Nonhost Resistance of Thinopyrum ponticum to Puccinia graminis f. sp. tritici and the Effects of the Sr24, Sr25, and Sr26 Genes Introgressed to Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Estimation of Stem Rust Development in Field and Laboratory Conditions
2.3. Cytological Methods
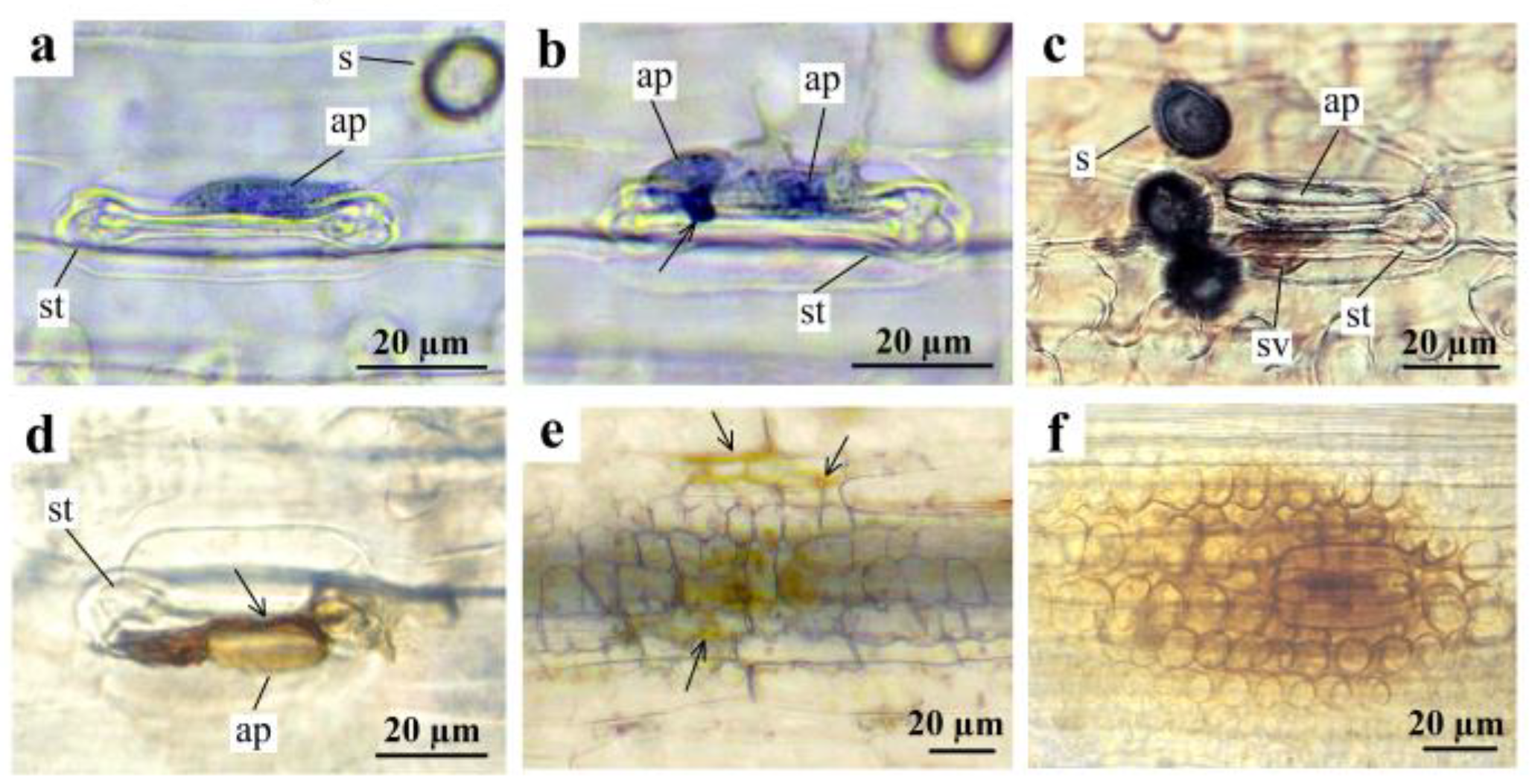
3. Results
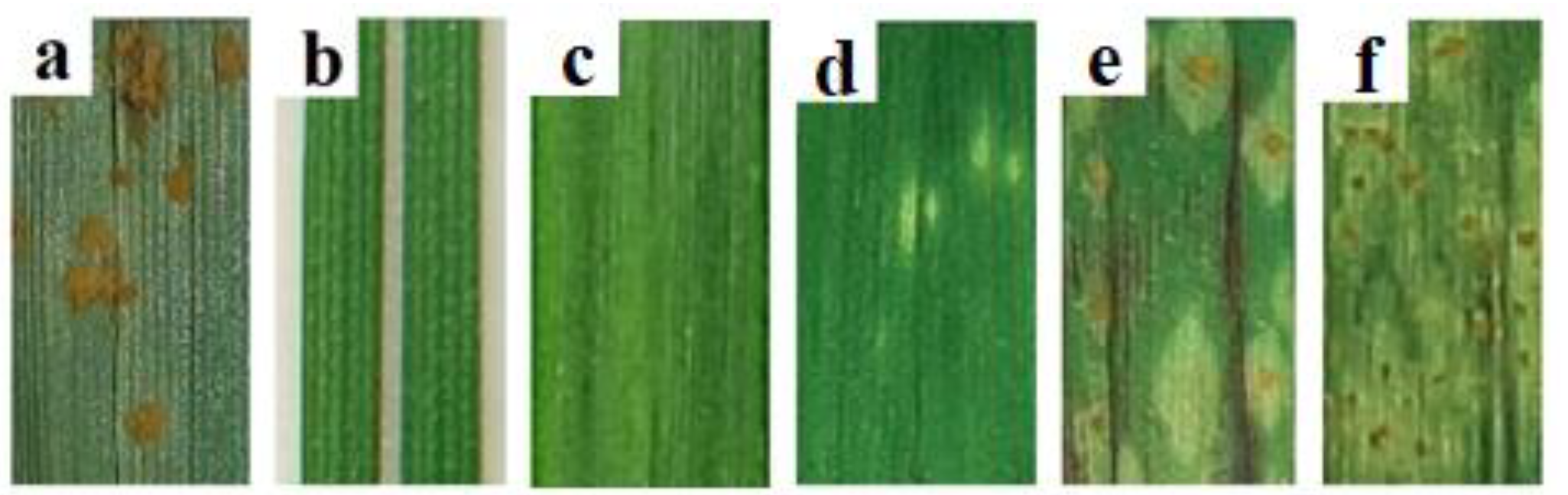
3.1. Estimation of Stem Rust Development in the Field Conditions and Laboratory
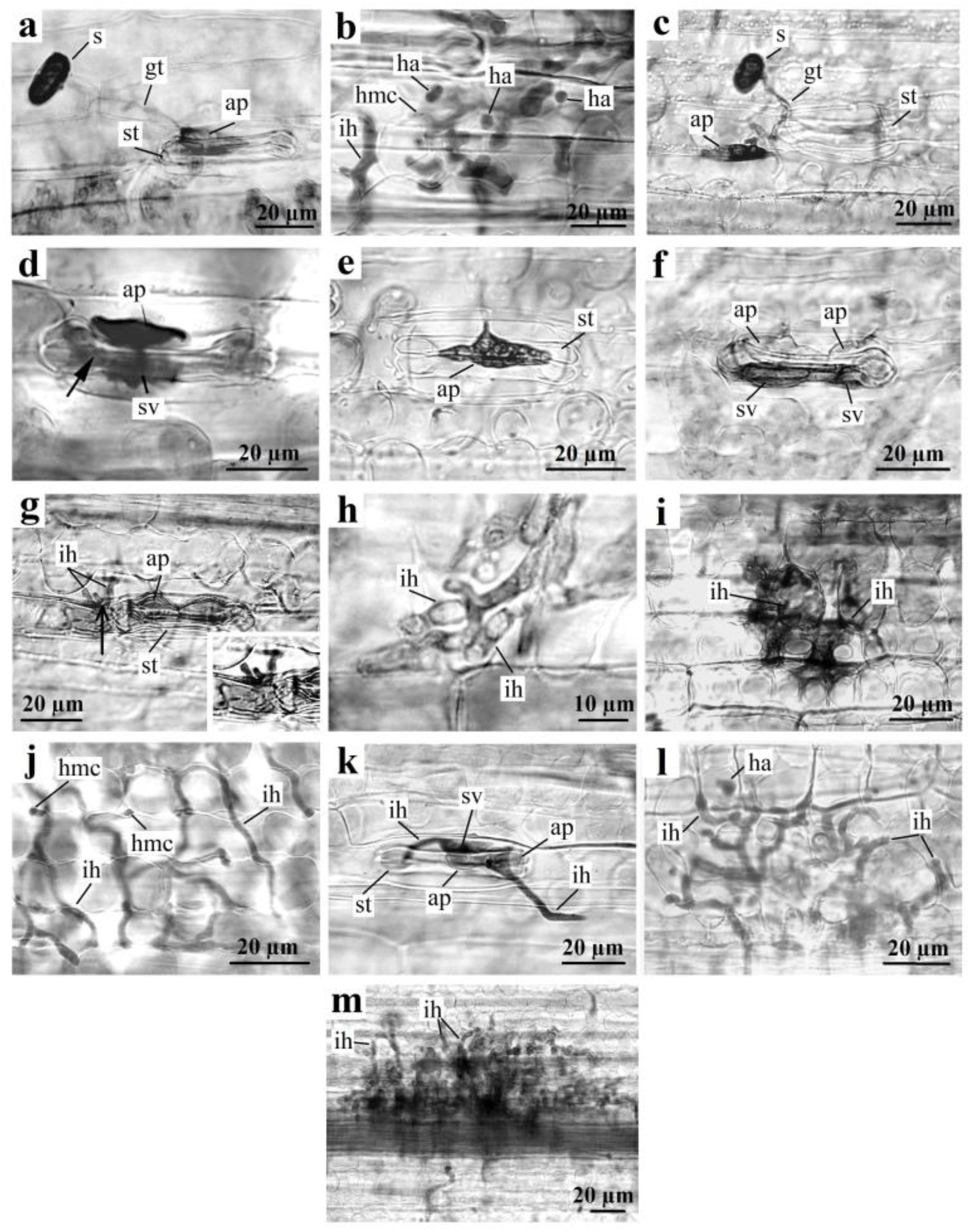
3.2. Interactions between P. graminis f. sp. tritici and Th. ponticum, and WWHs
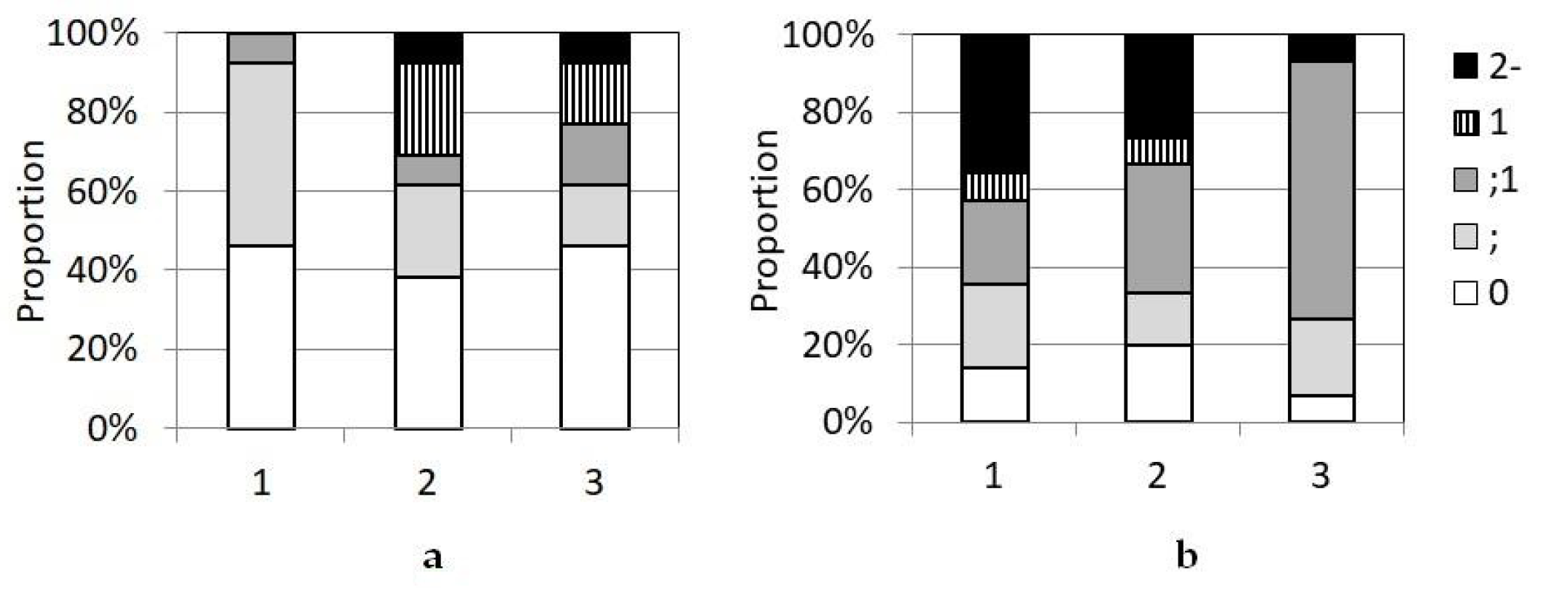
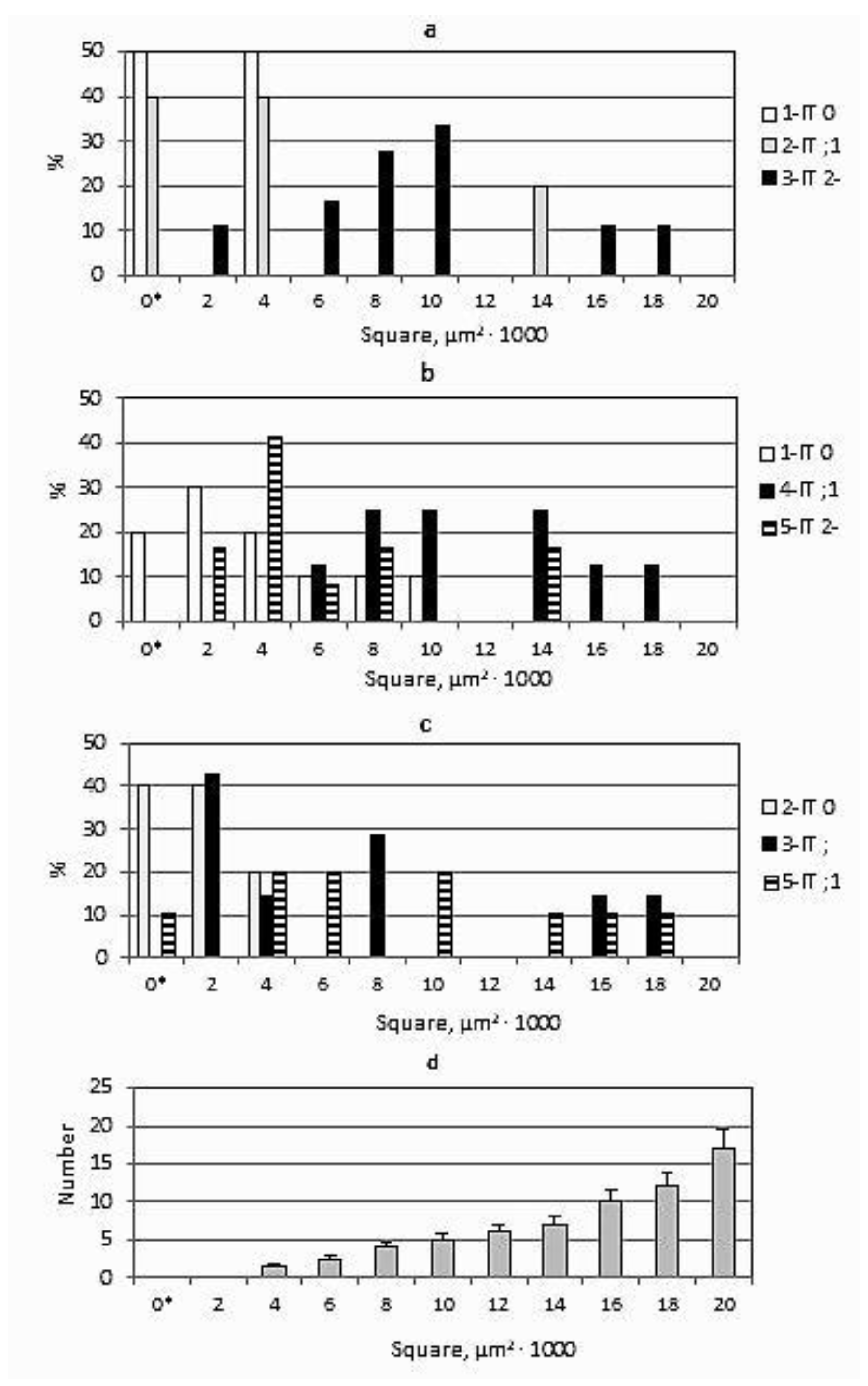
3.3. Interaction of P. graminis f. sp. tritici with Wheat Protected by the Sr24, Sr25, and Sr26 Genes
3.4. The Role of ROS in Interactions of P. graminis f. sp. tritici with Th. ponticum and the Line with the Sr25 Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| Abbreviations in the text | |
| cv. | cultivar |
| DAMPs | Damage-Associated Molecular Patterns |
| ETI | Effector-Triggered Immunity |
| HMC | haustorial mother cell |
| HR | hypersensitive reaction |
| IT | infection type |
| Lr | leaf rust resistance gene |
| MAMPs | Microbe-Associated Molecular Patterns |
| NHR | nonhost resistance |
| NIL | near-isogenic line |
| NLRs | Nucleotide-binding, Leucine-rich-repeat immune Receptors |
| Pgt | Puccinia graminis f. sp. tritici |
| PTI | PAMP-triggered immunity |
| ROS | reactive oxygen species |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PRRs | Pattern Recognition Receptors |
| SA | salicylic acid |
| Sr | stem rust resistance gene |
| SV | substomal vesicle |
| Tc | cultivar Thatcher |
| WWH | wheat–wheatgrass hybrid |
| Yr | stripe rust resistance gene |
| Abbreviations in Figure 1, Figure 3, and Figure 5 | |
| ap | appressorium |
| gt | growing tube |
| ha | haustoria |
| hmc | haustorial mother cell |
| HR | hypersensitive reaction |
| ih | infection hypha |
| s | urediniospore |
| st | stoma |
| sv | substomal vesicle |
| up | urediniopustule |
References
- FAOSTAT. 2019. Available online: https://www.fao.org/faostat (accessed on 12 December 2022).
- USDA. World Agricultural Production; USDA Foreign Agricultural Service: Washington, DC, USA, 2016; p. 20250. [Google Scholar]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jorgensen, L.N.; Hovmoller, M.S.; Huerta-Espino, J. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2005, 144, 31–43. [Google Scholar] [CrossRef]
- Huerta-Espino, J.; Singh, R.P.; Germán, S.S.; McCallum, B.D.; Park, R.F.; Chen, W.Q.; Bhardwaj, S.C.; Goyeau, H. Global Status of Wheat Leaf Rust Caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar] [CrossRef]
- Singh, R.P.; Hodson, D.P.; Jin, Y.; Lagudah, E.S.; Ayliffe, M.A.; Bhavani, S.; Rouse, M.N.; Pretorius, Z.A.; Szabo, L.J.; Huerta-Espino, J.; et al. Emergence and spread of new races of wheat stem rust fungus: Continued threat to food security and prospects of genetic control. Phytopathology 2015, 10, 872–884. [Google Scholar] [CrossRef]
- Pretorius, Z.A.; Bender, C.M.; Visser, B.; Terefe, T. First report of a Puccinia graminis f. sp. tritici race virulent to the Sr24 and Sr31 wheat stem rust resistance genes in South Africa. Plant Dis. 2010, 94, 784. [Google Scholar] [CrossRef] [PubMed]
- Patpour, M.; Hovmøller, M.S.; Rodriguez-Algaba, J.; Randazzo, B.; Villegas, D.; Shamanin, V.P.; Berlin, A.; Flath, K.; Czembor, P.; Hanzalova, A.; et al. Wheat Stem Rust Back in Europe: Diversity, Prevalence and Impact on Host Resistance. Front. Plant Sci. 2022, 13, 882440. [Google Scholar] [CrossRef]
- Fetch, T.G.; Park, R.F.; Pretorius, Z.A.; Depauw, R.M. Stem rust: Its history in Kenya and research to combat a global wheat threat. Can. J. Plant Pathol. 2021, 43, 275–297. [Google Scholar] [CrossRef]
