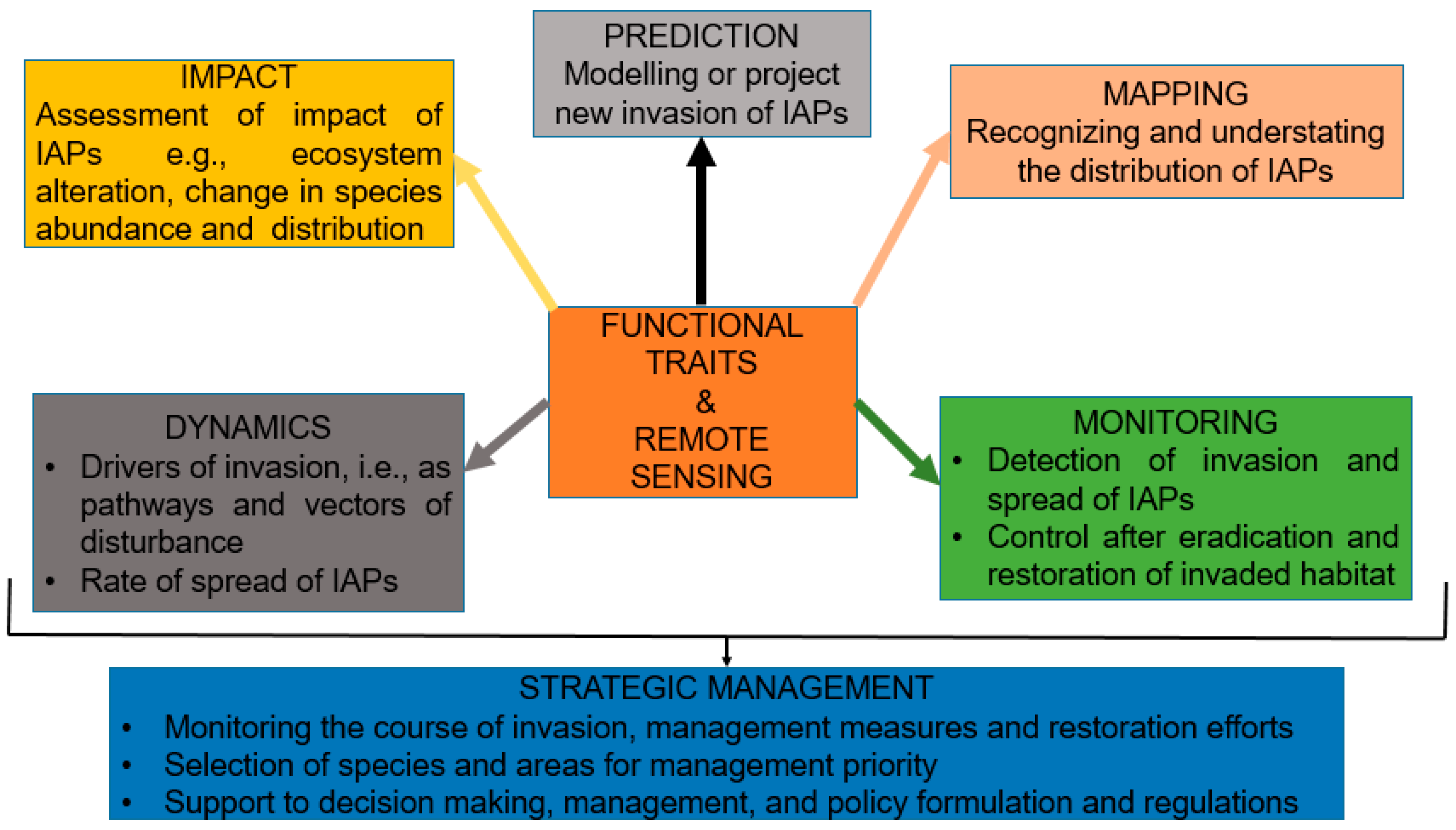
6. Importance of Functional Trait Remote Sensing-Based Research in Plant Invasions
7. Lessons Learned and Way Forward
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borokini, I.T.; Kortz, A.; Anibaba, Q.A.; Witt, A.; Aigbokhan, W.I.; Hejda, M.; Pyšek, P. Alien flora of Nigeria: Taxonomy, biogeography, habitats, and ecological impacts. Biol. Invasions 2023, 25, 3677–3696. [Google Scholar] [CrossRef]
- Sheergojri, I.A.; Rashid, I.; Rehman, I.U.; Rashid, I. Invasive species services-disservices conundrum: A case study from Kashmir Himalaya. J. Environ. Manag. 2022, 309, 114674. [Google Scholar] [CrossRef] [PubMed]
- Jocienė, L.; Krokaitė, E.; Rekašius, T.; Juškaitytė, E.; Ielciu, I.; Galanina, O.; Kupčinskienė, E. The Molecular Evidence for Invasive Climber Echinocystis lobata (Michx.) Torr. & A. Gray in Eastern and Central Europe. Diversity 2023, 15, 1084. [Google Scholar] [CrossRef]
- Petruzzellis, F.; Tordoni, E.; Tomasella, M.; Savi, T.; Tonet, V.; Palandrani, C.; Castello, M.; Nardini, A.; Bacaro, G. Functional differentiation of invasive and native plants along a leaf efficiency/safety trade-off. Environ. Exp. Bot. 2021, 188, 104518. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Munishi, L.K.; Ngondya, I.B. Realizing UN decade on ecosystem restoration through a nature-based approach: A case review of management of biological invasions in protected areas. PLoS Sustain Transform 2022, 1, e0000027. [Google Scholar] [CrossRef]
- Santolamazza-Carbone, S.; Durán-Otero, M.; Calviño-Cancela, M. Context dependency, co-introductions, novel mutualisms, and host shifts shaped the ectomycorrhizal fungal communities of the alien tree Eucalyptus globulus. Sci. Rep. 2019, 9, 7121. [Google Scholar] [CrossRef] [PubMed]
- Bacaro, G.; Maccherini, S.; Chiarucci, A.; Jentsch, A.; Rocchini, D.; Torri, D.; Gioria, M.; Tordoni, E.; Martellos, S.; Altobelli, A.; et al. Distributional patterns of endemic, native and alien species along a roadside elevation gradient in Tenerife, Canary Islands. Community Ecol. 2015, 16, 223–234. [Google Scholar] [CrossRef]
- Forey, E.; Lodhar, S.Y.F.; Galvin, S.D.; Lowry, J.H.; Gopaul, S.; Hanson, G.; Carboni, M.; Chauvat, M.; Boehmer, H.J. Alien palm invasion leads to selective biotic filtering of resident plant communities towards competitive functional traits. Biol. Invasions 2023, 25, 1489–1508. [Google Scholar] [CrossRef]
- Olden, J.D.; LeRoy Poff, N.; Douglas, M.R.; Douglas, M.E.; Fausch, K.D. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 2004, 19, 18–24. [Google Scholar] [CrossRef]
- Qian, H.; Guo, Q. Linking biotic homogenization to habitat type, invasiveness and growth form of naturalized alien plants in North America. Divers. Distrib. 2010, 16, 119–125. [Google Scholar] [CrossRef]
- Schirmel, J.; Buchholz, S. Invasive moss alters patterns in life-history traits and functional diversity of spiders and carabids. Biol. Invasions 2013, 15, 1089–1100. [Google Scholar] [CrossRef]
- Smith, T.C.; Bishop, T.B.B.; Duniway, M.C.; Villarreal, M.L.; Knight, A.C.; Munson, S.M.; Waller, E.K.; Jensen, R.; Gill, R.A. Biophysical factors control invasive annual grass hot spots in the Mojave Desert. Biol. Invasions 2023, 25, 3839–3858. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Stanek, M.; Majewska, M.L.; Nobisb, M.; Zubek, S. Invasive plant species identity affects soil microbial communities in a mesocosm experiment. Appl. Soil Ecol. 2019, 136, 168–177. [Google Scholar] [CrossRef]
- Garcia, R.A.; Clusella-Trullas, S. Impacts of invasive plants on animal diversity in South Africa: A synthesis. Bothalia-Afr. Biodivers. Conserv. 2017, 47, 1–12. [Google Scholar]
- Boy, G.; Witt, A. Invasive Alien Plants and Their Management in Africa, 1st ed.; CABI Africa: Nairobi, Kenya, 2013. [Google Scholar]
- Oh, M.; Heo, Y.; Lee, E.J.; Lee, H. Major environmental factors and traits of invasive alien plants determine their spatial distribution: A case study in Korea. J. Ecol. Environ. 2021, 45, 29. [Google Scholar] [CrossRef]
- Ribotta, S.; Liccari, F.; Muggia, L.; Pallavicini, A.; Bagnolini, F.; Tordoni, E.; Bacaro, G. Invasion at the Edge: The Case of Rosa rugosa (Rosaceae) in Italy. Diversity 2021, 13, 645. [Google Scholar] [CrossRef]
- Milanović, M.; Knapp, S.; Pyšek, P.; Kühn, I. Linking traits of invasive plants with ecosystem services and disservices. Ecosyst. Serv. 2020, 42, 101072. [Google Scholar] [CrossRef]
- Sheppard, C.S. Relative performance of co-occurring alien plant invaders depends on traits related to competitive ability more than niche differences. Biol. Invasions 2019, 21, 1101–1114. [Google Scholar] [CrossRef]
