Exploring the Role of Salicylic Acid in Regulating the Colonization Ability of Bacillus subtilis 26D in Potato Plants and Defense against Phytophthora infestans
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Microbe Material
2.2. Models of Plant–Microbe Interaction
2.3. Bacillus Motility
2.4. RNA Isolation and the Reverse Transcription Quantitative Polymerase Chain Reaction (qPCR)
2.5. Analysis of the Number of B. subtilis 26D Cells in Internal Plant Tissues
2.6. Statistical Analysis
3. Results
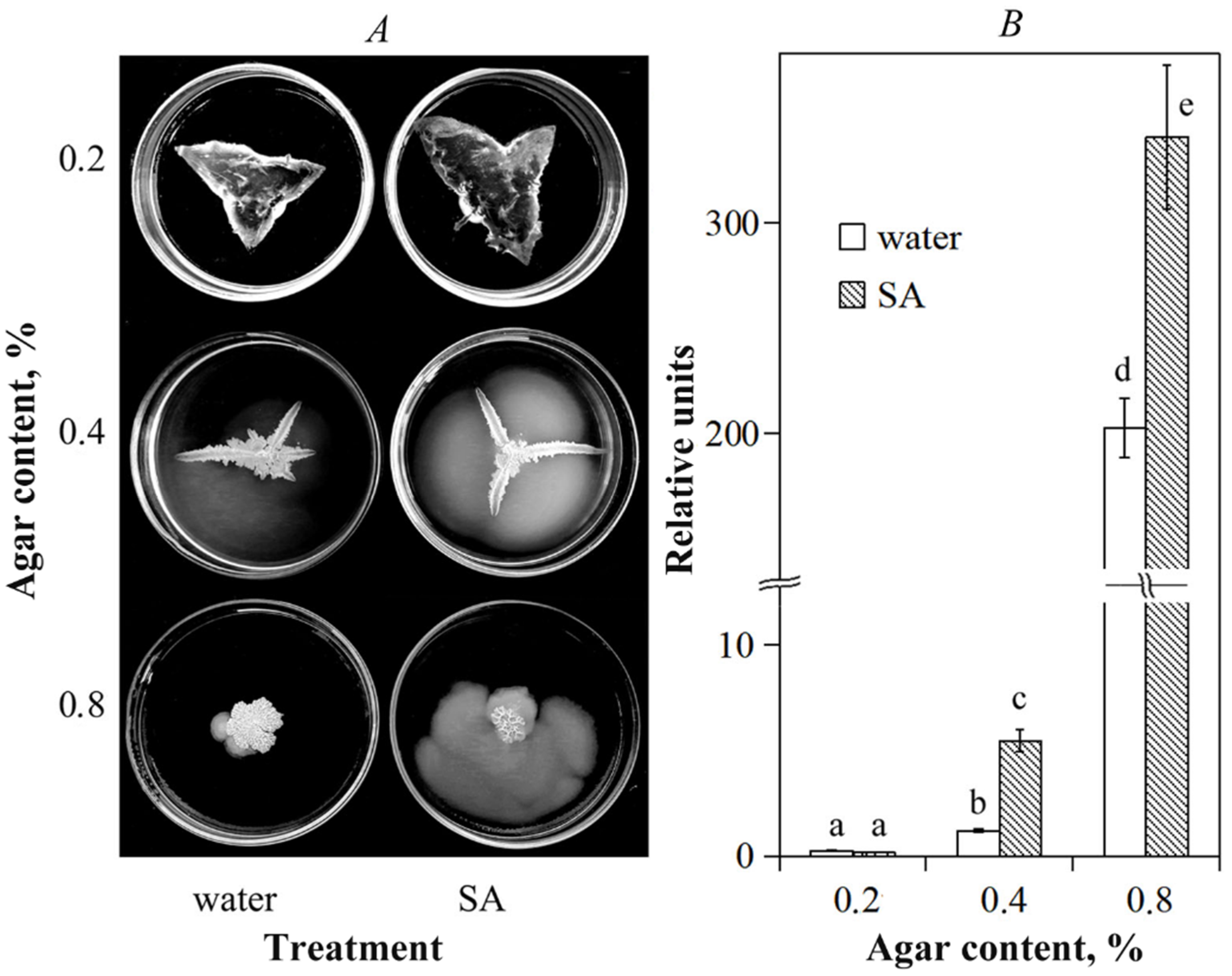
3.1. Influence of Salicylic Acid on B. subtilis 26D Motility and Surfactin Syntetase Transcription In Vitro
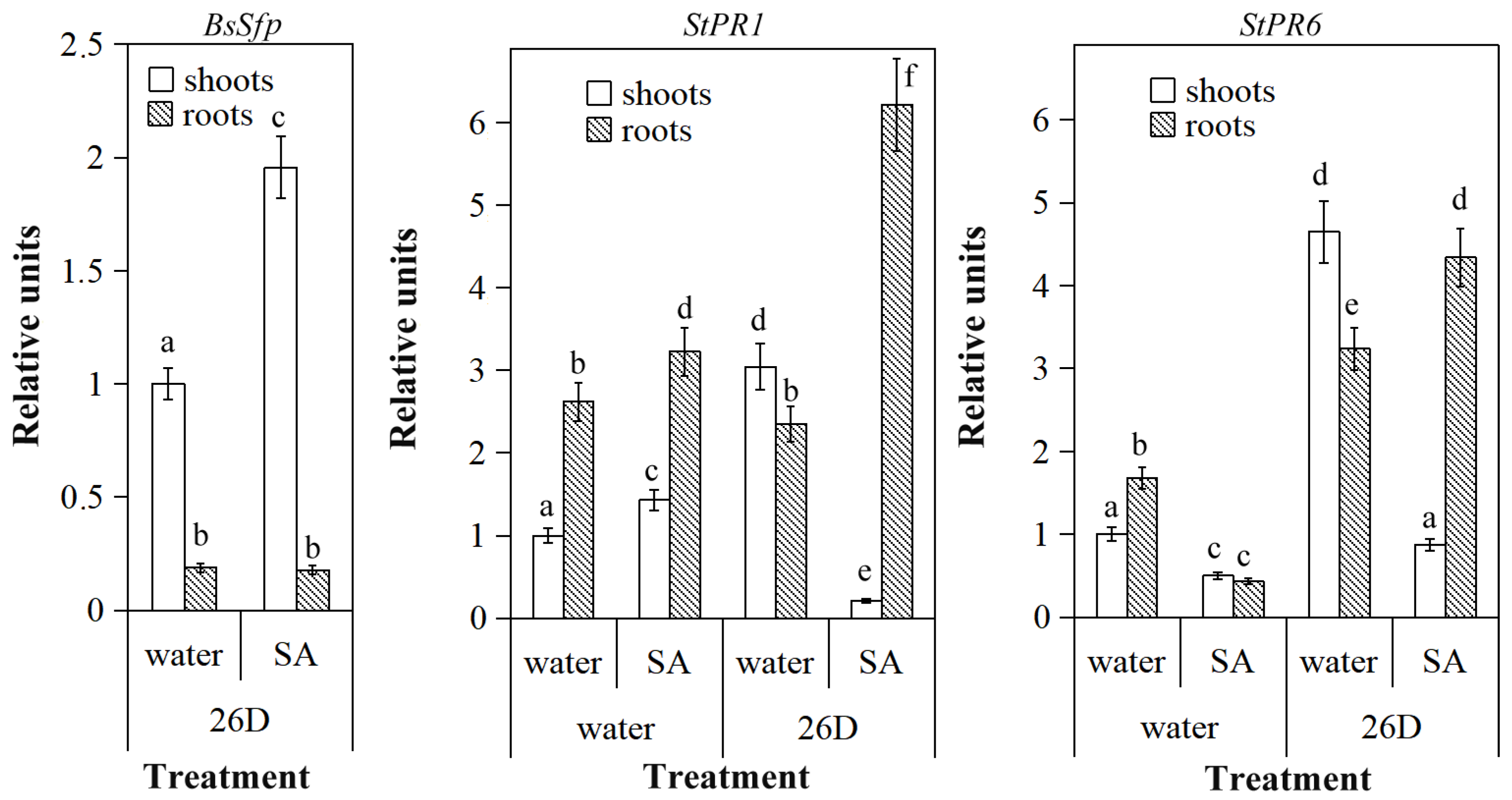
3.2. Impact of Salicylic Acid on Population of B. subtilis 26D
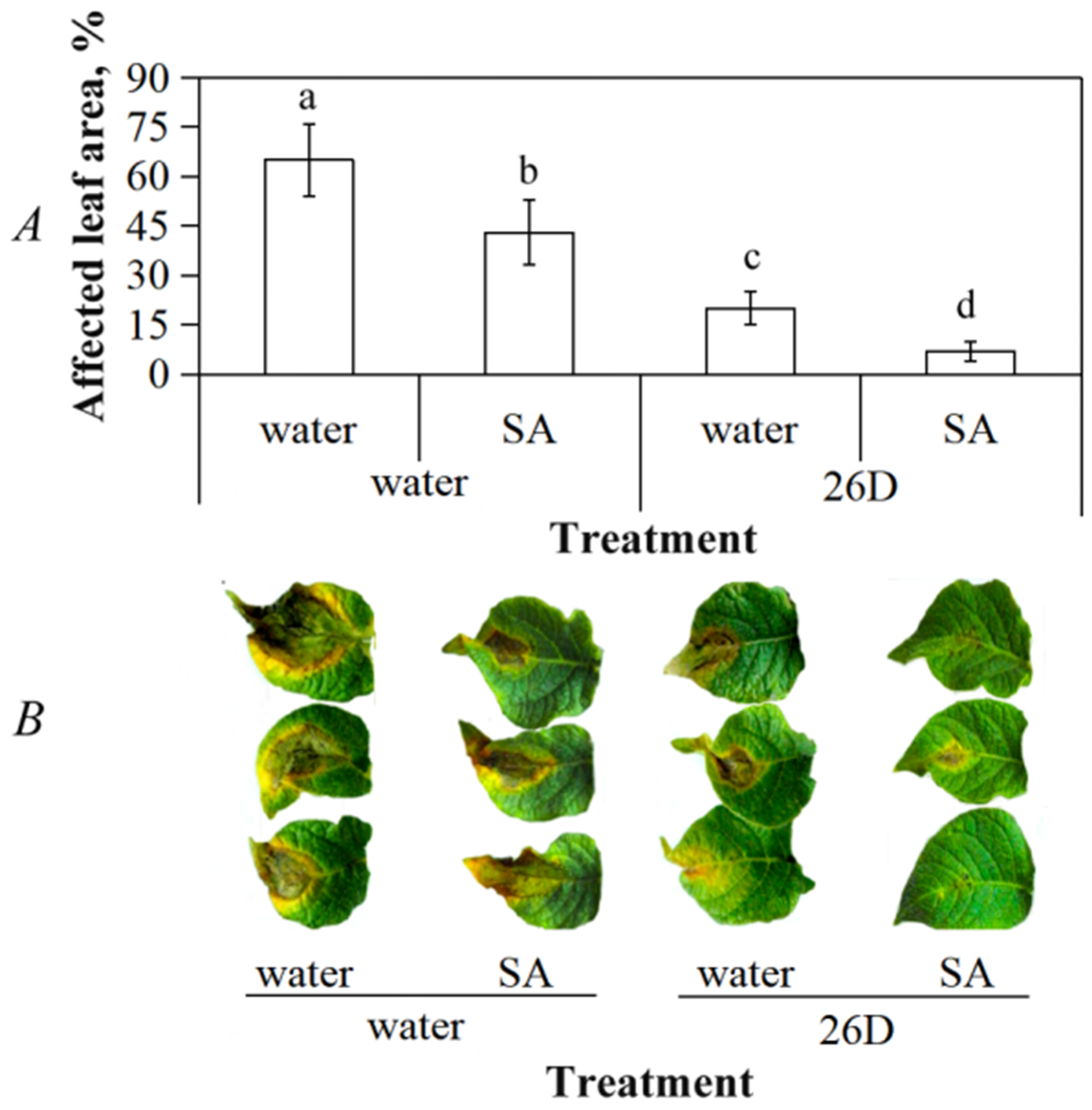
3.3. Salicylic Acid and B. subtilis 26D Enhance Plant Resistance to P. infestans
4. Discussion
5. Conclusions
- (1)
- Plants develop defense reactions on endophytic bacteria;
- (2)
- SA promotes endophytic colonization of shoots by down-regulation of plant defense reactions and up-regulation of the surfactin synthase gene of B. subtilis 26D in vitro and in planta;
- (3)
- High level of endophytic cells in SA+B. subtilis 26D treated plants leads to improvement in potato plants resistance to P. infestans;
- (4)
- Pathogen attack initiates activity of both plant PR genes and surfactin synthase gene of B. subtilis 26D and SA increase these parameters.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumawat, K.C.; Razdan, N.; Saharan, K. Rhizospheric Microbiome: Bio-based Emerging Strategies for Sustainable Agriculture Development and Future Perspectives. Microbiol. Res. 2022, 254, 126901. [Google Scholar] [CrossRef]
- Pinski, A.; Betekhtin, A.; Hupert-Kocurek, K.; Mur, L.A.J.; Hasterok, R. Defining the Genetic Basis of Plant–Endophytic Bacteria Interactions. Int. J. Mol. Sci. 2019, 20, 1947. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mallubhotla, S. Diversity, Antimicrobial Activity, and Antibiotic Susceptibility Pattern of Endophytic Bacteria Sourced from Cordia dichotoma L. Front. Microbiol. 2022, 13, 879386. [Google Scholar] [CrossRef] [PubMed]
