The Relationships between Climate, Tree-Ring Growth, and Cone Production in Longleaf Pine
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selection
- Escambia Experimental Forest in Southern Alabama (hereafter Escambia)
- Kisatchie National Forest in Louisiana (Kisatchie)
- Bladen Lakes State Forest in Eastern North Carolina (Bladen Lakes)
| Escambia | Kisatchie | Bladen Lakes | |
|---|---|---|---|
| Latitude, longitude | 31.0091, −87.0825 | 31.0249, −92.6359 | 34.7290, −78.5315 |
| Elevation (approx. m.) | 40 | 90 | 30 |
| Average air temperature (°C) | 19.8 | 19.5 | 17.6 |
| Average precipitation (cm) | 153.8 | 145.4 | 121.8 |
| Average sampled tree age (years) | 68.0 | 59.7 | 88.3 |
| Number of available cone count years | 64 | 53 | 41 |
2.2. Reproductive Data
2.3. Dendrochronological Data
2.4. Climate Data
2.5. Data Analysis
3. Results
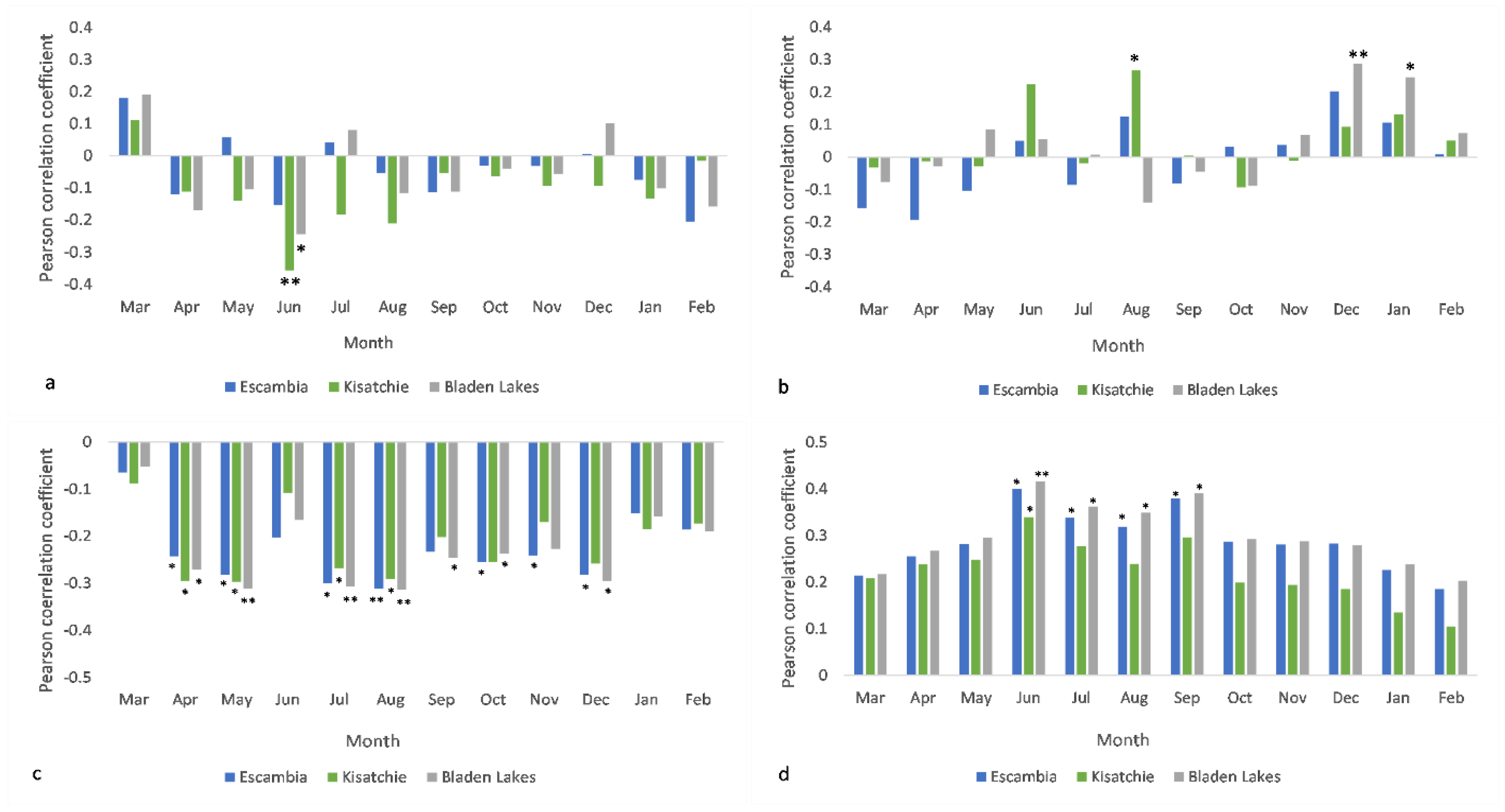
3.1. Radial Growth and Mean Climate Signals
3.2. Radial Growth and Extreme Climate Signals
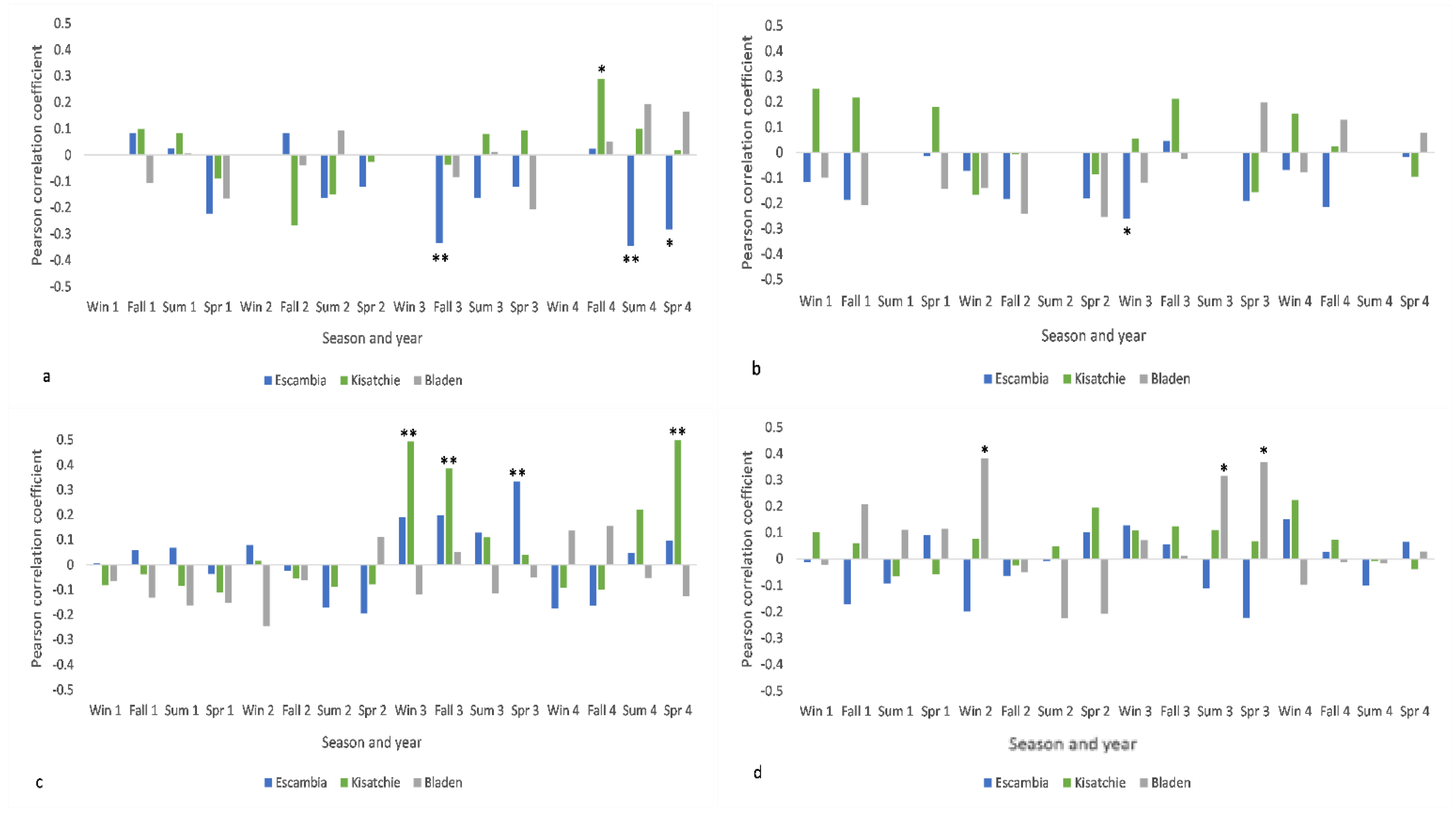
3.3. Cone Production and Mean Climate Signals
3.4. Cone Production and Extreme Climate Signals
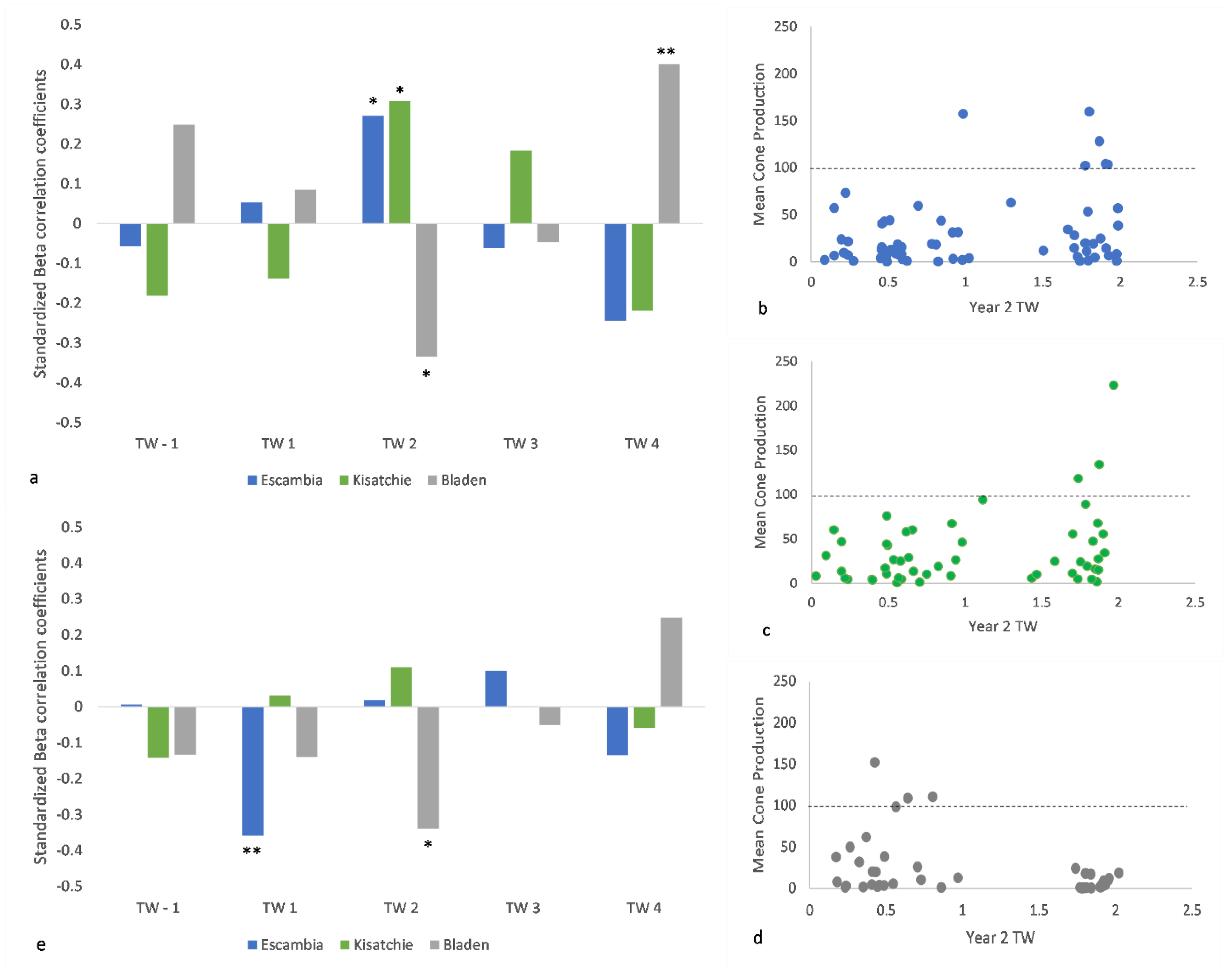
3.5. Cones and Radial Growth
4. Discussion
4.1. Intersite Variability
4.2. Mean Climate vs. Extreme Events
4.3. The Importance of Scale in Climate-Longleaf Analysis
4.4. Climate and Cone Production
4.5. Cone Production and Tree-Ring Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, C.; Breshears, D.; McDowell, N. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 129. [Google Scholar] [CrossRef]
- Lee, E.H.; Wickham, C.; Beedlow, P.; Waschmann, R.; Tingey, D. A likelihood-based time series modeling approach for application in dendrochronology to examine the growth-climate relations and forest disturbance history. Dendrochronologia 2017, 45, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Devictor, B.; Motard, E.; Machon, N.; Porcher, E. Short-term climate-induced change in French plant communities. Biol. Lett. 2019, 15, 20190280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Nat. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Guo, Q.; Bowman, K.A. Climate variation within the range of longleaf pine forests during the past century. Atmosphere 2022, 13, 465. [Google Scholar] [CrossRef]
- Gitau, M. Long-term seasonality of rainfall in the southwest Florida Gulf coastal zone. Climate Res. 2016, 69, 93–105. [Google Scholar] [CrossRef][Green Version]
- Maxwell, J.; Bregy, J.; Robeson, S.; Knapp, P.; Soulé, P.; Trouet, V. Recent increases in tropical cyclone precipitation extremes over the US east coast. Proc. Nat. Acad. Sci. USA 2021, 118, e2105636118. [Google Scholar] [CrossRef]
- Mudd, L.; Wang, Y.; Letchford, C.; Rosowsky, D. Assessing climate change Impact on the U.S. east coast hurricane hazard: Temperature, frequency, and track. Nat. Hazards Rev. 2014, 15, 04014001. [Google Scholar] [CrossRef]
- Wang, H.; Fu, R.; Kumar, A.; Li, W. Intensification of summer rainfall variability in the southeastern United States during recent decades. Am. Meteorol. Soc. 2010, 11, 1007–1018. [Google Scholar] [CrossRef]
- Cid-Serrano, L.; Ramírez, S.M.; Alfaro, E.J.; Enfield, D.B. Analysis of the Latin American west coast rainfall predictability using an ENSO index. Atmósfera 2015, 28, 191–203. [Google Scholar] [CrossRef][Green Version]
- González-Cásares, M.; Pompa-García, M.; Camarero, J.J. Differences in climate–growth relationship indicate diverse drought tolerances among five pine species coexisting in Northwestern Mexico. Trees 2017, 31, 531–544. [Google Scholar] [CrossRef]
- Wion, A.P.; Pearse, I.S.; Rodman, K.C.; Veblen, T.T.; Redmond, M.D. The effects of ENSO and the North American monsoon on mast seeding in two Rocky Mountain conifer species. Phil. Trans. R. Soc. B 2021, 376, 20200378. [Google Scholar] [CrossRef]