- Tordoni, E.; Petruzzellis, F.; Nardini, A.; Savi, T.; Bacaro, G. Make it simpler: Alien species decrease functional diversity of coastal plant communities. J. Veg. Sci. 2019, 30, 498–509. [Google Scholar] [CrossRef]
- Abadie, J.C.; Machon, N.; Muratet, A.; Porcher, E. Landscape disturbance causes small-scale functional homogenization, but limited taxonomic homogenization, in plant communities. J. Ecol. 2011, 99, 1134–1142. [Google Scholar] [CrossRef]
- Brice, M.; Pellerin, S.; Poulin, M. Does urbanization lead to taxonomic and functional homogenization in riparian forests? Divers. Distrib. 2017, 23, 828–840. [Google Scholar] [CrossRef]
- Lambdon, P.W.; Lloret, F.; Hulme, P.E. Do non-native species invasions lead to biotic homogenization at small scales? The similarity and functional diversity of habitats compared for alien and native components of Mediterranean floras. Divers. Distrib. 2008, 14, 774–785. [Google Scholar] [CrossRef]
- Milanović, M.; Kühn, I.; Pyšek, P.; Knapp, S. Functional diversity changes in native and alien urban flora over three centuries. Biol. Invasions 2021, 23, 2337–2353. [Google Scholar] [CrossRef]
- Kaushik, P.; Pati, P.K.; Khan, M.L.; Khare, P.K. Plant functional traits best explain invasive species’ performance within a dynamic ecosystem—A review. Trees For. People 2022, 8, 100260. [Google Scholar] [CrossRef]
- Carmona, C.P.; De Bello, F.; Mason, N.W.H.; Leps, J. Traits without borders: Integrating functional diversity across scales. Trends Ecol. Evol. 2016, 31, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Daehler, C.C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Funk, J.L.; Zachary, V.A. Physiological responses to short-term water and light stress in native and invasive plant species in southern California. Biol. Invasions 2010, 12, 1685–1694. [Google Scholar] [CrossRef]
- van Kleunen, M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Ojija, F.; Arnold, S.E.J.; Treydte, A.C. Impacts of alien invasive Parthenium hysterophorus on flower visitation by insects to co-flowering plants. Arthropod-Plant Interact. 2019, 13, 719–734. [Google Scholar] [CrossRef]
- Ruprecht, E.; Fenesi, A.; Nijs, I. Are plasticity in functional traits and constancy in performance traits linked with invasiveness? An experimental test comparing invasive and naturalized plant species. Biol. Invasions 2014, 16, 1359–1372. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Traits Associated with Invasiveness in Alien Plants: Where Do we Stand? In Biological Invasions; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 97–125. [Google Scholar]
- Rangi, D. Invasive Species: The Hidden Threat to Sustainable Development, 1st ed.; CABI: Nairobi, Kenya, 2018. [Google Scholar]
- Ferrero, M.C.; Tecco, P.A.; Gurvich, D.E. Is intraspecific variability an advantage in mountain invasions? Comparing functional trait variation in an invasive and a native woody species along multiple environmental gradients. Biol. Invasions 2022, 24, 1393–1412. [Google Scholar] [CrossRef]
- Funk, J.L. The physiology of invasive plants in low-resource environments. Conserv. Physiol. 2013, 1, cot026. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Vitousek, P.M. Resource-use efficiency and plant invasion in low-resource systems. Nature 2007, 446, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Standis, R.J.; Stock, W.D.; Valladares, F. Plant functional traits of dominant native and invasive species in mediterranean-climate ecosystems. Ecology 2016, 97, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Tecco, P.A.; Diaz, S.; Cabido, M.; Urcelay, C. Functional traits of alien plants across contrasting climatic and land-use regimes: Do aliens join the locals or try harder than them? J. Ecol. 2010, 98, 17–27. [Google Scholar] [CrossRef]
- Heberling, J.M.; Kichey, T.; Decocq, G.; Fridley, J.D. Plant functional shifts in the invaded range: A test with reciprocal forest invaders of Europe and North America. Funct. Ecol. 2016, 30, 875–884. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jenions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Godoy, O.; Vlladares, F.; Castro-Díez, P. Multispecies comparison reveals that invasive and native plants differ in their traits but not in their plasticity. Funct. Ecol. 2011, 25, 1248–1259. [Google Scholar] [CrossRef]
- Leishman, M.R.; Cooke, J.; Richardson, D.M.; Newman, J. Evidence for shifts to faster growth strategies in the new ranges of invasive alien plants. J. Ecol. 2014, 102, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.T.; Maxwell, B.D.; Pauchard, A.; Nunez, M.A.; Rew, L.J. Native versus non-native invasions: Similarities and differences in the biodiversity impacts of Pinus contorta in introduced and native ranges. Divers. Distrib. 2016, 22, 578–588. [Google Scholar] [CrossRef]
