Comparative Analysis of the Expression Profiles of Pathogenesis-Related Genes in Tomato Systemically Infected with Tobacco Mosaic and Cucumber Mosaic Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Tomato Seeds and Viruses
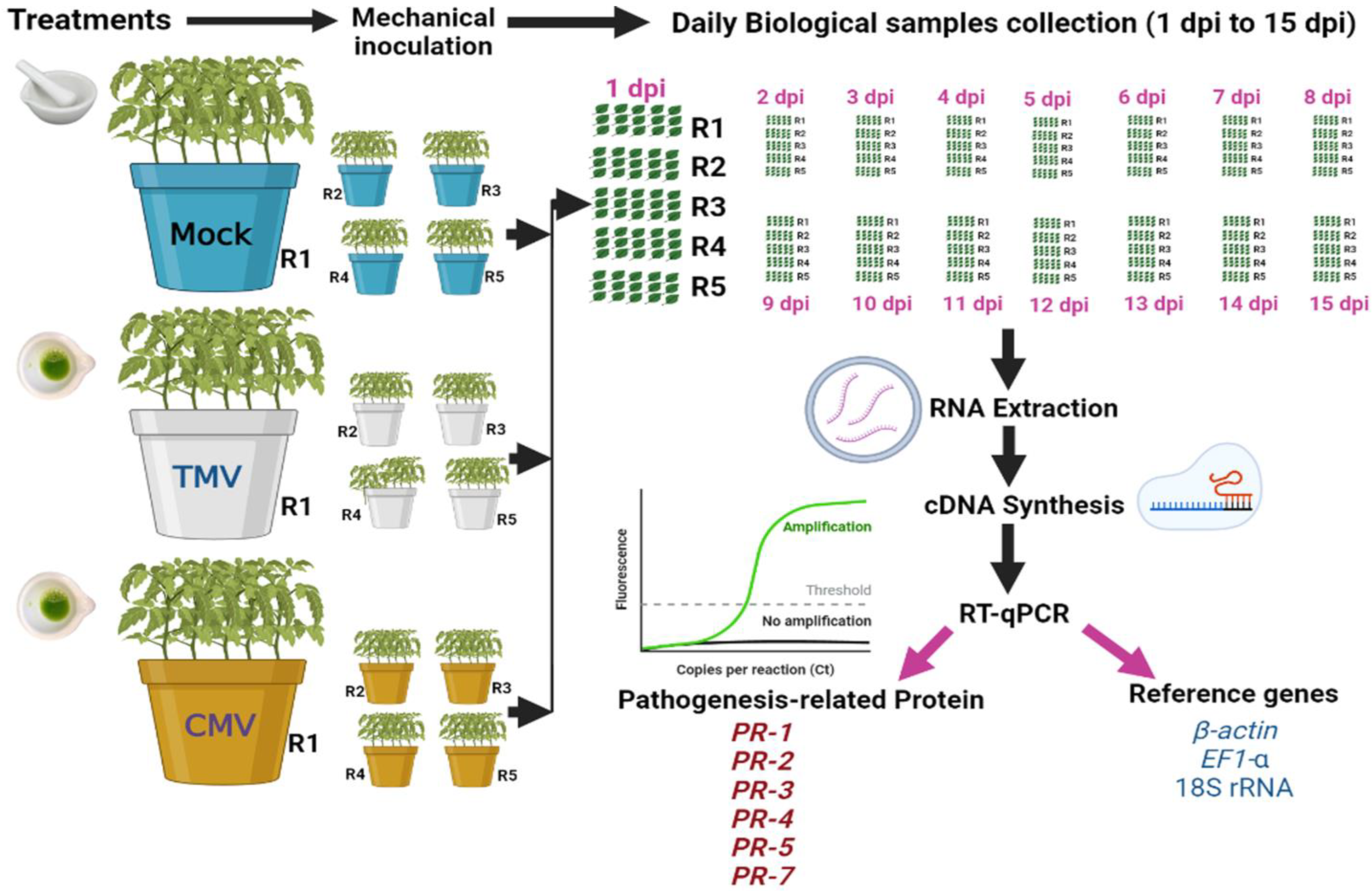
2.2. Greenhouse Experimental Design, Mechanical Inoculation and Sample Collection
2.3. Detection of TMV or CMV by Enzyme-Linked Immunosorbent Assay
2.4. RNA Extraction and cDNA Synthesis
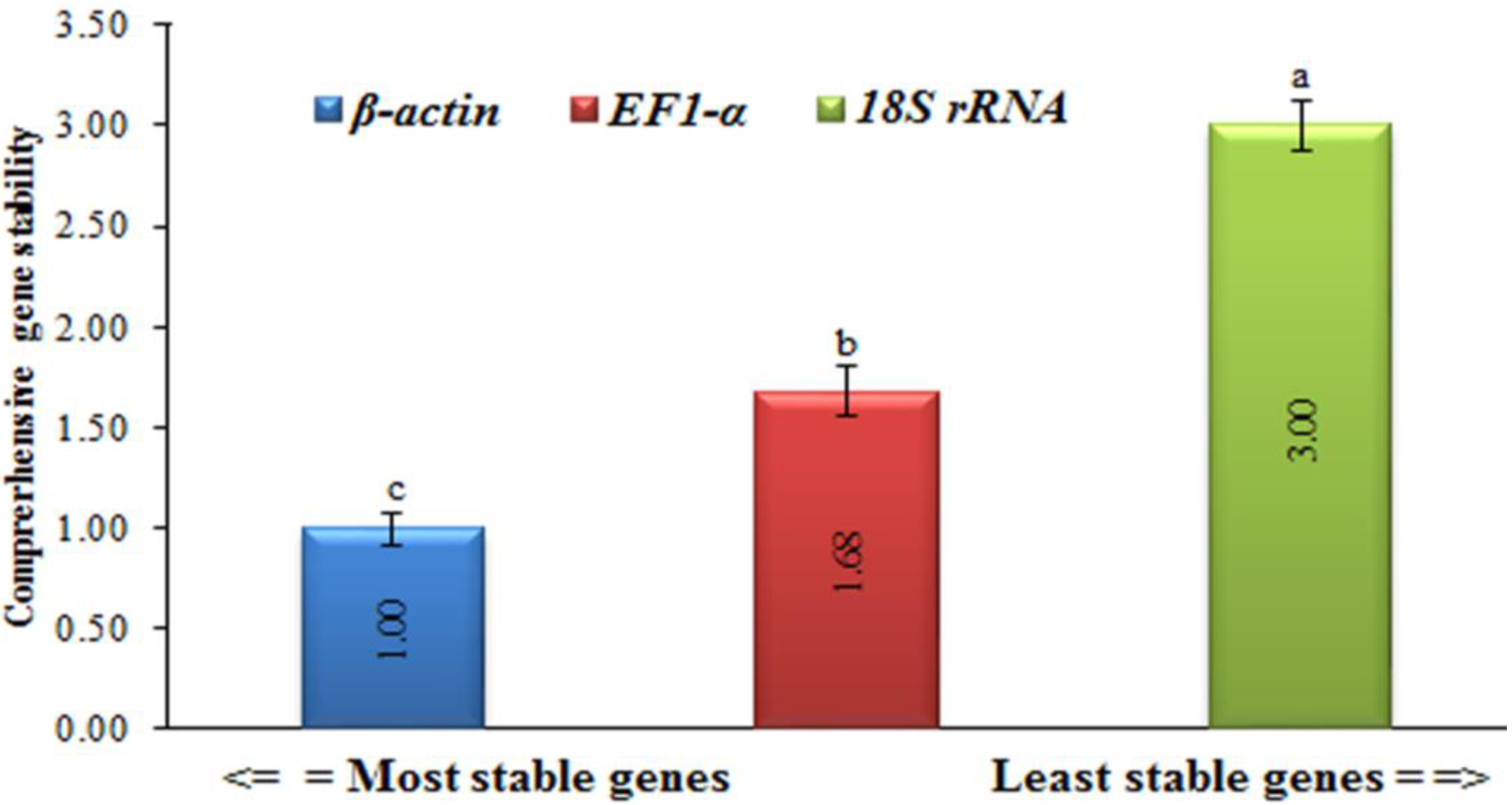
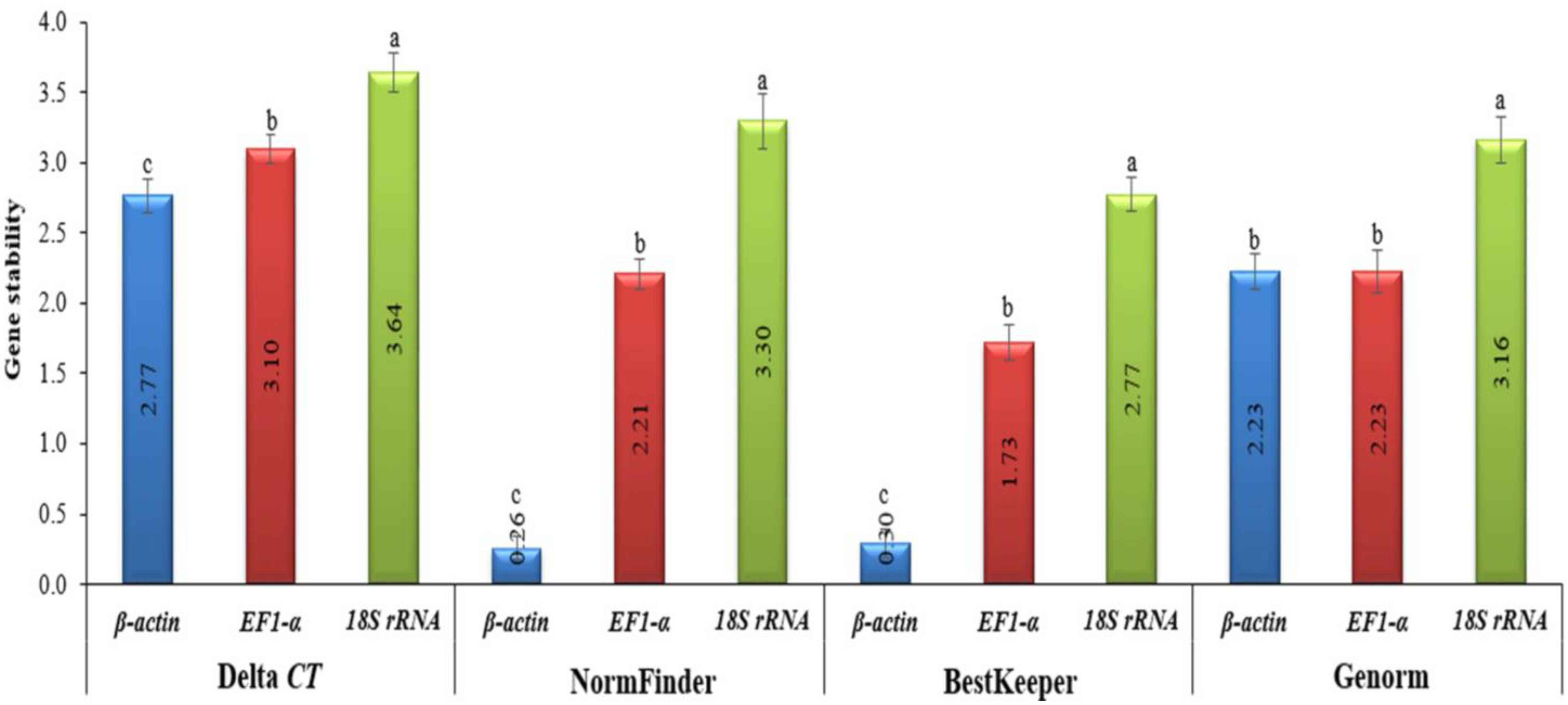
2.5. Normalization and Standardization of the Housekeeping Genes
2.6. RT-qPCR Assay
2.7. RT-qPCR Data Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Symptoms Development and Virus Detection
3.2. Determination and Standardization of the Housekeeping Genes
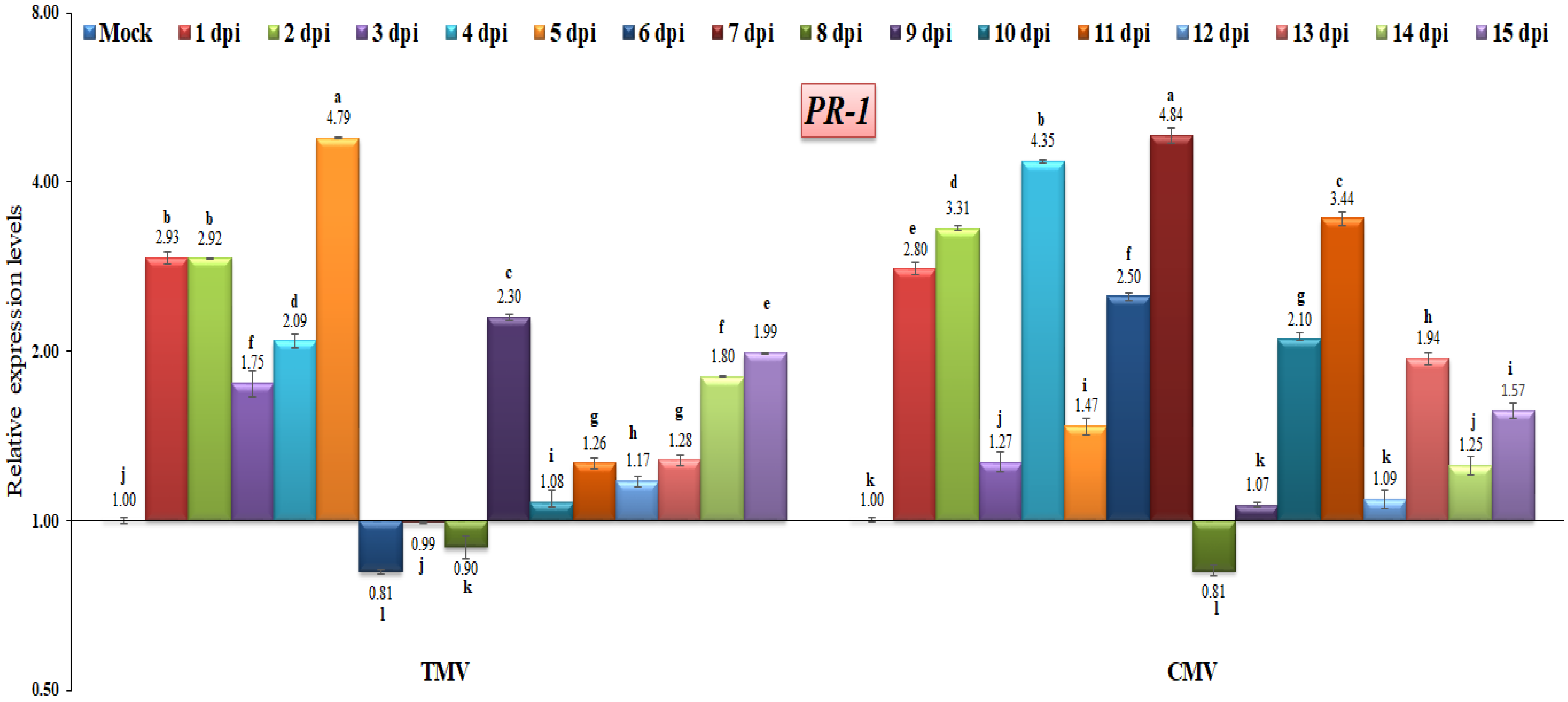
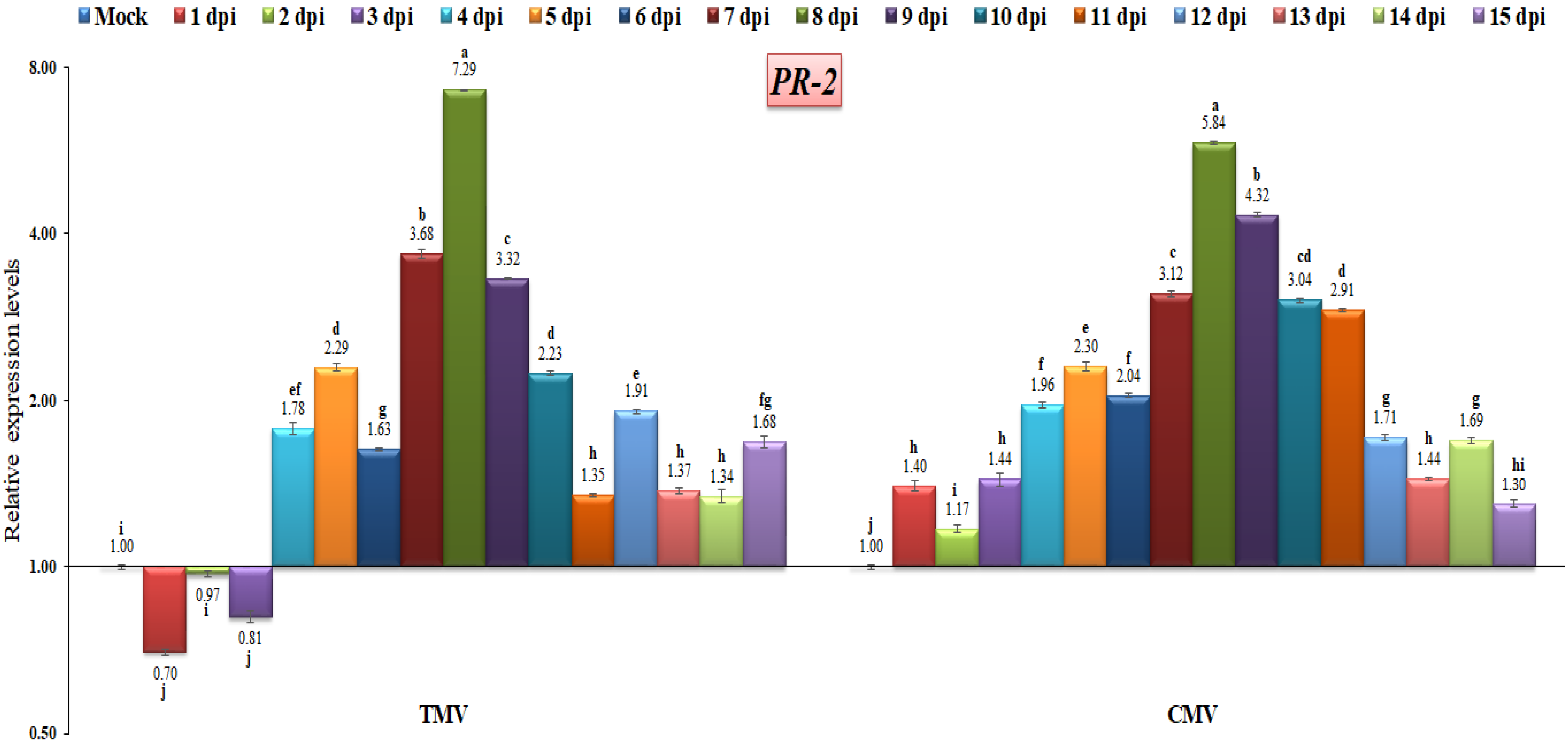
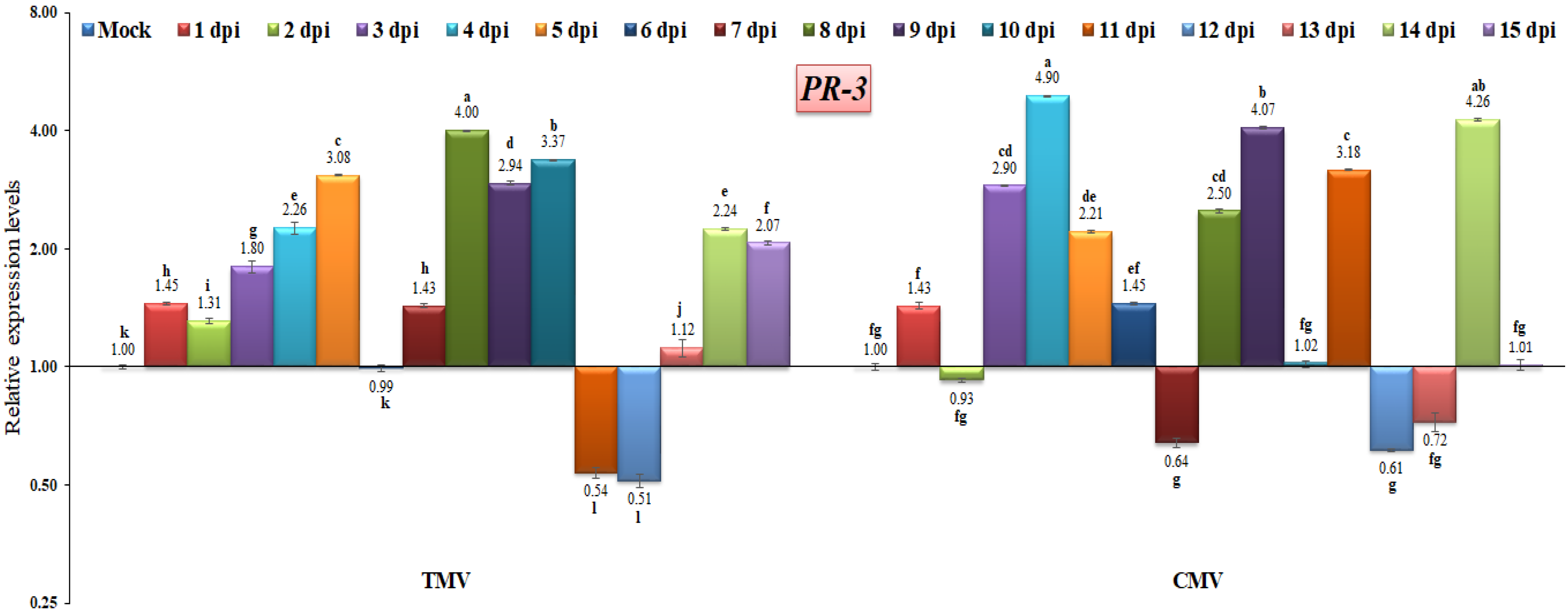
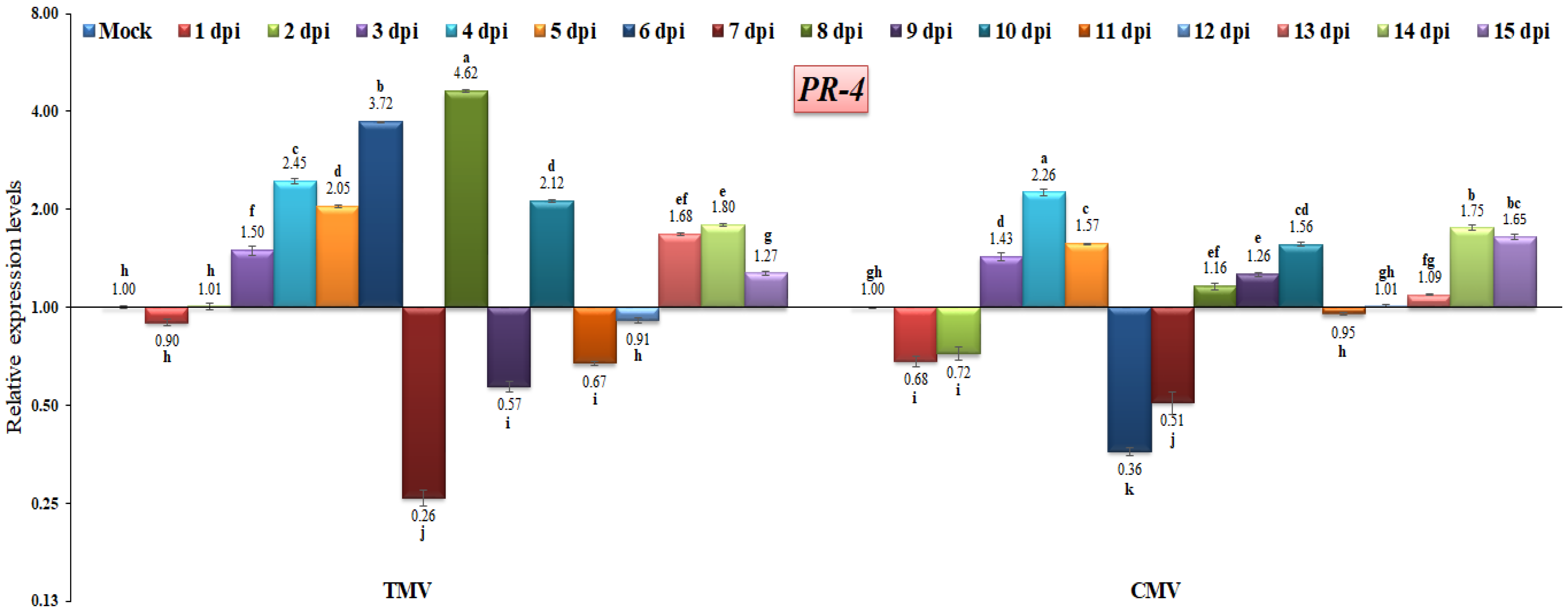
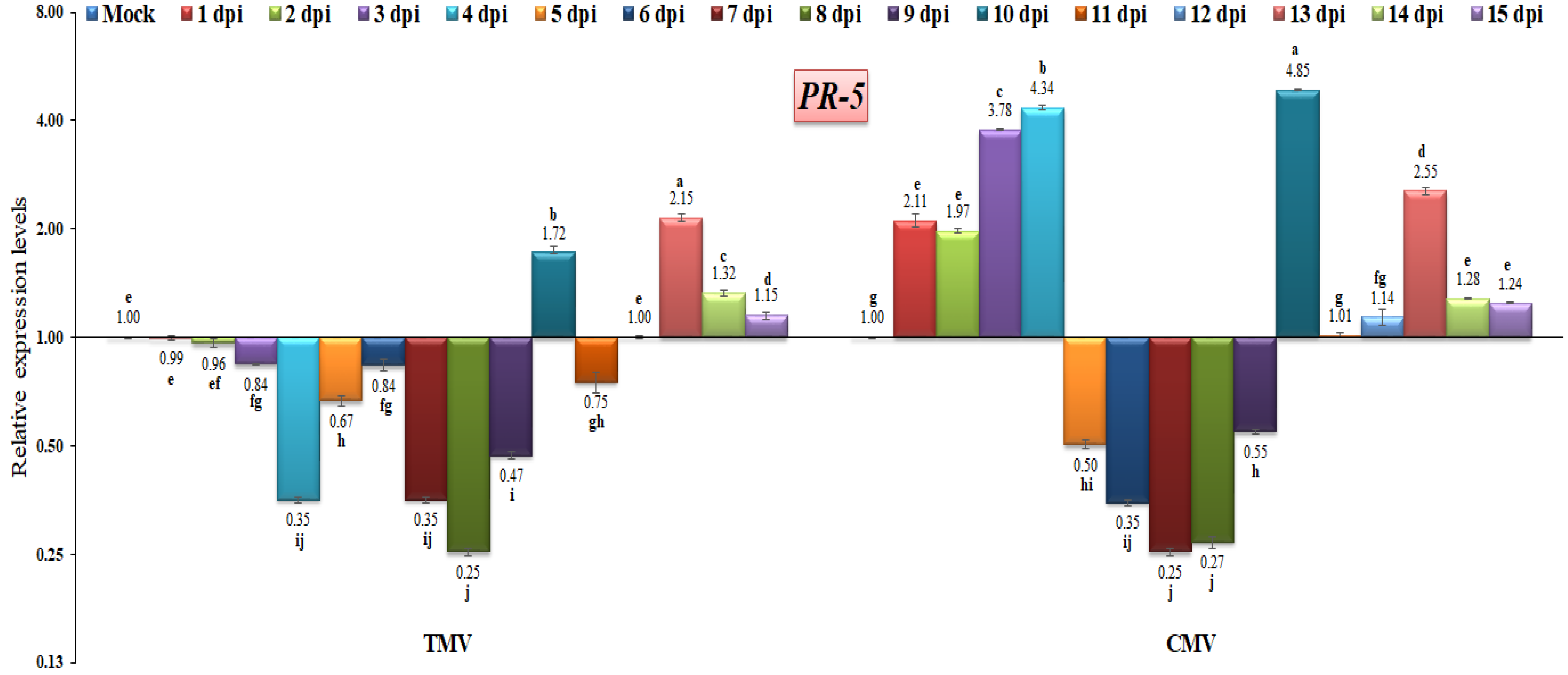
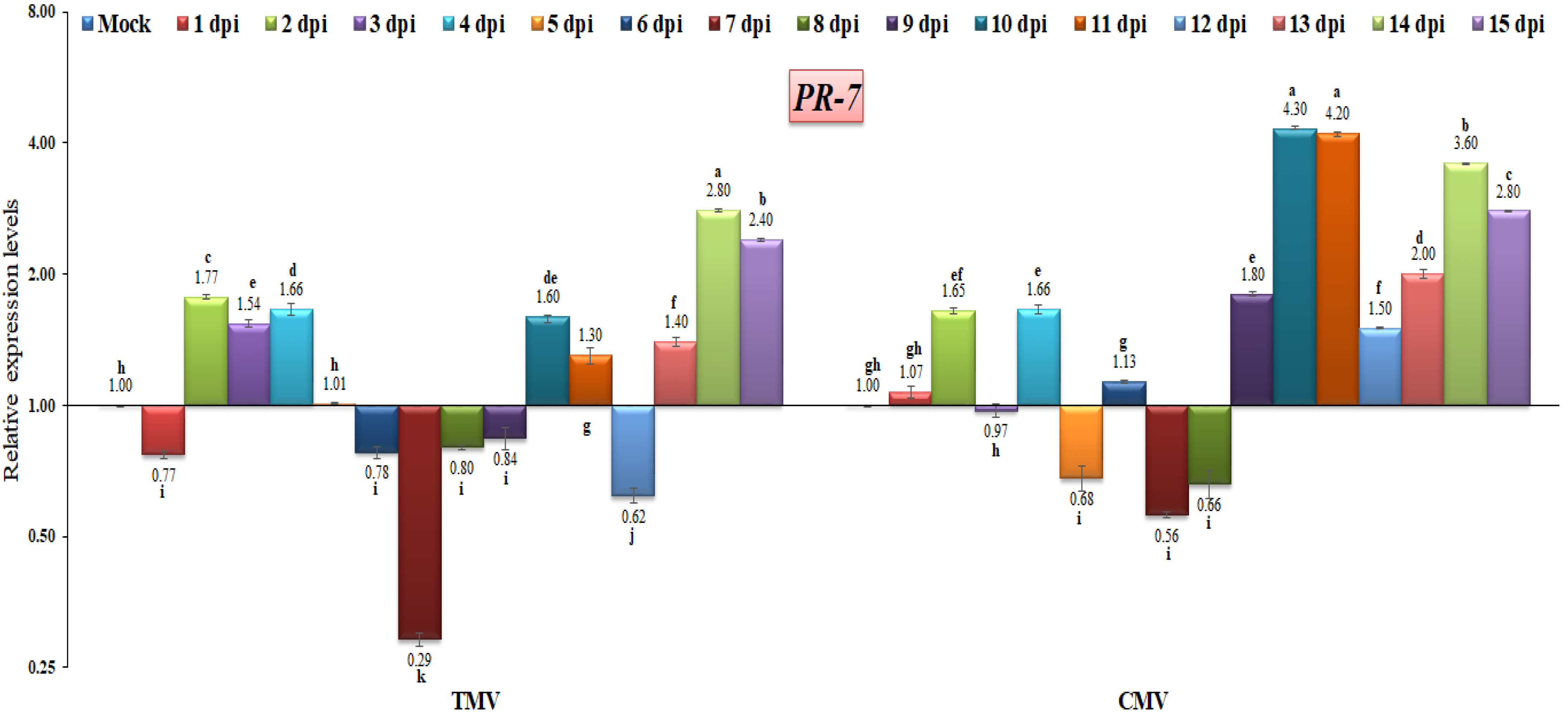
3.3. RT-qPCR Analysis of Pathogenesis-Related (PR) Gene Expressions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicaise, V. Crop immunity against viruses: Outcomes and future challenges. Front. Plant Sci. 2014, 5, 660. [Google Scholar] [CrossRef] [PubMed]
- Hančinský, R.; Mihálik, D.; Mrkvová, M.; Candresse, T.; Glasa, M. Plant Viruses Infecting Solanaceae Family Members in the Cultivated and Wild Environments: A Review. Plants 2020, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Mumford, R.A.; Macarthur, R.; Boonham, N. The role and challenges of new diagnostic technology in plant biosecurity. Food Secur. 2016, 8, 103–109. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, K.; Zhang, J.; Mu, S.; Hao, X. The limonoids and their antitobacco mosaic virus (TMV) activities from Munronia unifoliolata Oliv. J. Agric. Food Chem. 2012, 60, 4289–4295. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Sanan-Mishra, N. A comparative analysis of the suppressor activity of Tobacco mosaic virus proteins in the tomato plant. Jordan J. Biol. Sci. 2018, 11, 469–473. [Google Scholar]
- Scholthof, K.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Mochizuki, T.; Ohki, S.T. Cucumber mosaic virus: Viral genes as virulence determinants. Mol. Plant Pathol. 2012, 13, 217–225. [Google Scholar] [CrossRef]
- Palukaitis, P.; Roossinck, M.J.; Dietzgen, R.G.; Francki, R.I.B. Cucumber mosaic virus. Adv. Virus Res. 1992, 41, 281–348. [Google Scholar]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Whitham, S.A.; Yang, C.; Goodin, M.M. Global impact: Elucidating plant responses to viral infection. Mol. Plant-Microbe Interact. 2006, 19, 1207–1215. [Google Scholar] [CrossRef]