- Grothe, P.R.; Cobb, K.M.; Liguori, G.; Di Lorenzo, E.; Capotondi, A.; Lu, Y. Enhanced El Niño–Southern oscillation variability in recent decades. Geophys. Res. Lett. 2020, 47, e2019GL083906. [Google Scholar] [CrossRef]
- Bakkenes, M.; Alkemade, J.R.M.; Ihle, F.; Leemans, R.; Latour, J.B. Assessing effects of forecasted climate change on the diversity and distribution of European higher plants for 2050. Glob. Change Biol. 2002, 8, 390–407. [Google Scholar] [CrossRef]
- Becklin, K.M.; Medeiros, J.S.; Sale, K.R.; Ward, J.K. Evolutionary history underlies plant physiological responses to global change since the last glacial maximum. Ecol. Lett. 2014, 17. [Google Scholar] [CrossRef] [PubMed]
- Copenheaver, C.; Shumaker, K.; Butcher, B.; Hahn, G.; Perkins, L.; Dukes, C.; Thompson, E.; Pisaric, M. Dendroclimatology of sugar maple (Acer saccharum): Climate-growth response in a late-successional species. Dendrochronologia 2020, 63, 125747. [Google Scholar] [CrossRef]
- Abdelhakim, L.O.A.; Zhou, R.; Ottosen, C.O. Physiological Responses of Plants to Combined Drought and Heat under Elevated CO2. Agronomy 2002, 12, 2526. [Google Scholar] [CrossRef]
- Loka, D.; Harper, J.; Humphreys, M.; Gasior, D.; Wootton-Beard, P.; Gwynn-Jones, D.; Scullion, J.; Doonan, J.; Kingston-Smith, A.; Dodd, R.; et al. Impacts of abiotic stresses on the physiology and metabolism of cool-season grasses: A review. Food Energy Secur. 2019, 8, e00152. [Google Scholar] [CrossRef][Green Version]
- Mitchell, T.; Knapp, P.; Patterson, T. The importance of infrequent, high-intensity rainfall events for longleaf pine (Pinus palustris Mill.) radial growth and implications for dendroclimatic research. Trees For. People 2020, 1, 100009. [Google Scholar] [CrossRef]
- Bebre, I.; Annighöfer, P.; Ammer, C.; Seidel, D. Growth, morphology, and biomass allocation of recently planted seedlings of seven European tree species along a light gradient. IForest 2020, 13, 261–269. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Slot, J.C.; Visser, E.A.; Naidoo, S.; Sovic, M.G.; Conrad, A.O.; Kyre, B.; Vijayakumar, V.; Bonello, P. Mechanisms of pine disease susceptibility under experimental climate change. Front. For. Glob. Change 2022, 5, 872584. [Google Scholar] [CrossRef]
