1. Introduction
Increasing the resistance of plants to pathogens and unfavorable agro-climatic conditions is an urgent task of crop production, since under natural conditions, cultivated crops are simultaneously or sequentially exposed to stress factors of various nature. In this regard, microbiological approaches based on the use of the potential of plants and soil microorganisms seem to be the most promising. One such approach is the use of nonpathogenic rhizospheric bacteria (PGPR, plant growth promoting rhizobacteria), in particular, bacteria of the genus
Bacillus [
1]. Plant interactions with individual bacterial strains lead to induced systemic resistance (ISR). Metabolites of these microorganisms are recognized by plant receptors and can cause the development of resistance to a wide range of pathogens [
2]. The ISR then develops in a jasmonate- (JA) or ethylene-dependent pathway and has a broad spectrum of antipathogenic activity in general. At the same time, it has been shown that PGPR can activate plant systemic acquired resistance (SAR), providing long-term systemic immunity to pathogen infection, which depends on the synthesis and accumulation of salicylic acid [
3]. The ability of pathogens to invade and spread in plant tissues is largely determined by the activity of their hydrolytic enzymes. Interest in hydrolytic enzymes secreted by microorganisms is due to a number of reasons, among which one of the most important is their participation in the initiation and development of the pathological process in plant tissues.
The synthesis of inhibitors of hydrolytic enzymes is one of the most important defense responses of plants against the attack of pathogenic microorganisms and insect pests [
4]. The wide distribution of hydrolase inhibitors and their content in significant amounts in plant tissues allow us to speak of them as indicators of the activation of protective mechanisms and one of important links in the regulation of various physiological processes. In addition to performing protective functions, they serve as regulators of the activity of endogenous enzymes.
Among the abiotic factors that shape plant productivity, the most important is the presence of water [
5]. The growth and development of potatoes, as well as yields, are highly dependent on soil moisture. In potatoes, this dependence is much more pronounced than in other types of agricultural crops [
6]. It is known that rhizobacteria can significantly increase the drought resistance of plants [
7,
8]. It has been shown that exogenously applied salicylic acid, in addition to increasing resistance to pathogens, increases plant resistance to a number of physiological stresses [
9], similar data were obtained when treated with jasmonate [
10]. Thus, the combination of bacteria with salicylic and jasmonic acids can significantly expand the protective potential of drugs based on them. However, decreasing substrate moisture levels (i.e., increasing drought stress) can also predispose plants to oomycete pathogens, making them more susceptible to infection. Previous studies with water stress showed that tomato plants inoculated with
Phytophthora parasitica had higher disease severity than tomatoes grown under well-watered conditions. It was shown that tomato seedling mortality significantly increased when exposed to salt stress before inoculation with
Phytophthora capsici. Based on these studies, significant reductions in substrate moisture could facilitate the pathogen colonization of the host by increasing host susceptibility [
11].
The purpose of this work is to study the effect of Bacillus subtilis bacteria in combination with salicylic (SA) and jasmonic (JA) acids on the activity of hydrolytic enzymes (proteases, amylases, and cellulases) and their inhibitors caused by potato resistance to Phytophthora infestans and lack of moisture.
2. Materials and Methods
Research objects. The objects of the study were Solanum tuberosum plants of the susceptible cultivar Early Rose, grown from microtubers. The cultivation was carried out in pots with soil containing high-moor peat of varying degrees of decomposition, purified river sand, perlite, a complex of mineral fertilizers, and vermicompost. Soil pH was 6.0–6.5, planting depth was 3–4 cm. Cultivation was carried out on a light platform at an illumination of 8000–10,000 lux, a 16 h photoperiod, and temperatures of 20–22 °C.
The bacteria Bacillus subtilis, strain 26D, were cultivated in liquid LB medium for 24 h. The culture was diluted with distilled water.
Plants were infected with a highly aggressive P. infestans isolate obtained from potato plants in the Birsk region of the Republic of Bashkortostan (Birsk Experimental Station of the Bashkir Research Institute of Agriculture, 55°25′47.4′′ N 55°35′49.9′′ E) and stored in the collection of the laboratory of Biochemistry of Plant Immunity, Institute of Biochemistry and Genetics, Ufa Federal Research Center, Russian Academy of Sciences. Phytophthora culture was grown on potato agar with dextrose for 7 days. To restore the aggressiveness of phytophthora, the infection of mini-tubers was carried out and then the pathogen was re-isolated. The surface of P. infestans colonies was covered with distilled water and incubated at 4 °C for 30 min. The concentration of sporangia was assessed using a Fuchs–Rosenthal chamber, and the spore suspension was diluted to a titer of 1 × 105 spores/mL.