- Sinha, P.; Chen, X. Potential Infection Risks of the Wheat Stripe Rust and Stem Rust Pathogens on Barberry in Asia and Southeastern Europe. Plants 2021, 10, 957. [Google Scholar] [CrossRef]
- Markelova, T.S. Phytosanitary situation in the agrocenosis of grain crops in the Volga Region. Zashchita i Karantin Rasteniy = Plant Prot. Quar. 2015, 5, 22–23. (In Russian) [Google Scholar]
- Sibikeev, S.N.; Druzhin, A.E.; Badaeva, E.D.; Shishkina, A.A.; Dragovich, A.Y.; Gultyaeva, E.I.; Kroupin, P.Y.; Karlov, G.I.; Khuat, T.M.; Divashuk, M.G. Comparative analysis of Agropyron intermedium (Host) Beauv 6Agi and 6Agi2 chromosomes in bread wheat cultivars and lines with wheat-wheatgrass substitutions. Russ. J. Genet 2017, 53, 314–324. [Google Scholar] [CrossRef]
- Baranova, O.A.; Sibikeev, S.N.; Druzhin, A.E. Molecular identification of the stem rust resistance genes in the introgression lines of spring bread wheat. Vavilov J. Genet. Breed 2019, 23, 296–303. [Google Scholar] [CrossRef]
- Leonova, I.N.; Skolotneva, E.S.; Orlova, E.A.; Orlovskaya, O.A.; Salina, E.A. Detection of Genomic Regions Associated with Resistance to Stem Rust in Russian Spring Wheat Varieties and Breeding Germplasm. Int. J. Mol. Sci. 2020, 21, 4706. [Google Scholar] [CrossRef]
- Aidarbekova, T.J.; Syzdykova, G.T.; Malitskaya, N.V.; Nurgaziyev, R.E.; Husainov, A.T.; Zhabayeva, M.U.; Makhanova, S.K.; Shoykin, O.D. Comparative assessment of spring soft wheat lines (Triticum aestivum L.) in the steppe zone of the north Kazakhstan region. Sel’skokhozyaistvennaya Biologiya [Agric. Biol.] 2022, 57, 66–80. [Google Scholar] [CrossRef]
- Plotnikova, L.Y.; Aidosova, A.T.; Rispekova, A.N.; Myasnikov, A.Y. Introgressive lines of common wheat with genes of wheat grass Agropyron elongatum resistant to leaf diseases in the South West Siberia. Vestn. OmGAU 2014, 4, 3–7. (In Russian) [Google Scholar]
- Shamanin, V.P.; Salina, E.; Wanyera, R.; Zelenskiy, Y.; Olivera, P.; Morgunov, A. Genetic diversity of spring wheat from Kazakhstan and Russia for resistance to stem rust Ug99. Euphytica 2016, 212, 287–296. [Google Scholar] [CrossRef]
- Baranova, O.A. Molecular identification of stem rust resistance genes in new regional wheat varieties. Vestn. Zashchity Rasteniy = Plant Prot. News 2020, 103, 113–118. [Google Scholar] [CrossRef]
- Skolotneva, Å.S.; Kelbin, V.N.; Morgunov, A.I.; Boiko, N.I.; Shamanin, V.P.; Salina, E.A. Races composition of the Novosibirsk population of Puccinia graminis f. sp. tritici. Micol. Phytopatol 2020, 54, 49–58. [Google Scholar]
- Kelbin, V.N.; Skolotneva, E.S.; Salina, E.A. Challenges and prospects for developing genetic resistance in common wheat against stem rust in Western Siberia. Vavilovskii Zhurnal Genet. I Sel. = Vavilov J. Genet. Breed. 2020, 24, 821–828. [Google Scholar] [CrossRef]
- Bhavani, S.; Hodson, D.P.; Huerta-Espino, J.; Randhawa, M.S.; Singh, R.P. Progress in breeding for resistance to Ug99 and other races of the stem rust fungus in CIMMYT wheat germplasm. Front. Agric. Sci. Eng. 2019, 6, 210–224. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Chen, X.; Zhou, J. From plant immunity to crop disease resistance. J. Genet. Genom. 2022, 49, 693–703. [Google Scholar] [CrossRef]
- Kolmer, J. Leaf Rust of Wheat: Pathogen Biology, Variation and Host Resistance. Forests 2013, 4, 70–84. [Google Scholar] [CrossRef]
- McDonald, B.A.; Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef]
- Baker, L.; Grewal, S.; Yang, C.; Hubbart-Edwards, S.; Scholefield, D.; Ashling, S.; Burridge, A.; Przewieslik-Allen, A.; Wilkinson, P.; King, I.; et al. Exploiting the genome of Thinopyrum elongatum to expand the gene pool of hexaploid wheat. Theor. Appl. Genet 2020, 133, 2213–2226. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.A.R.; Arseniuk, E.; Börner, A. Genetic variability for resistance to fungal pathogens in bread wheat. Czech J. Genet. Plant Breed 2023, 59, 23–32. [Google Scholar] [CrossRef]
