Vimentin Methylation as a Potential Screening Biomarker for Colorectal Cancer in HIV-Helminth Co-Infected Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population and Sampling
2.3. Experimental Procedures
3. Results
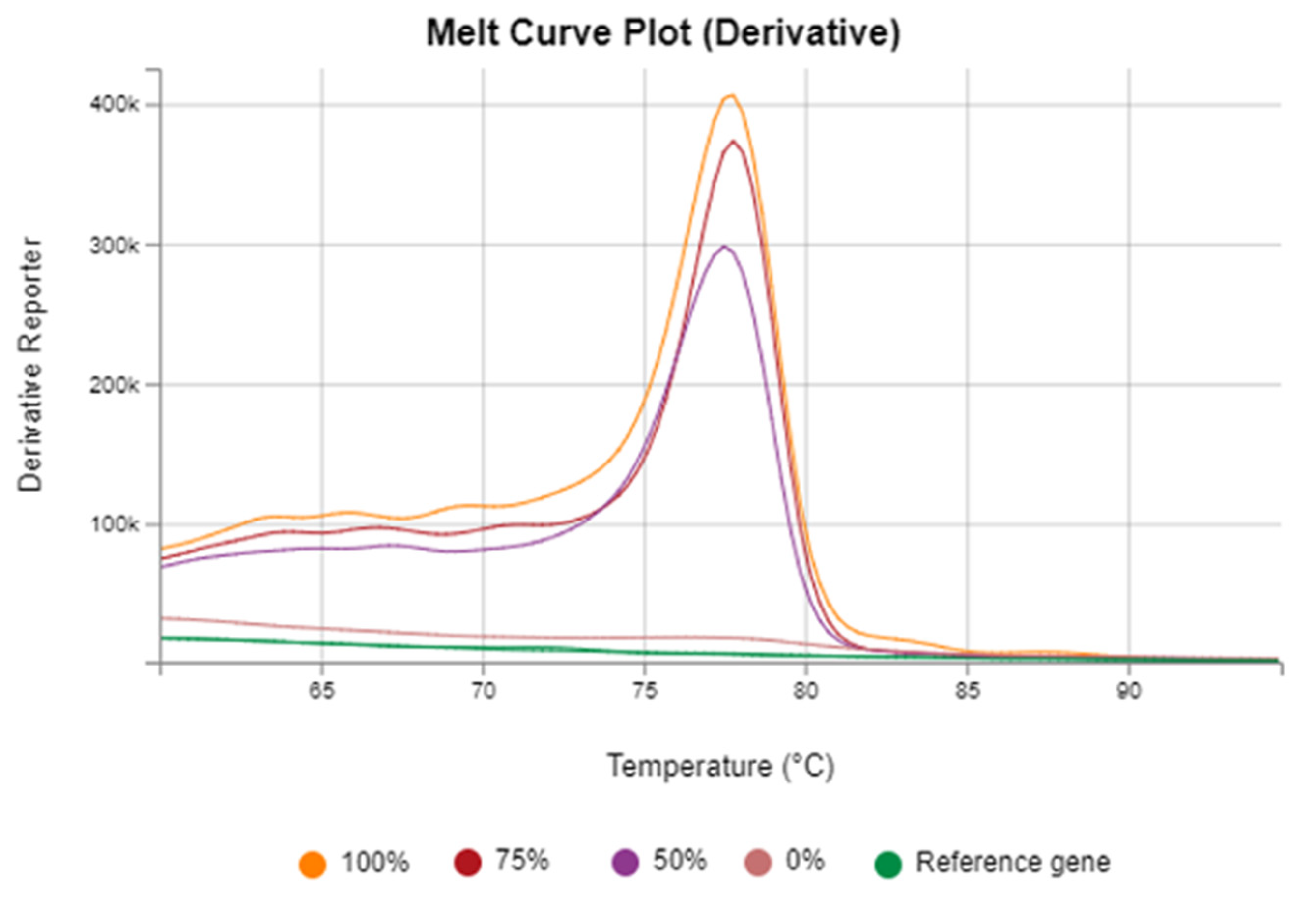
3.1. Assessment of Vimentin Methylation Across CRC and Infection-Related Groups
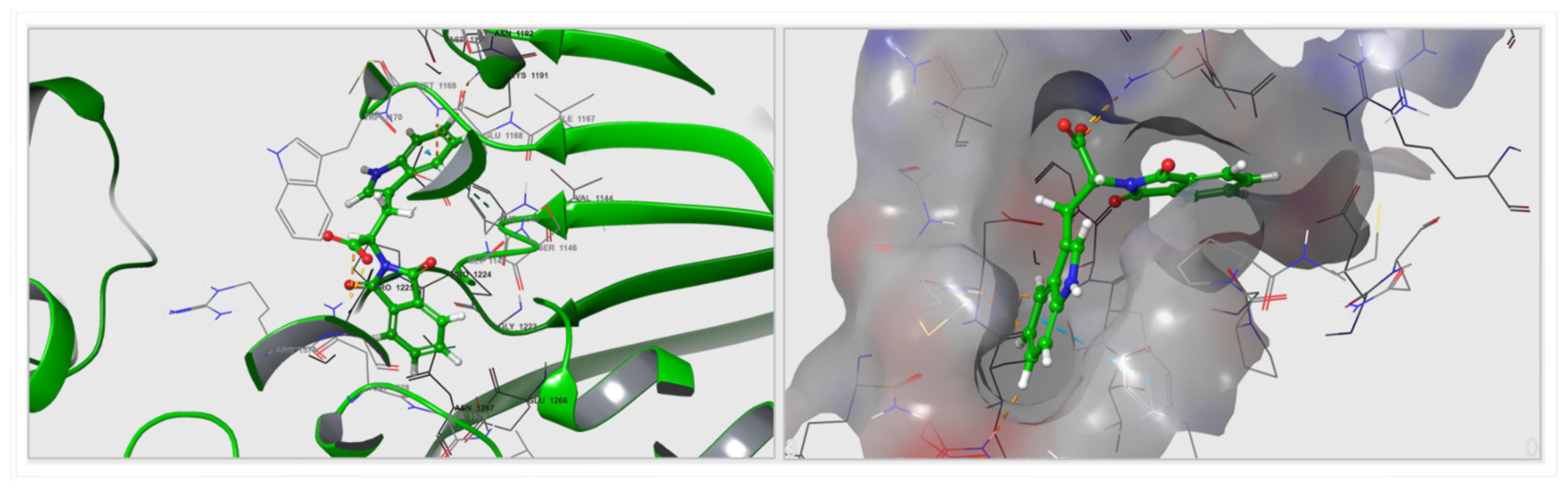
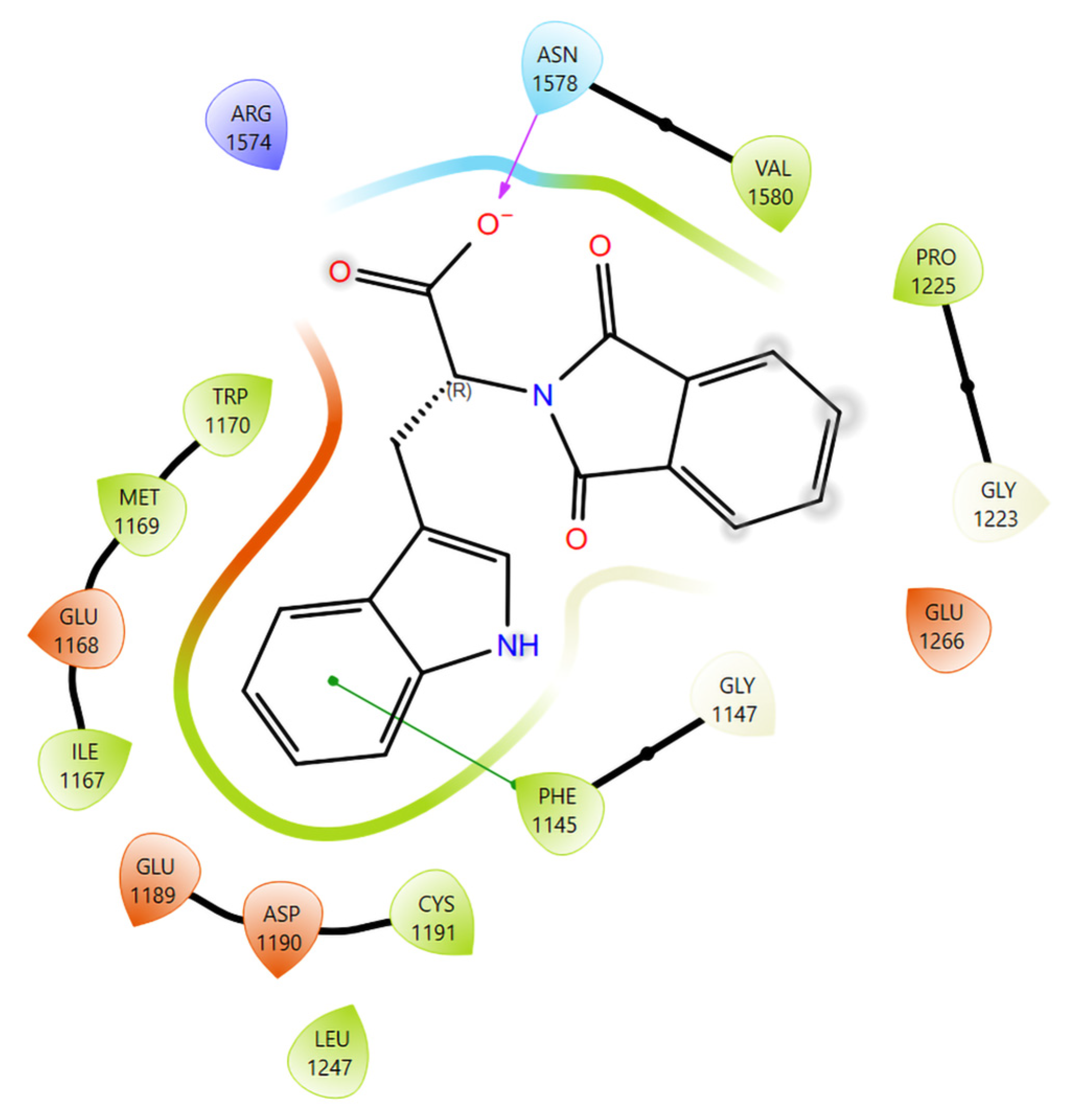
3.2. Computational Docking and Binding-Energy Results
3.3. Assessment of Fecal Occult Blood Test Across CRC and Infection-Related Groups
4. Discussion
4.1. Vimentin Methylation
4.2. Mechanistic and Translational Insights
5. Challenges and Limitations
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| CpG | Cytosine phosphate guanine dinucleotide |
| DNA | Deoxyribonucleic acid |
| HRM | High-resolution melt |
| HIV | Human immunodeficiency virus |
| qPCR | Quantitative polymerase chain reaction |
References
- Mostafa, M.H.; Sheweita, S.A.; O’Connor, P.J. Relationship between schistosomiasis and bladder cancer. Clin. Microbiol. Rev. 1999, 12, 97–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scholte, L.L.S.; Pascoal-Xavier, M.A.; Nahum, L.A. Helminths and Cancers from the Evolutionary Perspective. Front. Med. 2018, 5, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sánchez-Barrera, C.Á.; Fernandez-Muñoz, K.V.; Mendoza-Rodríguez, M.G.; Ortiz-Melo, M.T.; Carrillo-Pérez, J.A.; Rodríguez-Sosa, M.; Terrazas, L.I. The Impact of Helminths on Colorectal Cancer: From Infections to the Isolation of Biotherapeutics. Pathogens 2025, 14, 949. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jain, S.; Rana, M. From the discovery of helminths to the discovery of their carcinogenic potential. Parasitol. Res. 2024, 123, 47. [Google Scholar] [CrossRef]
- Li, H.; Shan, C.; Zhu, Y.; Yao, X.; Lin, L.; Zhang, X.; Qian, Y.; Wang, Y.; Xu, J.; Zhang, Y.; et al. Helminth-induced immune modulation in colorectal cancer: Exploring therapeutic applications. Front. Immunol. 2025, 16, 1484686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, M.; Mawa, P.A.; Kaleebu, P.; Elliott, A.M. Helminths and HIV infection: Epidemiological observations on immunological hypotheses. Parasite Immunol. 