- Linthorst, H.J.M.; Van Loon, L.C. Pathogenesis-related proteins of plants. CRC. Crit. Rev. Plant Sci. 1991, 10, 123–150. [Google Scholar] [CrossRef]
- Sehgal, O.P.; Mohamed, F. Pathogenesis-related proteins. In Plant Viruses; CRC Press: Boca Raton, FL, USA, 2018; pp. 65–83. ISBN 1351075780. [Google Scholar]
- Deepak, S.A.; Kottapalli, K.R.; Rakwal, R.; Oros, G.; Rangappa, K.S.; Iwahashi, H.; Masuo, Y.; Agrawal, G.K. Real-time PCR: Revolutionizing detection and expression analysis of genes. Curr. Genom. 2007, 8, 234–251. [Google Scholar] [CrossRef] [PubMed]
- Remans, T.; Keunen, E.; Bex, G.J.; Smeets, K.; Vangronsveld, J.; Cuypers, A. Reliable gene expression analysis by reverse transcription-quantitative PCR: Reporting and minimizing the uncertainty in data accuracy. Plant Cell 2014, 26, 3829–3837. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Dai, J.; Su, W.; Wu, H.; Shah, K.; Xing, L.; Ma, J.; Zhang, D.; Zhao, C. Selection and validation of reliable reference genes for gene expression studies in different genotypes and TRV-infected fruits of peach (Prunus persica L. Batsch) during Ripening. Genes 2022, 13, 160. [Google Scholar] [CrossRef]
- Abdelkhalek, A. Expression of tomato pathogenesis related genes in response to Tobacco mosaic virus. JAPS J. Anim. Plant Sci. 2019, 29, 1596–1602. [Google Scholar]
- Abdelkhalek, A.; Behiry, S.I.; Al-Askar, A.A. Bacillus velezensis PEA1 Inhibits Fusarium oxysporum Growth and Induces Systemic Resistance to Cucumber Mosaic Virus. Agronomy 2020, 10, 1312. [Google Scholar] [CrossRef]
- Kumar, S.; Udaya Shankar, A.C.; Nayaka, S.C.; Lund, O.S.; Prakash, H.S. Detection of Tobacco mosaic virus and Tomato mosaic virus in pepper and tomato by multiplex RT–PCR. Lett. Appl. Microbiol. 2011, 53, 359–363. [Google Scholar] [CrossRef]
- Khalil, A.M.; Behiry, S.I.; Abdelkhalek, A.; Younes, H.A. Isolation and purification of Alfalfa mosaic virus-infecting potato (Solanum tuberosum L.) in Beheira governorate. Middle East J. 2020, 9, 617–623. [Google Scholar]
- Kavroulakis, N.; Ehaliotis, C.; Ntougias, S.; Zervakis, G.I.; Papadopoulou, K.K. Local and systemic resistance against fungal pathogens of tomato plants elicited by a compost derived from agricultural residues. Physiol. Mol. Plant Pathol. 2005, 66, 163–174. [Google Scholar] [CrossRef]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Izbiańska, K.; Gzyl, J.; Jelonek, T. Implication of peroxynitrite in defence responses of potato to Phytophthora infestans. Plant Pathol. 2016, 65, 754–766. [Google Scholar] [CrossRef]
- Aseel, D.G.; Madian, R.A.; Aggag, S.A.; Elseehy, M.A. Evaluation of some defensin genes against tomv in different tomato cultivars using pathogenesis related protein genes. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 29–33. [Google Scholar]