- Friebe, B.G.; Jiang, J.; Raupp, W.J.; McIntosh, R.A.; Gill, B.S. Characterization of wheat translocations conferring resistance to diseases and pests: Current status. Euphytica 1996, 1, 9–87. [Google Scholar] [CrossRef]
- Wulf, B.B.H.; Moscou, J.M. Strategies for transferring resistance into wheat: From wide crosses to GM cassettes. Front. Plant Sci. 2014, 5, 692. [Google Scholar] [CrossRef]
- Ceoloni, C.; Kuzmanović, L.; Forte, P.; Gennaro, A.; Bitti, A. Targeted exploitation of gene pools of alien Triticeae species for sustainable and multi-faceted improvement of the durum wheat crop. Crop Pasture Sci. 2014, 65, 96–111. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Yamazaki, Y.; Dubcovsky, J.; Rogers, J.; Morris, C.; Appels, R.; Xia, X.C. Catalogue of Gene Symbols for Wheat. 2013. Available online: http://www.shigen.nig.ac.jp/wheat/komugi/genes/ (accessed on 15 January 2023).
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Morris, C.; Appels, R.; Xia, X.C. Catalogue of Gene Symbols for Wheat: 2015–2016 Supplement. In Annual Wheat Newsletter; KSU: USA, 2016; Volume 62, pp. 102–115. [Google Scholar]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Xia, X.C.; Raupp, W.J. Catalogue of Gene Symbols for Wheat: 2018 Supplement. In Annual Wheat Newsletter; KSU: USA, 2018; Volume 64, pp. 73–93. [Google Scholar]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Xia, X.C.; Raupp, W.J. Catalogue of Gene Symbols for Wheat: 2020 Supplement. In Annual Wheat Newsletter; KSU: USA, 2020; Volume 66, pp. 109–128. [Google Scholar]
- Zhang, J.; Hewitt, T.C.; Boshoff, W.H.P.; Dundas, I.; Upadhyaya, N.; Li, J.; Patpour, M.; Chandramohan, S.; Pretorius, Z.A.; Hovmøller, M.; et al. A recombined Sr26 and Sr61 disease resistance gene stack in wheat encodes unrelated NLR genes. Nat. Commun. 2021, 12, 3378. [Google Scholar] [CrossRef] [PubMed]
- Kroupin, P.Y.; Kuznetsova, V.M.; Nikitina, E.A.; Martirosyan, Y.T.; Karlov, G.I.; Divashuk, M.G. Development of new cytogenetic markers for Thinopyrum ponticum (Podp.) Z.-W. Liu & R.-C. Wang. Comp. Cytogenet. 2019, 13, 231–243. [Google Scholar] [CrossRef]
- Li, H.; Boshoff, W.H.P.; Pretorius, Z.A.; Zheng, Q.; Li, B.; Li, Z. Establishment of wheat-Thinopyrum ponticum translocation lines with resistance to Puccinia graminis f. sp. tritici Ug99. J. Genet. Genom. 2019, 46, 405–407. [Google Scholar] [CrossRef]
- Sheng, D.; Liu, M.; Zhang, X.; Qiao, L.; Chang, L.; Guo, H.; Zhang, S.; Chen, F.; Yuan, Z.; Liu, C.; et al. Characterization of leaf rust resistance in a set of wheat-Thinopyrum amphiploid-derived hexaploid breeding lines. Crop Prot. 2022, 156, 105956. [Google Scholar] [CrossRef]
- Kolmer, T.; Flowers, T.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot 2006, 57, 1059–1078. [Google Scholar] [CrossRef]
- Taeb, M.; Koebner, R.; Forster, B. Genetic variation for waterlogging tolerance in the Triticeae and the chromosomal location of genes conferring waterlogging tolerance in Thinopyrum elongatum. Genome 1993, 36, 825–830. [Google Scholar] [CrossRef]
- Tsitsin, N.V. Problems of distant hybridization. In Problems of Distant Hybridization; Tsitsin, N.V., Ed.; Kolos: Moscow, Russia, 1979; pp. 5–20. [Google Scholar]
- Hang, A.; Bockelman, H.E.; Burton, C.S. Cytological and seed morphological investigation of 250 accessions from the W.J. Sando collection. In Proceedings of the Agronomy Society of America, Crop Science Society of America, Soil Science Society of America Meeting, Salt Lake, UT, USA, 6–10 November 2005. [Google Scholar]
- Li, H.; Wang, X. Thinopyrum ponticum and Th. intermedium: The promising source of resistance to fungal and viral diseases of wheat. J. Genet. Genom. 2009, 36, 557–565. [Google Scholar] [CrossRef]