2006, 28, 613–623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esperante, D.; Gutiérrez, M.I.M.; Issa, M.E.; Schcolnik-Cabrera, A.; Mendlovic, F. Similarities and divergences in the metabolism of immune cells in cancer and helminthic infections. Front. Oncol. 2023, 13, 1251355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Mapanga, W.; Norris, S.A.; Chen, W.C.; Blanchard, C.; Graham, A.; Baldwin-Ragaven, L.; Boyles, T.; Donde, B.; Greef, L.; Huddle, K.; et al. Consensus study on the health system and patient-related barriers for lung cancer management in South Africa. PLoS ONE 2021, 16, e0246716. [Google Scholar] [CrossRef] [PubMed]
- Sepassi, A.; Li, M.; Zell, J.A.; Chan, A.; Saunders, I.M.; Mukamel, D.B. Rural-urban disparities in colorectal cancer screening, diagnosis, treatment, and survivorship care: A systematic review and meta-analysis. Oncologist 2024, 29, e431–e446. [Google Scholar] [CrossRef]
- Lala, V.G.; Mohamed, A.; Tate, D.J.; Seabi, N.M.; Mokgoko, D. Colorectal cancer screening: An update and South African perspective. Wits J. Clin. Med. 2024, 6, 95–102. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Turnbull, B.A.; Ross, M.E. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N. Engl. J. Med. 2004, 351, 2704–2714. [Google Scholar] [CrossRef]
- Khakimov, N.; Khasanova, G.; Ershova, K.; Gibadullina, L.; Vetkina, T.; Lobisheva, G.; Chumakova, A. Screening for colon cancer: A test for occult blood. Int. J. Risk Saf. Med. 2015, 27 (Suppl. S1), S110–S111. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Levin, T.R.; Lavin, P.; Lidgard, G.P.; Ahlquist, D.A.; Berger, B.M. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 2014, 370, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; He, K.X.; Huang, Y.; Lou, Q.Q.; He, T.; Xu, X. A New Method for the Detection of Colorectal Cancer and the Precancerous Lesions: Occult Blood Testing Combination with Promoter Methylation in the Fecal Sample. J. Cancer 2021, 12, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Pakbaz, B.; Jabinin, R.; Soltani, N.; Ayatollahi, H.; Farzanehfar, M.R. Quantitative study of vimentin gene methylation in stool samples for colorectal cancer screening. J. Adv. Pharm. Technol. Res. 2019, 10, 121–125. [Google Scholar] [CrossRef]