- Jankowski, A.; Wyka, T.P.; Żytkowiak, R.; Danusevičius, D.; Oleksyn, J. Does climate-related in situ variability of Scots pine (Pinus sylvestris L.) needles have a genetic basis? Evidence from common garden experiments. Tree Physiol. 2019, 39, 573–589. [Google Scholar] [CrossRef]
- Obeso, J. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Reznick, D. Costs of reproduction: An evaluation of the empirical evidence. Oikos 1985, 44, 257–267. [Google Scholar] [CrossRef]
- Berdanier, A.; Clark, J. Divergent reproductive allocation trade-offs with canopy exposure across tree species in temperate forests. Ecosphere 2016, 7, E01313. [Google Scholar] [CrossRef][Green Version]
- Lauder, J.; Moran, E.; Hart, S. Fight or flight? Potential tradeoffs between drought defense and reproduction in conifers. Tree Physiol. 2019, 39, 1071–1085. [Google Scholar] [CrossRef]
- Liu, W.; Pennings, S.C. Self-thinning and size-dependent flowering of the grass Spartina alterniflora across space and time. Func. Ecol. 2019, 33, 1830–1841. [Google Scholar] [CrossRef]
- Merganičová, K.; Merganič, J.; Lehtonen, A.; Vacchiano, G.; Ostrogović, S.M.Z.; Augustynczik, A.; Grote, R.; Kyselová, I.; Mäkelä, A.; Yousefpour, R.; et al. Forest carbon allocation modelling under climate change. Tree Physiol. 2019, 39, 1937–1960. [Google Scholar] [CrossRef]
- Wenk, E.; Falster, D. Quantifying and understanding reproductive allocation schedules in plants. Ecol. Evol. 2015, 5, 5521–5538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hacket-Pain, A.; Ascoli, D.; Berretti, R.; Mencuccini, M.; Motta, R.; Nola, P.; Piussi, P.; Ruffinatto, F.; Vacchiano, G. Temperature and masting control Norway spruce growth, but with high individual tree variability. For. Ecol. Manag. 2019, 438, 142–150. [Google Scholar] [CrossRef][Green Version]