- Wang, X.; El Hadrami, A.; Adam, L.R.; Daayf, F. Differential activation and suppression of potato defence responses by Phytophthora infestans isolates representing US-1 and US-8 genotypes. Plant Pathol. 2008, 57, 1026–1037. [Google Scholar] [CrossRef]
- Dixit, R.; Agrawal, L.; Gupta, S.; Kumar, M.; Yadav, S.; Chauhan, P.S.; Nautiyal, C.S. Southern blight disease of tomato control by 1-aminocyclopropane-1-carboxylate (ACC) deaminase producing Paenibacillus lentimorbus B-30488. Plant Signal. Behav. 2016, 11, e1113363. [Google Scholar] [CrossRef] [PubMed]
- Mascia, T.; Santovito, E.; Gallitelli, D.; Cillo, F. Evaluation of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in infected tomato plants. Mol. Plant Pathol. 2010, 11, 805–816. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Alsubaie, M.M.; Behiry, S.I. First Report of Protective Activity of Paronychia argentea Extract against Tobacco mosaic virus Infection. Plants 2021, 10, 2435. [Google Scholar] [CrossRef]
- Deloko, D.C.T.; Chofong, N.G.; Ali, I.M.; Kachiwouo, I.G.; Songolo, F.O.; Manock, A.R.N.; Kamgaing, M.; Fonkou, T.; Njukeng, A.P. Detection of Cucumber mosaic virus on Solanum lycopersicum L. and Capsicum annuum L. in the Western region of Cameroon. J. Agric. Food Res. 2022, 8, 100294. [Google Scholar]
- Pfitzner, A.J.P. Resistance to Tobacco mosaic virus and Tomato mosaic virus in tomato. In Natural Resistance Mechanisms of Plants to Viruses; Springer: Berlin/Heidelberg, Germany, 2006; pp. 399–413. [Google Scholar]
- Abdelkhalek, A.; Sanan-Mishra, N. Differential expression profiles of tomato miRNAs induced by Tobacco mosaic virus. J. Agric. Sci. Technol. 2019, 21, 475–485. [Google Scholar]
- El-Helaly, H.S.; Ahmed, A.A.; Awad, M.A.; Soliman, A.M. Biological and molecular characterization of potato infecting Alfalfa mosaic virus in Egypt. Int. J. Virol. 2012, 8, 106–113. [Google Scholar] [CrossRef][Green Version]
- Al-Saleh, M.A.; Amer, M.A. Biological and molecular variability of Alfalfa mosaic virus affecting alfalfa crop in Riyadh region. Plant Pathol. J. 2013, 29, 410–417. [Google Scholar] [CrossRef]
- Wood, A.J.; Joel Duff, R.; Oliver, M.J. The translational apparatus of Tortula ruralis: Polysomal retention of transcripts encoding the ribosomal proteins RPS14, RPS16 and RPL23 in desiccated and rehydrated gametophytes. J. Exp. Bot. 2000, 51, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Jarošová, J.; Kundu, J.K. Validation of reference genes as internal control for studying viral infections in cereals by quantitative real-time RT-PCR. BMC Plant Biol. 2010, 10, 146. [Google Scholar] [CrossRef]