- Praca, L.B.; Gomes, A.C.M.M.; Cabral, G.; Martins, E.S.; Sujii, E.H.; Monnerat, R.G. Entophytic Colonization by Brazilian Strains of Bacillus thuringiensis on Cabbage Seedlings Grown in vitro. Bt Res. 2012, 3, 11–19. [Google Scholar] [CrossRef]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Glavina del Rio, T.; Jones, C.D.; Tringe, S.G.; et al. Salicylic Acid Modulates Colonization of the Root Microbiome by Specific Bacterial Taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, N.; Kumar, A.; Munjal, V.; Nadakkakath, A.V.; Eapen, S.J. Pseudomonas putida BP25 Alters Root Phenotype and Triggers Salicylic Acid Signaling as a Feedback Loop in Regulating Endophytic Colonization in Arabidopsis thaliana. Physiol. Mol. Plant. Pathol. 2016, 93, 99–111. [Google Scholar] [CrossRef]
- Iniguez, A.L.; Dong, Y.; Carter, H.D.; Ahmer, B.M.; Stone, J.M.; Triplett, E.W. Regulation of Enteric Endophytic Bacterial Colonization by Plant Defenses. Mol. Plant Microbe Interact. 2005, 18, 169–178. [Google Scholar] [CrossRef]
- Trda, L.; Fernandez, O.; Boutrot, F.; Heloir, M.C.; Kelloniemi, J.; Daire, X. The Grapevine Flagellin Receptor VvFLS2 Differentially Recognizes Flagellin-derived Epitopes from the Endophytic Growth-promoting Bacterium Burkholderia phytofirmans and Plant Pathogenic Bacteria. New Phytol. 2014, 201, 1371–1384. [Google Scholar] [CrossRef]
- Martínez-Gil, M.; Yousef-Coronado, F.; Espinosa-Urgel, M. LapF, the Second Largest Pseudomonas putida Protein, Contributes to Plant Root Colonization and Determines Biofilm Architecture. Mol. Microbiol. 2010, 77, 549–561. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Singh, B.P.; Cherepanova, E.A.; Burkhanova, G.F.; Khairullin, R.M. Prospects and Applications of Lipopeptide-producing Bacteria for Plant Protection (Review). App. Biochem. Microbiol. 2020, 56, 15–28. [Google Scholar] [CrossRef]