- Fréjaville, T.; Vizcaíno-Palomar, N.; Fady, B.; Kremer, A.; Benito, G.M. Range margin populations show high climate adaptation lags in European trees. Glob. Chang. Biol. 2020, 26, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Mundo, I.A.; Sanguinetti, J.; Kitzberger, T. Multi-centennial phase-locking between reproduction of a South American conifer and large-scale drivers of climate. Nature Plants 2021, 7, 1560–1570. [Google Scholar] [CrossRef]
- Patterson, T.; Knapp, T. Longleaf pine cone–radial growth relationships in the southeastern U.S.A. Dendrochronologia 2018, 50, 134–141. [Google Scholar] [CrossRef]
- Zhao, Z.; Kang, D.; Guo, W.; Zhao, L.; Cui, L.; Li, J. Climate sensitivity of purple cone spruce (Picea purpurea) across an altitudinal gradient on the eastern Tibetan Plateau. Dendrochronologia 2019, 56, 125586. [Google Scholar] [CrossRef]
- Jose, S.; Jokela, E.; Miller, D. The Longleaf Pine Ecosystem. In The Longleaf Pine Ecosystem; Springer Series on Environmental Management; Jose, S., Jokela, E.J., Miller, D.L., Eds.; Springer: New York, NY, USA, 2006; pp. 3–8. [Google Scholar] [CrossRef]
- Oswalt, C.; Cooper, J.; Brockway, D.; Brooks, H.; Walker, J.; Connor, K.; Oswalt, S.; Conner, R. History and Current Condition of Longleaf Pine in the Southern United States; General Technical Report; U.S. Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2012; p. 51.
- Addington, R.; Donovan, L.; Mitchell, R.; Vose, J.; Pecot, S.; Jack, S.; Hacke, U.; Sperry, J.; Oren, R. Adjustments in hydraulic architecture of Pinus palustris maintain similar stomatal conductance in xeric and mesic habitats. Plant Cell Environ. 2006, 29, 535–545. [Google Scholar] [CrossRef]
- Noss, R.; LaRoe, E.; Scott, J. Endangered Ecosystems of the United States: A Preliminary Assessment of Loss and Degradation; Biological Report; USDI National Biological Service: Washington, DC, USA, 1995; Volume 28.