- Kruppa, K.; Türkösi, E.; Mayer, M.; Tóth, V.; Vida, G.; Szakács, É.; Molnár-Láng, M. McGISH identification and phenotypic description of leaf rust and yellow rust resistant partial amphiploids originating from a wheat × Thinopyrum synthetic hybrid cross. J. Appl. Genet. 2016, 57, 427–437. [Google Scholar] [CrossRef]
- Mo, Q.; Wang, C.Y.; Chen, C.H.; Wang, Y.J.; Zhang, H.; Liu, X.L.; Ji, W.Q. Molecular Cytogenetic Identification of a Wheat-Thinopyrum ponticum Substitution Line with Stripe Rust Resistance. Cereal Res. Commun. 2017, 45, 564–573. [Google Scholar] [CrossRef]
- Pei, Y.; Cu, Y.; Zhang, Y.; Wang, H.; Bao, Y.; Li, X. Molecular cytogenetic identification of three rust-resistant wheat-Thinopyrum ponticum partial amphiploids. Mol. Cytogenet. 2018, 11, 27. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Wang, R.R.C. Genome analysis of Elytrigia caespitosa, Lophopyrum nodosum, Pseudoroegneria geniculata ssp. scythica, and Thinopyrum intermedium (Triticeae: Gramineae). Genome 1993, 36, 102–111. [Google Scholar] [CrossRef]
- Martynov, S.P.; Dobrotvorskaya, T.V.; Krupnov, V.A. Genealogical Analysis of the Use of Two Wheatgrass (Agropyron) Species in Common Wheat (Triticum aestivum L.) Breeding for Disease Resistance. Genetika 2016, 52, 179–188. [Google Scholar] [CrossRef]
- Flath, K.; Miedaner, T.; Olivera, P.D.; Rouse, M.N.; Jin, Y. Genes for wheat stem rust resistance postulated in German cultiv`ars and their efficacy in seedling and adult-plant field tests. Plant Breed 2018, 1, 12. [Google Scholar] [CrossRef]
- Sibikeev, S.N.; Baranova, O.A.; Druzhin, A.E. A prebreeding study of introgression spring bread wheat lines carrying combinations of stem rust resistance genes, Sr22+Sr25 and Sr35+Sr25. Vavilovskii Zhurnal Genet. I Sel. = Vavilov J. Genet. Breed. 2021, 25, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Niks, R.E. How Specific is Non-Hypersensitive Host and Nonhost Resistance of Barley to Rust and Mildew Fungi? J. Integr. Agric. 2014, 13, 244–254. [Google Scholar] [CrossRef]
- Lee, S.; Hutton, S.; Whitaker, V. Mini Review: Potential Applications of Non-host Resistance for Crop Improvement. Front. Plant Sci. 2016, 7, 997. [Google Scholar] [CrossRef] [PubMed]
- Van Baarlen, P.; van Belkum, A.; Summerbell, R.C.; Crous, P.W.; Thomma, B.P.H.J. Molecular mechanisms of pathogenicity: How do pathogenic microorganisms develop cross-kingdom host jumps? FEMS Microbiol. Rev. 2007, 31, 239–277. [Google Scholar] [CrossRef] [PubMed]
- Ellingboe, A.H. Genetics of Host-Parasite Interactions. In Encyclopedia of Plant Physiology; Heitefuss, R., Williams, P.H., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1976; pp. 761–778. [Google Scholar]
- Heath, M.C. Reaction of Nonsuscepts to fungal pathogens. Curr. Opin. Plant Biol. 1980, 18, 211–236. [Google Scholar] [CrossRef]
- Ayliffe, M.; Jin, Y.; Kang, Z.; Persson, M.; Steffenson, B.; Wang, S.; Leung, H. Determining the basis of nonhost resistance in rice to cereal rusts. Euphytica 2011, 179, 33–40. [Google Scholar] [CrossRef]
- Heath, M.C. Nonhost resistance and nonspecific plant defenses. Curr. Opin. Plant Biol. 2000, 3, 315–319. [Google Scholar] [CrossRef]
- Flor, H.H. Current status of the gene-for-gene concept. Annu. Rev. Phytopathol. 1971, 9, 275–296. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Lee, H.-A.; Seo, E.; Lee, J.; Kim, S.-B.; Oh, S.; Choi, E.; Choi, E.; Lee, S.E.; Choi, D. Current Understandings of Plant Nonhost Resistance. Mol. Plant Microbe Interact. 2017, 30, 5–15. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Gill, U.S.; Lee, S.; Mysore, K.S. Host versus nonhost resistance: Distinct wars with similar arsenals. Phytopathology 2015, 105, 580–587. [Google Scholar] [CrossRef]