- ElMorsi, A.; Abdelkhalek, A.; Alshehaby, O.; Hafez, E. Pathogenesis-related genes as tools for discovering the response of onion defence system against Iris yellow spot virus infection. Botany 2015, 93, 735–744. [Google Scholar] [CrossRef]
- Mafra, V.; Kubo, K.S.; Alves-Ferreira, M.; Ribeiro-Alves, M.; Stuart, R.M.; Boava, L.P.; Rodrigues, C.M.; Machado, M.A. Reference genes for accurate transcript normalization in citrus genotypes under different experimental conditions. PLoS ONE 2012, 7, e31263. [Google Scholar] [CrossRef] [PubMed]
- Maltseva, D.V.; Khaustova, N.A.; Fedotov, N.N.; Matveeva, E.O.; Lebedev, A.E.; Shkurnikov, M.U.; Galatenko, V.V.; Schumacher, U.; Tonevitsky, A.G. High-throughput identification of reference genes for research and clinical RT-qPCR analysis of breast cancer samples. J. Clin. Bioinform. 2013, 3, 13. [Google Scholar] [CrossRef]
- Bokhale, M.; Mwaba, I.; Allie, F. Real-time PCR data for reference candidate gene selection in tomato infected with Tomato curly stunt virus. Data Brief 2020, 31, 105750. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Cutt, J.R.; Harpster, M.H.; Dixon, D.C.; Carr, J.P.; Dunsmuir, P.; Klessig, D.F. Disease response to Tobacco mosaic virus in transgenic tobacco plants that constitutively express the pathogenesis-related PR1b gene. Virology 1989, 173, 89–97. [Google Scholar] [CrossRef]
- Vuorinen, A.L.; Gammelgård, E.; Auvinen, P.; Somervuo, P.; Dere, S.; Valkonen, J.P.T. Factors underpinning the responsiveness and higher levels of virus resistance realised in potato genotypes carrying virus-specific R genes. Ann. Appl. Biol. 2010, 157, 229–241. [Google Scholar] [CrossRef]
- Niderman, T.; Genetet, I.; Bruyere, T.; Gees, R.; Stintzi, A.; Legrand, M.; Fritig, B.; Mosinger, E. Pathogenesis-related PR-1 proteins are antifungal (isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans). Plant Physiol. 1995, 108, 17–27. [Google Scholar] [CrossRef]
- Luo, X.; Tian, T.; Feng, L.; Yang, X.; Li, L.; Tan, X.; Wu, W.; Li, Z.; Treves, H.; Serneels, F. Pathogenesis-related protein 1 suppresses oomycete pathogen by targeting against AMPK kinase complex. J. Adv. Res. 2023, 43, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Klessig, D.F.; Tian, M.; Choi, H.W. Multiple targets of salicylic acid and its derivatives in plants and animals. Front. Immunol. 2016, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging Insights into the Functions of Pathogenesis-Related Protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Otulak, K.; Garbaczewska, G. Cellular localisation of calcium ions during potato hypersensitive response to Potato virus Y. Micron 2011, 42, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, V.A.; Meins, F.; Meins, F., Jr. Movement of plant viruses is delayed in a β-1, 3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J. 2000, 21, 157–166. [Google Scholar] [CrossRef]
