Inhibitory Effect of CUSTOS, a Formulated Allium-Based Extract, on the Growth of Some Selected Plant Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Assays of CUSTOS Effects on Pathogen Growth
2.2. Inoculum Preparation and in Planta Pathogen Assays
2.3. Experimental Procedure for Carrot Plants and Pathogenic Fungi
2.4. Treatment of Strawberry Fields with CUSTOS
2.5. Fungal DNA Quantification in Plant
2.6. RNA Extraction
2.7. Quantitative PCR (qPCR)
3. Results
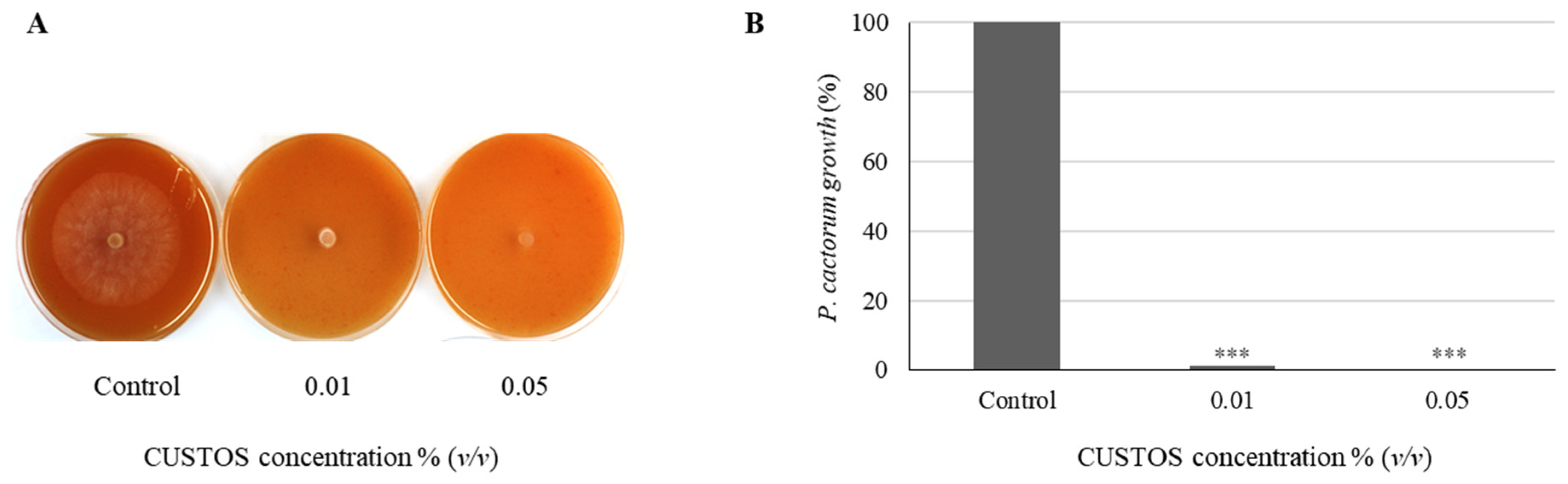
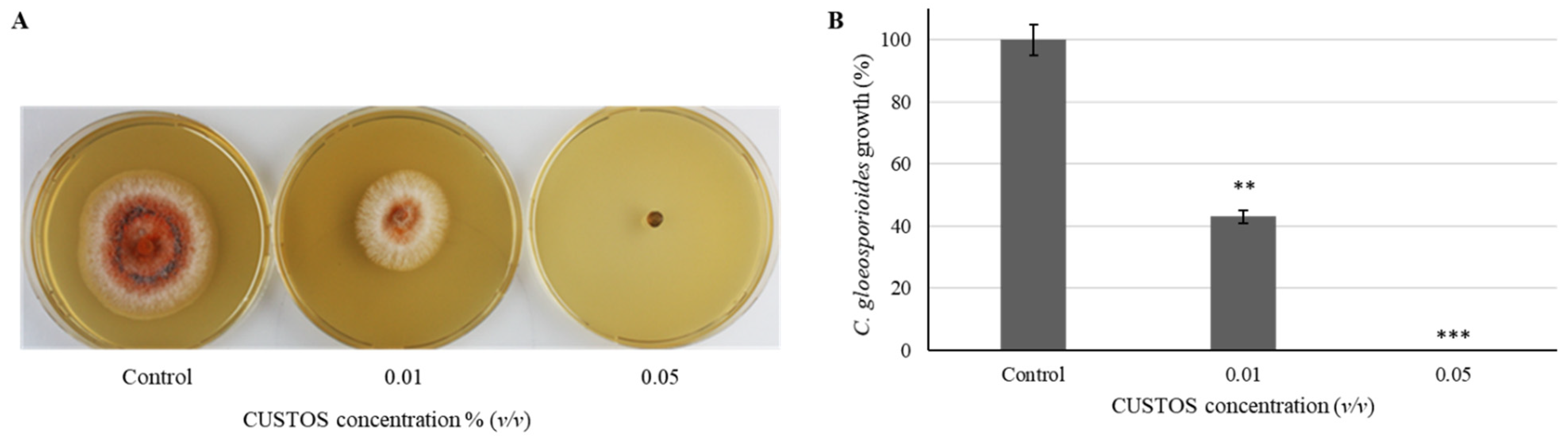
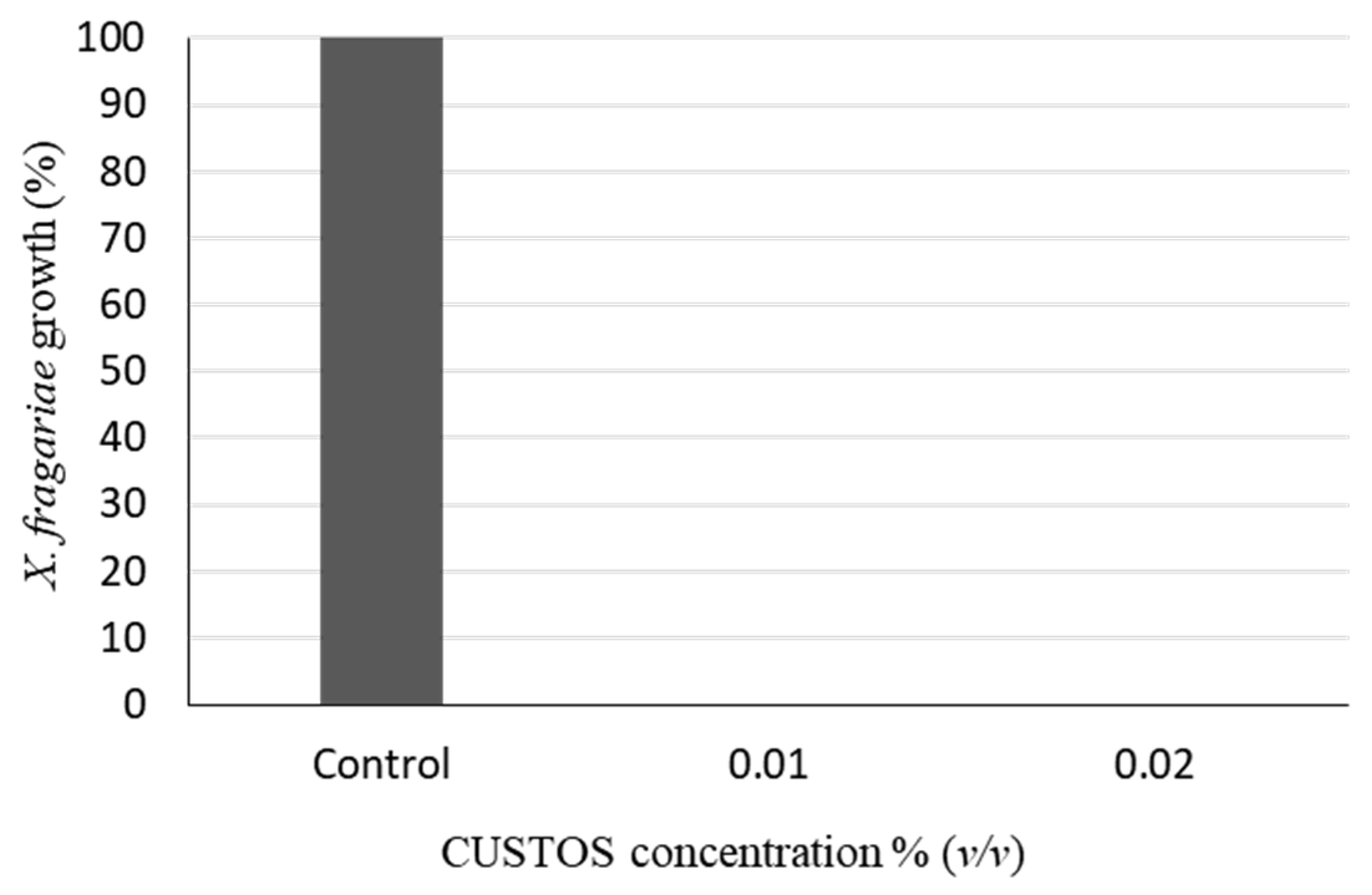
3.1. CUSTOS Has Strong Antimicrobial Effects against Various Microbial Pathogens in In Vitro Assays
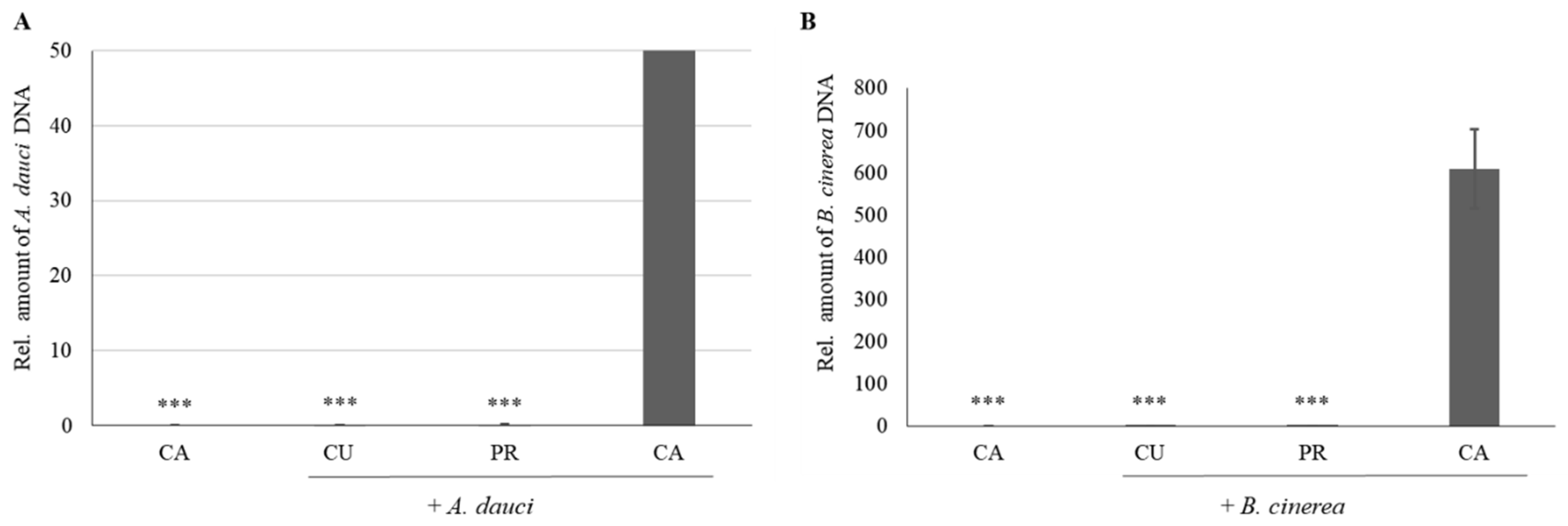
3.2. Evaluation of Fungal Growth and PR Gene Expression upon Curative and Preventive CUSTOS Treatments
3.3. Pre-Harvest CUSTOS Application on a Strawberry Prevented Post-Harvest Pathogenic Growth
4. Discussion
4.1. In Vitro Inhibition Assays
4.2. The Curative and Preventive Effects of CUSTOS on Carrot Plants
4.3. Pre-Harvest Application of CUSTOS in a Strawberry Field
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osusky, M.; Zhou, G.; Osuska, L.; Hancock, R.E.; Kay, W.W.; Misra, S. Transgenic plants expressing cationic peptide chimeras exhibit broad-spectrum resistance to phytopathogens. Nat. Biotechnol. 2000, 18, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Cook, J. Towards cropping systems that enhance productivity and sustainability. Proc. Natl. Acad. Sci. USA 2006, 103, 18389–18394. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.G.M.; Allinson, G.; Stagnatti, F.; Tanaka, A.; Westbrooke, M. Arsenic contamination in Bangladesh groundwater: A major environmental and social disaster. Int. J. Environ. Health Res. 2002, 12, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Schmid, M.; Rothballer, M.; Hause, G.; Zuccaro, A.; Imani, J.; Kämpfer, P.; Domann, E.; Schäfer, P.; Hartmann, A.; et al. Detection and identification of mycorrhiza helper bacteria intimately associated with representatives of the order Sebacinales. Cell. Microbiol. 