- Boller, T.; He, S.Y. Innate immunity in plants: An arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 2009, 324, 742–744. [Google Scholar] [CrossRef]
- Schulze-Lefert, P.; Panstruga, R. A molecular evolutionary concept connecting nonhost resistance, pathogen host range, and pathogen speciation. Trends Plant Sci. 2011, 16, 117–125. [Google Scholar] [CrossRef]
- Peng, Y.; Wersch, R.; Zhang, Y. Convergent and Divergent Signaling in PAMP-Triggered Immunity and Effector-Triggered Immunity. Mol. Plant-Microbe Interact. 2018, 31, 403–409. [Google Scholar] [CrossRef]
- Yuan, M.; Pok, B.; Ngou, M.; Ding, P.; Xin, X.-F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, J. Regulation of plant responses to biotic and abiotic stress by receptor-like cytoplasmic kinases. Stress Biol. 2022, 2, 25. [Google Scholar] [CrossRef]
- Littlefield, L.J.; Heath, M.C. Ultrastructure of Rust Fungi; Academic Press: New York, NY, USA; San Francisco, CA, USA; London, UK, 1979; 277p. [Google Scholar]
- Mendgen, K.; Struck, C.; Voegele, R.T.; Hahn, M. Biotrophy and rust haustoria. Physiol. Plant Pathol 2000, 56, 141–145. [Google Scholar] [CrossRef]
- Anker, C.C.; Niks, R.E. Prehaustorial resistance to the wheat leaf rust fungus Puccinia triticina in Triticum monococcum (s.s.). Euphytica 2001, 117, 209–215. [Google Scholar] [CrossRef]
- Plotnikova, L.Y. Influence of the surface features and physiological reactions of non-host species on the development of cellular structures of rust fungi. Tcitologija 2008, 50, 439–446. [Google Scholar]
- Elmhirst, J.F.; Heath, M.C. Interactions of the bean rust and cowpea rust fungi with species of the Phaseolus-Vigna plant complex. I. Fungal growth and development. Can. J. Bot. 1987, 65, 1096–1107. [Google Scholar] [CrossRef]
- Plotnikova, L.Y.; Seryukov, G.M.; Shvarts, Y.K. Cytophysiological resistance mechanisms to leaf rust in Wheat-Agropyron hybrids created on the base of Agropyron elongatum. Mikol. Fitopatol. 2011, 45, 443–454. (In Russian) [Google Scholar]
- Roelfs, A.P.; Singh, R.P.; Saari, E.E. Rust Diseases of Wheat: Concepts and Methods of Disease Management; International Maize and Wheat Improvement Center: Mexico City, Mexico, 1992. [Google Scholar]
- Koyshybaev, M. Wheat Diseases; FAO: Ankara, Turkey, 2018; 394p. [Google Scholar]
- Roelfs, A.P.; Martens, J.W. An international system of nomenclature for Puccinia graminis f. sp. tritici. Phytopathology 1988, 78, 526–533. [Google Scholar] [CrossRef]
- Plotnikova, L.Y.; Meshkova, L.V. Evolution of cytophysiological relationships between leaf rust causal agent and common wheat in the process of overcoming of resistance determined by the gene Lr19. Mikol. Fitopatol. 2009, 43, 343–357. [Google Scholar]
- Plotnikova, L.Y.; Pozherukova, V.Y.; Mitrofanova, O.P.; Degtyarev, A.I. The Effect of Oxidative Burst Suppression or Induction on the Interaction between Brown Rust Fungus and Timopheevi Wheat. Appl. Biochem. Microbiol. 2016, 52, 61–70. [Google Scholar] [CrossRef]
- Gultyaeva, E.; Shaydayuk, E.; Kosman, E. Virulence Diversity of Puccinia striiformis f. sp. tritici in Common Wheat in Russian Regions in 2019–2021. Agriculture 2022, 12, 1957. [Google Scholar] [CrossRef]
- Prank, M.; Kenaley, S.C.; Bergstrom, G.C.; Acevedo, M.; Mahowald, N.M. Climate change impacts the spread potential of wheat stem rust, a significant crop disease. Environ. Res. Lett. 2019, 14, 10. [Google Scholar] [CrossRef]
- Park, R.F.; Golegaonkar, P.G.; Derevnina, L.; Sandhu, K.S.; Karaoglu, H.; Elmansour, H.M.; Dracatos, P.M.; Singh, D. Leaf rust of cultivated barley: Pathology and control. Annu Rev. Phytopathol 2015, 53, 565–589. [Google Scholar] [CrossRef]
- Pathotype Tracker—Where is Ug99?/RustTracker.org. Available online: http://rusttracker.cimmyt.org/ (accessed on 1 December 2022).