- Oide, S.; Bejai, S.; Staal, J.; Guan, N.; Kaliff, M.; Dixelius, C. A novel role of PR 2 in abscisic acid (ABA) mediated, pathogen-induced callose deposition in Arabidopsis thaliana. New Phytol. 2013, 200, 1187–1199. [Google Scholar] [CrossRef]
- Šindelářová, M.; Šindelář, L. Isolation of pathogenesis-related proteins from TMV-infected tobacco and their influence on infectivity of TMV. Plant Prot. Sci 2005, 41, 52–57. [Google Scholar] [CrossRef]
- Otulak-Kozieł, K.; Kozieł, E.; Lockhart, B. Plant cell wall dynamics in compatible and incompatible potato response to infection caused by Potato virus Y (PVYNTN). Int. J. Mol. Sci. 2018, 19, 862. [Google Scholar] [CrossRef]
- Bucher, G.L.; Tarina, C.; Heinlein, M.; Di Serio, F.; Meins, F., Jr.; Iglesias, V.A. Local expression of enzymatically active class I β-1, 3-glucanase enhances symptoms of TMV infection in tobacco. Plant J. 2001, 28, 361–369. [Google Scholar] [CrossRef]
- Dobnik, D.; Baebler, Š.; Kogovšek, P.; Pompe-Novak, M.; Štebih, D.; Panter, G.; Janež, N.; Morisset, D.; Žel, J.; Gruden, K. β-1, 3-glucanase class III promotes spread of PVY NTN and improves in planta protein production. Plant Biotechnol. Rep. 2013, 7, 547–555. [Google Scholar] [CrossRef]
- Kørner, C.J.; Klauser, D.; Niehl, A.; Domínguez-Ferreras, A.; Chinchilla, D.; Boller, T.; Heinlein, M.; Hann, D.R. The immunity regulator BAK1 contributes to resistance against diverse RNA viruses. Mol. Plant-Microbe Interact. 2013, 26, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Jannoey, P.; Channei, D.; Kotcharerk, J.; Pongprasert, W.; Nomura, M. Expression Analysis of Genes Related to Rice Resistance Against Brown Planthopper, Nilaparvata lugens. Rice Sci. 2017, 24, 163–172. [Google Scholar] [CrossRef]
- Abo-Zaid, G.A.; Matar, S.M.; Abdelkhalek, A. Induction of Plant Resistance against Tobacco MOSAIC Virus Using the Biocontrol Agent Streptomyces cellulosae Isolate Actino 48. Agronomy 2020, 10, 1620. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Hafez, E. Differential induction and suppression of the potato innate immune system in response to Alfalfa mosaic virus infection. Physiol. Mol. Plant Pathol. 2020, 110, 101485. [Google Scholar] [CrossRef]
- Busam, G.; Kassemeyer, H.-H.; Matern, U. Differential expression of chitinases in Vitis vinifera L. responding to systemic acquired resistance activators or fungal challenge. Plant Physiol. 1997, 115, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Singh, R.P.; Kushwaha, G.S.; Iqbal, N.; Singh, A.; Kaushik, S.; Kaur, P.; Sharma, S.; Singh, T.P. Current overview of allergens of plant pathogenesis related protein families. Sci. World J. 2014, 2014, 543195. [Google Scholar] [CrossRef]
