1. Introduction
The management of fungal diseases, including ripe rot, gray mold, powdery mildew, and downy mildew, has a direct impact on fruit yield and quality in viticulture. Owing to excessive rainfall during the growing season in Japan, the humidity surrounding grapevines tends to be high, and the frequency of disease occurrence is very high. For example, climate simulation models have demonstrated an increased risk of heavy rainfall in early summer (May to July) in Japan [
1]. The humid climate of Japan, exacerbated by the prolonged concentration of extremely moist air masses over the country due to global warming [
2], has contributed to increased damage to grapevines by a wide variety of phytopathogens, making effective disease control extremely difficult in the face of climate change. Although fungal diseases can be managed with chemical pesticides, no chemical agents are currently available in Japan to control crown gall disease, a bacterial disease affecting grapevines.
Crown gall disease, caused by soil-borne bacterial pathogens, is a general term for diseases characterized by the formation of galls on trunks and branches, leading to reduced tree vigor. Grapevine crown gall disease is widespread throughout Japan. In a global context, crown gall disease is recognized as a major threat to viticulture in many grape-growing regions, including North America, Europe, and the Middle East, causing significant economic losses due to reduced vigor, delayed bud break, and decreased fruit production [
3]. In Japan, bacterial crown gall is particularly prevalent in Hokkaido, an island located in the northernmost part of Japan and a region known for heavy snowfall [
4,
5]. Although crown gall disease has been reported to spread through soil-borne transmission or the planting of infected nursery stock, with no alternative infection routes identified [
6], there is growing evidence that secondary infections occur in commercial vineyards in Hokkaido [
4]. Therefore, a symptomatic control strategy similar to chemical pesticides is required against grapevine crown gall disease.
Active research is being conducted on microbial pesticides to combat crown gall disease. Microbial pesticides, an alternative disease control strategy to chemical pesticides, primarily utilize microorganisms isolated from natural environments [
7]. Compared to chemical pesticides, microbial pesticides offer a significant advantage—they alleviate concerns related to the increasing impact of chemical pesticides. However, microbial pesticides also have limitations, the most notable being their typically narrow spectrum of activity. For example, agrocin 84 [
8], an antibacterial compound produced by
Agrobacterium radiobacter strain K84 (commercially known as Bacterose), effectively inhibits rose crown gall pathogens but not grapevine crown gall pathogens [
9]. One promising discovery is the non-pathogenic strains of
Allorhizobium vitis ARK-1 [
10] and VAR03-1 [
11], which exhibit antagonistic activity against several crown gall pathogens. However, to date, none of the identified microbial strains have been put to practical use as commercial microbial pesticides against a wide variety of crown gall diseases. Given this background, identifying microorganisms with antagonistic activity against multiple crown gall pathogens is a critical strategy for developing more practical microbial pesticides. To address these challenges, we focused on the hypothesis that soil-derived microorganisms previously identified as antagonists against major fungal pathogens may also exhibit broad-spectrum antagonistic activity against crown gall pathogens. Despite increasing recognition of microbial biocontrol agents, there remains a critical knowledge gap: very few strains have demonstrated efficacy against multiple crown gall pathogens affecting different host plants, and none have progressed to practical application. Therefore, this study aimed to identify and characterize a single microbial strain capable of providing broad-spectrum biocontrol against grapevine and rose crown gall pathogens. We have established a library of soil bacteria [
12,
13] consisting of isolates with verified antagonistic activity against
Botrytis cinerea (the causal pathogen of grapevine gray mold) and
Colletotrichum gloeosporioides (the major pathogen causing grapevine ripe rot). Through screening of this library, we identified
Pseudomonas sp. strain YU44, which exhibits antagonistic activity against grapevine and rose crown gall pathogens.
2. Materials and Methods
2.1. Pathogens
Two strains of the grapevine crown gall pathogen, Allorhizobium vitis, and one strain of the rose crown gall pathogen, Rhizobium radiobacter, were used in this study. One vitopine-type strain of A. vitis, isolated from Vitis vinifera cv. Zweigeltrebe cultivated in Hokkaido, and one octopine-type strain, isolated from V. vinifera cv. Riesling grown in Hiroshima, were designated as vito1 and octo2, respectively. One strain of R. radiobacter, isolated from Rosa cv. Jubilé du Prince de Monaco grown in Yamanashi, was designated as Rr1.