- Otto, S.B.; Martin, M.; Schäfer, D.; Hartmann, R.; Drescher, K.; Brix, S.; Kovács, Á.T. Privatization of Biofilm Matrix in Structurally Heterogeneous Biofilms. mSystems 2020, 5, e00425-20. [Google Scholar] [CrossRef] [PubMed]
- Zeriouh, H.; de Vicente, A.; Pérez-García, A.; Romero, D. Surfactin Triggers Biofilm Formation of Bacillus subtilis in Melon Phylloplane and Contributes to the Biocontrol Activity. Environ. Microbiol. 2014, 16, 2196–2211. [Google Scholar] [CrossRef]
- Munakata, Y.; Heuson, E.; Daboudet, T.; Deracinois, B.; Duban, M.; Hehn, A.; Coutte, F.; Slezack-Deschaumes, S. Screening of Antimicrobial Activities and Lipopeptide Production of Endophytic Bacteria Isolated from Vetiver Roots. Microorganisms 2022, 10, 209. [Google Scholar] [CrossRef]
- Stoll, A.; Salvatierra-Martínez, R.; González, M.; Araya, M. The Role of Surfactin Production by Bacillus velezensis on Colonization, Biofilm Formation on Tomato Root and Leaf Surfaces and Subsequent Protection (ISR) against Botrytis cinerea. Microorganisms 2021, 9, 2251. [Google Scholar] [CrossRef] [PubMed]
- Hoff, G.; Arguelles Arias, A.; Boubsi, F.; Pršić, J.; Meyer, T.; Ibrahim, H.M.M.; Steels, S.; Luzuriaga, P.; Legras, A.; Franzil, L.; et al. Surfactin Stimulated by Pectin Molecular Patterns and Root Exudates Acts as a Key Driver of the Bacillus-Plant Mutualistic Interaction. mBio 2021, 12, e0177421. [Google Scholar] [CrossRef]
- Cherepanova, E.A.; Galyautdinov, I.V.; Burkhanova, G.F.; Maksimov, I.V. Isolation and Identification of Lipopeptides of Bacillus subtilis 26D. App. Biochem. Microbiol. 2021, 57, 636–642. [Google Scholar] [CrossRef]