- Lamb, Y.N.; Dhillon, S. Epi proColon® 2.0 CE: A blood-based screening test for colorectal cancer. Mol. Diagn. Ther. 2017, 21, 225–232. [Google Scholar] [CrossRef]
- Redwood, D.G.; Dinh, T.A.; Kisiel, J.B.; Borah, B.J.; Moriarty, J.P.; Provost, E.M.; Sacco, F.D.; Tiesinga, J.J.; Ahlquist, D.A. Cost-Effectiveness of Multitarget Stool DNA Testing vs Colonoscopy or Fecal Immunochemical Testing for Colorectal Cancer Screening in Alaska Native People. Mayo Clin. Proc. 2021, 96, 1203–1217. [Google Scholar] [CrossRef]
- Rui, M.; Wang, Y.; You, J.H.S. Novel Noninvasive Tests for Colorectal Cancer Screening—A Cost-Effectiveness Analysis. Cancer Epidemiol. Biomark. Prev. 2025, 34, 1111–1121. [Google Scholar] [CrossRef]
- Ladabaum, U.; Mannalithara, A.; Weng, Y.; Schoen, R.E.; Dominitz, J.A.; Desai, M.; Lieberman, D. Comparative Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening with Blood-Based Biomarkers (Liquid Biopsy) vs. Fecal Tests or Colonoscopy. Gastroenterology 2024, 167, 378–391. [Google Scholar] [CrossRef]
- Mpaka-Mbatha, M.N.; Naidoo, P.; Islam, M.; Singh, R.; Mkhize-Kwitshana, Z.L. Demographic profile of HIV and helminth-coinfected adults in KwaZulu-Natal, South Africa. S. Afr. J. Infect. Dis. 2023, 38, 466. [Google Scholar] [CrossRef]
- Damane, B.P.; Mulaudzi, T.V.; Kader, S.S.; Naidoo, P.; Dlamini, Z.; Mkhize-Kwitshana, Z.L. HIV-Helminth Co-Infections and Immune Checkpoints: Implications for Cancer Risk in South Africa. Viruses 2025, 17, 451. [Google Scholar] [CrossRef]
- Tanaka, K.; Okamoto, A. Degradation of DNA by bisulfite treatment. Bioorg. Med. Chem. Lett. 2007, 17, 1912–1915. [Google Scholar] [CrossRef]
- Hong, S.R.; Shin, K.J. Bisulfite-converted DNA quantity evaluation: A multiplex quantitative real-time PCR system for evaluation of bisulfite conversion. Front. Genet. 2021, 12, 618955. [Google Scholar] [CrossRef] [PubMed]
