Abstract
The species of Artemisia are well known in the Mediterranean region, especially in Morocco, for their traditional uses and health benefit. In this study, we were interested in two species of Artemisia, namely A. herba alba Asso and A. mesatlantica Maire. These species were collected from different soils of the Central Middle Atlas (loamy, stony, limestone and rocky soil) with different atmospheres. Extraction of essential oils from the leaves and flowering tops was carried out by hydrodistillation in Clevenger apparatus. Chemical composition analysis was further carried out using gas chromatography coupled with mass spectrometry (GC-MS). Principal component analysis (PCA) was performed to determine the similarities and dissimilarities in the chemical compositions of these six essential oils. The results obtained showed that the essential oil contents extracted from the flowering tops vary from one species to another according to the place of harvest, altitude, soil type and climate. The essential oil yield is between 0.84% and 2.19% (mL/100 g). Chemical analysis revealed that the chemotype of A. herba alba in limestone soil with a subhumid to humid atmosphere is trans-thujone (33.78%), while camphor (46.19%) is for limestone soil with a semi-arid atmosphere, vetivenic acid (14.91%) and davana ether (14.64%) are for limestone soil with a semi-arid and arid atmosphere and camphor (18.39%) is for loamy and stony soil with a semi-arid atmosphere. As for A. mesatlantica from a rocky soil on limestone with a subhumid to humid atmosphere, the main component is camphor (44.86%), and that of limestone soil with a subhumid to the humid atmosphere trans-thujone (41.08%). In addition, HCA affirmed the PCA and allowed us to distinguish between four groups. Our findings observed differences in the chemical compositions of the isolated essential oils most likely related to many factors such as the climates in the regions of the samples collected, altitudes and soil types.
Keywords:
chemotypes; essential oil; hydrodistillation; GC/MS; chemical composition; PCA; HCA; plant diversity; abiotic factors 1. Introduction
Morocco has several Artemesia called “Chih” in Arabic and “Izri” or “ifssi” in Tamazighte. Artemisia are part of the Asteraceae family, which contains between 200 and 400 herbaceous species in arid, semi-arid, sub-humid and humid zones [1]. There are 19 species in Morocco [2], the most important of which are A. alba chitachensis, A. atlantica var. maroccana, A. flahauti, A. mesatlantica, A. negrei, A ifranensis, A. herba alba, A. arborescens and A. absinthium. Studies of the chemical composition of essential oils (EOs) and chemotypes have revealed a high degree of polymorphism [3]. In Morocco, 16 chemotypes have been identified, with monoterpenes as the major constituents [4]. We also note the presence of sesquiterpene alcohols, sesquiterpene acids, flavonoids, coumarins, polyenes, sterols and triterpenes [5]. The major components of Artemisia EOs are trans-thujone, cis-thujone, camphor, 1, 8-cineole, α-terpineol, chrysanthenone, camphene, α-pinene and davanone. Variations in chemical composition are the result of many factors such as the geographic region, season and environmental and climatic conditions [1], along with genetic factors [6]. Most actors (herbalists, cooperatives, associations, etc.) market local products (plants, hydrosols, etc.) with numbered labels, without any scientific denomination of the exact species, nor of the region of harvest. It should be noted that the chemical composition differs between species or even very similar subspecies of the same genus. It is, therefore, imperative to ensure the botanical identity of the species and determine its chemical composition to avoid any risk of toxicity. Morocco supplies 90% of the sagebrush essential oils to the world market, used in the cosmetics and perfume industries [7]. To enhance the essential oil of sagebrush, two spontaneous species collected at five sites in the Fez-Meknes-Morocco region were chosen. The purpose of this study was to participate in the socioeconomic development of this region’s population, to enable them to promote and market their local products. Our contribution is to provide answers as to the scientific names of the sagebrush species, the yields of the essential oils and their qualitative and quantitative chemical compositions.
2. Materials and Methods
2.1. Study Zone
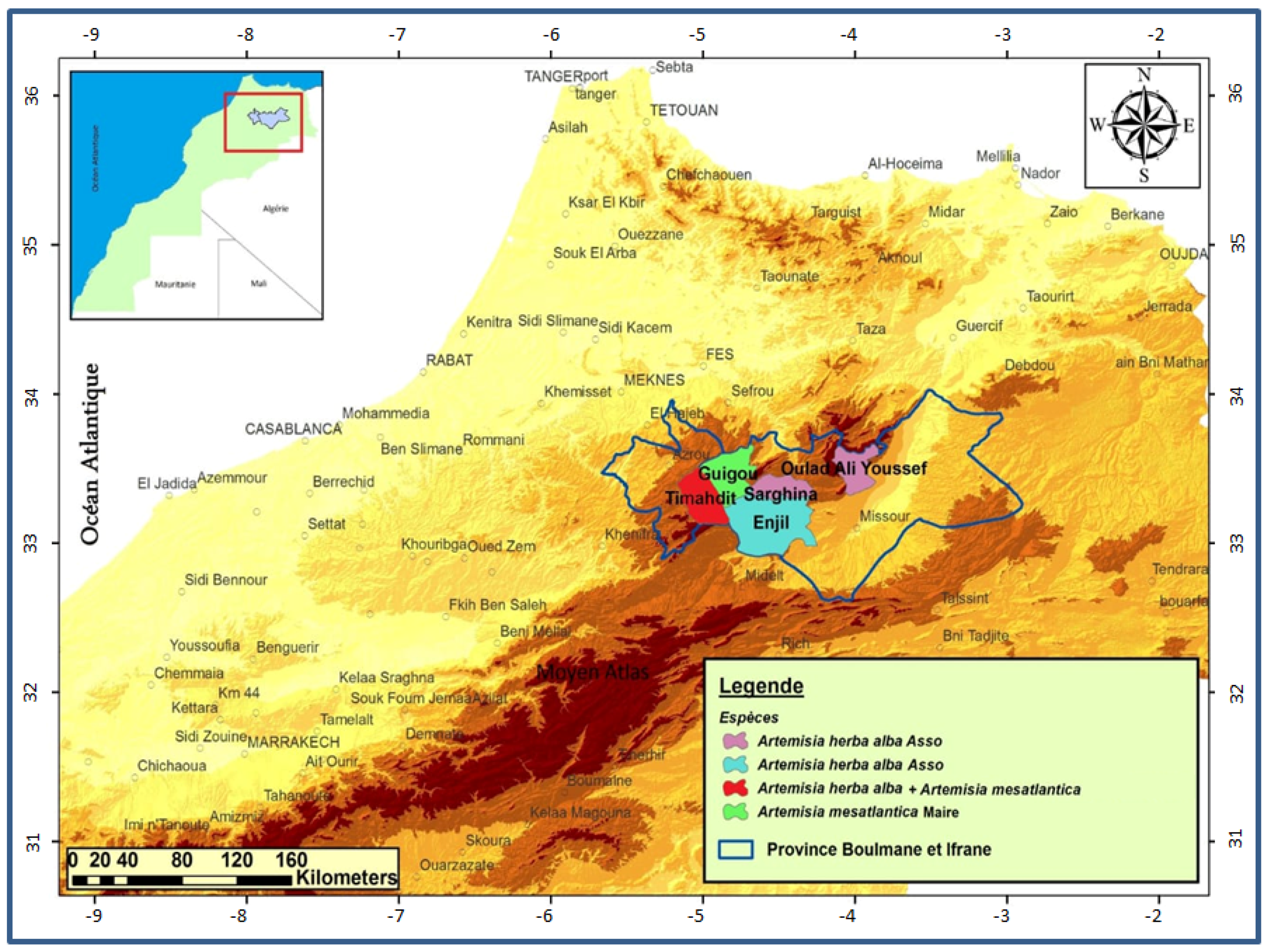
The central Middle Atlas of Morocco has a very varied bioclimate (climate, soil, relief, etc.), with forest cover and remarkable flora. This constitutes a protective and productive heritage, as well as a genetic reservoir of biodiversity. This regional flora, thus rich in aromatic and medicinal plants, can be developed as a source of products with high added value for the rural populations of the regions of Boulemane and Ifrane. The territory of our study comprised five sites: Serghina, Oulad aliyoussef, Enjil, Guigou and Timahdit. These sites are grouped in the central Middle Atlas mountain zone (Figure 1).