- Jin, Y.; Szabo, L.J.; Pretorius, Z.A.; Singh, R.P.; Ward, R.; Fetch, T., Jr. Detection of virulence to resistance gene Sr24 with in race TTKS of Puccinia graminis f. sp. tritici. Plant Dis. 2008, 92, 923–926. [Google Scholar] [CrossRef]
- Pruitt, R.N.; Locci, F.; Wanke, F.; Zhang, L.; Saile, S.C.; Joe, A.; Karelina, D.; Hua, C.; Fröhlich, K.; Wan, W.-L.; et al. The EDS1-PAD4-ADR1 node mediates Arabidopsis pattern-triggered immunity. Nature 2021, 598, 495–499. [Google Scholar] [CrossRef]
- Zhai, K.; Liang, D.; Li, H.; Jiao, F.; Yan, B.; Liu, J.; Lei, Z.; Huang, L.; Gong, X.; Wang, X.; et al. NLRs guard metabolism to coordinate pattern- and effector-triggered immunity. Nature 2022, 601, 245–251. [Google Scholar] [CrossRef]
- Liu, X.; Ao, K.; Yao, J.; Zhang, Y.; Li, X. Engineering plant disease resistance against biotrophic pathogens. Curr. Opin. Plant Biol. 2021, 60, 101987. [Google Scholar] [CrossRef]
- Frazer, R.S.S. Special aspects of resistance to viruses. In Mechanisms of Resistance to Plant Diseases; Slusarenko, A.J., Frazer, R.S.S., van Loon-Kluewer, L.S., Eds.; Academic Publisher: Amsterdam, The Netherlands, 2000; pp. 479–518. [Google Scholar] [CrossRef]
- Zhou, H.; Lin, J.; Johnson, A.; Morgan, R.L.; Zhong, W.; Ma, W. Pseudomonas syringae type III effector HopZ1 targets a host enzyme to suppress isoflavone biosynthesis and promote infection in soybean. Cell Host Microbe 2011, 9, 177–186. [Google Scholar] [CrossRef]
- Peng, P.; Li, R.; Chen, Z.-.H.; Wang, Y. Stomata at the crossroad of molecular interaction between biotic and abiotic stress responses in plants. Front. Plant Sci. 2022, 13, 1031891. [Google Scholar] [CrossRef]
- McDonald, M.C.; Solomon, P.S. Just the surface: Advances in the discovery and characterization of necrotrophic wheat effectors. Curr. Opin. Microbiol. 2018, 46, 14–18. [Google Scholar] [CrossRef]
- Shafiei, R.; Hang, C.; Kang, J.-G.; Loake, G.J. Identification of loci controlling non-host disease resistance in Arabidopsis against the leaf rust pathogen Puccinia triticina. Mol. Plant Pathol. 2007, 8, 773–784. [Google Scholar] [CrossRef]
- Schulze-Lefert, P.; Panstruga, R. Establishment of Biotrophy by Parasitic Fungi and Reprogramming of Host Cells for Disease Resistance. Annu. Rev. Phytopathol. 2003, 41, 641–667. [Google Scholar] [CrossRef]
- Chen, S.; Guo, Y.; Briggs, J.; Dubach, F.; Chao, S.; Zhang, W.; Rouse, M.N.; Dubcovsky, J. Mapping and characterization of wheat stem rust resistance genes SrTm5 and Sr60 from Triticum monococcum. Theor. Appl. Genet. 2018, 131, 625–635. [Google Scholar] [CrossRef]
- Chen, J.P.; Upadhyaya, N.M.; Ortiz, D.; Sperschneider, J.; Li, F.; Bouton, C.; Breen, S.; Dong, C.M.; Xu, B.; Zhang, X.X.; et al. Loss of AvrSr50 by somatic exchange in stem rust leads to virulence for Sr50 resistance in wheat. Science 2017, 358, 1607–1610. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Zipfel, C. Molecular mechanisms of early plant pattern triggered immune signaling. Mol. Cell. 2021, 81, 3449–3467. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.C. Apoptosis, programmed cell death and the hypersensitive response. Eur. J. Plant Pathol. 1998, 104, 117–124. [Google Scholar] [CrossRef]
- Yarullina, L.G.; Kasimova, R.I.; Burkhanova, G.F.; Akhatova, A.R. The effect of salicylic and jasmonic acids on the activity and range of protective proteins during the infection of wheat by the septoriosis pathogen. Biol. Bull. 2015, 42, 27–33. [Google Scholar] [CrossRef]
- Boller, T.; Keen, N.T. Perception and Transduction of Elicitor Signals in Host-Pathogen Interactions. In Mechanisms of Resistance to Plant Diseases; Slusarenko, A.J., Frazer, R.S.S., van Loon, L.S., Eds.; Kluwer: Dordrecht, The Netherlands, 2000; pp. 189–230. [Google Scholar]
- Bi, G.; Zhou, J.M. Regulation of cell death and signaling by pore-forming resistosomes. Annu. Rev. Phytopathol. 2021, 59, 239–263. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Plotnikova, L.Y.; Shtubey, T.Y. Influence of salicylic and succinic acids on the cytophysiological reactions of wheat infected by brown rust. Tcitologija 2009, 51, 43–52. [Google Scholar]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.-M.; He, S.Y.; Xin, X.-F. Pattern-recognition receptors are required for NLR mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Plotnikova, L.; Pozherukova, V.; Knaub, V.; Kashuba, Y. What was the reason for the durable effect of Sr31 against wheat stem rust? Agriculture 2022, 12, 2116. [Google Scholar] [CrossRef]
- Plotnikova, L.Y. The involvement of reactive oxygen species in defense of wheat lines with the genes introgressed from Agropyron species contributing the resistance against brown rust. Rus. J. Plant Physiol. 2009, 56, 200–209. [Google Scholar] [CrossRef]
- Plotnikova, L.Y. Cellular features of immune reaction of common wheat with Lr19 gene to brown rust fungus infection. Tsitologiia 2008, 50, 124–131. [Google Scholar]