2008, 10, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- Carlos, E.S.; Jesus, A.B.C.; Guadalupe, R.S.; Carmen, O.S. Biologically Active and Antimicrobial Peptides from Plants. Biomedical. Res. Int. 2015, 10, 21–29. [Google Scholar] [CrossRef]
- Gutierrez, J.; Rodriguez, G.; Barry-Ryan, C.; Bourke, P. Efficacy of plant essential oils against foodborne pathogens and spoilage bacteria associated with ready-to-eat vegetables: Antimicrobial and sensory screening. J. Food Prot. 2008, 71, 1846–1854. [Google Scholar] [CrossRef]
- Gurjar, M.S.; Ali, S.; Akhtar, M.; Singh, K.S. Efficacy of plant extracts in plant disease management. Agric. Sci. 2012, 3, 425–433. [Google Scholar] [CrossRef]
- Amadioha, A.C. Evaluation of some plant leaf extracts against Colletotrichum lindemuthianum in cowpea. Acta Phytopathol. Entomol. Hung. 2003, 38, 259–265. [Google Scholar] [CrossRef]
- Opara, E.U.; Wokocha, R.C. Efficacy of some plant extracts on the in vitro and in vivo control of Xanthomonas campestris pv. vesicatoria. Agric. J. 2008, 3, 163–170. [Google Scholar]
- Dong, Y.; Zhao, M.; Zhao, T.; Feng, M.; Chen, H.; Zhuang, M.; Lin, L. Bioactive profiles, antioxidant activities, nitrite scavenging capacities and protective effects on H2O2-injured PC12 cells of Glycyrrhiza glabra L. leaf and root extracts. Molecules 2014, 19, 9101–9113. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.H.; Ranjbar, R.; Eshraghi, S.; Sadeghi, G.; Jonaidi, N.; Bazzaz, N.; Izadi, M.; Sadeghifard, N. An evaluation of antibacterial activity of Glycyrrhiza glabra extract on the growth of Salmonella, Shigella and ETEC E. coli. J. Biol. Sci. 2007, 7, 827–829. [Google Scholar] [CrossRef]