- Petruzzellis, F.; Peng, G.; Tyree, M.T.; Tonet, V.; Savi, T.; Torboli, V.; Pallavicini, V.; Bacaro, G.; Nardini, A. Plasticity of functional traits of tree of heaven is higher in exotic than in native habitats. Trees 2019, 33, 411–420. [Google Scholar] [CrossRef]
- Thompson, K.; Davis, M.A. Why research on traits of invasive plants tells us very little. Trends Ecol. Evol. 2011, 26, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Ojija, F. Eco-friendly management of Parthenium hysterophorus. Sci. Prog. 2022, 105, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Minuto, L. Management of an invasive plant in a Mediterranean protected area: The experience of Senecio deltoideus in Italy. Ann. Bot. 2021, 11, 1–12. [Google Scholar]
- Tassin, J.; Kull, C.A. Facing the broader dimensions of biological invasions. Land Use Policy 2015, 42, 165–169. [Google Scholar] [CrossRef]
- Chacón-Madrigal, E.; Wanek, W.; Hietz, P.; Dullinger, S. Traits indicating a conservative resource strategy are weakly related to narrow range size in a group of neotropical trees. Perspect. Plant Ecol. Evol. Syst. 2018, 32, 30–37. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Müllerová, J.; Brundu, G.; Große-Stoltenberg, A.; Kattenborn, T.; Richardson, D.M. Pattern to process, research to practice remote sensing of plant invasions. Biol. Invasions 2023, 25, 3651–3676. [Google Scholar] [CrossRef]
- Evangelista, P.; Stohlgren, T.; Morisette, J.; Kumar, S. Mapping invasive Tamarisk (Tamarix): A comparison of single-scene and time-series analyses of remotely sensed data. Remote Sens. 2009, 1, 519–533. [Google Scholar] [CrossRef]
- Hall, S.J.; Asner, G.P. Biological invasion alters regional nitrogen-oxide emissions from tropical rainforests. Glob. Chang. Biol. 2007, 13, 2143–2160. [Google Scholar] [CrossRef]
- Mallmann, C.L.; Filho, W.P.; Dreyer, J.B.B.; Tabaldi, L.A.; Durgante, F.M. Leaf-level field spectroscopy to discriminate invasive species (Psidium guajava L. and Hovenia dulcis Thunb.) from native tree species in the Southern Brazilian Atlantic Forest. Remote Sens. 2023, 15, 791. [Google Scholar] [CrossRef]
- Mielczarek, D.; Sikorski, P.; Archiciński, P.; Ciezkowski, W.; Zaniewska, E.; Chormanski, J. The use of an airborne laser scanner for rapid identification of invasive tree species Acer negundo in riparian forests. Remote Sens. 2022, 15, 212. [Google Scholar] [CrossRef]
- Naupari, J.A.; Vierling, L.A.; Eitel, J.U.H. Delineating native and invasive plant functional groups in shrub-steppe vegetation using bidirectional reflectance. J. Appl. Remote Sens. 2013, 7, 073563. [Google Scholar] [CrossRef]
- Van Cleemput, E.; Van Meerbeek, K.; Helsen, K.; Honnay, O.; Somers, B. Remotely sensed plant traits can provide insights into ecosystem impacts of plant invasions: A case study covering two functionally different invaders. Biol. Invasions 2020, 22, 3533–3550. [Google Scholar] [CrossRef]
- Bordbar, F.; Meerts, P. Patterns in the alien flora of the Democratic Republic of the Congo: A comparison of Asteraceae and Fabaceae. Plant Ecol. Evol. 2020, 153, 373–389. [Google Scholar] [CrossRef]
- Foxcroft, L.C.; Richardson, D.M.; Rejmánek, M.; Pysek, P. Alien plant invasions in tropical and sub-tropical savannas: Patterns, processes and prospects. Biol. Invasions 2010, 12, 3913–3933. [Google Scholar] [CrossRef]
- Ojija, F. Allelopathic effects of Sphaeranthus suaveolens (Forssk.) DC. and Argemone mexicana L leaf crude extract on Zea mays L germination and growth. AGBIR 2023, 39, 651–656. [Google Scholar]
- Ali, A.M.; Darvishzadeh, R.; Shahi, K.R.; Skidmore, A. Validating the predictive power of statistical models in retrieving leaf dry matter content of a coastal wetland from a Sentinel-2 Image. Remote Sens. 2019, 11, 1936. [Google Scholar] [CrossRef]
- Gavier-Pizarro, G.I.; Kuemmerle, T.; Hoyos, L.E.; Stewart, S.I.; Huebner, C.D.; Keuler, N.S.; Radeloff, V.C. Monitoring the invasion of an exotic tree (Ligustrum lucidum) from 1983 to 2006 with Landsat TM/ETM+ satellite data and Support Vector Machines in Córdoba, Argentina. Remote Sens. Environ. 2012, 122, 134–145. [Google Scholar] [CrossRef]
- Hantson, W.; Kooistra, L.; Slim, P.A. Mapping invasive woody species in coastal dunes in the Netherlands: A remote sensing approach using LIDAR and high-resolution aerial photographs. Appl. Veg. Sci. 2012, 15, 536–547. [Google Scholar] [CrossRef]
- Maruthi Sridhar, B.B.; Vincent, R.K.; Clapham, W.B.; Sritharan, S.I.; Osterberg, J.; Neale, C.M.U.; Watts, D.R. Mapping saltcedar Tamarix ramosissima and other riparian and agricultural vegetation in the Lower Colorado River region using multi-spectral Landsat TM imagery. Geocarto Int. 2010, 25, 649–662. [Google Scholar] [CrossRef]