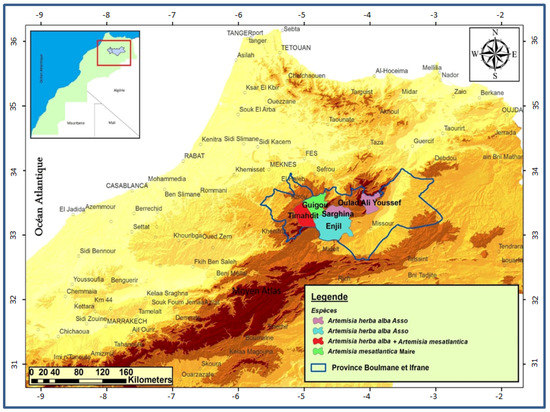
Figure 1.
Map showing the harvesting sites of the two sagebrush species.
2.2. Plant Material
The studied samples were collected at the time of their flowering (months of March and April 2017 and 2018) (Table 1). Then, they were dried in the shade for ten days. The botanical identification of species was carried out at the Floristics Laboratory of the Scientific Institute in Rabat by Hamid Khamar. Voucher specimens were deposited in the National Herbarium (RAB) of the Scientific Institute RABAT, with the code number of A. herba alba as RAB113451 and that of A. mesatlantica as RAB113452. Morphological aspects of A. herba alba Asso (A) and A. mesatlantica Maire (B) are presented below (Figure 2).

Figure 2.
Morphological aspects of A. herba alba Asso (A) and A. mesatlantica Maire (B).
2.3. Determination of the Humidity Level
The water contents of the samples collected were determined by the oven-drying process, through measuring the mass of the fresh plant and its mass after drying. Three repetitions were performed.
The humidity rate was calculated by the following relationship:
where M0 is the initial mass of the plant and M1 is the mass after drying.
2.4. Extraction of Essential Oils and Determination of Yields
The extraction of essential oils (EOs) was carried out by hydrodistillation, from 100 g of flowering tops of each species, for four hours using a Clevenger-type device. Next, the recovered EOs were dried with anhydrous sodium sulfate and then quantified and stored at a temperature of 4 °C, protected from light until their use [8]. This operation was repeated three times.
The EO yield was calculated from 100 g plant material by the formula:
where V (HE) is the volume of HE recovered (mL) and TH (%) is the moisture rate.
2.5. Analysis and Identification of the Chemical Composition of EO
Analysis of the chemical composition of sagebrush EOs was carried out at the Faculty of Sciences research center of Meknes-Morocco.
We used a gas chromatograph of the Thermo Electron type (Trace GC Ultra), coupled to a mass spectrometer of the Thermo Electron Trace MS system type (Thermo Electron: Trace GC Ultra; Polaris Q MS). Fragmentation was carried out by electronic impact with an intensity of 70 eV; the chromatograph was equipped with a DB-5-type column (5% phenyl-methyl-siloxane) (30 m × 0.25 mm × 0.25 μm film thickness) of a flame ionization detector (FID) supplied with a mixture of H2 gas/air. The temperature of the column was programmed at the rate increase of 4 °C/min from 50 to 200 °C for 5 min. The injection mode was split (leakage ratio: 1/70, flow mL/min), and the carrier gas used was nitrogen with a flow rate of 1 mL/min.
Identification of the EO chemical composition was performed through the determination and comparison of compounds’ Kovats (IK) indices with those of standard products known from the literature [9,10,11]. The identification of each compound was made using the Kovats index by comparing peak retention times with those of known authentic standards available in the authors’ laboratory, and comparing their reported RI and MS data with those stored in the standard mass spectral databases of Wiley and NIST 14 and the published literature. Kovats indices compare the retention time of any product with the retention time of a linear alkane containing the same carbon number. They are determined by co-injecting a mixture of the alkanes (C7-C40 standard) under the same operating conditions.
2.6. Statistical Analysis
In this study, all statistical analyses were performed by GraphPad Prism 7.03 software (GraphPad Software Inc., San Diego, CA, USA) and XLSTAT v.2017 (Addinsoft, New York, NY, USA), respectively, for statistical comparisons by one-way analysis of variance (ANOVA) and for principal component analysis (PCA) and hierarchical cluster analysis (HCA). Briefly, the ANOVA was performed with multiple Bonferroni and Tukey comparisons tests to compare the extraction yields of essential oils. Any statistical difference was noted if the p-value < 0.5 in Table 1 (*: p < 0.5; **: p < 0.1; ***: p < 0.01). The chemical compositions were subjected to PCA and HCA to determine the degree of correlation of the main compounds identified with particular essential oils (and through this correlation, to deduce the similarity or dissimilarity between species from different localities). HCA was made to group similar compositions of oils using Ward’s method and Euclidian distances.
3. Results and Discussion
3.1. Extraction Yields of Essential Oils
The EO yields of each species obtained are presented in Table 1. We note that the essential oil contents of the different species vary from one to another and within the same species, depending on the location of harvest, altitude, soil type and climate; they are included between 0.8% and 2.2% (mL/100 g) (Table 1).

Table 1.
Yield (%) in EO of the different species of sagebrush studied.
Table 1.
Yield (%) in EO of the different species of sagebrush studied.
| Species | Codification | Site | EO (Yield %) | Geographical Coordinates and Altitude | Type of Soil | Bioclimatic Atmosphere |
|---|---|---|---|---|---|---|
| Artemisia herba alba Asso | AHAass-OA | Oulad aliyoussef | 0.9 ± 0.04 *** | 32°24′8.86″ N 3°58′25.98″ W 1507 m | Poor loamy and stony soil | Semi-arid |
| Artemisia herba alba Asso | AHAass-SA | Serghina | 1.0 ± 0.04 *** | 33°26′40.955″ N 4°58′8.006″ W 1532 m | Limestone | Semi-arid, arid |
| Artemisia herba alba Asso | AHAass-ENJ | Enjil | 1.1 ± 0.05 *** | 33°11′22.068″ N 4°32′0.065″ W 1552 m | Limestone | Semi-arid |
| Artemisia herba alba Asso | AHAass-TI | Timahdit | 1.9 ± 0.09 *** | 33°19′36.686″ N 45°51′4.078″ W 1599 m | Limestone | Subhumid to humid |
| Artemisia mesatlantica Maire | AMma-GU | Guigou | 2.2 ± 0.10 *** | 33°19′44.637″ N 4°54′31.54″ W 1731 m | Rocky soil on limestone | Subhumid to humid |
| Artemisia mesatlantica | AM-TI | Timahdit | 0.8 ± 0.02 *** | 33°24′27.53″ N 4°52′13.49″ W 1498 m | Limestone | Subhumid to humid |
***: p < 0.01 at least one time by performing comparison between two essential oils yields.
The best EO yield (2.2 ± 0.1%) was given by the AMma-GU sample, followed by AHAass-TI (1.9 ± 0.09%) and finally by AHAass-ENJ (1.1 ± 0.05%). Low levels were recorded for AHAass from the two provinces of Oulad aliyoussef and Serghina, of 1 ± 0.04% and 0.9 ± 0.04%, respectively. Finally, AM-TI provided the lowest EO yield (0.8 ± 0.02%).