- Abulikemu, S.; Yesilyurt, A.; Gencer, D.; Usta, M.; Nalcacioglu, R. Comparison of the potential activities of viral and bacterial chitinases. Egypt. J. Biol. Pest Control 2021, 31, 91. [Google Scholar] [CrossRef]
- Abo-Zaid, G.; Abdelkhalek, A.; Matar, S.; Darwish, M.; Abdel-Gayed, M. Application of Bio-Friendly Formulations of Chitinase-Producing Streptomyces cellulosae Actino 48 for Controlling Peanut Soil-Borne Diseases Caused by Sclerotium rolfsii. J. Fungi 2021, 7, 167. [Google Scholar] [CrossRef]
- Zhang, Y. Studies of Pathogenesis-Related Proteins in the Strawberry Plant: Partial Purification of a Chitinase-Containing Protein Complex and Analysis of an Osmotin-like Protein Gene; Louisiana State University and Agricultural & Mechanical College: Baton Rouge, LA, USA, 2006; ISBN 9798802747094. [Google Scholar]
- Mandadi, K.K.; Pyle, J.D.; Scholthof, K.-B.G. Comparative analysis of antiviral responses in Brachypodium distachyon and Setaria viridis reveals conserved and unique outcomes among C3 and C4 plant defenses. Mol. Plant-Microbe Interact. 2014, 27, 1277–1290. [Google Scholar] [CrossRef]
- Broekaert, I.; Lee, H.; Kush, A.; Chua, N.-H.; Raikhel, N. Wound-induced accumulation of mRNA containing a hevein sequence in laticifers of rubber tree (Hevea brasiliensis). Proc. Natl. Acad. Sci. USA 1990, 87, 7633–7637. [Google Scholar] [CrossRef]
- Neuhaus, J.-M.; Fritig, B.; Linthorst, H.J.M.; Meins, F.; Mikkelsen, J.D.; Ryals, J. A revised nomenclature for chitinase genes. Plant Mol. Biol. Rep. 1996, 14, 102–104. [Google Scholar] [CrossRef]
- Franco, F.P.; Dias, R.O.; Toyama, D.; Henrique-Silva, F.; Moura, D.S.; Silva-Filho, M.C. Structural and functional characterization of PR-4 SUGARWINs from sugarcaneand their role in plant defense. Front. Plant Sci. 2019, 9, 1916. [Google Scholar] [CrossRef]
- Guevara-Morato, M.A.; García de Lacoba, M.; García-Luque, I.; Serra, M.T. Characterization of a pathogenesis-related protein 4 (PR-4) induced in Capsicum chinense L3 plants with dual RNase and DNase activities. J. Exp. Bot. 2010, 61, 3259–3271. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, S.; Pan, W.; Li, Q.; Xia, Z.; Guan, E.; Zheng, M.; Pang, G.; Yang, Y.; Yi, Z. Strategy of tobacco plant against black shank and Tobacco mosaic virus infection via induction of PR-1, PR-4 and PR-5 proteins assisted by medicinal plant extracts. Physiol. Mol. Plant Pathol. 2018, 101, 127–145. [Google Scholar] [CrossRef]