- Devin, S.; Beisel, J.-N. Biological and ecological characteristics of invasive species: A gammarid study. Biol. Invasions 2006, 9, 13–24. [Google Scholar] [CrossRef]
- Agha, S.B.; Alvarez, M.; Becker, M.; Fèvre, E.M.; Junglen, S.; Borgemeister, C. Invasive alien plants in Africa and the potential emergence of mosquito-borne arboviral diseases—A review and research outlook. Viruses 2020, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef] [PubMed]
- Uyi, O.; Mukwevho, L.; Ejomah, A.J.; Toews, M. Invasive alien plants in Sub-Saharan Africa: A review and synthesis of their insecticidal activities. Front. Agron. 2021, 3, 725895. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Callaway, R.M. Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef]
- Witt, A.; Beale, T.; Van Wilgen, B.W. An assessment of the distribution and potential ecological impacts of invasive alien plant species in eastern Africa. Trans. R. Soc. S. Afr. 2018, 73, 217–236. [Google Scholar] [CrossRef]
- Afzal, M.R.; Naz, M.; Ashraf, W.; Du, D. The Legacy of Plant Invasion: Impacts on Soil Nitrification and Management Implications. Plants 2023, 12, 2980. [Google Scholar] [CrossRef]
- Goncalves, E.; Herrera, I.; Duarte, M.; Bustamante, R.O.; Lampo, M.; Velasquez, G.; Sharma, G.P.; Garcıa-Rangel, S. Global invasion of Lantana camara: Has the climatic niche been conserved across continents? PLoS ONE 2014, 9, e111468. [Google Scholar] [CrossRef]
- Kilawe, C.J.; Baltazary, I.S.; Malila, B.P.; Lyimo, P.J.; Mwakalukwa, E.E. Replacement of native trees by the neotropical invasive tree Cedrela odorata L. in the Kimboza Forest Reserve, Tanzania. Biol. Invasions 2023, 25, 3697–3710. [Google Scholar] [CrossRef]
- Nyasembe, V.O.; Cheseto, X.; Kaplan, F.; Foster, W.A.; Teal, P.E.A.; Tumlinson, J.H.; Borgemeister, C.; Torto, B. The invasive american weed Parthenium hysterophorus can negatively impact malaria control in Africa. PLoS ONE 2015, 10, e0137836. [Google Scholar] [CrossRef] [PubMed]
- Ojija, F.; Ngimba, C. Suppressive abilities of legume fodder plants against the invasive weed Parthenium hysterophorus (Asteraceae). Environ. Sustain. Indic. 2021, 10, 100111. [Google Scholar] [CrossRef]
- Landmann, T. Invasive plants and food security in Africa: The potential of earth observation data. S. Afr. Inst. Int. Aff. 2017, 1, 1–4. [Google Scholar]
- Pellegrini, E.; Buccheri, M.; Martini, F.; Boscutti, F. Agricultural land use curbs exotic invasion but sustains native plant diversity at intermediate levels. Sci. Rep. 2021, 11, 8385. [Google Scholar] [CrossRef]
- Egoh, B.N.; Ntshotsho, P.; Maoela, M.A.; Blanchard, R.; Ayompe, L.M.; Rahlao, S. Setting the scene for achievable post-2020 convention on biological diversity targets: A review of the impacts of invasive alien species on ecosystem services in Africa. J. Environ. Manag. 2020, 261, 110171. [Google Scholar] [CrossRef]
- Diagne, C.; Turbelin, A.J.; Moodley, D.; Novoa, A.; Leroy, B.; Angulo, E.; Adamjy, T.; Dia, C.A.K.M.; Taheri, A.; Tambo, J.; et al. The economic costs of biological invasions in Africa: A growing but neglected threat? NeoBiota 2021, 67, 11–51. [Google Scholar] [CrossRef]
- Mologni, F.; Bellingham, P.J.; Cameron, E.K.; Dinh, K.; Wright, A.E.; Burns, K.C. Functional traits explain non-native plant species richness and occupancy on northern New Zealand islands. Biol. Invasions 2022, 24, 2135–2154. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Dawson, W.; Dostal, P. Research on invasive-plant traits tells us a lot. Trends Ecol. Evol. 2011, 26, 317. [Google Scholar] [CrossRef] [PubMed]
- Korpelainen, H.; Pietiläinen, M. What makes a good plant invader? Life 2023, 13, 1596. [Google Scholar] [CrossRef] [PubMed]
- Mathakutha, R.; Steyn, C.; Le Roux, P.C.; Blom, I.J.; Chown, S.L.; Daru, B.H.; Ripley, B.S.; Louw, A.; Greve, M. Invasive species differ in key functional traits from native and non-invasive alien plant species. J. Veg. Sci. 2019, 30, 994–1006. [Google Scholar] [CrossRef]
- Lamarque, L.J.; Delzon, S.; Lortie, C.J. Tree invasions: A comparative test of the dominant hypotheses and functional traits. Biol. Invasions 2011, 13, 1969–1989. [Google Scholar] [CrossRef]
- Moravcová, L.; Pyšek, P.; Jarošík, V.; Pergl, J. Getting the right traits: Reproductive and dispersal characteristics predict the invasiveness of herbaceous plant species. PLoS ONE 2015, 10, e0123634. [Google Scholar] [CrossRef]
- Smith, M.D.; Knapp, A.K. Physiological and morphological traits of exotic, invasive exotic, and native plant species in tallgrass prairie. Int. J. Plant Sci. 2001, 162, 785–792. [Google Scholar] [CrossRef]
- Milanović, M.; Knapp, S.; Pyšek, P.; Kühn, I. Trait–environment relationships of plant species at different stages of the introduction process. NeoBiota 2020, 58, 55–74. [Google Scholar] [CrossRef]