- Chen, X.; Guo, Q.; Brockway, D. Power laws in cone production of longleaf pine across its native range in the United States. Sust. Agri. Res. 2017, 6, 64–73. [Google Scholar] [CrossRef][Green Version]
- Boyer, W. Long-term changes in flowering and cone production by longleaf pine. In Proceedings of the Ninth Biennial Southern Silvicultural Research Conference; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 1997. [Google Scholar]
- Chen, X.; Brockway, D.; Guo, Q. Characterizing the dynamics of cone production for longleaf pine forests in the southeastern United States. For. Ecol. Manag. 2018, 429, 1–6. [Google Scholar] [CrossRef]
- Guo, Q.; Zarnoch, S.; Chen, X.; Brockway, D. Life cycle and masting of a recovering keystone indicator species under climate fluctuation. Ecosys. Health Sust. 2016, 2, e01226. [Google Scholar] [CrossRef][Green Version]
- Bowman, K.A.; Chen, X. Current standing of longleaf pine trees under climate change. J. Bot. Res. 2022, 4, 28–39. [Google Scholar] [CrossRef]
- Boyer, W. Pinus palustris, Mill. Longleaf pine. In Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; Volume 1, pp. 405–412. [Google Scholar]
- Chen, X.; Brockway, D.; Guo, Q. Temporal patterns of pollen shedding for longleaf pine (Pinus palustris) at the Escambia Experimental Forest in Alabama, USA. Dendrobiology 2020, 84, 30–38. [Google Scholar] [CrossRef]
- Guo, Q.; Brockway, D.; Chen, X. Temperature-related sex allocation shifts in a recovering keystone species, Pinus palustris. Plant Ecol. Diversity 2017, 10, 303–310. [Google Scholar] [CrossRef]
- Patterson, T. Longleaf Pine Cone Production and the Influence of Super-Producing Trees. Southeast. Geographer. 2020, 60, 332–344. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Q.; Brockway, D. Analyzing the complexity of cone production in longleaf pine by multiscale entropy. J. Sust. For. 2016, 35, 172–182. [Google Scholar] [CrossRef]
- Patterson, T.; Knapp, P. Longleaf pine masting, northern bobwhite quail, and tick-borne diseases in the southeastern United States. Appl. Geogr. 2018, 98, 1–8. [Google Scholar] [CrossRef]
- Patterson, T.; Knapp, P. Stand dynamics Influence masting/radial growth relationships in Pinus palustris Mill. Castanea 2016, 81, 314–322. [Google Scholar] [CrossRef]
- Henderson, J.; Grissino-Mayer, H. Climate-tree growth relationships of longleaf pine (Pinus palustris Mill.) in the Southeastern Coastal Plain, USA. Dendrochronologia 2009, 27, 31–43. [Google Scholar] [CrossRef]
- Stambaugh, M.; Bigelow, S.; Abadir, E. Linkages between forest growth, climate, and agricultural production are revealed through analysis of seasonally-partitioned longleaf pine (Pinus palustris Mill.) tree rings. Dendrochronologia 2021, 65, 125801. [Google Scholar] [CrossRef]
- Soulé, P.; Knapp, P.; Maxwell, J.; Mitchell, T. A comparison of the climate response of longleaf pine (Pinus palustris Mill.) trees among standardized measures of earlywood, latewood, adjusted latewood, and totalwood radial growth. Trees 2021, 35, 1065–1074. [Google Scholar] [CrossRef]
- Patterson, T.; Cummings, L.; Knapp, P. Longleaf Pine (Pinus palustris Mill.) Morphology and climate/growth responses along a physiographic gradient in North Carolina. Prof. Geogr. 2015, 68, 238–248. [Google Scholar] [CrossRef]
- Rother, M.; Huffman, J.M.; Harley, G.; Verkerk, P.J. Cambial phenology informs tree-ring analysis of fire seasonality in coastal plain pine savannas. Fire Ecol. 2018, 14, 164–185. [Google Scholar] [CrossRef][Green Version]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2011. [Google Scholar]
- Crawford, C.J.; Griffin, D.; Kipfmueller, K.F. Capturing season specific precipitation signals in the northern Rocky Mountains, USA, using earlywood and latewood tree rings. JGR Biogeosci. 2015, 120, 428–440. [Google Scholar] [CrossRef]