- Sorokan, A.; Benkovskaya, G.; Burkhanova, G.; Blagova, D.; Maksimov, I. Endophytic Strain Bacillus subtilis 26DCryChS Producing Cry1Ia Toxin from Bacillus thuringiensis Promotes Multifaceted Potato Defense against Phytophthora infestans (Mont.) de Bary and Pest Leptinotarsa decemlineata Say. Plants 2020, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.V.; Blagova, D.K.; Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Cherepanova, E.A.; Sarvarova, E.R.; Rumyantsev, S.D.; Alekseev, V.Y.; Khayrullin, R.M. Recombinant Bacillus subtilis 26DCryChS Line with Gene Btcry1Ia Encoding Cry1Ia Toxin from Bacillus thuringiensis Prom otes Integrated Wheat Defense Against Pathogen Stagonospora nodorum Berk. and Greenbug Schizaphis graminum Rond. BioControl 2020, 144, 104242. [Google Scholar] [CrossRef]
- Veselova, S.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Blagova, D.K.; Maksimov, I.V. Strains of Bacillus ssp. Regulate Wheat Resistance to Greenbug Schizaphis graminum Rond. App. Biochem. Microbiol. 2019, 55, 46–52. [Google Scholar] [CrossRef]
- Riera, N.; Wang, H.; Li, Y.; Li, J.; Pelz-Stelinski, K.; Wang, N. Induced Systemic Resistance Against Citrus Canker Disease by Rhizobacteria. Phytopathology 2018, 108, 1038–1045. [Google Scholar] [CrossRef]
- De Souza, A.R.; De Souza, S.; De Oliveira, M.; Ferraz, T.; Figueiredo, F.; Da Silva, N.; Rangel, P.; Panisset, C.; Olivares, F.; Campostrini, E. Endophytic Colonization of Arabidopsis thaliana by Gluconacetobacter diazotrophicus and its Effect on Plant Growth Promotion, Plant Physiology, and Activation of Plant Defense. Plant Soil 2016, 399, 257–270. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.-J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed]
- Mosher, S.; Moeder, W.; Nishimura, N.; Jikumaru, Y.; Joo, S.H.; Urquhart, W.; Klessig, D.F.; Kim, S.K.; Nambara, E.; Yoshioka, K. The Lesion-Mimic Mutant cpr22 Shows Alterations in Abscisic Acid Signaling and Abscisic Acid Insensitivity in a Salicylic Acid-Dependent Manner. Plant Physiol. 2010, 152, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.; Borges, A.; Teodósio, J.; Araújo, P.; Mergulhão, F.; Melo, L.; Simões, M. The Effects of Ferulic and Salicylic Acids on Bacillus cereus and Pseudomonas fluorescens Single- and Dual-species Biofilms. Int. Biodeterior. Biodegrad. 2014, 86, 42–51. [Google Scholar] [CrossRef]