- Hassan, S.M.; Byrd, J.A.; Cartwright, A.L.; Bailey, C.A. Hemolytic and antimicrobial activities differ among saponin-rich extracts from guar, quillaja, yucca, and soybean. Appl. Biochem. Biotechnol. 2010, 162, 1008–1017. [Google Scholar] [CrossRef]
- Shenge, K.C.; Mabagala, R.B.; Mortensen, C.N. Identification and characterization of strains of Xanthomonas campestris pv. vesicatoria from Tanzania by biological system and sensitivity to antibiotics. Afr. J. Biotechnol. 2007, 6, 015–022. [Google Scholar]
- Mbega, E.R.; Mortensen, C.N.; Mabagala, R.B.; Wulff, E.G. The effect of plant extracts as seed treatments to control bacterial leaf spot of tomato in Tanzania. J. Gen. Plant Pathol. 2012, 78, 277–286. [Google Scholar] [CrossRef]
- Srivasata, S.; Singh, V.P.; Kumar, R.; Srivasata, M.; Sinha, A.; Simon, S. In vitro evaluation of Carbendazim 50 % WP, antagonists and botanicals against Fusarium oxysporum f. sp. psidii associated with rhizosphere soil of guava. Asian J. Plant Pathol. 2011, 5, 46–53. [Google Scholar]
- Das, K.; Tiwari, R.K.; Shrivastava, D.K. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J. Med. Plant Res. 2010, 4, 104–111. [Google Scholar]
- Leontiev, R.; Hohaus, N.; Jacob, C.; Gruhlke, M.C.H.; Slusarenko, A.J. A Comparison of the Antibacterial and Antifungal Activities of Thiosulfinate Analogues of Allicin. Sci. Rep. 2018, 8, 6763. [Google Scholar] [CrossRef]
- Koch, E.; Slusarenko, A. Arabidopsis is susceptible to infection by a Downy mildew fungus. Plant Cell 1990, 2, 437–445. [Google Scholar] [PubMed]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- Samadi, S.; Ghasemnezhad, A.; Naz, S.; Imani, J. An approved protocol for Cynara scolymus and Momordica charantia seed surface sterilization for pre-explant in-vitro culture using Allium extract. J. Biol. Nat. 2015, 4, 47–55. [Google Scholar]