- Niphadkar, M.; Nagendra, H. Remote sensing of invasive plants: Incorporating functional traits into the picture. Int. J. Remote Sens. 2016, 37, 3074–3085. [Google Scholar] [CrossRef]
- Moyano, J.; Dickie, I.A.; Rodriguez-Cabal, M.A.; Nuñez, M.A. Patterns of plant naturalization show that facultative mycorrhizal plants are more likely to succeed outside their native Eurasian ranges. Ecography 2020, 43, 648–659. [Google Scholar] [CrossRef]
- Díaz De León Guerrero, S.D.; González-Rebeles Guerrero, G.; Ibarra-Montes, T.M.; Bastarrachea, A.R.; Cobos, R.S.; Bullock, S.H.; Sack, L.; Mendez-Alonzo, R. Functional traits indicate faster resource acquisition for alien herbs than native shrubs in an urban Mediterranean shrubland. Biol. Invasions 2020, 22, 2699–2712. [Google Scholar] [CrossRef]
- Santos, M.J.; Hestir, E.L.; Khanna, S.; Ustin, S.L. Image spectroscopy and stable isotopes elucidate functional dissimilarity between native and nonnative plant species in the aquatic environment. New Phytol. 2012, 193, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.-T.; Gao, Y.; Xie, X.; Guo, H.; Zhang, T.; Zhao, B. Spectral Discrimination of the Invasive Plant Spartina alterniflora at multiple phenological stages in a Saltmarsh Wetland. PLoS ONE 2013, 8, e67315. [Google Scholar] [CrossRef] [PubMed]
- Divíšek, J.; Chytrý, M.; Beckage, B.; Gotelli, N.J.; Lososová, Z.; Pyšek, P.; Richardson, D.M.; Molofsky, J. Similarity of introduced plant species to native ones facilitates naturalization, but differences enhance invasion success. Nat. Commun. 2018, 9, 4631. [Google Scholar] [CrossRef] [PubMed]
- Marchini, G.L.; Maraist, C.A.; Cruzan, M.B. Trait divergence, not plasticity, determines the success of a newly invasive plant. Ann. Bot. 2019, 123, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Gränzig, T.; Clasen, A.; Fassnacht, F.E.; Cord, A.; Förster, M. Combining remote sensing, habitat suitability models and cellular automata to model the spread of the invasive shrub Ulex europaeus. Biol. Invasions 2023, 25, 3711–3736. [Google Scholar] [CrossRef]
- Vaz, A.S.; Alcaraz-Segura, D.; Campos, J.C.; Vicente, J.R.; Honrado, J.P. Managing plant invasions through the lens of remote sensing: A review of progress and the way forward. Sci. Total Environ. 2018, 642, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Beccari, E.; Carmona, C.P.; Tordoni, E.; Petruzzellis, F.; Martinucci, D.; Casagrande, G.; Pavanetto, N.; Rocchini, D.; D’Antraccoli, M.; Ciccarelli, D.; et al. Plant spectral diversity from high-resolution multispectral imagery detects functional diversity patterns in coastal dune communities. J. Veg. Sci. 2024, 35, e13239. [Google Scholar] [CrossRef]
- Hacker, P.W.; Coops, N.C.; Laliberté, E.; Michaletz, S.T. Variations in accuracy of leaf functional trait prediction due to spectral mixing. Ecol. Indic. 2022, 136, 108687. [Google Scholar] [CrossRef]
- Tordoni, E.; Petruzzellis, F.; Nardini, A.; Bacaro, G. Functional divergence drives invasibility of plant communities at the edges of a resource availability gradient. Diversity 2020, 12, 148. [Google Scholar] [CrossRef]
- Rahman, M.M.; Zhang, X.; Ahmed, I.; Iqbal, Z.; Zeraatpisheh, M.; Kanzaki, M.; Xu, M. Remote sensing-based mapping of senescent leaf C:N ratio in the sundarbans reserved forest using machine learning tech-niques. Remote Sens. 2020, 12, 1375. [Google Scholar] [CrossRef]
- Ramsey, E.W.; Nelson, G.A.; Sapkota, S.K.; Seeger, E.B.; Martella, K.D. Mapping Chinese Tallow with Color-Infrared Photography. Photogramm. Eng. 2002, 68, 251–255. [Google Scholar]
- Brenner, J.C.; Christman, Z.; Rogan, J. Segmentation of landsat thematic mapper imagery improves buffelgrass (Pennisetum ciliare) pasture mapping in the Sonoran Desert of Mexico. Appl. Geogr. 2012, 34, 569–575. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Friedman, M.S.; McMillan, N.A.; Hammond, W.M.; Hassani, K.; Sams, A.V.; Charles, M.D.; Garrett, D.R.; Joshi, O.; Hamilton, R.G.; et al. Mapping invasive alien species in grassland ecosystems using airborne imaging spectroscopy and remotely observable vegetation functional traits. Remote Sens. Environ. 2022, 271, 112887. [Google Scholar] [CrossRef]
- Underwood, E.C.; Ustin, S.L.; Ramirez, C.M. A Comparison of Spatial and Spectral Image Resolution for Mapping Invasive Plants in Coastal California. Environ. Manag. 2007, 39, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Van Cleemput, E.; Adler, P.B.; Suding, K.N. Making remote sense of biodiversity: What grassland characteristics make spectral diversity a good proxy for taxonomic diversity? Glob. Ecol. Biogeogr. 2023, 32, 2177–2188. [Google Scholar] [CrossRef]