From these results, we noted that:
- -
- The value of the sagebrush EO yield increased with the altitude of the sampling site;
- -
- The soil profile was characterized by a dominance of limestone at the levels of AHAass-SA, AHAass-ENJ, AMma-GU, AHAass-TI and AM-TI, while the site of Oulad aliyoussef was of a poor silty and stony type;
- -
- The spontaneous species of sagebrush in humid and subhumid stages exceeded the spontaneous species in arid and semi-arid stages.
A comparison with the literature showed that the yield of AMma-GU (2.2%) from the Boulemane region was higher than that given by Rouani et al. (0.9%) for the same species from the Ifrane region [12]. The EO yields of AHAass from the four provinces Oulad aliyoussef (0.8%), Serghina (0.9%), Timahdit (1.1%) and Enjil (1.9%) were in agreement with those found by Ghanmi et al. (0.8%) [13] for A. herba alba of Guercif (Eastern Morocco), by Ourid et al. (1.1%) from the Imouzzer Marmoucha region [14] and close to those obtained by Hinane et al., who showed that A. herba alba from Amskroude provided an EO yield of approximately 1.6% [15]. Finally, for AM-TI (Ifrane region), the EO content was higher than that of the Ifrane-Boulmane region (0.5%) [16].
We found that the EO yields obtained for other sagebrush species from western and southwestern Turkey, such as A. vulgaris L. (0.4%), A. santonicum L. (0.4%) and A. campestris (0.7%) [17], remained significantly lower than those of the sagebrush species in the present study.
From these results, it can be concluded that intra-specific variations in yields appear to be correlated with abiotic factors such as the climate specific to the sample collection region and geographic factors such as the elevation and soil type. This agrees with the results reported by El Idrissi et al. (2016), according to which variations in content can be caused by several factors including the climate, altitude and soil type. Several studies have also shown the influence of the vegetative cycle, nature of the plant (dried or fresh), period of picking and extraction method on the yield and quality of the essential oil [18,19,20].
3.2. Chemical Composition of the Studied Sagebrush Essential Oils
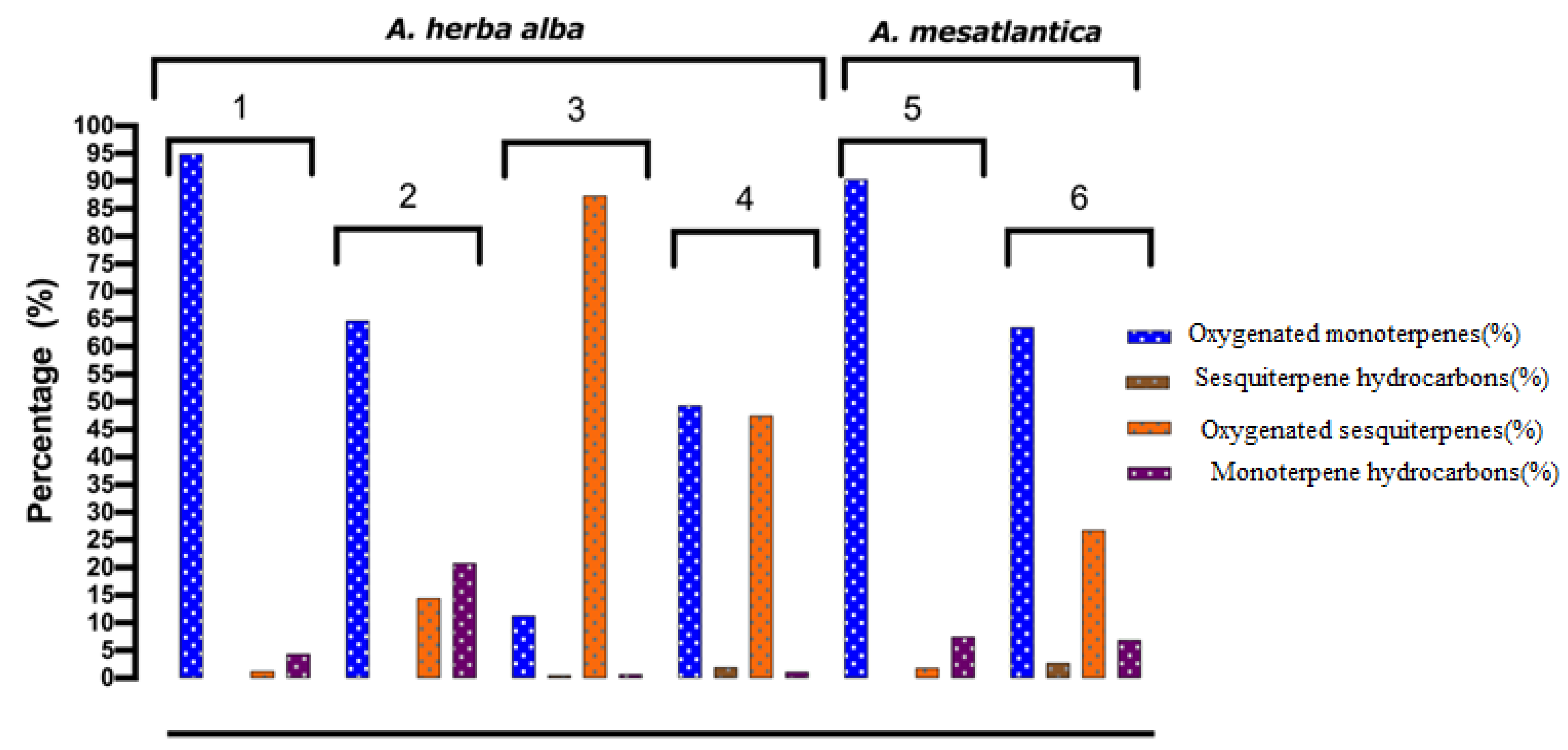
Table 2 and Table 3 and the histogram in Figure 3 present the results related to the chemical compositions of the essential oils of the six Artemisia, determined by GC/SM.

Table 2.
The chemical composition of essential oil of A. herba alba Asso from different locations.

Table 3.
The chemical composition of essential oil of A. mesatlantica from different locations.
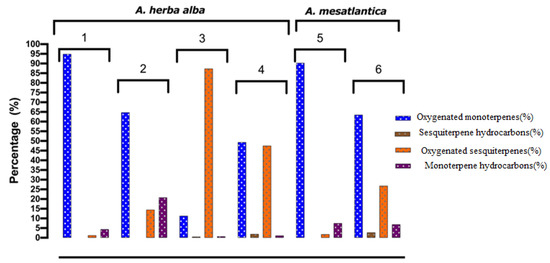
Figure 3.
Different classes of compounds identified in the EOs of A. herba alba Asso: (1) Limestone/Semi-arid; (2) Poor loamy and stony soil/Semi-arid; (3) Limestone/Semi-arid, arid; (4) Limestone/Subhumid to humid, A. mesatlantica; (5) Rocky soil on limestone/Subhumid to humid; (6) Limestone/Subhumid to humid.