- Srirungruang, S.; Mahajindawong, B.; Nimitpanya, P.; Bunkasem, U.; Ayuyoe, P.; Nuchprayoon, S.; Sanprasert, V. Comparative study of DNA extraction methods for the PCR detection of intestinal parasites in human stool samples. Diagnostics 2022, 12, 2588. [Google Scholar] [CrossRef] [PubMed]
- Kresse, S.H.; Brandt-Winge, S.; Pharo, H.; Flatin, B.T.B.; Jeanmougin, M.; Vedeld, H.M.; Lind, G.E. Evaluation of commercial kits for isolation and bisulfite conversion of circulating cell-free tumor DNA from blood. Clin. Epigenetics 2023, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Davidović, R.S.; Božović, A.M.; Mandušić, V.L.; Krajnović, M.M. Methylation-specific PCR: Four steps in primer design. Cent. Eur. J. Biol. 2014, 9, 1127–1139. [Google Scholar] [CrossRef]
- Chen, J.; Sun, H.; Tang, W.; Zhou, L.; Xie, X.; Qu, Z.; Chen, M.; Wang, S.; Yang, T.; Dai, Y.; et al. DNA methylation biomarkers in stool for early screening of colorectal cancer. J. Cancer 2019, 10, 5264–5271. [Google Scholar] [CrossRef]
- Diakite, M.; Shaw-Saliba, K.; Lau, C.Y. Malignancy and viral infections in sub-Saharan Africa: A review. Front. Virol. 2023, 3, 1103737. [Google Scholar] [CrossRef]
- Chen, W.D.; Han, Z.J.; Skoletsky, J.; Olson, J.; Sah, J.; Myeroff, L.; Platzer, P.; Lu, S.; Dawson, D.; Willis, J.; et al. Detection in fecal DNA of colon cancer–specific methylation of the nonexpressed vimentin gene. J. Natl. Cancer Inst. 2005, 97, 1124–1132. [Google Scholar] [CrossRef]
- Tristan-Flores, F.E.; de la Rocha, C.; Pliego-Arreaga, R.; Cervantes-Montelongo, J.A.; Silva-Martínez, G.A. Epigenetic changes induced by infectious agents in cancer. In Pathogens Associated with the Development of Cancer in Humans: Omics, Immunological, and Pathophysiological Studies; Velázquez-Márquez, N., Paredes-Juárez, G.A., Vallejo-Ruiz, V., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 411–457. [Google Scholar]
- Das, D.; Karthik, N.; Taneja, R. Crosstalk Between Inflammatory Signaling and Methylation in Cancer. Front. Cell Dev. Biol. 2021, 9, 756458. [Google Scholar] [CrossRef]
- Arumugam, T.; Ramphal, U.; Adimulam, T.; Chinniah, R.; Ramsuran, V. Deciphering DNA Methylation in HIV Infection. Front. Immunol. 2021, 12, 795121. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, A.; Sánchez, O.F.; Zhao, H.; Lin, L.; Min, A.; Yuan, C. Development and application of novel BiFC probes for cell sorting based on epigenetic modification. Cytom. Part A 2022, 101, 339–350. [Google Scholar] [CrossRef]