- Schweiger, A.K.; Cavender-Bares, J.; Townsend, P.A.; Hobbie, S.E.; Madritch, M.D.; Wang, R.; Tilman, D.; Gamon, J.A. Plant spectral diversity integrates functional and phylogenetic components of biodiversity and predicts ecosystem function. Nat. Ecol. Evol. 2018, 2, 976–982. [Google Scholar] [CrossRef]
| Publisher | Number of Reviewed Articles | Indexing Database | Number of Reviewed Articles |
|---|---|---|---|
| Springer | 20 | Scopus | 33 |
| Elsevier | 32 | Directorate of Open Access Journals | 17 |
| Taylor & Francis | 10 | African Journal Online | 11 |
| SAGE | 8 | Web of Science | 49 |
| Wiley-Blackwell | 6 | ||
| PLOS ONE | 9 | ||
| MDPI | 21 | ||
| Hindawi | 4 | ||
| Total reviewed articles | 110 | 110 |
| Scientific Name | Common Name(s) | Native Range | Impacts | References |
|---|---|---|---|---|
| Acacia mearnsii De Wild (Family: Fabaceae) | Green wattle, Black wattle, or Late black wattle | South-eastern Australia and Tasmania | Averts native species growth, decreases rangeland productivity and surface water, raises the amount of soil nitrogen, and alters its physicochemical characteristics. | [16,74] |
| Acacia melanoxylon R. Br (Family: Fabaceae) | Blackwood acacia, or Blackwood | South eastern Australia | Drives away native plant species, and modifies soil nutrients by adding nitrogen. | [16,70,74] |
| Argemone mexicana L. (Family: Papaveraceae) | Mexican poppy or Mexican prickly poppy | Central America and the Caribbean | Poisonous to livestock; it is rarely eaten, causes health disorders, and exerts allelopathic effects on native plants. | [16,64,70,74] |
| Bidens pilosa L. (Family: Asteraceae) | Blackjack | Tropical America | Hinders the growth and establishment of native plant species. Also, it competes with crops for resources (water, nutrients, light, and spaces) | [16,74] |
| Caesalpinia decapetala (Roth) Alston (Family: Fabaceae) | Cat’s claw, Mauritius, or Mysore thorn | Asia mainly India | Its impenetrable thickets prevent peoples’ and animals’ free movement; its massive spines on the stems hinder the management of forests, also harm wildlife, livestock, and people. | [16,74] |
| Calotropis procera (Aiton) W.T.Aiton (Family: Apocynaceae) | Sodom apple, king’s crown, rubber bush, or rubber tree | South and Western Asia, North Africa, and Tropical Africa | Displaces native plants, grows into dense thickets, and its sap irritates the eyes severely. When consumed, it makes people sick. | [16,74] |
| Chromolaena odorata (L.) R. M. King & H. Rob. (Family: Asteraceae) | Siam weed, Rouge plant, Christmas bush, or Devil weed | South and North America | Lowers rangelands productivity, suppresses native plants leading homogenization, toxic to animals including human, and intensifies fires. | [16,74] |
| Clidemia hirta (L.) D. Don (Family: Melastomataceae) | Clidemia, Soapbush, or Koster’s curse | Tropical America | Harmful to livestock, suppresses native vegetation, and forms thick or dense stands. | [16,74] |
| Datura stramonium L. (Family: Solanaceae) | Thorn apple, Jimson weed, devil’s trumpet, or devil’s weed | Central, South and North America | Forms monospecific dense stands that replace native species, and it is harmful to animals and plants. | [16,70,74] |
| Lantana camara L. (Family: Verbenaceae) | Lantana | South America and Central America | Lowers the production of fodder, inhibits the growth of vegetation, and destroys or leads to biodiversity loss. | [16,74,76] |
| Leucaena leucocephala (Lam.) de Wit (Family: Fabaceae) | White leadtree, River tamarind, Pearl wattle, or Jumbay | Southern Mexico and Northern Central America | Displaces native flora and fauna species, changes the ecosystems structure, disrupts primary succession processes, and decreases environmental quality. | [16,74] |
| Mimosa diplotricha C. Wright ex Sauvalle (Family: Fabaceae) | Giant false sensitive plant or Giant sensitive plant | Tropical America | Produces shadows that stop light-demanding plant species from regenerating; thick stands make it difficult for animals and wildlife to roam freely. Additionally, it poisons animals. | [16,70,74] |
| Mimosa pigra L. (Family Fabaceae) | Giant sensitive tree | South America | Decreases native biodiversity, blocks the open habitats used by wildlife, modifies the ecosystem, and reduces native resources and grazing areas for livestock and wildlife. | [16,72,74] |
| Parthenium hysterophorus L. (Family: Asteraceae) | Carrot weed, or Whitetop weed | North and South America | Toxic invasive plant; it rapidly suppresses native vegetation through allelopathy and resource competition. Alters native plant community structure to monospecific stands, reduces rangeland productivity and crop yields, and causes health problems to people and animals. | [33,50,72,78,79] |