- Dai, L.; Wang, D.; Xie, X.; Zhang, C.; Wang, X.; Xu, Y.; Wang, Y.; Zhang, J. The novel gene VpPR4-1 from Vitis pseudoreticulata increases powdery mildew resistance in transgenic Vitis vinifera L. Front. Plant Sci. 2016, 7, 695. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Gao, L.; Zhang, W.-H.; Liu, J.-K.; Zhang, Y.-J.; Wang, H.-Y.; Liu, D.-Q. Characteristic expression of wheat PR5 gene in response to infection by the leaf rust pathogen, Puccinia triticina. J. Plant Interact. 2015, 10, 132–141. [Google Scholar] [CrossRef]
- Padmanabhan, C.; Ma, Q.; Shekasteband, R.; Stewart, K.S.; Hutton, S.F.; Scott, J.W.; Fei, Z.; Ling, K.-S. Comprehensive transcriptome analysis and functional characterization of PR-5 for its involvement in tomato Sw-7 resistance to tomato spotted wilt tospovirus. Sci. Rep. 2019, 9, 7673. [Google Scholar] [CrossRef]
- Yang, L.; Xu, Y.; Liu, Y.; Meng, D.; Jin, T.; Zhou, X. HC-Pro viral suppressor from tobacco vein banding mosaic virus interferes with DNA methylation and activates the salicylic acid pathway. Virology 2016, 497, 244–250. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Teng, K.; Lai, J.; Zhang, Y.; Huang, Y.; Li, Y.; Liang, L.; Wang, Y.; Chu, C. Up-regulation of LSB1/GDU3 affects geminivirus infection by activating the salicylic acid pathway. Plant J. 2010, 62, 12–23. [Google Scholar] [CrossRef]
- Wang, X.; Zafian, P.; Choudhary, M.; Lawton, M. The PR5K receptor protein kinase from Arabidopsis thaliana is structurally related to a family of plant defense proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 2598–2602. [Google Scholar] [CrossRef]
- Satoh, K.; Shimizu, T.; Kondoh, H.; Hiraguri, A.; Sasaya, T.; Choi, I.-R.; Omura, T.; Kikuchi, S. Relationship between symptoms and gene expression induced by the infection of three strains of Rice dwarf virus. PLoS ONE 2011, 6, e18094. [Google Scholar] [CrossRef] [PubMed]
- Roylawar, P.; Panda, S.; Kamble, A. Comparative analysis of BABA and Piriformospora indica mediated priming of defence-related genes in tomato against early blight. Physiol. Mol. Plant Pathol. 2015, 91, 88–95. [Google Scholar] [CrossRef]

| Gene Name | Specific Class | Accession Number | Nucleotide Sequence (5′-3′) | Reference |
|---|---|---|---|---|
| PR-1 | Pathogenesis related protein-1 | AJ011520 | Forward: CCAAGACTATCTTGCGGTTC Reverse: GAACCTAAGCCACGATACCA | [20] |
| PR-2 | β-1, 3-glucanases | M80604 | Forward: TATAGCCGTTGGAAACGAAG Reverse: CAACTTGCCATCACATTCTG | |
| PR-3 | Chitinase | AF043248 | Forward: ACTGGAGGATGGGCTTCAGCA Reverse: TGGATGGGGCCTCGTCCGAA | [21] |
| PR-4 | Chitin-binding proteins Classes I (hevein-like domain) | Forward: GACAACAATGCGGTCGTCAAGG Reverse: AGCATGTTTCTGGAATCAGGCTG | [22] | |
| PR-5 | Thaumatin-Like Protein | X67244F | Forward: ATGGGGTAAACCACCAAACA Reverse: GTTAGTTGGGCCGAAAGACA | [23] |
| PR-7 | Endoproteinase | Y17275 | Forward: AACTGCAGAACAAGTGAAGG Reverse: AACGTGATTGTAGCAACAGG | [24] |