- Grimmer, M.K.; Foulkes, M.J.; Paveley, N.D. Foliar pathogenesis and plant water relations: A Review. J. Exp. Bot. 2012, 63, 4321–4331. [Google Scholar] [CrossRef]
- Murata, Y.; Mori, I.C.; Munemasa, S. Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 2015, 66, 369–392. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef]
- Qi, J.; Wang, J.; Gong, Z.; Zhou, J.M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol. 2017, 38, 92–100. [Google Scholar] [CrossRef]
- Thor, K.; Jiang, S.; Michard, E.; George, J.; Scherzer, S.; Huang, S.; Dindas, J.; Derbyshire, P.; Leitao, N.; DeFalco, T.A.; et al. The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature 2020, 585, 569–573. [Google Scholar] [CrossRef]
- Melotto, M.; Zhang, L.; Oblessuc, P.R.; He, S.Y. Stomatal Defense a Decade Later. Plant Physiol. 2017, 174, 561–571. [Google Scholar] [CrossRef]
- Frailie, T.B.; Innes, R.W. Engineering healthy crops: Molecular strategies for enhancing the plant immune system. Curr. Opin. Biotechnol. 2021, 70, 151–157. [Google Scholar] [CrossRef]
- Tucker, S.L.; Talbot, N.J. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 2001, 39, 385–417. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Sharma, N.; Gautam, A.K. Early Pathogenicity events in Plant Pathogenic Fungi: A Comprehensive Review. Biol. Forum—Int. J. 2019, 11, 24–34. [Google Scholar]
- Mendgen, K.; Schneider, A.; Sterk, M.; Fink, W. The differentiation of infection structure as a result of recognition events between some biotrophic parasites and their hosts. J. Phytopathol. 1988, 123, 259–272. [Google Scholar] [CrossRef]
- Mendgen, K.; Hahn, M. Plant infection and the establishment of fungal biotrophy. Trends Plant Sci. 2002, 7, 352–356. [Google Scholar] [CrossRef]
- Mendgen, K.; Wiesel, S.G.R.; Jux, A.; Hoffmann, J.; Boland, W. Volatiles modulate the development of plant pathogenic rust fungi. Planta 2006, 224, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.; Troshina, N.; Surina, O.; Cherepanova, E. Salicylic acid increases the defense reaction against bunt and smut pathogens in wheat calli. J. Plant Interact. 2014, 9, 306–314. [Google Scholar] [CrossRef]
| Sample | Field, Severity, and IT | Seedling Test, IT * | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | |||||||||
| 15.08 | 25.08 | 05.08 | 15.08 | 15.08 | 25.08 | 15.08 | 25.08 | 14.08 | 25.08 | 15.08 | 25.08 | 25.08 | 2020 | 2022 | |
| Pamyati Azieva | 60S | 100S | 30S | 80S | 30S | 50S | 60S | 100S | 50S | 100S | 20S | 50S | 20S | 4 | 4 |
| Chernyava 13 | 60S | 100S | 40S | 80S | 40S | 60S | 60S | 100S | 60S | 100S | 20S | 50S | 40S | 4 | 4 |
| Th. ponticum Russia, Africa | R | R | R | R | R | R | R | R | R | R | R | R | R | 0 | 0 |
| IH ** | R | R | R | R | R | R | R | R | R | R | R | R | R | ; | 0 |
| WWH-1 *** | R | R | R | R | R | R | R | R | R | R | R | R | R | ; | 0 |
| WWH-2 | R | R | R | R | R | R | R | R | R | R | R | R | R | 0 | ; |
| WWH-3 | R | R | R | R | R | R | R | R | R | R | R | R | R | ; | ; |
| WWH-4 | R | R | R | R | R | R | R | R | R | R | R | R | R | 0 | 0 |
| WWH-5 | R | R | R | R | R | R | R | R | R | R | R | R | R | ; | ; |
| WWH-6 | R | R | R | R | R | R | R | R | R | R | R | R | R | 0 | 0 |
| LMPG-Sr24 | 20MS | 50MS | 10MR | 30MS | 5MR | 30MS | 5MR | 30M | R | 30MS | 5MR | 10S | R | ;, ;1, 2 | ;1, 2- |
| TcLr24/Sr24 | 10MS | 40S | 10MR | 30MS | R | 10MS | R | 10MR | R | 20M | R | 10MR | R | ;, ;1 | ;1, 2- |
| LMPG-Sr25 | 20MS | 50S | 10MR | 20MR | 5M | 10M | 5M | 10M | 5MR | 20M | 5MR | 20M | 5MR | ;1, 2- | ;, ;1 |
| TcLr19/Sr25 | 10MS | 30MS | R | 5MR | R | 5M | R | 5M | 5MR | 10M | R | 10M | R | ;1, 2- | ;1, 2- |
| Eagle-Sr26 | 5MR | 40S | 5MR | 30MS | R | 10MR | R | 10M | R | 10M | R | 5MS | R | ;1, 2- | ;1, 2- |
| LMPG-Sr26 | 10MS | 40S | 10M | 50MS | R | 5MR | R | 5M | R | 5M | R | 10MR | R | ;1, 2- | ;1, 2- |
| Sample | IT | Proportion of Germinated Spores, % | Proportion of Growing Tubes with Appressoria, % | Proportion of Appressoria, % | Proportion of Inoculum Penetrated into Stomata, % | |
|---|---|---|---|---|---|---|
| On Stomata from the Total Number | Germinated from the Number on the Stomata | |||||
| Chernyava 13—control | 4 | 91.0 ± 3.6 | 87.0 ± 4.5 | 89.0 ± 3.4 | 85.0 ± 0.9 | 59.9 |
| Th. ponticum, Russia | 0 | 87.1 ± 4.4 | 14.1 ± 0.2 | 39.3 ± 3.5 | 0.0 | 0.0 |
| Th. ponticum, Africa | ; | 88.4 ± 4.4 | 27.2 ± 1.4 | 40.0 ± 4.0 | 7.1 ± 4.6 | 0.7 |
| IH | 0 | 82.6 ± 4.1 | 26.0 ± 1.3 | 58.6 ± 2.9 | 13.5 ± 4.3 | 1.7 |
| WWH-1 | 0 | 77.9 ± 3.9 | 55.9 ± 2.8 | 60.2 ± 3.0 | 11.9 ± 4.4 | 3.1 |
| WWH-2 | ; | 76.8 ± 3.8 | 42.0 ± 2.1 | 63.6 ± 3.2 | 9.2 ± 2.5 | 1.9 |
| WWH-3 | ; | 79.1 ± 3.9 | 32.0 ± 1.6 | 70.3 ± 3.5 | 5.4 ± 4.7 | 1.0 |
| WWH-4 | 0 | 85.3 ± 4.3 | 34.0 ± 1.7 | 63.1 ± 3.2 | 11.0 ± 4.5 | 2.3 |
| WWH-5 | ; | 83.8 ± 4.2 | 31.7 ± 1.6 | 67.3 ± 3.4 | 10.8 ± 4.5 | 1.9 |
| WWH-6 | 0 | 78.8 ± 3.9 | 46.2 ± 2.3 | 70.5 ± 3.5 | 0.0 | 0.0 |