2.2. Plants
Grapevine and rose cultivars used in this study were selected on the basis of previous reports [
14,
15], which confirmed their low susceptibility to crown gall disease. Grapevine seedlings (
V. vinifera cvs. Pinot Noir and Cabernet Sauvignon) were grown on their own roots in pots at 27 °C under light irradiation (11.8 Wm
−2/16 h/d). One-year-old seedlings were used in the in vivo bioassay. Seedlings of
Rosa canina, prickly wild rose, were grown on their own roots in pots at 27 °C under light irradiation (11.8 Wm
−2/16 h/d), and 60-day-old seedlings were used in the in vivo bioassay.
2.3. Screening Antagonistic Bacteria Against Crown Gall Pathogen
Fifty-five strains from the soil bacterial library, which were previously confirmed to exhibit antagonistic effects against gray mold and ripe rot pathogens [
11,
12], were tested. Glycerol stocks of
A. vitis octo2 and the 55 strains were inoculated onto soybean casein digest (SCD) agar plates using a platinum loop and cultured at 25 °C. Single colonies were transferred to 20 mL of SCD liquid medium in 100 mL flasks and incubated in a shaking incubator at 25 °C and 130 rpm for 24 h. The absorbance at 600 nm was measured using a spectrophotometer, and each culture was diluted with sterile water to adjust the OD
600 to 1.0–1.3. A total of 100 μL of
A. vitis octo2 culture was spread onto new SCD agar plates using a spreader. Two wells (7 mm in diameter) were made at opposite sides of the plates using a cork borer. A 50 μL volume of each bacterial culture was dispensed into one of the wells, while an equal volume of SCD liquid medium was added to the opposite well as a control. After incubation at 25 °C for 2 d, the diameter of the inhibition zone, where
A. vitis octo2 growth was suppressed, was measured with a ruler. As mentioned in
Section 3, we found that strain YU44, which exhibits antagonistic activity against
A. vitis octo2.
2.4. In Vitro Bioassay with YU 44 Culture Filtrate
SCD agar plates were prepared by spreading A. vitis octo2 over the surface and creating two opposite wells, as mentioned above. The supernatant from YU44 SCD liquid cultures was obtained by centrifugation at 3000 rpm for 10 min, followed by filtration through a 0.22 μm PTFE syringe filter (Millipore, Bedford, MA, USA). A 50 μL volume of the culture filtrate was dispensed into one of the wells, while an equal volume of SCD liquid medium was added to the opposite well as a control. After incubation at 25 °C for 2 d, the inhibition zone, where A. vitis octo2 growth was suppressed, was measured.
2.5. Antagonistic Activity of YU44 Against Other Crown Gall Pathogens
To confirm whether YU44 would form an inhibition zone against other crown gall pathogens, SCD agar plates were prepared by spreading A. vitis vito1 and R. radiobacter Rr1 over the surface and creating two opposite wells, as mentioned above. A 50 μL volume of YU44 culture (OD600 = 1.0) was dispensed into one of the wells, while an equal volume of SCD liquid medium was added to the opposite well as a control. After incubation at 25 °C for 2 d, the inhibition zone where A. vitis vito1 and R. radiobacter Rr1 growth was suppressed was observed.