| β-actin | Housekeeping gene | BT013707 | Forward: AGGCAGGATTTGCTGGTGATGATGCT Reverse: ATACGCATCCTTCTGTCCCATTCCGA | [25] |
| EF1-α | Housekeeping gene | AB061263 | Forward: ATTGGAAATGGATATGCTCCA Reverse: TCCTTACCTGAACGCCTGTCA | |
| 18S rRNA | Housekeeping gene | X51576 | Forward: GGGCATTCGTATTTCATAGTCAGA Reverse: GTTCTTGATTAATGAAAACATCCT |
| Days Post-Inoculation (dpi) | Absorbance ELISA Values at 405 nm | |||
|---|---|---|---|---|
| TMV | CMV | |||
| ELISA Value | Result | ELISA Value | Result | |
| 1 | 0.212 ± 0.024 | - | 0.208 ± 0.035 | - |
| 2 | 0.223 ± 0.037 | - | 0.217 ± 0.029 | - |
| 3 | 0.243 ± 0.049 | - | 0.255 ± 0.041 | - |
| 4 | 0.267 ± 0.019 | - | 0.287 ± 0.052 | - |
| 5 | 0.347 ± 0.027 | - | 0.315 ± 0.063 | - |
| 6 | 0.424 ± 0.036 | + | 0.417 ± 0.043 | + |
| 7 | 0.517 ± 0.044 | + | 0.491 ± 0.079 | + |
| 8 | 0.580 ± 0.025 | + | 0.538 ± 0.095 | + |
| 9 | 0.654 ± 0.068 | + | 0.576 ± 0.082 | + |
| 10 | 0.750 ± 0.024 | + | 0.638 ± 0.076 | + |
| 11 | 0.808 ± 0.043 | + | 0.781 ± 0.097 | + |
| 12 | 0.926 ± 0.079 | + | 0.856 ± 0.081 | + |
| 13 | 0.997 ± 0.087 | + | 0.894 ± 0.099 | + |
| 14 | 1.174 ± 0.089 | + | 0.975 ± 0.098 | + |
| 15 | 1.561 ± 0.103 | + | 1.269 ± 0.111 | + |
| Mock-inoculated plants (healthy) | 0.187 ± 0.011 | - | 0.187 ± 0.011 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aseel, D.G.; Sobhy, S.; Samy, M.A.; Hamdy, E.; Behiry, S.I.; Abdelkhalek, A. Comparative Analysis of the Expression Profiles of Pathogenesis-Related Genes in Tomato Systemically Infected with Tobacco Mosaic and Cucumber Mosaic Viruses. Int. J. Plant Biol. 2023, 14, 458-473. https://doi.org/10.3390/ijpb14020035
Aseel DG, Sobhy S, Samy MA, Hamdy E, Behiry SI, Abdelkhalek A. Comparative Analysis of the Expression Profiles of Pathogenesis-Related Genes in Tomato Systemically Infected with Tobacco Mosaic and Cucumber Mosaic Viruses. International Journal of Plant Biology. 2023; 14(2):458-473. https://doi.org/10.3390/ijpb14020035
Chicago/Turabian StyleAseel, Dalia G., Sherien Sobhy, Marwa A. Samy, Esraa Hamdy, Said I. Behiry, and Ahmed Abdelkhalek. 2023. "Comparative Analysis of the Expression Profiles of Pathogenesis-Related Genes in Tomato Systemically Infected with Tobacco Mosaic and Cucumber Mosaic Viruses" International Journal of Plant Biology 14, no. 2: 458-473. https://doi.org/10.3390/ijpb14020035
APA StyleAseel, D. G., Sobhy, S., Samy, M. A., Hamdy, E., Behiry, S. I., & Abdelkhalek, A. (2023). Comparative Analysis of the Expression Profiles of Pathogenesis-Related Genes in Tomato Systemically Infected with Tobacco Mosaic and Cucumber Mosaic Viruses. International Journal of Plant Biology, 14(2), 458-473. https://doi.org/10.3390/ijpb14020035