- Puhm, M.; Hendrikson, J.; Kivisaar, M.; Teras, R. Pseudomonas putida Biofilm Depends on the vWFa-Domain of LapA in Peptides-Containing Growth Medium. Int. J. Mol. Sci. 2022, 23, 5898. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Marszałkowska, M.; Reinhold-Hurek, B. Jasmonic Acid, Not Salicylic Acid Restricts Endophytic Root Colonization of Rice. Front. Plant. Sci. 2020, 10, 3–15. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Oláh, J.; Popp, J. Losses in the Grain Supply Chain: Causes and Solutions. Sustainability 2020, 12, 2342. [Google Scholar] [CrossRef]
- Ramos, O.F.; Smith, C.M.; Fritz, A.K.; Madl, R.L. Salicylic Acid-Mediated Synthetic Elicitors of Systemic Acquired Resistance Administered to Wheat Plants at Jointing Stage Induced Phenolics in Mature Grains. Crop Sci. 2017, 57, 3122–3129. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of Inducible Defense-related Proteins in Infected Plants. Annu. Rev. Phytopathol. 2006, 44, 135–151. [Google Scholar] [CrossRef]
- Breen, S.; Williams, S.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging Insights Into the Functions of Pathogenesis-Related Protein1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef]
- Kattupalli, D.; Srinivasan, A.; Soniya, E.V. A Genome-wide Analysis of Pathogenesis-Related Protein-1 (PR-1) Genes from Piper nigrum Reveals its Critical Role During Phytophthora capsici Infection. Genes 2021, 12, 1007. [Google Scholar] [CrossRef]
- Shin, S.H.; Pak, J.-H.; Kim, M.J.; Kim, H.J.; Oh, J.S.; Choi, H.K.; Jung-Hun, P.; Chung, Y.S. An Acidic PATHOGENESIS-RELATED1 Gene of Oryza grandiglumis Is Involved in Disease Resistance Response Against Bacterial Infection. Plant Pathol. J. 2014, 30, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, J.E.; Sanchez, J.P.; Zumstein, K.; Gilchrist, D.G. Plant and Animal PR1 Family Members Inhibit Programmed Cell Death and Suppress Bacterial Pathogens in Plant Tissues. Mol. Plant Pathol. 