- Andrus, R.A.; Harvey, B.J.; Hoffman, A.; Veblen, T.T. Reproductive maturity and cone abundance vary with tree size and stand basal area for two widely distributed conifers. Ecosphere 2020, 11, e03092. [Google Scholar] [CrossRef]
- Dengle, S.; Aeby, D.; Grace, J. A relationship between galactic cosmic radiation and tree rings. New Phytol. 2009, 184, 545–551. [Google Scholar] [CrossRef]
- Knapp, P.A.; Maxwell, J.T.; Soulé, P.T. Tropical cyclone rainfall variability in coastal North Carolina derived from longleaf pine (Pinus palustris Mill.): AD 1771–2014. Clim. Change 2016, 135, 311–323. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Yamane, K.; Islam, M.A.; Oribe, Y.; Ko, J.H.; Jin, H.; Funada, R. A rapid decrease in temperature induces latewood formation in artificially reactivated cambium of conifer stems. Ann. Bot. 2012, 110, 875–885. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rahman, M.H.; Begum, S.; Nakaba, S.; Yamagishi, Y.; Kudo, K.; Nabeshima, E.; Nugroho, W.D.; Oribe, Y.; Funada, R. Relationship between the earlywood-to-latewood transition and changes in levels of stored starch around the cambium in locally heated stems of the evergreen conifer Chamaecyparis pisifera. Trees 2016, 30, 1619–1631. [Google Scholar] [CrossRef]
- Fritts, H.C.; Smith, D.G.; Stokes, M.A. The biological model for paleoclimatic interpretation of Mesa Verde tree-ring series. Am. Antiq. Mem. Soc. Am. Archaeol. 1965, 31, 101–121. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L.W.; et al. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Anderegg, W.R.L.; Guo, J.S.; Ogle, K. Contemporary tree growth shows altered climate memory. Ecol. Lett. 2022, 25, 2663–2674. [Google Scholar] [CrossRef]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef][Green Version]
- Rozas, V.; Le Quesne, C.; Rojas-Badilla, M.; González-Reyes, Á.; Donoso, S.; Olano, J.M. Climatic cues for secondary growth and cone production are sex-dependent in the long-lived dioecious conifer Araucaria Araucana. Agric. For. Meteorol. 2019, 274, 132–143. [Google Scholar] [CrossRef]
- Herrera-Ramirez, D.; Andreu-Hayles, L.; del Valle, J.I.; Santos, G.M.; Gonzalez, P.L.M. Nonannual tree rings in a climate-sensitive Prioria copaifera chronology in the Atrato River, Colombia. Ecol. Evol. 2017, 7, 6334–6345. [Google Scholar] [CrossRef] [PubMed]
- Davi, H.; Cailleret, M.; Restoux, G.; Amm, A.; Pichot, C.; Fady, B. Disentangling the factors driving tree reproduction. Ecosphere 2016, 7, e01389. [Google Scholar] [CrossRef]



| Escambia | ||||||||||||
| Earlywood | Latewood | Totalwood | ||||||||||
| Tem. | Pre. | SOI | SST | Tem. | Pre. | SOI | SST | Tem. | Pre. | SOI | SST | |
| Spr. | 0.067 | −0.333 | −0.217 | 0.262 | 0.026 | −0.176 | −0.155 | 0.051 | 0.069 | −0.235 | −0.231 | 0.267 |
| 0.568 | 0.004 | 0.071 | 0.107 | 0.826 | 0.132 | 0.200 | 0.758 | 0.555 | 0.043 | 0.055 | 0.101 | |
| Sum. | −0.010 | −0.042 | −0.304 | 0.306 | −0.066 | −0.024 | −0.168 | 0.212 | −0.080 | 0.042 | −0.313 | 0.364 |
| 0.935 | 0.721 | 0.010 | 0.058 | 0.574 | 0.836 | 0.164 | 0.195 | 0.497 | 0.722 | 0.008 | 0.023 | |
| Fall | −0.042 | −0.151 | −0.211 | 0.241 | −0.091 | −0.146 | −0.154 | 0.174 | −0.076 | −0.026 | −0.269 | 0.313 |
| 0.720 | 0.197 | 0.080 | 0.140 | 0.439 | 0.213 | 0.203 | 0.290 | 0.516 | 0.825 | 0.024 | 0.052 | |
| Win. | −0.117 | 0.062 | −0.167 | 0.166 | −0.201 | 0.110 | −0.119 | 0.121 | −0.122 | 0.157 | −0.228 | 0.238 |
| 0.317 | 0.596 | 0.167 | 0.312 | 0.083 | 0.347 | 0.325 | 0.464 | 0.299 | 0.179 | 0.057 | 0.144 | |
| Year | −0.066 | −0.262 | −0.255 | 0.270 | −0.166 | −0.143 | −0.171 | 0.164 | −0.099 | −0.057 | −0.302 | 0.337 |
| 0.573 | 0.023 | 0.033 | 0.096 | 0.155 | 0.221 | 0.156 | 0.318 | 0.400 | 0.625 | 0.011 | 0.036 | |
| Kisatchie | ||||||||||||
| Earlywood | Latewood | Totalwood | ||||||||||
| Tem. | Pre. | SOI | SST | Tem. | Pre. | SOI | SST | Tem. | Pre. | SOI | SST | |