Plant processing. Fifteen days after germination, the plants were treated with
B. subtilis (spraying with a suspension of 10
8 cells/mL, 5 mL per plant) and a mixture of bacteria with salycilic acid (10
−6 M) and jasmonic acid (10
−7 M) [
12]. Control plants were sprayed with distilled water. Three days later, the plants were sprayed with phytophthora (10
5 spores/mL, 5 mL per plant).
The lack of moisture was created by reducing irrigation until the soil moisture reached 40% ± 5% of the total moisture capacity [
12]. Seven days after infection with phytophthora, when symptoms of infection (wilting) began to appear, the activity of hydrolases and hydrolase inhibitors in the leaves was determined. Normal soil moisture was 75 ± 5% of the total moisture capacity. Relative humidity was 50 ± 5%.
Damage degree was assessed as the percentage of the affected area of the leaf blade on the 7th day after infection. The leaves were photographed, and the resulting images were processed using ImageJ software (
imagej.nih.gov). The total area of the leaf blade and the area of the damaged part were measured. The degree of damage was defined as the percentage of damaged area. The repetition rate for determining the degree of damage was at least 5.
Protein content. The protein content in the samples was determined by the Bradford method using bovine serum albumin as a standard [
13]. Absorbance was measured at 595 nm.
Enzymatic activity. The activity of amylases, proteases, and cellulases was determined by the hydrolysis of immobilized starch, bovine serum albumin, and carboxymethyl cellulose, respectively [
14]. Enzyme substrates with a final concentration of 1% were immobilized in 4% polyacrylamide gel. Solutions with enzymatic activity were applied to polyacrylamide gel, kept for 20 min at 37 °C, and then stained with Lugol’s solution, Coomassie G-250 of Congo reg. Enzyme activity was determined by densitometry using calibration curves constructed using standard preparations of amylase, cellulase, and trypsin. Enzymatic activity was expressed as µmol substrate/(mg protein min). The content of soluble protein in the samples was determined through the Bradford method.
The activity of hydrolase inhibitors was similarly determined by adding a known amount of a standard solution of the enzyme to the reaction mixture.
Statistical analysis. The experiments included 5 biological replicates for biochemical parameters. The histograms show the sample means and their 95% confidence intervals.
3. Results
The analysis of the development of late blight symptoms on the leaves of the susceptible cultivar Early Rose revealed that the treatment with
B. subtilis bacteria, including its combinations with signal molecules, contributed to a decrease in the degree of pathogen infestation of plants with a lack of moisture (
Figure 1). Thus, in the control variant under drought, the degree of leaf damage was about 65%, and after pretreatment with
B. subtilis in a mixture with SA and JA, leaf damage decreased by 15–30% (
Figure 1). In control, water deficiency also led to a decrease in damage.
Under the conditions of normal humidity, the infection of plants with
P. infestans led to a decrease in the activity of amylases in the leaves (
Figure 2). In conditions of moisture deficiency, on the contrary, the activity of amylases increased upon infection with
P. infestans. The treatment of plants with
B. subtilis, including in various combinations with signal molecules, did not cause changes in the activity of amylases in infected plants grown under normal humidity conditions, with the exception of the
B. subtilis + SA + JA variant, where a significant increase in amylase activity was noted. Under the conditions of moisture deficiency, treatment with