2018, 19, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, W.; Yang, J.; Xu, J.; Meng, Y.; Shan, W. Two Phytophthora parasitica Cysteine Protease Genes, PpCys44 and PpCys45, Trigger Cell Death in Various Nicotiana spp. and Act as Virulence Factors. Mol. Plant. Pathol. 2020, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef] [PubMed]
- Hotson, A.; Mudgett, M.B. Cysteine Proteases in Phytopathogenic Bacteria: Identification of Plant Targets and Activation of Innate Immunity. Curr. Opin. Plant. Biol. 2004, 7, 384–390. [Google Scholar] [CrossRef]
- IBG UFRC RAS Endophytes Catalogue. Available online: http://ibg.anrb.ru/wp-content/uploads/2019/04/Katalog-endofit.doc (accessed on 2 February 2023).
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial Interactions within the Plant Holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- Balsanelli, E.; Tuleski, T.R.; de Baura, V.A.; Yates, M.G.; Chubatsu, L.S.; de Oliveira Pedrosa, F.; de Souza, E.M.; Monteiro, R.A. Maize Root Lectins Mediate the Interaction with Herbaspirillum seropedicae via N-acetyl Glucosamine Residues of Lipopolysaccharides. PLoS ONE 2013, 8, e77001. [Google Scholar] [CrossRef]
- Meneses, C.H.S.G.; Rouws, L.F.M.; Simoes-Araujo, J.L.; Vidal, M.S.; Baldani, J.I. Exopolysaccharide Production Is Required for Biofilm Formation and Plant Colonization by the Nitrogen-fixing Endophyte Gluconacetobacter diazotrophicus. Mol. Plant Microbe Interact. 2011, 24, 1448–1458. [Google Scholar] [CrossRef]
- Fernández-Llamosas, H.; Díaz, E.; Carmona, M. Motility, Adhesion and c-di-GMP Influence the Endophytic Colonization of Rice by Azoarcus sp. CIB. Microorganisms 2021, 9, 554. [Google Scholar] [CrossRef]
- Yeung, A.T.; Torfs, E.C.; Jamshidi, F.; Bains, M.; Wiegand, I.; Hancock, R.E.; Overhage, J. Swarming of Pseudomonas aeruginosa Is Controlled by a Broad Spectrum of Transcriptional Regulators, Including MetR. J. Bacteriol. 2009, 191, 5592–5602. [Google Scholar] [CrossRef]