- Ou, Y.; Zhang, Q.; Tang, Y.; Lu, Z.; Lu, X.; Zhou, X.; Liu, C. DNA methylation enzyme inhibitor rg108 suppresses the radioresistance of esophageal cancer. Oncol. Rep. 2018, 39, 993–1002. [Google Scholar] [CrossRef]
- Lascano, S.; Lopez, M.; Arimondo, P.B. Natural products and chemical biology tools: Alternatives to target epigenetic mechanisms in cancers. Chem. Rec. 2018, 18, 1854–1876. [Google Scholar] [CrossRef] [PubMed]
- Assis, R.I.; Wiench, M.; Silvério, K.G.; Silva, R.A.D.; Feltran, G.D.S.; Sallum, E.A.; Casati, M.Z.; Nociti, F.H.; Andia, D.C. Rg108 increases nanog and oct4 in bone marrow-derived mesenchymal cells through global changes in DNA modifications and epigenetic activation. PLoS ONE 2018, 13, e0207873. [Google Scholar] [CrossRef]
- Launoy, G.D.; Bertrand, H.J.; Berchi, C.; Talbourdet, V.Y.; Guizard, A.V.; Bouvier, V.M.; Caces, E.R. Evaluation of an immunochemical fecal occult blood test with automated reading in screening for colorectal cancer in a general average-risk population. Int. J. Cancer 2005, 115, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Doshi, P.; Sievers, C. Understanding the utility of fecal occult blood testing in hospitalized patients with suspected GI bleeding. Cureus 2024, 16, e57406. [Google Scholar] [CrossRef]
- Gómez-Molina, R.; Suárez, M.; Martínez, R.; Chilet, M.; Bauça, J.M.; Mateo, J. Utility of stool-based tests for colorectal cancer detection: A comprehensive review. Healthcare 2024, 12, 1645. [Google Scholar] [CrossRef]
- Gomez, F.; Hirbo, J.; Tishkoff, S.A. Genetic variation and adaptation in Africa: Implications for human evolution and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a008524. [Google Scholar] [CrossRef] [PubMed]
- Papaiakovou, M.; Waeschenbach, A.; Ajibola, O.; Ajjampur, S.S.; Anderson, R.M.; Bailey, R.; Benjamin-Chung, J.; Cambra-Pellejà, M.; Caro, N.R.; Chaima, D.; et al. Global diversity of soil-transmitted helminths reveals population-biased genetic variation that impacts diagnostic targets. Nat. Commun. 2025, 16, 6374. [Google Scholar] [CrossRef] [PubMed]