B. subtilis, as with
B. subtilis + SA + JA, led to an increase in the activity of amylases in infected plants; treatment with
B. subtilis + SA led to a decrease; and
B. subtilis + JA resulted in no changes.
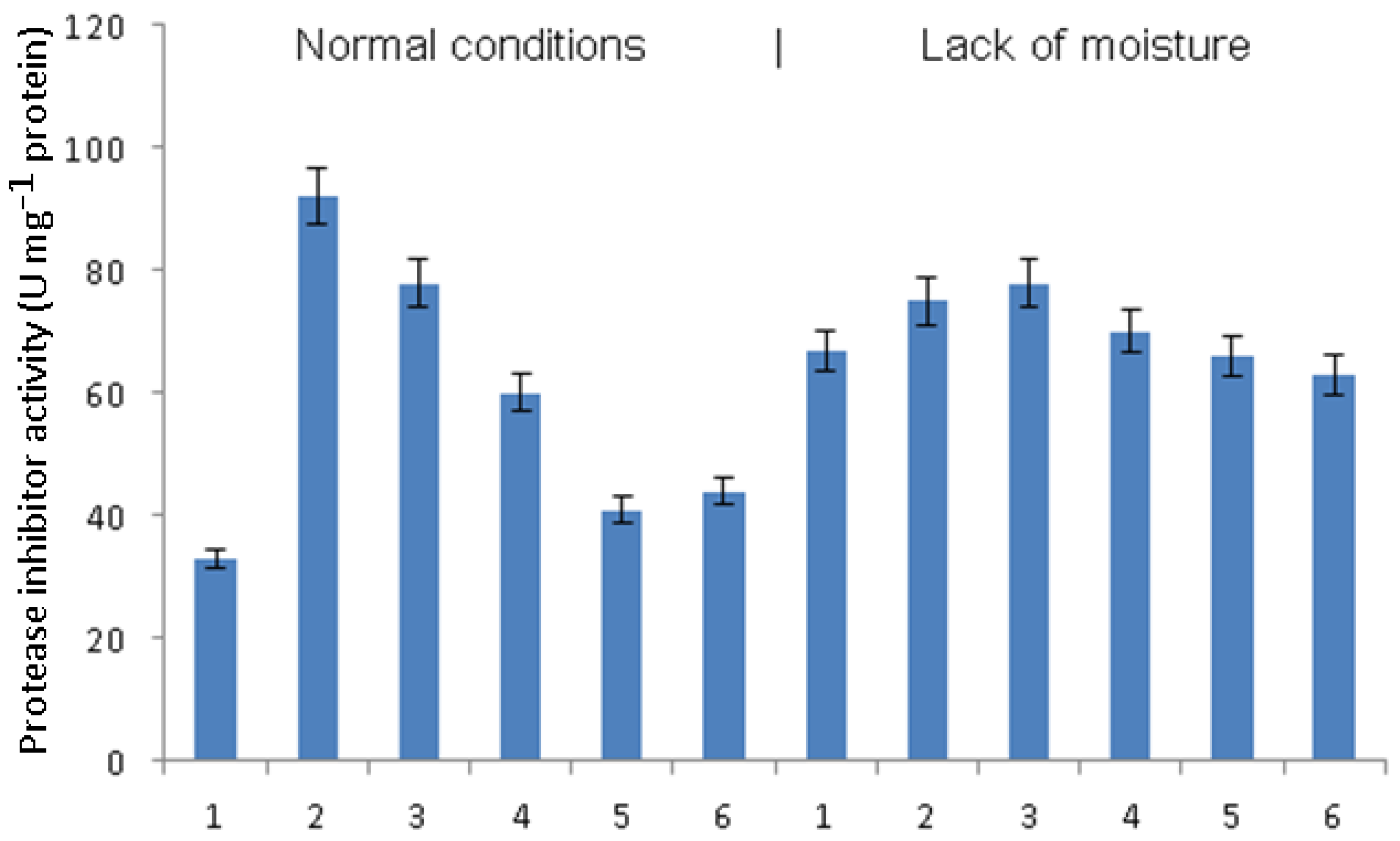
The activity of proteases under the conditions of normal humidity did not change significantly either under the influence of phytophthora infection or due to pretreatment with bacteria or signal molecules (
Figure 3). Under conditions of moisture deficiency, phytophthora infection led to an increase in protease activity. In infected plants pretreated with
B. subtilis, a decrease in protease activity occurred, which manifested itself to a lesser extent in the combination of
B. subtilis + SA and to an even lesser extent in the combination of
B. subtilis + JA. Protease activity in infected plants pretreated with
B. subtilis + SA + JA did not differ significantly from that in untreated infected plants.
In plants grown under normal humidity conditions, phytophthora infection did not lead to a change in cellulase activity (
Figure 4). Pretreatment with
B. subtilis in the absence of signal molecules caused a significant decrease in cellulase activity, while other treatments did not change it. Under conditions of moisture deficiency, phytophthora infection, as well as the treatment of infected plants with bacteria, did not lead to a change in the activity of cellulases. In plants treated with