| Opuntia ficus-indica (L.) Mill. (Family: Cactaceae) | Sweet prickly pear, India fig opuntia, Barbary fig, or Cactus pear | North America | Its spines hinder access to pasture and harm humans, animals, and wildlife, and drives out native species. | [16,74] |
| Opuntia stricta (Haw.) Haw. (Family: Cactaceae) | Erect prickly pear | Tropical America | Its spines hinder access to pasture and injure people, animals, and wildlife, and displaces native species. | [16,74] |
| Pinus patula Schiede ex Schltdl. & Cham. (Family: Pinaceae) | Patula pine or Spreading-leaved pine | Central America, e.g., Mexico | Its dense stands displace and/or inhibit the growth and germination of native plants and reduce drainage or water run-off. | [16,74] |
| Pistia stratiotes L. (Family: Araceae) | Water cabbage, Nile cabbage, Water lettuce, or Shellflower | Probably Tropical America or Africa | Impedes fishing, obstructs waterways, slows down water flow, destroys fish rookeries (breeding colonies), increases nutrient loading and siltation rates (thus, depresses water quality), and threatens fish and other species survival. | [16,74] |
| Prosopis juliflora (Sw.) DC. (Family: Fabaceae) | Mesquite | Tropical America | Depletes groundwater, lowers ecosystems and rangelands ability to support wildlife, and eradicates native species from invaded areas. | [16,72,74] |
| Psidium guajava L. (Family: Myrtaceae) | Common guava | Central America and the Caribbean | Makes thick stands that hinder or displaces native species; uses allelopathy to negatively impact plants and crops. | [16,58,74] |
| Ricinus communis L. (Family: Euphorbiaceae) | Castor bean | East Africa | Forms thick stands that, especially in riparian areas, supplant native plants. | [16,74] |
| Rubus niveus Thunb. (Family: Rosaceae) | Ceylon raspberry, Mysore raspberry, or hill raspberry | East and South Asia, Australia, or the Himalayas | Transforms the plant community and forms dense thickets that interfere with or impede the growth and rejuvenation of native plants. | [16,72,74] |
| Senna spectabilis (DC.) H.S. Irwin & Barneby (Family: Fabaceae) | Golden wonder tree | Tropical America | Inhibits the growth and rejuvenation of native plants using allelopathy. | [16,74] |
| Tagetes minuta L. (Family: Asteraceae) | Wild marigold | South America | Forms monospecific stands by suppressing the growth and germination of native plant species. | [16,72,74] |
| Tephrosia vogelli Hook. f. (Family: Fabaceae) | Fish bean or Fish-poison bean | Tropical Africa | Its leaves are toxic to animals including fishes, worms, insects, molluscs, toads, and frogs. | [16,70,74] |
| Tithonia diversifolia (Hemsl.) A. Gray (Family: Asteraceae) | Mexican sunflower, Tree marigold, or Japanese sunflower | Mexico and Central America | Decreases the productivity of rangelands, modifies the organization of plant communities, and leads to the loss of some native species. | [16,74] |
| IAP Functional Trait Categories | Ecological Importance | References |
|---|---|---|
| Reproduction, spread and growth rate (seed mass, seed number, dispersal method, relative growth rate) | IAPs have a higher fecundity (or reproduction rate). They make significant reproductive investments, resulting in a large number of seeds that can swiftly invade new areas. Their high fecundity makes them compete more successfully than native species. | [4,17,18,19,20,21,34,37,84,87,88] |
| IAPs have efficient and different seed dispersal mechanisms. Propagule size, weight, shape, water dispersal (hydrochory), wind dispersal (anemochory), dispersal, animal dispersal (epizoochory) are examples of seed dispersal traits. | [84,87,88,89] | |
| As IAPs devote a greater number of resources to growth and reproduction, they grow faster than native species. | [18,37,84,87,90] | |
| Phenology | In contrast to native species, IAPs can alter their phenological patterns. This makes it possible for them to take advantage of resources when there is little competition. IAPs also begin flowering earlier and continue flowering for longer than native plants. | [19,21,66,91,92] |
| Resource acquisition and utilization (leaf economic spectrum traits) | IAPs’ functional traits related to resource (water, nutrients, and light) acquisition include leaf area (LA), specific leaf area (SLA), vein length per unit area (VLA), leaf mass per area (LMA), leaf area, rapid growth rate, height, and extensive and deep root system. These traits indicate IAP’s ability to capture and use resources efficiently, which enables them to surpass native species. | [4,17,18,19,20,21,37] |
| Hydraulic traits (vein traits and drought tolerance traits) and water use | Most IAPs are able to grow in a variety of environmental conditions because they possess traits associated with severe drought and/or temperature resilience or tolerance. For instance, most IPAs show high venation, leaf water potential at turgor loss point, and leaf osmotic potential at full turgor. But some IAPs may have lower drought resistance than natives. | [4,7,19,21,48,93] |