- Gopal, P.; Ahmed, Z.; Kant, V.V.R.; Rao, G.V.; Rebala, P. Circulating tumor DNA for monitoring colorectal cancer: A prospective observational study to assess the presence of methylated SEPT9 and VIM promoter genes and its role as a biomarker in colorectal cancer management. Turk. J. Surg. 2023, 39, 107–114. [Google Scholar] [CrossRef]
- Mpaka-Mbatha, M.N.; Naidoo, P.; Bhengu, K.N.; Islam, M.M.; Singh, R.; Nembe-Mafa, N.; Mkhize-Kwitshana, Z.L. Cytokine Gene Expression Profiles during HIV and Helminth Coinfection in Underprivileged Peri-Urban South African Adults. Diagnostics 2023, 13, 2475. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Jandorf, L.; Brand, R.; Rabeneck, L.; Schroy, P.C., III; Sontag, S.; Johnson, D.; Skoletsky, J.; Durkee, K.; Markowitz, S.; et al. Improved fecal DNA test for colorectal cancer screening. Clin. Gastroenterol. Hepatol. 2007, 5, 111–117. [Google Scholar] [CrossRef]
- Porcaro, F.; Voccola, S.; Cardinale, G.; Porcaro, P.; Vito, P. DNA methylation biomarkers in stool samples: Enhancing colorectal cancer screening strategies. Oncol. Rev. 2024, 18, 1408529. [Google Scholar] [CrossRef]
- Su, W.; Du, Y.; Lian, F.; Wu, H.; Zhang, X.; Yang, W.; Duan, Y.; Pan, Y.; Liu, W.; Wu, A.; et al. Standards for collection, preservation, and transportation of fecal samples in TCM clinical trials. Front. Cell Infect. Microbiol. 2022, 12, 783682. [Google Scholar] [CrossRef]
- Momo Cabrera, P.; Bokulich, N.A.; Zimmermann, P. Evaluating stool microbiome integrity after domestic freezer storage using whole-metagenome sequencing, genome assembly, and antimicrobial resistance gene analysis. Microbiol. Spectr. 2025, 13, e0227824. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Wynn, T.A.; Donaldson, D.D.; Urban, J.F. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infections. Curr. Opin. Immunol. 1999, 11, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Gray, E.H.; Azarian, S.; Zamalloa, A.; McPhail, M.J.W.; Vincent, R.P.; Williams, R.; Chokshi, S.; Patel, V.C.; Edwards, L.A. Faecal cytokine profiling as a marker of intestinal inflammation in acutely decompensated cirrhosis. JHEP Rep. 2020, 2, 100151. [Google Scholar] [CrossRef]