B. subtilis + SA or
B. subtilis + JA, cellulase activity decreased, which was more pronounced in the
B. subtilis + JA variant. The combination of
B. subtilis + SA + JA showed a much less pronounced, although significant, decrease in cellulase activity.
The activity of amylase inhibitors in plants grown under conditions of normal humidity doubled when infected with phytophthora (
Figure 5). The treatment of such plants with
B. subtilis did not lead to a significant change in the activity of inhibitory amylases. Treatment with bacteria together with signaling molecules, in any combination, caused an equal decrease in the activity of amylase inhibitors. Under the conditions of a lack of moisture, the activity of amylase inhibitors was also higher in plants infected with phytophthora compared to healthy plants. Treatment with
B. subtilis or
B. subtilis + SA was characterized by the greatest increase in the activity of amylase inhibitors, while treatment with
B. subtilis + JA or
B. subtilis + SA + JA also resulted in an increase but to a lesser extent.
The activity of protease inhibitors, as well as amylase inhibitors, was significantly increased in late blight-infected plants compared to healthy plants (
Figure 6). Treatment with
B. subtilis significantly reduced this activity in infected plants, and treatment with bacteria together with SA led to a stronger decrease; finally, the greatest decrease in the activity of amylase inhibitors in plants infected with phytophthora was noted during pretreatment with
B. subtilis + JA and
B. subtilis + SA + J.A. Under conditions of moisture deficiency, phytophthora infection did not lead to a significant increase in the activity of protease inhibitors. This was observed only in infected plants pretreated with
B. subtilis. A significant decrease in the activity of protease inhibitors with a lack of moisture was observed when treating
B. subtilis + JA and
B. subtilis + SA + JA.
The activity of cellulase inhibitors under normal humidity conditions was also higher in late blight-infected plants relative to uninfected ones (
Figure 7). In infected plants treated with
B. subtilis together with signaling molecules, the activity of cellulase inhibitors was lower. The greatest decrease under normal humidity conditions was observed in the treatment of
B. subtilis + SA + JA. With a lack of moisture, phytophthora infection also caused an increase in the activity of cellulase inhibitors, but it was less pronounced. Pretreatment with bacteria under the conditions of moisture deficiency did not lead to changes in the activity of cellulase inhibitors in infected plants. Pretreatment with
B. subtilis + SA increased this activity, and pretreatment with
B. subtilis + JA and
B. subtilis + SA + JA decreased it.
4. Discussion
Treatment with
B. subtilis bacteria, especially in combination with JA under water deficiency, led to essential decrease in the plants damage degree by the pathogen. Previously, it was shown that treatment with JA and
B. subtilis 26D reduced the development of
P. infestans on potato tubers [
15]. The decrease in the degree of damage by phytophthora in conditions with a lack of moisture can be explained by unfavorable conditions for the development of the pathogen.
We made an assumption that the formation of a protective reaction of potato plants to the causative agent of late blight under the action of bacteria under the conditions of a lack of moisture is carried out with the participation of the jasmonate signaling system. This agrees with our earlier data on test-tube potato plants [
16]. It is known that the development of ISR caused by rhizobacteria occurs with the participation of JA and ethylene [
14].
The synthesis of inhibitors of hydrolytic enzymes is one of the most important defense reactions of plants in the attack of pathogenic microorganisms and insect pests. The wide distribution of hydrolase inhibitors and their content in significant amounts in plant tissues allow us to speak of them as one of the important links in the regulation of various physiological processes. In addition to performing protective functions, they serve as regulators of the activity of endogenous enzymes in ontogeny. An increase in the content of hydrolase inhibitors in a plant does not occur, as a rule, due to an increase in the concentration of constitutive compounds but due to the synthesis of new forms of inhibitors [
17]. The most common in plants are inhibitors of proteases and amylases [
18]. Thus, the activity of hydrolytic enzymes and their inhibitors is an important indicator of the activation of defense mechanisms in plant tissues under the influence of pathogen infection, treatment with endophytes and signaling molecules, and abiotic stress.
The activity of amylases under normal humidity conditions decreased upon infection with phytophthora, including various treatments. An exception was the treatment with B. subtilis + SA + JA, which increased the activity of amylases to the level of the control plants. With a lack of moisture, the combined action of B. subtilis + SA + JA also led to the greatest increase in the activity of amylases. A very similar picture was also observed for proteases, where the greatest increase in activity corresponded to the treatment with B. subtilis + SA + JA. The activity of cellulases, as well as amylases, under conditions with a lack of moisture was the highest in plants treated with B. subtilis alone and B. subtilis + SA + JA.