| Mycorrhizal associations | IAPs’ access to nutrients may be influenced by their mycorrhizal associations. Some IAPs may exhibit flexibility in forging associations or have a variety of mycorrhizal partners. | [7,18,93] |
| Allelopathy and chemical defense | Some IAPs produce secondary metabolites or allelochemicals that inhibit the growth of native plants. They use these allelochemicals to influence their invasiveness and reduce competition with their nearby native species. | [64,72,89] |
| Functional Traits | Uses in Remote Sensing | RS Technique | Country | References |
|---|---|---|---|---|
| Leaves phenology (green or dry leaves) | Monitoring the invasion of Ligustrum lucidum W.T. Aiton. | Satellite | Argentina | [66] |
| SLA, LDMC, and LNC | Assessing impacts of plant invasions (Impatiens glandulifera Royle and S. gigantea) on ecosystem. | Field spectrometer | Belgium | [61] |
| Sum of leaf magnesium and calcium contents (leaf Ca + Mg) | Assessing impacts of plant invasions (I. glandulifera and S. gigantea) on ecosystem. | Field spectrometer | Belgium | [61] |
| LDMC | Verifying statistical models’ predictive power using IAPs Festuca rubra L. Elytriagia atherica (Link) Kerguélen, and Puccinellia maritima (Jacq.) Parl. | Satellite | Netherlands | [65] |
| Flowering periods (flower phenology) | Tamarix spp. invasion detection and mapping using spectral signatures acquired during flowering periods | Satellite | US | [56] |
| Leaf C:N | Comparing carbon-to-nitrogen ratios of senescent leaf in plants. | Satellite | Bangladesh | [104] |
| Plant leaf colour (leaf phenology) | Mapping occurrences of IAP Sapium sebiferum (L.) Roxb. | UAV (aircraft) | US | [105] |
| Patches or clumps (Structural traits) | Mapping of IAP Pennisetum ciliare (L.) Link | Satellite | Mexico | [106] |
| Total nitrogen, magnesium, canopy height, potassium, and total chlorophyll (Chla + Chlb) | Mapping IAP Lespedeza cuneata (Dum. Cours.) G.Don in grassland ecosystems. | Field spectrometer + UAV (aircraft) | US | [107] |
| Plant height, inflorescence, flowering, germination, and vegetative growth | Mapping of IAP Spartina alterniflora (Loisel.) P.M.Peterson & Saarela | Field spectrometer | China | [96] |
| Chlorophyll, anthocyanin, and carotene concentrations | Elucidate functional dissimilarity between IAPs (e.g., Egeria densa Planch., Myriophyllum spicatum L, etc.) and native (e.g., Elodea Canadensis Michx., Stuckenia pectinate (L.) Böerner, etc.). | Field spectrometer + UAV (aircraft) | US | [95] |
| Canopy structure, senesced leaves, eight, biochemical and biophysical features, and inflorescences | Detecting three different IAP species (Carpobrotus edulis (L.) N.E. Br, Eucalyptus globulus Labill., and Cortaderia jubata (Lem.) Stapf). | UAV (aircraft) | US | [108] |
| Leaf orientation | Delineating IAP (Taeniatherum caput-medusae L) and native plants. | Field spectrometer | US | [60] |
| Canopy leaf nitrogen content | Assessing impact of IAP (Morella faya Ait.) invasion on nitrogen-oxide emissions. | UAV (aircraft) | US | [57] |
| SLA, LMA, water content, carotenoid (Car) content, Chla, Chlb, total chlorophyll (Chla + Chlb), chlorophyll a:b ratio (Chla/Chlb), carotenoid:total chlorophyll ratio (Car/Chl) | Distinguishing IAPs (Hovenia dulcis and P. guajava), from native plant species (Luehea divaricata Mart and Psidium cattleianum Sabine). | Field spectrometer | Brazil | [58] |
| Canopy, tree diameter, and height | Identification of IAP Acer negundo L in forests. | UAV | Poland | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojija, F.; Petruzzellis, F.; Bacaro, G. Review of Invasive Plant Functional Traits and Management Using Remote Sensing in Sub-Saharan Africa. Int. J. Plant Biol. 2024, 15, 358-374. https://doi.org/10.3390/ijpb15020029
Ojija F, Petruzzellis F, Bacaro G. Review of Invasive Plant Functional Traits and Management Using Remote Sensing in Sub-Saharan Africa. International Journal of Plant Biology. 2024; 15(2):358-374. https://doi.org/10.3390/ijpb15020029
Chicago/Turabian StyleOjija, Fredrick, Francesco Petruzzellis, and Giovanni Bacaro. 2024. "Review of Invasive Plant Functional Traits and Management Using Remote Sensing in Sub-Saharan Africa" International Journal of Plant Biology 15, no. 2: 358-374. https://doi.org/10.3390/ijpb15020029
APA StyleOjija, F., Petruzzellis, F., & Bacaro, G. (2024). Review of Invasive Plant Functional Traits and Management Using Remote Sensing in Sub-Saharan Africa. International Journal of Plant Biology, 15(2), 358-374. https://doi.org/10.3390/ijpb15020029