| Spr. | 0.005 | −0.074 | −0.254 | 0.255 | 0.017 | −0.069 | −0.251 | 0.290 | −0.039 | −0.039 | −0.261 | 0.248 |
| 0.970 | 0.579 | 0.054 | 0.117 | 0.899 | 0.608 | 0.057 | 0.074 | 0.770 | 0.772 | 0.048 | 0.129 | |
| Sum. | −0.243 | −0.279 | −0.250 | 0.231 | −0.255 | 0.231 | −0.267 | 0.239 | −0.306 | 0.305 | −0.260 | 0.293 |
| 0.066 | 0.034 | 0.058 | 0.157 | 0.053 | 0.082 | 0.042 | 0.142 | 0.019 | 0.020 | 0.048 | 0.070 | |
| Fall | −0.082 | −0.039 | −0.145 | 0.157 | −0.063 | −0.075 | −0.184 | 0.153 | −0.111 | −0.069 | −0.230 | 0.226 |
| 0.540 | 0.771 | −0.278 | 0.338 | 0.638 | 0.576 | 0.167 | 0.353 | 0.405 | 0.608 | 0.083 | 0.167 | |
| Win. | −0.120 | 0.110 | −0.138 | 0.075 | −0.134 | 0.081 | −0.147 | 0.072 | −0.116 | 0.087 | −0.229 | 0.146 |
| 0.371 | 0.410 | 0.303 | 0.648 | 0.317 | 0.545 | 0.270 | 0.663 | 0.385 | 0.517 | 0.083 | 0.374 | |
| Year | −0.158 | 0.112 | −0.219 | 0.209 | −0.158 | 0.060 | −0.238 | 0.195 | −0.198 | 0.118 | −0.285 | 0.252 |
| 0.238 | 0.403 | 0.098 | 0.208 | 0.237 | 0.656 | 0.072 | 0.234 | 0.136 | 0.377 | 0.030 | 0.122 | |
| Bladen Lakes | ||||||||||||
| Earlywood | Latewood | Totalwood | ||||||||||
| Tem. | Pre. | SOI | SST | Tem. | Pre. | SOI | SST | Tem. | Pre. | SOI | SST | |
| Spr. | −0.011 | −0.004 | −0.199 | 0.213 | −0.077 | −0.063 | −0.196 | 0.169 | 0.017 | −0.009 | −0.246 | 0.277 |
| 0.919 | 0.967 | 0.099 | 0.194 | 0.480 | 0.561 | 0.104 | 0.303 | 0.877 | 0.933 | 0.040 | 0.088 | |
| Sum. | −0.117 | −0.109 | −0.254 | 0.296 | −0.147 | −0.138 | −0.080 | 0.052 | −0.143 | −0.061 | −0.304 | 0.389 |
| 0.282 | 0.316 | 0.034 | 0.067 | 0.174 | 0.201 | 0.510 | 0.754 | 0.185 | 0.572 | 0.010 | 0.014 | |
| Fall | −0.136 | 0.010 | −0.207 | 0.268 | −0.180 | 0.004 | −0.085 | 0.015 | −0.104 | −0.053 | −0.262 | 0.320 |
| 0.208 | 0.930 | 0.086 | 0.099 | 0.095 | 0.973 | 0.482 | 0.930 | 0.340 | 0.624 | 0.028 | 0.047 | |
| Win. | −0.117 | 0.274 | −0.199 | 0.175 | −0.157 | 0.089 | −0.045 | −0.053 | −0.077 | 0.301 | −0.231 | 0.246 |
| 0.281 | 0.010 | 0.098 | 0.287 | 0.146 | 0.414 | 0.714 | 0.749 | 0.479 | 0.005 | 0.054 | 0.132 | |
| Year | −0.156 | 0.060 | −0.250 | 0.270 | −0.226 | −0.057 | −0.110 | 0.032 | −0.118 | 0.058 | −0.302 | 0.350 |
| 0.150 | 0.583 | 0.037 | 0.096 | 0.035 | 0.601 | 0.364 | 0.846 | 0.278 | 0.593 | 0.011 | 0.029 | |
| Escambia | Kisatchie | Bladen Lakes | ||||
|---|---|---|---|---|---|---|
| Earlywood | Latewood | Earlywood | Latewood | Earlywood | Latewood | |
| Year 1 | 0.099 | 0.104 | −0.117 | −0.095 | 0.057 | 0.011 |
| 0.440 | 0.416 | 0.408 | 0.501 | 0.729 | 0.945 | |
| Year 2 | 0.236 | 0.178 | 0.281 | 0.270 | −0.340 | −0.402 |
| 0.060 | 0.160 | 0.044 | 0.053 | 0.029 | 0.009 | |
| Year 3 | −0.098 | −0.089 | 0.150 | 0.106 | 0.013 | −0.068 |
| 0.441 | 0.482 | 0.289 | 0.455 | 0.937 | 0.671 | |
| Year 4 | −0.083 | −0.031 | −0.167 | −0.200 | 0.408 | 0.449 |
| 0.516 | 0.810 | 0.237 | 0.155 | 0.008 | 0.003 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowman, K.A.; Chen, X. The Relationships between Climate, Tree-Ring Growth, and Cone Production in Longleaf Pine. Int. J. Plant Biol. 2023, 14, 418-434. https://doi.org/10.3390/ijpb14020033
Bowman KA, Chen X. The Relationships between Climate, Tree-Ring Growth, and Cone Production in Longleaf Pine. International Journal of Plant Biology. 2023; 14(2):418-434. https://doi.org/10.3390/ijpb14020033
Chicago/Turabian StyleBowman, Kimberly A., and Xiongwen Chen. 2023. "The Relationships between Climate, Tree-Ring Growth, and Cone Production in Longleaf Pine" International Journal of Plant Biology 14, no. 2: 418-434. https://doi.org/10.3390/ijpb14020033
APA StyleBowman, K. A., & Chen, X. (2023). The Relationships between Climate, Tree-Ring Growth, and Cone Production in Longleaf Pine. International Journal of Plant Biology, 14(2), 418-434. https://doi.org/10.3390/ijpb14020033