Analysis of the results of the chemical compositions of essential oils of sagebrush from the Boulemane and Ifrane regions revealed the presence of 34 chemical compounds for the two species A. mesatlantica Maire (Guigou) and A. herba alba Asso (Enjil), 33 for A. herba alba Asso (Oulad aliyoussef) and A. mesatlantica (Timahdit), 31 for A. herba alba Asso (Timahdit) and 28 for A. herba alba Asso (Serghina), whose total chemical composition varies between 99.7% and 100% of the total EO (Table 3 and Table 4). These compounds divide into four main chemical families:
- -
- Oxygenated monoterpenes (11.4 to 94.9%), of which the major compound is camphor (0.82 to 46.19%) followed by trans-thujone (0.4 to 46.2%). Then come 1,8-cineole (0.9 to 13.8%), and in a small percentage, cis-thujone (3.5 to 5.5%);
- -
- Hydrocarbon monoterpenes (0.7 to 20.8%), which are mainly represented by camphene as the major compound (0.3 to 4.5%);
- -
- Hydrocarbon sesquiterpenes (0.51 to 2.74%), the most important of which are present in AM-TI Artemisia with a percentage of 2.74%. However, we note the absence of the latter in the species AHAass-NJ, AMma-GU and AHAass-OA;
- -
- Oxygenated sesquiterpenes, which reach (87.4%) of the EO of AHAass-SA, represented by vetivenic acid (14.9%), davana ether (14.6%) and davanone (13.1%). We were the first to identify the two compounds vetivenic acid and davana ether within the sagebrush species. The latter is also present in the EOs of AHAvar-TI (47.6%), AHAass-OA (14.5%) and AM-TI (26.8%), and with lower percentages, in AHAass-NJ (1.2%) and AMma-GU (1.8%).

Table 4.
Overall percentages (%) of the different chemical families identified in the EO of A. herba alba Asso: Oulad aliyoussef, Serghina, Enjil and Timahdit; A. mesatlantica Maire: Guigou and Timahdit.
Table 4.
Overall percentages (%) of the different chemical families identified in the EO of A. herba alba Asso: Oulad aliyoussef, Serghina, Enjil and Timahdit; A. mesatlantica Maire: Guigou and Timahdit.
| Components | A. herba alba Serghina | A. herba alba Oulad Ali Youssef | A. herba alba Enjil | A. herba alba Timahdit | A. mesatlantica Timahdit | A. mesatlantica Guigou |
|---|---|---|---|---|---|---|
| Alcohols (%) | 6.9 | 26.6 | 24.5 | 20.8 | 32.8 | 22.7 |
| Aldehydes (%) | 0 | 2.3 | 3.2 | 0 | 0.1 | 1.0 |
| Ketones (%) | 31.3 | 39.9 | 50.7 | 55.4 | 49.5 | 51.7 |
| Esters (%) | 16.4 | 6.3 | 2.9 | 0.4 | 2.9 | 0.8 |
| Oxides (%) | 4.1 | 5.1 | 13.8 | 12.9 | 13.2 | 5.7 |
| Phenols (%) | 0 | 0 | 0 | 1.76 | 0 | 0 |
We noted the presence of certain oxygenated monoterpenes in the EO of AHAass-NJ but at lower contents: santolina alcohol (7.3%), chrysanthenol (4.6%), yomogi alcohol (3.8%) and borneol (2.8%).
Other monoterpene hydrocarbons were also identified, such as ο-cymene (5.9%) and sabinene (4.3%), in the EO of AHAass-OA. It is important to note that the percentages of the chemical compounds of AHAass-NJ and AMma-GU are similar to the other studied species collected in the same region.
A. herba alba has four different chemotypes: trans-thujone and iso-acorone extracted from the plant from Timahdit and camphor and 1,8-cineole from Enjil. However, A. herba alba Asso combines camphor and trans-thujone from Oulad aliyoussef, and conversely, Serghina is predominated by vetivenic acid and davana ether.
In A. mesatlantica of Guigou, camphor and 1,8-cineole predominate, while A. mesatlantica from Timahdit is trans-thujone and occidenol.
Overall, the constituents of our isolated oils are complex mixtures of monoterpene hydrocarbons, alcohols, aldehydes, ketones, oxides, esters and a very low rate of phenols, which are characterized by the predominance of terpene products with ketone structures (camphor and thujone, in particular).
The results obtained show remarkable variations in the chemical composition of different species, within the same genus, within the same species and between provinces. These differences are remarkable, in particular, due to the nature of the most abundant compound and the other constituents identified; even if we find some similar elements, this variability from one locality to another confirms that the chemical profile of the species studied is very variable depending on the origin of the plant, the altitude, the nature of the soil and the climate specific to the region. Other factors influence the chemical composition, affected by factors such as the season, environmental conditions [1] and genetic makeup [6].
3.3. Analysis of the Similarity between Species by Ascending Hierarchical Analysis (HCA) and Principal Components (PCA)
Analysis of the chemical composition of the essential oils of the sagebrush studied (A. mesatlantica Maire, A. herba alba Asso) showed a high diversity of the compounds identified, both qualitatively and quantitatively. For statistical description of our sampling and to be able to highlight a possible chemical variability, as well as to identify the possible relationships between the abundance of volatile organic compounds and the abiotic and geographical factors, we used two common tests of multidimensional descriptive statistics: bottom-up hierarchical analysis and principal component analysis (PCA).
3.4. Hierarchical Bottom-Up Analysis
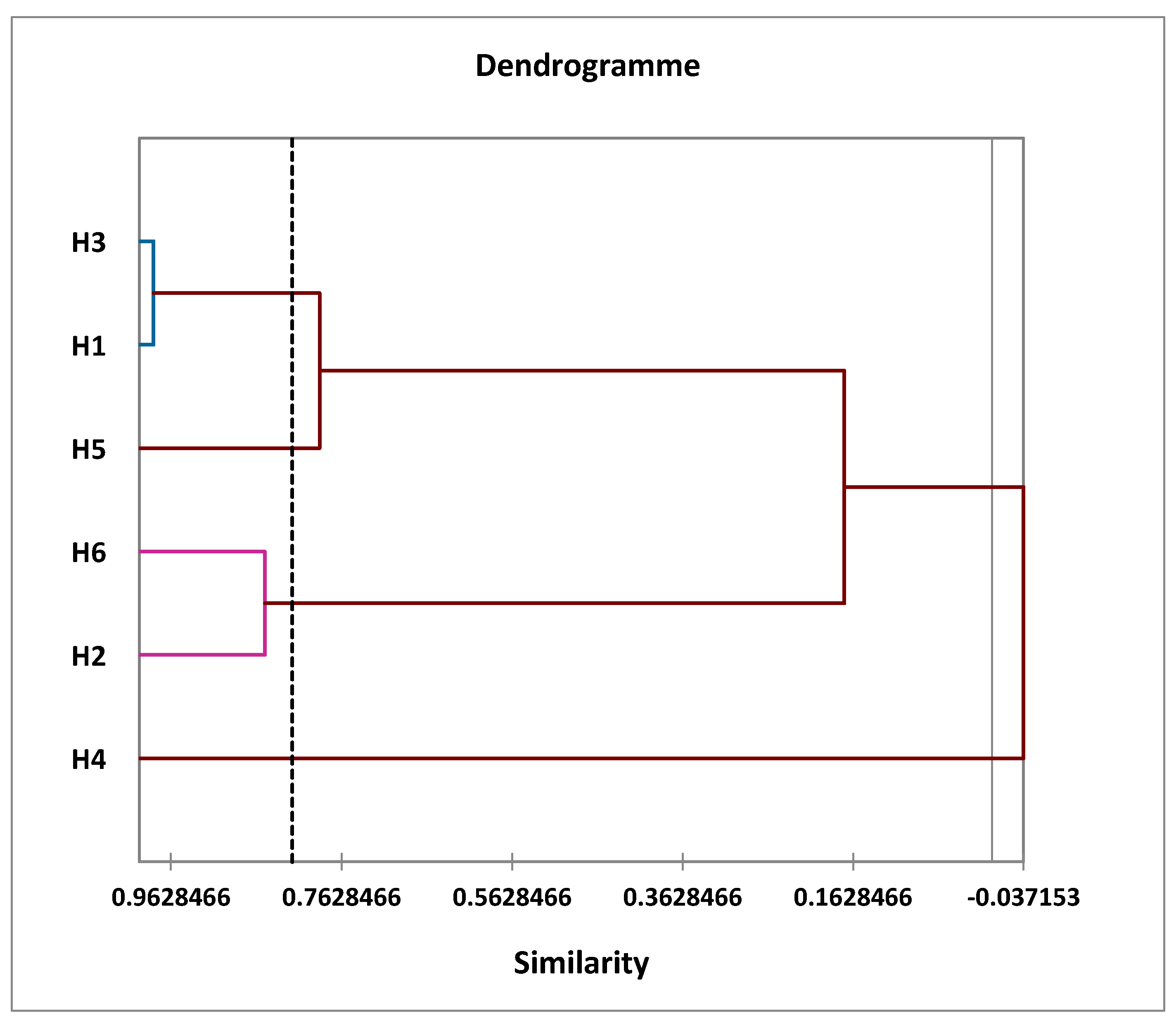
First, a bottom-up hierarchical analysis was performed to divide the sample into groups of homogeneous observations, each group being well-differentiated from the others. This analysis then brought the samples together to produce a dendrogram or classification tree (Figure 4).