- Sarvarova, E.R.; Khairullin, R.M.; Maksimov, I.V. Effect of Ferulic Acid on the Colony Growth and Cell Reproduction of the Endophytic Bacterial Strain Bacillus subtilis 26D. Appl. Biochem. Microbiol. 2021, 57, 508–513. [Google Scholar] [CrossRef]
- Bordiec, S.; Paquis, S.; Lacroix, H.; Dhondt, S.; Ait Barka, E.; Kauffmannx, S.; Jeandet, P.; Mazeyrat-Gourbeyre, F.; Clément, C.; Baillieul, F.; et al. Comparative Analysis of Defence Responses Induced by the Endophytic Plant Growth-promoting Rhizobacterium Burkholderia phytofirmans Strain PsJN and the Non-host Bacterium Pseudomonas syringae pv. pisi in Grapevine Cell Suspensions. J. Exp. Bot. 2011, 62, 595–603. [Google Scholar] [CrossRef]
- Lastochkina, O.; Baymiev, A.; Shayahmetova, A.; Garshina, D.; Koryakov, I.; Shpirnaya, I.; Pusenkova, L.; Mardanshin, I.; Kasnak, C.; Palamutoglu, R. Effects of Endophytic Bacillus subtilis and Salicylic Acid on Postharvest Diseases (Phytophthora infestans, Fusarium oxysporum) Development in Stored Potato Tubers. Plants 2020, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Garshina, D.; Allagulova, C.; Fedorova, K.; Koryakov, I.; Vladimirova, A. Application of Endophytic Bacillus subtilis and Salicylic Acid to Improve Wheat Growth and Tolerance under Combined Drought and Fusarium Root Rot Stresses. Agronomy 2020, 10, 1343. [Google Scholar] [CrossRef]
- Islam, M.N.; Ali, M.S.; Choi, S.J.; Park, Y.I.; Baek, K.H. Salicylic Acid-Producing Endophytic Bacteria Increase Nicotine Accumulation and Resistance against Wildfire Disease in Tobacco Plants. Microorganisms 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]

| Gene | NCBI Access Number | Gene Product | Primers |
|---|---|---|---|
| StPR1 | AY050221 | SA-responsive pathogenesis related protein 1 (PR1 protein) of potato | F: 5′_tgggtggtggttcatttcttgt_3′ R: 5′_catttaattccttacacatcataag_ |