- Okuhara, P.; Kumar, N.; Hohenwarter, L.; Micknass, U.; Kogel, K.H.; Imani, J.; Graham, D.; Doty, S.L. Inhibition of fungal and bacterial plant pathogens by CUSTOS®, an Allium-BASED antimicrobial formulation. J. Biol. Nat. 2017, 8, 40–51. [Google Scholar]
- Samadi, S.; Ghasemnezhad, A.; Imani, J. Extending shelf life of strawberry using some pre-storage treatments. Acta Hortic. 2017, 1156, 643–652. [Google Scholar] [CrossRef]
- Imani, J.; Kumar, N.; Sand, B.; Kogel, K.H. VEG‘LYS, a product from allium, has a strong potential for plant disease control. In Proceedings of the ADIVK Aktuell, 26th General Assembly of German In Vitro Culture Working Group (ADVIK), Lünen, Germany, 2013; Available online: http://www.adivk.de/index.php?id=4 (accessed on 22 March 2022).
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Hughes, B.G.; Lawson, L.D. Antimicrobial effects of Allium sativum L. (garlic), Allium ampeloprasum L. (elephant garlic), and Allium cepa L. (onion), garlic compounds and commercial garlic supplement products. Phytother. Res. 1991, 5, 154–158. [Google Scholar] [CrossRef]
- Wilson, C.L.; Solar, J.M.; El Ghaouth, S.; Wisniewski, M.E. Rapid Evaluation of Plant Extracts and Essential Oils for Antifungal Activity Against Botrytis cinerea. Plant Dis. 1997, 81, 204–210. [Google Scholar] [CrossRef]
- Negri, M.; Salci, T.P.; Shinobu-Mesquita, C.S.; Capoci, I.R.; Svidzinski, T.I.; Kioshima, E.S. Early state research on antifungal natural products. Molecules 2014, 19, 2925–2956. [Google Scholar] [CrossRef]
- Khan, S.; Imran, M.; Imran, M.; Pindari, N. Antimicrobial activity of various ethanolic plant extracts against pathogenic multi drug resistan Candida spp. Bioinformation 2017, 13, 67. [Google Scholar] [CrossRef]