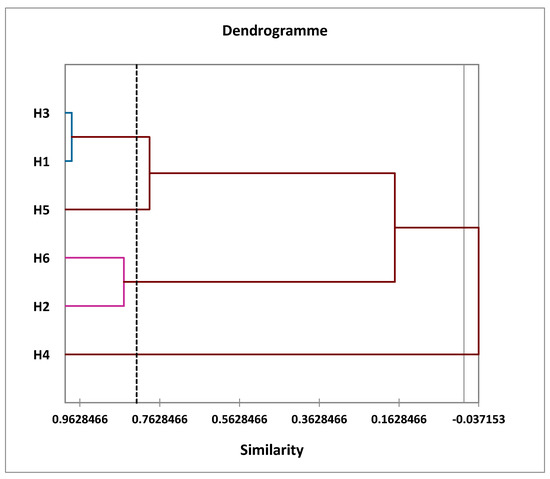
Figure 4.
Dendrogram obtained by a hierarchical bottom-up analysis (HCA) of the six samples studied.
Figure 4 shows the similarity between the EOs of the six samples studied. This analysis shows that the samples can be grouped into four distinct groups depending on the distance between them:
- The first group (I) includes two oil samples: H1 and H3;
- The second group (II) also contains two samples: H2 and H6;
- The third group (III) comprises a single sample: H4;
- The fourth group (IV) also contains a single sample: H5.
At the dendrogram level, we noted that the EOs of group (I) included two samples of oils and those were grouped within the same class, so there was intra-class homogeneity; this was confirmed by the small distance between the two populations, which explained why the two taxa were similar or very close in terms of the chemical profile example:
- H3 has 98.31% similarity with H1;
Likewise, Group (II) included two samples of oils that were similar or very similar in their chemical profiles:
- H6 has 85.25% similarity with H2;
In contrast, for group (III) and (IV), these consisted of a single oil, H4 or H5. They were heterogeneous compared to other classes, with a significant distance.
- H6 has 17.32% similarity with H1 and H3.
Note:
H1:
A. herba alba from the Enjil region;
H2:
A. herba alba from the Timahdit region;
H3:
A. mesatlantica from the Guigou region;
H4:
A. herba alba from the Serghina region;
H5:
A. herba alba from the Oulad Ali region;
H6:
A. mesatlantica from the Timahdit region.
3.5. Principal Component Analysis (PCA)
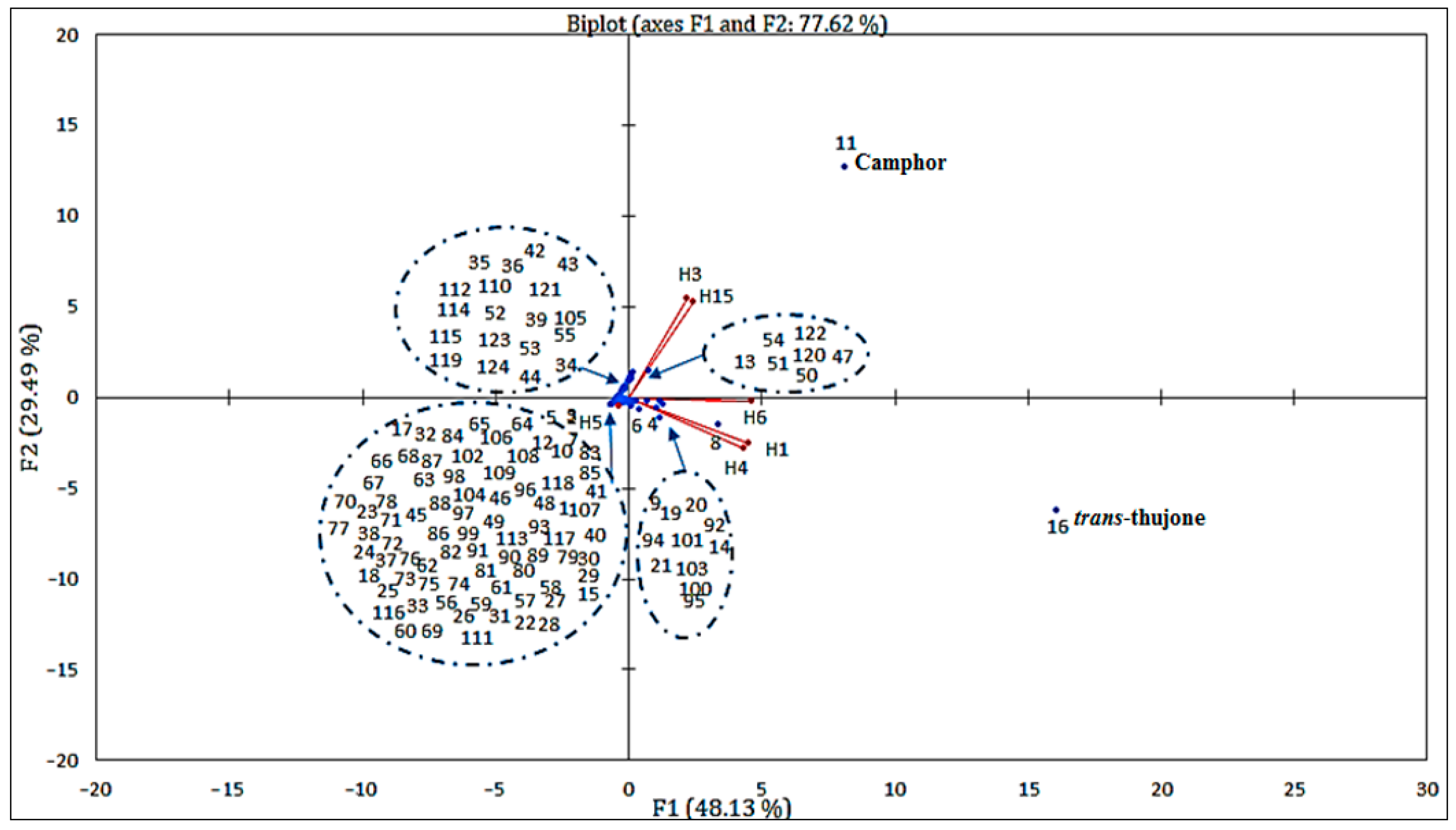
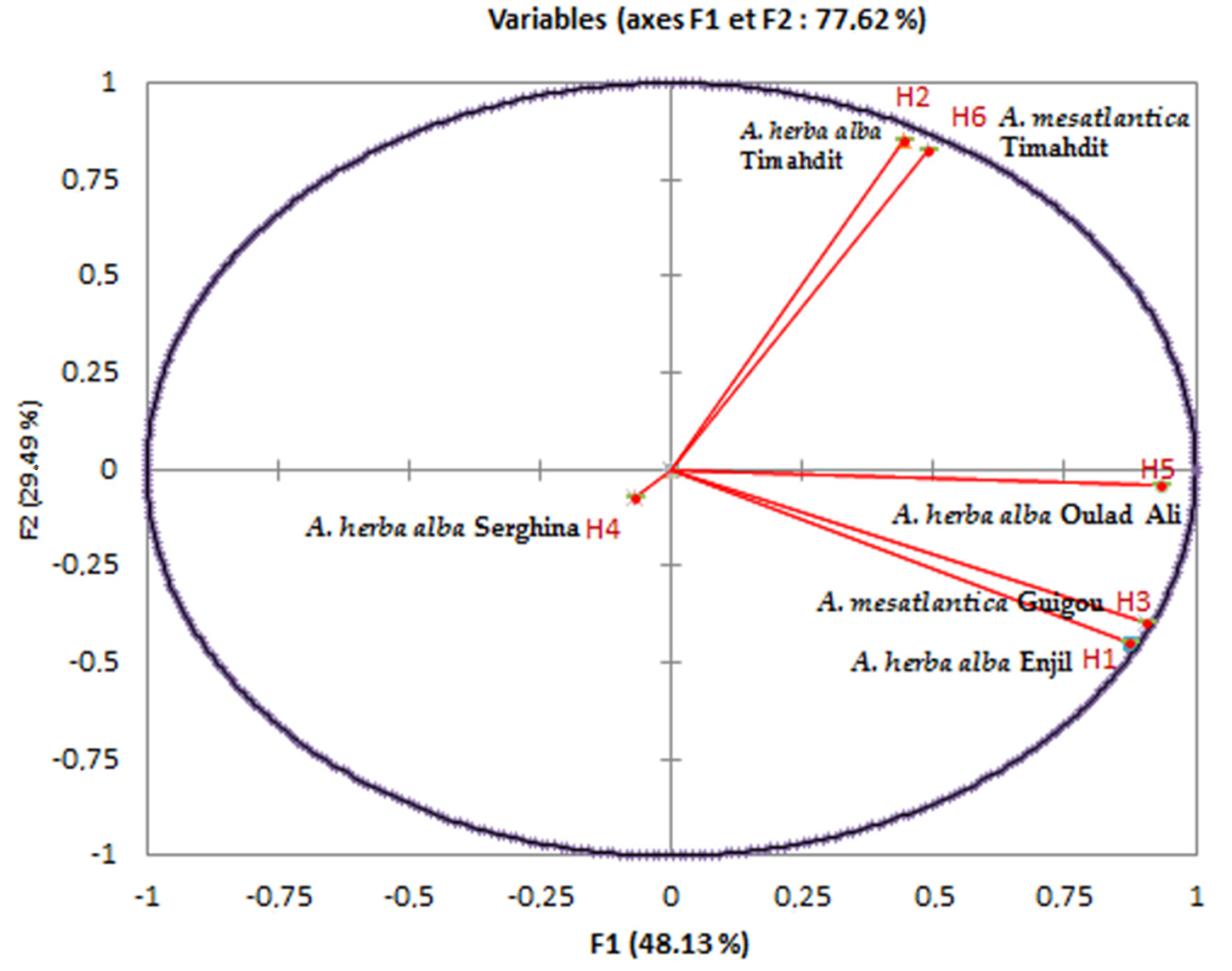
Principal component analysis (PCA) is a method that allows for the simplification of data by studying the relationships between all variables, to determine the similarities and dissimilarities between individuals (Figure 5 and Figure 6).