- Walusimbi, B.; Lawson, M.A.E.; Nassuuna, J.; Kateete, D.P.; Webb, E.L.; Grencis, R.K.; Elliott, A.M. The effects of helminth infections on the human gut microbiome: A systematic review and meta-analysis. Front. Microbiomes 2023, 2, 1174034. [Google Scholar] [CrossRef]
- Harnett, W.; Harnett, M.M. Epigenetic changes induced by parasitic worms and their excretory-secretory products. Biochem. Soc. Trans. 2024, 52, 55–63. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer/Probe | Primer Sequences | References |
|---|---|---|---|
| Vimentin | Forward | 5′TCGTTTCGAGGTTTTCGCGTTAGAGAC-3′ | [17] |
| Reverse | 5′CGACTAAAACTCGACCG ACTCGCGA-3′ | ||
| ACTB | Forward | GAAAGGGTGTAGTTTTGGGAGGTTAG | [29] |
| Reverse | AATAACCCAAATAAATAACCCACTACCTC |
| Step | Temperature (°C) | Time | Notes |
|---|---|---|---|
| Initial denaturation | 95 | 10 min | Polymerase activation |
| Denaturation | 95 | 15 s | |
| Annealing/Extension | 60 | 60 s | Data collected during this step |
| Steps 2–3 were repeated | 40 cycles | ||
| HRM preconditioning | 95 | 10 s | |
| HRM step | 60 | Ramp at 0.1 °C | Continuous ramp with data acquisition |
| Group, (n) | Methylation, n (%) | Non-Methylation, n (%) | p Value | Odds Ratio (95% CI) |
|---|---|---|---|---|
| Uninfected controls (n = 10) | 1 (10) | 9 (90) | <0.0001 * | |
| HIV-infected (n = 14) | 13 (92.9) | 1 (7.1) | ||
| Helminth-infected (n = 15) | 14 (93.3) | 1 (6.7) | ||
| Coinfection (n = 13) | 10 (76.9) | 3 (20) | ||
| CRC confirmed patients (n = 10) | 9 (90) | 1 (10) | ||
| HIV and helminth uninfected, single-infected and coinfected individuals versus CRC confirmed patients (Positive Control) | ||||
| Uninfected controls versus CRC confirmed patients | 0.0011 | 0.01 (0.00–0.23) | ||
| HIV-infected versus CRC confirmed patients | 1.0000 | 1.44 (0.08–26.25) | ||
| Helminth-infected versus CRC confirmed patients | 1.0000 | 1.56 (0.09–28.17) | ||
| Coinfection versus CRC confirmed patients | 0.6036 | 0.37 (0.03–4.23) | ||
| HIV and helminth single and coinfected groups Versus Uninfected controls (Negative Control) | ||||