It is known that amylolytic activity is characteristic of the representatives of most taxonomic groups of fungi, and these enzymes are almost always represented by constitutive proteins. However, amylase is absent in oomycetes, in particular, in fungi of the genus Phytophthora, which use potato enzymes to cleave starch, activating their biosynthesis in affected tissues [
19].
High proteolytic activity in infected tissues not only provides amino acids for the growth and development of a pathogenic microorganism but can also neutralize protective potato proteins, such as lectins, and hydrolase inhibitors. Thus, it was shown that the extracellular metalloproteinase of the phytopathogenic bacterium
Erwinia carotovora (Jones) Waldee cleaves the potato lectin involved in plant protection [
20]. An increase in the activity of protease inhibitors is aimed at suppressing the activity of exogenous proteases and contributes to an increase in potato resistance.
Phytopathogenic fungi have a large set of enzymes that destroy the cell walls of plants. With the help of these enzymes, the parasite enters the cell and feeds on its contents. Destroying the median lamina, the parasites acquire the ability to move in the infected tissue of the plant. The high activity of fungal cellulases makes it possible to cleave a large part of the cell wall, leading to the death of plant cells [
21].
The increased activity of hydrolytic enzymes in potato plants grown under conditions with a lack of moisture and infection with P. infestans is apparently a manifestation of systemic resistance associated with the activation of metabolic processes in response to joint abiotic and biotic stress. As can be seen from our results, both salicylate and jasmonate signaling pathways play an important role in the activation of these mechanisms. The commonality of changes in the activity of proteases and amylases can be associated with the presence of common inhibitors of these enzymes in plant tissues. It is interesting to note that plant resistance to phytophthora damage in the absence of moisture significantly correlates with cellulase activity: the lowest cellulase activity is observed in the variant with B. subtilis + JA treatment, which also corresponds to the lowest degree of plant damage.
The membrane receptor systemin, a protein with a molecular weight of about 160 kDa, plays a key role in initiating the formation of defense reaction protease inhibitors. In response to injury, it causes membrane depolarization, which leads to the opening of ion channels and a sharp increase in the level of intracellular calcium ions. As a result, MAP kinases and phospholipases are activated, and in the course of a series of reactions, jasmonic acid is formed, which probably serves as an activator of the transcription of protective protein genes [
22].
It is assumed that PGPR have individual compounds for each strain, which are produced and secreted into the extracellular environment. For example, peptides with antibiotic properties, as well as universal signal molecules ethylene, and salicylic and jasmonic acids [
23]. For example, in the formation of resistance of pepper plants to bacterial rot
Xantomonas axonopodis pv. vesicatoria under the influence of the
B. cereus BS107 strain, genes of protective proteins (PR proteins) activated during pathogenesis were involved, some of which, for example, PR-1, are induced by salicylic acid and some (PR-4, PR-10) by jasmonic acid and ethylene and part-H
2O
2 [
24]. It has been shown that the inoculation of tomato plants with the
B. subtilis BEB-DN strain leads to an increase in the expression of a number of PR genes, in particular, genes of the inhibitors of proteases and enzymes of lignin synthesis [
25].
Protease and cellulase inhibitors, when infected with phytophthora, showed higher activity than in healthy plants, both under conditions of normal humidity and with a lack of moisture. Various treatments generally led to a decrease in the activity of inhibitors; however, the greatest effect was observed when treated with B. subtilis + JA or B. subtilis + SA + JA, which correlates both with the indicators of plant damage and with the activity of the corresponding enzymes. Separately, it should be noted the activity of amylase inhibitors, which, under conditions with a lack of moisture, did not change significantly when infected with phytophthora. This may be one of the manifestations of the effect of phytophthora on the plant in order to activate the amylolytic enzymes of the plant, which manifested itself in our experiments under conditions of abiotic stress. Treatment with bacteria and signaling molecules stimulated the increased activity of inhibitors as part of the plant’s defense mechanisms. In this case, treatment with B. subtilis or B. subtilis + SA achieved the greatest effect.
The results indicate that the mechanism of activation of defense systems in potato plants by Bacillus subtilis bacterium in combination with signal molecules is associated with their modulating effect on the activity of hydrolytic enzymes and a stimulating effect on the activity of hydrolase inhibitors. The revealed differences in the degree of activation of hydrolase inhibitors under the influence of B. subtilis bacteria and signal molecules suggest different paths for the formation of potato resistance to P. infestans under drought conditions.