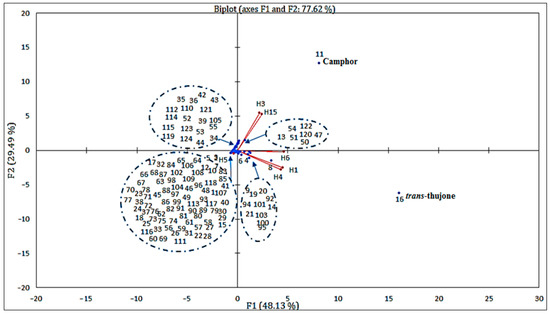
Figure 5.
Correlation between the principal compounds and the essential oils of the six samples from different regions studied.
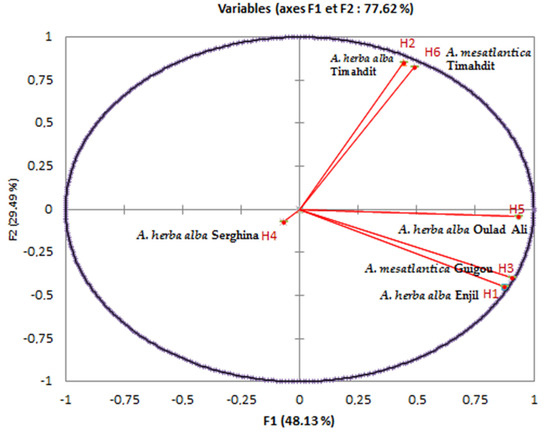
Figure 6.
Principal component analysis for the EO of the six samples from the different regions studied.
To perform this analysis, we chose the first two factor axes. The dispersion of the Artemisia L. species in the plane formed by these two axes concerning the chosen variables explained 77.62% of the variability, including 48.13% on the first axis and 29.49% on the second one (Figure 6). This figure confirmed the results of the bottom-up hierarchical analysis and highlighted the main quantitative differences between the four chemical composition groups. Furthermore, it showed that the chemical composition of essential oils from different populations is heterogeneous.
On the other hand, some chemical compounds were positively correlated, for example, essential oils (H6, H2) that were rich in trans-thujone contained less camphor, iso-acorone, α-bisabolol oxide β and α-bisabolol oxide A. Thus, as essential oils (H1, H3) that are rich in camphor, 1,8 cineoles are also rich in davanone, cis-thujone and camphene. We noted that certain species had similar compositions, such as H2 and H6. On the other hand, others presented a significant difference in terms of chemical composition, such as H4 and H5. In addition, we noted that there were several correlations between chemical compounds with certain EOs. We can cite compound 16 (camphor), which strongly correlated with EOs H1 and H3 and very weakly correlated with EOs H2 and H6. Regarding compound 11 (trans-thujone), contrary to compound 16 (Camphor), it relatively highly correlated with EOs H6 and H2 and very weakly correlated with EOs H1 and H3.