| Sample | Isolate | IT | Proportion of Germinated Spores, % | Proportion of Growing Tubes with Appressoria, % | Proportion of Appressoria, % | Proportion of Inoculum Penetrated into Stomata, % | |
|---|---|---|---|---|---|---|---|
| On Stomata from the Total Number | Germinated from the Number on the Stomata | ||||||
| Chernyava 13—control * | 1, 2, 3, 4 | 4 | 93.5 ± 3.2 | 85.0 ± 4.5 | 87.0 ± 3.6 | 88.0 ± 1.2 | 60.8 |
| TcLr24/Sr24 | 1 | 0 | 89.6 ± 2.4 | 27.6 ± 2.1 | 38.3 ± 2.9 | 3.8 ± 1.5 | 0.4 |
| 2 | ;1 | 88.7 ± 3.5 | 39.4 ± 6.1 | 83.8 ± 4.9 | 5.4 ± 1.2 | 1.6 | |
| 3 | 2- | 92.2 ± 1.6 | 57.7 ± 4.5 | 91.4 ± 1.2 | 21.2 ± 3.5 | 11.2 | |
| TcLr19/Sr25 | 1 | 0 | 93.8 ± 4.4 | 21.8 ± 2.3 | 86.3 ± 5.2 | 3.7 ± 1.3 | 0.7 |
| 4 | ;1 | 87.6 ± 4.6 | 30.3 ± 2.9 | 45.0 ± 8.3 | 24.2 ± 3.2 | 3.3 | |
| 5 | 2- | 85.4 ± 2.4 | 65.3 ± 5.1 | 87.2 ± 3.2 | 25.6 ± 2.6 | 14.6 | |
| LMPG-Sr26 | 2 | 0 | 89.8 ± 2.3 | 29.3 ± 3.5 | 66.9 ± 6.3 | 3.5 ± 1.1 | 0.7 |
| 3 | ; | 93.4 ± 1.8 | 35.3 ± 5.1 | 88.5 ± 2.4 | 12.3 ± 2.3 | 3.8 | |
| 5 | ;1 | 93.3 ± 2.8 | 34.2 ± 2.3 | 88.1 ± 2.3 | 13.5 ± 1.4 | 3.9 | |
| Isolate | Proportion of Growing Tubes with Appressoria | Proportion of Appressoria, % | |
|---|---|---|---|
| On Stomata from the Total Number | Germinated from the Number on the Stomata | ||
| Sr24 and Sr25 | |||
| 1 | + | − | + |
| Sr24 and Sr26 | |||
| 3 | ± | + | − |
| 2 | + | ± | + |
| Sr25 and Sr26 | |||
| 5 | ± | + | ± |
| Isolate | Variant | IT | Proportion of Growing Tubes with Appressoria, % | Proportion Appressoria, % | Proportion of Structures Penetrated into the Stomata, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| On Stomata from the Total Number | Germinated from the Number on the Stomata | SV | Colony Square, μm2 | |||||||||||
| 2001–4000 | 4001–6000 | 6001–8000 | 8001–10,000 | 10,001–12,000 | 14,001–16,000 | 16,001–20,000 | ˃25,000 | |||||||
| Chernyava 13—control | ||||||||||||||
| 1 | Control | 4 | 87.5 ± 4.6 | 90.1 ± 3.5 | 90.2 ± 3.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 |
| Verapamil | ; | 84.6 ± 3.7 | 87.6 ± 4.4 | 87.0 ± 3.8 | 0.0 | 0.0 | 0.0 | 0.0 | 20.0 | 26.7 | 40.0 | 13.3 | 0.0 | |
| SA | 2+ | 86.3 ± 4.1 | 91.2 ± 3.7 | 88.6 ± 4.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0,0 | 20.0 | 33.3 | 46.7 | 0.0 | |
| Th. ponticum | ||||||||||||||
| 1 | Control | 0 | 14.1 ± 0.2 | 39.3 ± 3.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Verapamil | 0 | 13.9 ± 0.2 | 40.2 ± 3.2 | 19.1 ± 1.3 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| SA | 0 | 14.3 ± 0.3 | 41.0 ± 4.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| TcLr19/Sr25 | ||||||||||||||
| 1 | Control | 0 | 21.8 ± 2.3 | 86.3 ± 5.2 | 3.7 ± 1.3 | 20.0 | 30.0 | 20.0 | 10.0 | 10.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Verapamil | 0 | 24.1 ± 2.1 | 83.1 ± 3.8 | 10.2 ± 2.1 | 33.3 | 33.3 | 22.2 | 11.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| SA | 0 | 23.3 ± 3.2 | 85.0 ± 4.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 4 | Control | ;1 | 30.3 ± 0.9 | 45.0 ± 8.3 | 24.2 ± 3.2 | 0.0 | 0.0 | 0.0 | 13.2 | 25.3 | 25.5 | 25.0 | 11.2 | 0.0 |
| Verapamil | ; | 32.5 ± 1.5 | 42.3 ± 5.2 | 30.1 ± 5.1 | 0.0 | 0.0 | 0.0 | 30.0 | 40.0 | 30.0 | 0.0 | 0.0 | 0.0 | |
| SA | ; | 33.1 ± 2.6 | 43.0 ± 3.9 | 7.2 ± 2.3 | 19.2 | 11.5 | 14.0 | 24.7 | 23.1 | 7.5 | 0.0 | 0.0 | 0.0 | |
| 5 | Control | 2- | 29.3 ± 3.5 | 66.9 ± 6.3 | 25.6 ± 2.7 | 0.0 | 17.5 | 32.5 | 11.4 | 22.8 | 0.0 | 25.7 | 0.0 | 0.0 |
| Verapamil | ; | 25.6 ± 3.1 | 63.1 ± 4.5 | 28.5 ± 3.5 | 0.0 | 22.0 | 35.0 | 28.0 | 15.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| SA | ; | 28.1 ± 2.9 | 68.5 ± 4.3 | 9.8 ± 1.8 | 21.4 | 21.4 | 21.4 | 28.6 | 7.14 | 0 | 0 | 0.0 | 0.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotnikova, L.; Knaub, V.; Pozherukova, V. Nonhost Resistance of Thinopyrum ponticum to Puccinia graminis f. sp. tritici and the Effects of the Sr24, Sr25, and Sr26 Genes Introgressed to Wheat. Int. J. Plant Biol. 2023, 14, 435-457. https://doi.org/10.3390/ijpb14020034
Plotnikova L, Knaub V, Pozherukova V. Nonhost Resistance of Thinopyrum ponticum to Puccinia graminis f. sp. tritici and the Effects of the Sr24, Sr25, and Sr26 Genes Introgressed to Wheat. International Journal of Plant Biology. 2023; 14(2):435-457. https://doi.org/10.3390/ijpb14020034
Chicago/Turabian StylePlotnikova, Lyudmila, Valeria Knaub, and Violetta Pozherukova. 2023. "Nonhost Resistance of Thinopyrum ponticum to Puccinia graminis f. sp. tritici and the Effects of the Sr24, Sr25, and Sr26 Genes Introgressed to Wheat" International Journal of Plant Biology 14, no. 2: 435-457. https://doi.org/10.3390/ijpb14020034
APA StylePlotnikova, L., Knaub, V., & Pozherukova, V. (2023). Nonhost Resistance of Thinopyrum ponticum to Puccinia graminis f. sp. tritici and the Effects of the Sr24, Sr25, and Sr26 Genes Introgressed to Wheat. International Journal of Plant Biology, 14(2), 435-457. https://doi.org/10.3390/ijpb14020034