- Corbu, V.M.; Gheorghe, I.; Marinas, I.C.; Geanǎ, E.I.; Moza, M.I.; Csutak, O.; Chifiriuc, M.C. Demonstration of Allium sativum Extract Inhibitory Effect on Biodeteriogenic Microbial Strain Growth, Biofilm Development, and Enzymatic and Organic Acid Production. Molecules 2021, 26, 7195. [Google Scholar] [CrossRef] [PubMed]
- Miron, T.; Rabinkov, A.; Mirelman, D.; Wilchek, M.; Weiner, L. The mode of action of allicin: Its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim. Biophys. Acta 2000, 1463, 20–30. [Google Scholar] [CrossRef]
- Müller, A.; Eller, J.; Albrecht, F.; Prochnow, P.; Kuhlmann, K.; Bandow, J.E.; Slusarenko, A.J.; Leichert, L.I.O. Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J. Biol. Chem. 2016, 291, 11477–11490. [Google Scholar] [CrossRef] [PubMed]
- Okuhara, P.A.; Arroyo-Garcia, R.; Shen, K.A.; Mazier, M.; Meyers, B.C.; Ochoa, O.E.; Kim, S.; Yang, C.H.; Michelmore, R.W. A transgenic mutant of Lactuca sativa (lettuce) with a T-DNA tightly linked to loss of downy mildew resistance. Mol. Plant-Microbe Interact. 1997, 10, 970–977. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castillo, L.; Plaza, V.; Larrondo, L.F.; Canessa, P. Recent advances in the study of the plant pathogenic fungus Botrytis cinerea and its interaction with the Environment. Curr. Protein Pept. Sci. 2017, 18, 976–989. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Kumar, K.; Rani, A.; Mishra, V. Signaling Pathways and Downstream Effectors of Host Innate Immunity in Plants. Int. J. Mol. Sci. 2021, 22, 9022. [Google Scholar] [CrossRef]
- Terefe, N.S.; Matihaies, K.; Simons, L.; Versteeg, C. Combined high pressure-mild temperature processing for optimal retention of physical and nutritional quality of strawberries. Innov. Food Sci. Emerg. Technol. 2009, 10, 297–307. [Google Scholar] [CrossRef]