We deduced that the chemical compositions of the six samples of essential oils collected in different regions of Morocco presented heterogeneity; indeed, some samples contained camphor as the major compound, while others contain trans-thujone. The contents of other compounds 1,8-cineole, vetivenic acid, davana ether, occidenol and iso-acorone varied substantially. Although the compositions of these samples were largely dominated by camphor and trans-thujone, statistical analysis distinguished four groups:
- The first group (I) was rich in camphor (compound 11) and 1,8-cineole (compound 8); this group contained two samples, one from Enjil: AHAass-ENJ (1552 m), and another from Guigou: AMma-GU (1731 m);
- The second group (II) contained two samples from the same region (Timahdit), AHAass-TI (1599 m) and AM-TI (1498 m), characterized by an abundance of trans-thujone (compound 16);
- The third group (III) comprised a single sample from Serghina: AHAass-SA (1532 m), characterized by particular abundances of vetivenic acid and davana ether;
- The fourth group (IV) also contained a single sample from Oulad aliyoussef, AHAass-OA (1507 m), characterized by particular abundances of camphor and trans-thujone.
Previous studies found similar chemical compositions for the sagebrush essential oils in our study, where camphor and trans-thujone were the primary compounds.
The presence of camphor as a major constituent in the EOs of A. herba alba Asso d’Oulad Ali, A. herba alba from Enjil and A. mesatlantica Maire de Guigou has already been mentioned in certain populations of A. herba alba in Morocco [21,22], Spain [23] and Algeria [24] and for other Artemisia species worldwide: A. absinthium. L in Brazil [25], A. sieberi in Iran [26] and A. nilagirica in India [27].
Similarly, for A. mesatlantica (Timahdit) and A. herba alba Asso (Timahdit), the chemical compositions were found to be similar to those in other work carried out in Morocco on A. mesatlantica [12,28], in India on A. nilagirica [29] and in Tunisia on A. herba alba [30], where trans-thujone is the major constituent. However, in other works, the results were quite different. EOs of A. anethoides were dominated by 1,8-cineole [31] in China, A. judaica was the predominant piperitone in Jordan [32] and α-thujone was most prevalent in the EO of A. herba alba in Tunisia [33]. Meanwhile, davanone has been described as an important component in EO of A. herba alba from Morocco [34].
4. Conclusions
Essential oils are substances with a very complex chemical composition. Within the same genus Artemisia, our study has shown qualitative and quantitative variations in the yields and chemical compositions of the essential oils studied.
In general, the observed differences clearly show the influence of abiotic factors such as the climate specific to the regions where samples were collected and geographical factors such as the altitude and nature of the soil.
We also noted in this study a slight modification of the chemical compositions of the studied species. This modification was remarkable at the level of the major compounds, i.e., trans-thujone (41.1%), camphor (46.2%) vetivenic acid (14.9%) or 1,8-cineole (13.8%). HCA affirmed the findings of PCA and allowed us to distinguish four groups. Thus, at the end of this study, it appears that the Artemisia species are rich in terpene products of ketone structures (camphor and thujone, in particular), which are bioactive molecules responsible for various biological activities and highly sought after in the pharmaceutical, cosmetic and industrial fields. The promotion of these plants in the aforementioned sectors can contribute to the promotion and social and sustainable development of the medicinal and aromatic plants (MAP) sector. In fact, the MAP sector in this locality needs promotion to support its marketing, processing techniques, labeling and certification. Such promotion would be of great interest to the regions studied and could even be a source of significant income. This would allow, among other things, the local population to market their local products and develop them to be healthier. Yet, such progress can only be made possible by ethnopharmacological, phytochemical and biological studies determining the scientific names of the Artemisia species, the yields of EOs, their qualitative and quantitative chemical compositions and the biological properties of the chemical compounds of sagebrush species in the regions studied.
Author Contributions
Conceptualization, S.A. (Sanae Amine) and T.Z.; methodology, M.B. and H.M.; validation, A.A., M.R., S.A. (Smail Amalich) and S.S.; data curation, S.A. (Sanae Amine); writing—original draft preparation, S.A. (Sanae Amine), M.B. and H.M.; supervision, M.M. and T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ouyahya, A.; Negre, R.; Viano, J.; Lozano, Y.F.; Gaydou, E.M. Essential oils from Moroccan Artemisia negrei, A. mesatlantica and A. herba alba. Leb. Technol. 1990, 23, 528–530. [Google Scholar]
- Ibn-Tattou, M.; Fennane, M. Flore vasculaire du Maroc: Inventaire et chorologie. Trav. L’institut Sci. Série Bot. 2008, 39, 1–309. [Google Scholar]
- Paolini, J.; El Ouariachi, M.; Bouyanzer, A.H.; Hammouti, B.; Desjobert, J.M.; Costa, J.; Muselli, A. Chemical variability of Artemisia herba-alba Asso essential oils from East Morocco. Chem. Pap. 2010, 64, 550–556. [Google Scholar] [CrossRef]
- Mrabet, N.; Zrira, S.; Ismaïli-Alaoui, M.; Lahlou, H.; Benjilali, B. Plantes aromatiques et médicinales et leurs huiles essentielles. Dans Actes Congrès Int. PAM Actes Ed. Rabat 1997, 51, 111–114. [Google Scholar]
- Tilaoui, M.; Mouse, H.A.; Jaafari, A.; Aboufatima, R.; Chait, A.; Zyad, A. Chemical composition and antiproliferative activity of essential oil from aerial parts of a medicinal herb Artemisia herba-alba. Rev. Bras. Farmacogn. 2011, 21, 781–785. [Google Scholar] [CrossRef] [Green Version]
- El Ajjouri, M.; Satrani, B.; Ghanmi, M.; Aafi, A.; Farah, A.; Rahouti, M.; Amarti, F.; Aberchane, M. Activité antifongique des huiles essentielles de Thymus bleicherianus Pomel et Thymus capitatus (L.) Hoffm. & Link contre les champignons de pourriture du bois d’œuvre. Biotechnol. Agron. Soc. Environ. 2008, 12, 345–351. Available online: https://popups.uliege.be/1780-4507/index.php?id=2996&usg=__9Pii3kcge4cKzWIKx6w2daOYZas=&h=602&w=325&sz=120&hl=fr&lang=nl (accessed on 23 April 2021).
- USAID USAID E—Government Update: FY 2006 Final October 2006. Available online: https://www.usaid.gov/sites/default/files/USAID_E-Government_Update_FY2006.pdf (accessed on 12 March 2021).
- ou AFNOR, A.P.A. Normes des références pour les rapports scientifiques et le site WEB du Centre PsyCLE. Psychol. Française 2000, 45, 279–284. [Google Scholar]
- Kovats, E.S. Gas chromatographic characterization of organic substances in the retention index system. Adv. Chromatogr. 1965, 1, 229–247. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Hübschmann, H.-J. Handbook of GC-MS: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 3527334742. [Google Scholar]
- Rouani, M. Contribution à la Valorisation du Potentiel Aromatique et Médicinal des Plantes Marocaines: Genre Artemisia. Valorisation par Combinaison des Méthodes Phytochimiques, de Synthèse Organique et D’activité Biologique. 2015. Available online: https://thesesenafrique.imist.ma/handle/123456789/1788 (accessed on 10 April 2021).