| HIV-infected versus Uninfected controls | <0.0001 | 117 (6.44–2126) | ||
| Helminth-infected versus Uninfected controls | <0.0001 | 126 (6.96–2281) | ||
| Coinfection versus Uninfected controls | <0.0001 | 30 (2.62–342.9) | ||
| Comparison between infected groups | ||||
| HIV-infected versus Helminth-infected | 1.0000 | 0.93 (0.05–16.43) | ||
| Coinfection versus HIV-infected | 0.3259 | 0.26 (0.02–2.85) | ||
| Coinfection versus Helminth-infected | 0.3111 | 0.24 (0.02–2.64) | ||
| Residue | ΔG (kcal mol−1) | Interaction Type |
|---|---|---|
| Glu1266 | −5.84 | Hydrogen bond/electrostatic |
| Asn1578 | −4.19 | Hydrogen bond |
| Phe1145 | −3.71 | π–π stacking/hydrophobic |
| Pro1225 | −3.26 | Hydrophobic/backbone contact |
| Cys1226 | −2.88 | Catalytic loop stabilization |
| Trp1170 | −2.33 | Hydrophobic |
| Arg1574 | −1.95 | Polar/solvent-exposed |
| Total ΔG_bind | −49.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damane, B.P.; Kader, S.; Alaouna, M.; Naidoo, P.; Dlamini, Z.; Mkhize-Kwitshana, Z.L. Vimentin Methylation as a Potential Screening Biomarker for Colorectal Cancer in HIV-Helminth Co-Infected Individuals. Microbiol. Res. 2025, 16, 236. https://doi.org/10.3390/microbiolres16110236
Damane BP, Kader S, Alaouna M, Naidoo P, Dlamini Z, Mkhize-Kwitshana ZL. Vimentin Methylation as a Potential Screening Biomarker for Colorectal Cancer in HIV-Helminth Co-Infected Individuals. Microbiology Research. 2025; 16(11):236. https://doi.org/10.3390/microbiolres16110236
Chicago/Turabian StyleDamane, Botle Precious, Shakeel Kader, Mohammed Alaouna, Pragalathan Naidoo, Zodwa Dlamini, and Zilungile Lynette Mkhize-Kwitshana. 2025. "Vimentin Methylation as a Potential Screening Biomarker for Colorectal Cancer in HIV-Helminth Co-Infected Individuals" Microbiology Research 16, no. 11: 236. https://doi.org/10.3390/microbiolres16110236
APA StyleDamane, B. P., Kader, S., Alaouna, M., Naidoo, P., Dlamini, Z., & Mkhize-Kwitshana, Z. L. (2025). Vimentin Methylation as a Potential Screening Biomarker for Colorectal Cancer in HIV-Helminth Co-Infected Individuals. Microbiology Research, 16(11), 236. https://doi.org/10.3390/microbiolres16110236