- Wright, K.P.; Kader, A.A. Effect of slicing and controlled-atmosphere storage on the ascorbate content and quality of strawberries and persimmons. Postharvest Biol. Technol. 1997, 10, 39–48. [Google Scholar] [CrossRef]
- Boyraz, N.; Ozcan, M. Antifungal effect of some spice hydrosols. Fitoterapia 2005, 76, 661–665. [Google Scholar] [CrossRef]

| Primer’s Names | Sequence (5′->3′) |
|---|---|
| qpcr1.01_F | CTTGACATACGACTCCACCG |
| qpcr1.01_R | TATGGCCGTGGTCTTGGTA |
| qpcr1.02_F | TGAGGTTGAGGCTCCTTCC |
| qpcr1.02_R | CTGACGGTTCCAACACCAC |
| qpcr1.03_F | AGACAGCCTTGTGGTACTGGA |
| qpcr1.03_R | GCTTCCACTGTTGCACTCAA |
| Bc1-β-tubulin_F | GTTACTTGACATGCTCTGCCATT |
| Bc1-β-tubulin_R | CACGGCTACAGAAAGTTAGTTTCTACAA |
| qpcr-Ubiquitin_F | AAGCCCAAGAAGATCAAGCA |
| qpcr-Ubiquitin_R | TCAAAATGATTGGCCATGAA |
| Gpd_F | AAGCTTCCCCAAGCACTCACAA |
| Gpd_R | CTGCGTTCTGCAGCTGTAGAGA |
| qpcrActin_F | CCACTGAACCCAAAAGCAA |
| qpcrActin_R | CCGTGTGGCTAACACCATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oladejo, O.; Imani, J. Inhibitory Effect of CUSTOS, a Formulated Allium-Based Extract, on the Growth of Some Selected Plant Pathogens. Int. J. Plant Biol. 2022, 13, 44-54. https://doi.org/10.3390/ijpb13020006
Oladejo O, Imani J. Inhibitory Effect of CUSTOS, a Formulated Allium-Based Extract, on the Growth of Some Selected Plant Pathogens. International Journal of Plant Biology. 2022; 13(2):44-54. https://doi.org/10.3390/ijpb13020006
Chicago/Turabian StyleOladejo, Oluwashina, and Jafargholi Imani. 2022. "Inhibitory Effect of CUSTOS, a Formulated Allium-Based Extract, on the Growth of Some Selected Plant Pathogens" International Journal of Plant Biology 13, no. 2: 44-54. https://doi.org/10.3390/ijpb13020006
APA StyleOladejo, O., & Imani, J. (2022). Inhibitory Effect of CUSTOS, a Formulated Allium-Based Extract, on the Growth of Some Selected Plant Pathogens. International Journal of Plant Biology, 13(2), 44-54. https://doi.org/10.3390/ijpb13020006