- Ghanmi, M.; Satrani, B.; Aafi, A.; Isamili, M.R.; Houti, H.; El Monfalouti, H.; Benchakroun, K.H.; Aberchane, M.; Harki, L.; Boukir, A. Effet de la date de récolte sur le rendement, la composition chimique et la bioactivité des huiles essentielles de l’armoise blanche (Artemisia herba-alba) de la région de Guerçif (Maroc oriental). Phytothérapie 2010, 8, 295–301. [Google Scholar] [CrossRef]
- Btisam, O.; Mohamed, G.; Badr, S.; Lahsen, E.G.; Mariam, F.; Mounyr, B.; Elhassan, E.H.; Benaissa, K.; Eddine, B.Y.S. Effect of harvest date on yield, chemical composition and antimicrobial activity of Artemisia herba-alba essential oil. Int. J. Sci. Eng. Res. 2016, 7, 600–606. [Google Scholar]
- Hinane, D.; Oubaha, S.; Satrani, B.; Ghanmi, M.; Bourkhiss, B. Domestication of the endemic plant of Morocco and high added value: Artemisia herba alba. J. Mater. Environ. Sci. 2020, 11, 283–292. [Google Scholar]
- Bencheqroun, H.K.; Ghanmi, M.; Satrani, B.; Aafi, A.; Chaouch, A. Activité antimicrobienne des huiles essentielles d’Artemisia mesatlantica, plante endémique du Maroc Antimicrobial activity of the essential oil of an endemic plant in Morocco Artemisia mesatlantica. Bull. société R. Sci. Liège 2012, 81, 4–21. [Google Scholar]
- EREL, Ş.B.; Reznicek, G.; Şenol, S.G.; YAVAŞOĞLU, N.Ü.K.; Konyalioğlu, S.; Zeybek, A.U. Antimicrobial and antioxidant properties of Artemisia L. species from western Anatolia. Turkish J. Biol. 2012, 36, 75–84. [Google Scholar]
- Fellah, S.; Romdhane, M.; Abderraba, M. Extraction et étude des huiles essentielles de la Salvia officinalis. l cueillie dans deux régions différentes de la Tunisie. J.-Soc. Alger. Chim. 2006, 16, 193. [Google Scholar]
- Bendahou, M.; Muselli, A.; Grignon-Dubois, M.; Benyoucef, M.; Desjobert, J.-M.; Bernardini, A.-F.; Costa, J. Antimicrobial activity and chemical composition of Origanum glandulosum Desf. essential oil and extract obtained by microwave extraction: Comparison with hydrodistillation. Food Chem. 2008, 106, 132–139. [Google Scholar] [CrossRef]
- Elazzouzi, H.; Khabbal, Y.; Bouachrine, M.; Zair, T.; Alaoui El Belghiti, M. Chemical composition and in vitro antibacterial activity of Artemisia ifranensis J. Didier essential oil Growing Wild in Middle Moroccan Atlas. J. Essent. Oil Res. 2018, 30, 142–151. [Google Scholar] [CrossRef]
- Benjilali, B.; Sarris, J.; Richard, H. Nouveaux Chemotypes D’artemisia Herba Alba. Sci. Aliment. 1982, 2, 515–527. [Google Scholar]
- Boutkhil, S.; El Idrissi, M.; Chakir, S.; Derraz, M.; Amechrouq, A.; Chbicheb, A.; El Badaoui, K. Antibacterial and antifungal activity of extracts and essential oils of Seriphidium herba-alba (Asso) Soják and their combination effects with the essential oils of Dysphania ambrosioides (L) Mosyakin & Clemants. Acta Bot. Gall. 2011, 158, 425–433. [Google Scholar]
- Salido, S.; Valenzuela, L.R.; Altarejos, J.; Nogueras, M.; Sánchez, A.; Cano, E. Composition and infraspecific variability of Artemisia herba-alba from southern Spain. Biochem. Syst. Ecol. 2004, 32, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Belhattab, R.; Amor, L.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C. Essential oil from Artemisia herba-alba Asso grown wild in Algeria: Variability assessment and comparison with an updated literature survey. Arab. J. Chem. 2014, 7, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Vieira, T.M.; Dias, H.J.; Medeiros, T.C.T.; Grundmann, C.O.; Groppo, M.; Heleno, V.C.G.; Martins, C.H.G.; Cunha, W.R.; Crotti, A.E.M.; Silva, E.O. Chemical composition and antimicrobial activity of the essential oil of Artemisia absinthium Asteraceae leaves. J. Essent. Oil Bear. Plants 2017, 20, 123–131. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Akbarzadeh, A.; Hashemi-Moghaddam, H.; Nafchi, A.M.; Mashayekhi, H.A.; Aryanpour, A. Chemical composition of the essential oils from the aerial parts of Artemisia sieberi by using conventional hydrodistillation and microwave assisted hydrodistillation: A comparative study. J. Essent. Oil Bear. Plants 2016, 19, 32–45. [Google Scholar] [CrossRef]
- Sandip, S.; Reena, P.; Santilata, S.; Sanghamitra, N.; Sujata, M. High radical scavenging activity of camphor rich Artemisia nilagirica essential oil growing in eastern plain areas of India. Res. J. Biotechnol. 2014, 9, 63–65. [Google Scholar]
- Boumhara, K.; Bentiss, F.; Tabyaoui, M.; Costa, J.; Desjobert, J.M.; Bellaouchou, A.; Guenbour, A.; Hammouti, B.; Al-Deyab, S.S. Use of Artemisia mesatlantica essential oil as green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution. Int. J. Electrochem. Sci. 2014, 9, 1187–1206. [Google Scholar]
- Mishra, T.; Srivastava, M.; Kumar, A.; Pal, M.; Tewari, S.K. Chemical composition and termiticidal activity of Artemisia nilagirica essential oil growing in Southern Hilly Regions of India. J. Essent. Oil Bear. Plants 2017, 20, 247–252. [Google Scholar] [CrossRef]
- Younsi, F.; Trimech, R.; Boulila, A.; Ezzine, O.; Dhahri, S.; Boussaid, M.; Messaoud, C. Essential oil and phenolic compounds of Artemisia herba-alba (Asso.): Composition, antioxidant, antiacetylcholinesterase, and antibacterial activities. Int. J. Food Prop. 2016, 19, 1425–1438. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Wang, W.; Zheng, Y.; Zhang, D.; Wang, J.; Guo, S.; Zhang, W.; Du, S.; Zhang, J. Bioactivities and chemical constituents of essential oil extracted from Artemisia anethoides against two stored product insects. J. Oleo Sci. 2017, 66, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Zulfiqar, A.; Khan, I.A.; Efferth, T.; Salgueiro, L. Chemical composition and biological activities of Artemisia judaica essential oil from southern desert of Jordan. J. Ethnopharmacol. 2016, 191, 161–168. [Google Scholar] [CrossRef]
- Bellili, S.; Jazi, S.; Hrira, M.Y.; Lamari, A.; Dhifi, W.; Diouani, M.F.; Araújo, M.E.; Cioni, P.L.; Flamini, G.; Cherif, A. Phytochemical identification of volatile fraction, essential oil and screening of antioxidant, antibacterial, allelopathic and insecticidal potential from Artemisia herba-alba leaves. Main Group Chem. 2017, 16, 95–109. [Google Scholar] [CrossRef]
- Hannour, K.; Boughdad, A.; Maataoui, A.; Bouchelta, A. Chemical composition and toxicity of Moroccan Rosmarinus officinalis (Lamiaceae) essential oils against the potato tuber moth, Phthorimaea operculella (Zeller, 1873) Zeller (Lepidoptera, Gelechiidae). J. Mater. Environ. Sci. 2017, 8, 758–769. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).