| StPR6 | NW_006239045.1 | Proteinase inhibitor of potato (PR6) | F: 5′_gggaaagaatatgctcaagttat_3′ R: 5′_aattctccatcatcttccactg_3′ |
| BsSrf | EU882341.1 | Surfactin synthetase of B. subtilis | F: 5′_atcttcccgacgctcatttc_3′ R: 5′_atctcaaggctgatcggtttc |
| StAct | X55749.1 | Actin, potato housekeeping gene | F: 5′_gatggtgtcagccacac_3′ R: 5′_attccagcagcttccattcc_3′ |
| Bs16S | NR_112116.2 | 16S rRNA, B. subtilis housekeeping gene | F: 5′_accagaaagccacggctaactac_3′ R: 5′_ggcggaaaccccctaacact_3′ |
| Content of CFU × 104/g in Internal Plant Tissues | Treatment | |
|---|---|---|
| 26D | 26D+SA | |
| shoots | 44.9 ± 10.0 a | 75.0 ± 10.42 b |
| roots | 19.8 ± 5.15 a | 21.4 ± 7.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorokan, A.; Burkhanova, G.; Gordeev, A.; Maksimov, I. Exploring the Role of Salicylic Acid in Regulating the Colonization Ability of Bacillus subtilis 26D in Potato Plants and Defense against Phytophthora infestans. Int. J. Plant Biol. 2023, 14, 242-253. https://doi.org/10.3390/ijpb14010020
Sorokan A, Burkhanova G, Gordeev A, Maksimov I. Exploring the Role of Salicylic Acid in Regulating the Colonization Ability of Bacillus subtilis 26D in Potato Plants and Defense against Phytophthora infestans. International Journal of Plant Biology. 2023; 14(1):242-253. https://doi.org/10.3390/ijpb14010020
Chicago/Turabian StyleSorokan, Antonina, Guzel Burkhanova, Andrew Gordeev, and Igor Maksimov. 2023. "Exploring the Role of Salicylic Acid in Regulating the Colonization Ability of Bacillus subtilis 26D in Potato Plants and Defense against Phytophthora infestans" International Journal of Plant Biology 14, no. 1: 242-253. https://doi.org/10.3390/ijpb14010020
APA StyleSorokan, A., Burkhanova, G., Gordeev, A., & Maksimov, I. (2023). Exploring the Role of Salicylic Acid in Regulating the Colonization Ability of Bacillus subtilis 26D in Potato Plants and Defense against Phytophthora infestans. International Journal of Plant Biology, 14(1), 242-253. https://doi.org/10.3390/ijpb14010020