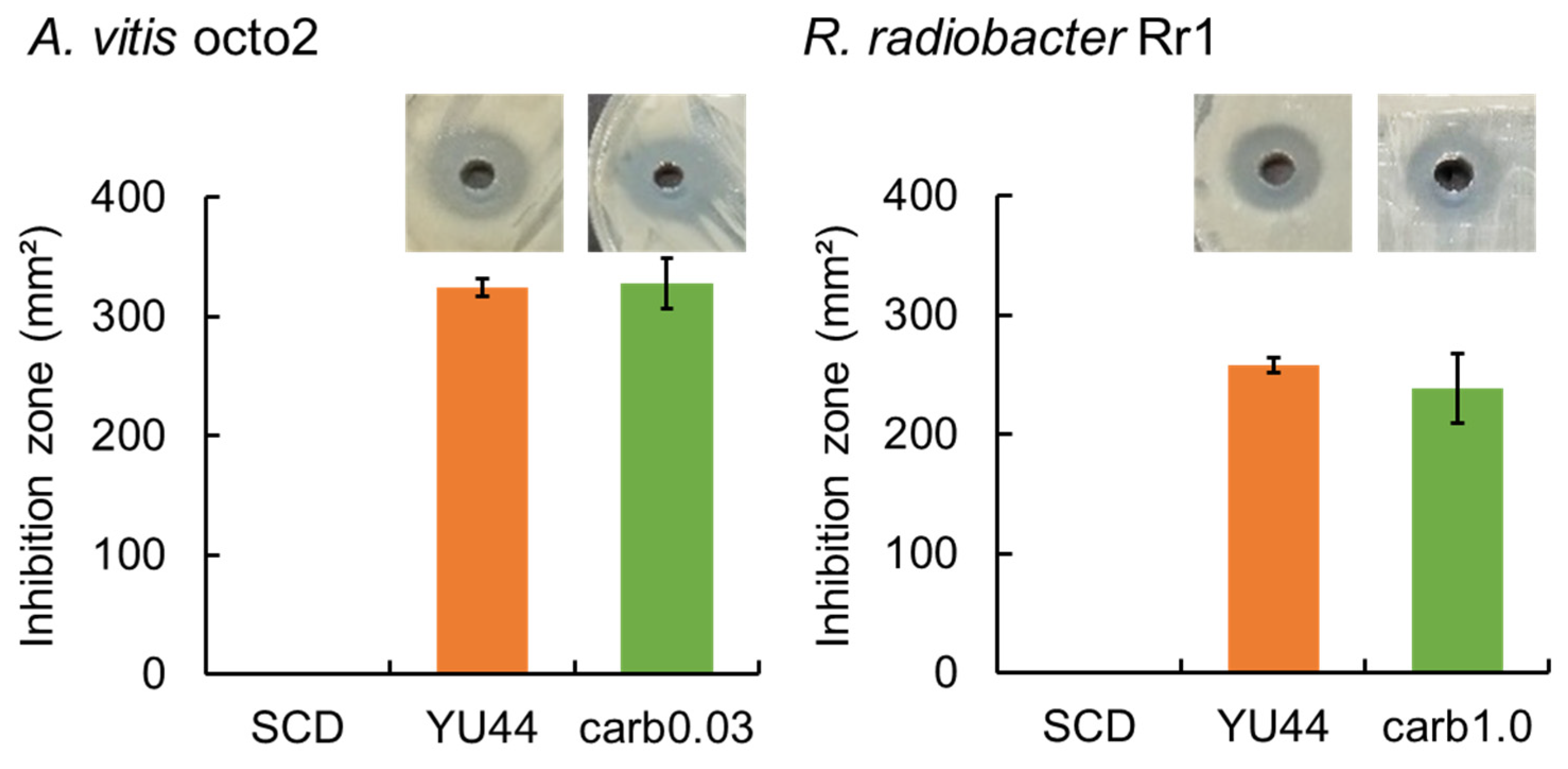
2.6. Comparison of the Antagonistic Activity of YU44 Culture Filtrate with Carbenicillin
The antagonistic activity of bioactive compounds secreted by YU44 was compared with that of carbenicillin, a bactericidal antibiotic against crown gall pathogens. SCD agar plates were prepared by spreading
A. vitis octo2 and
R. radiobacter Rr1 over the surface and creating two opposite wells, as mentioned above. A 50 μL volume of YU44 culture filtrate or serially diluted carbenicillin (Nacalai Tesque, Kyoto, Japan) was dispensed into one of the wells, while an equal volume of SCD liquid medium was added to the opposite well as a control. The inhibition zone formed on the SCD agar plates was measured using ImageJ ver. 1.54 (
https://imagej.net/ij/ (accessed on 15 March 2025)). The concentration of the carbenicillin solution that produced an inhibition zone equal in size to that created by the YU44 culture filtrate was determined.
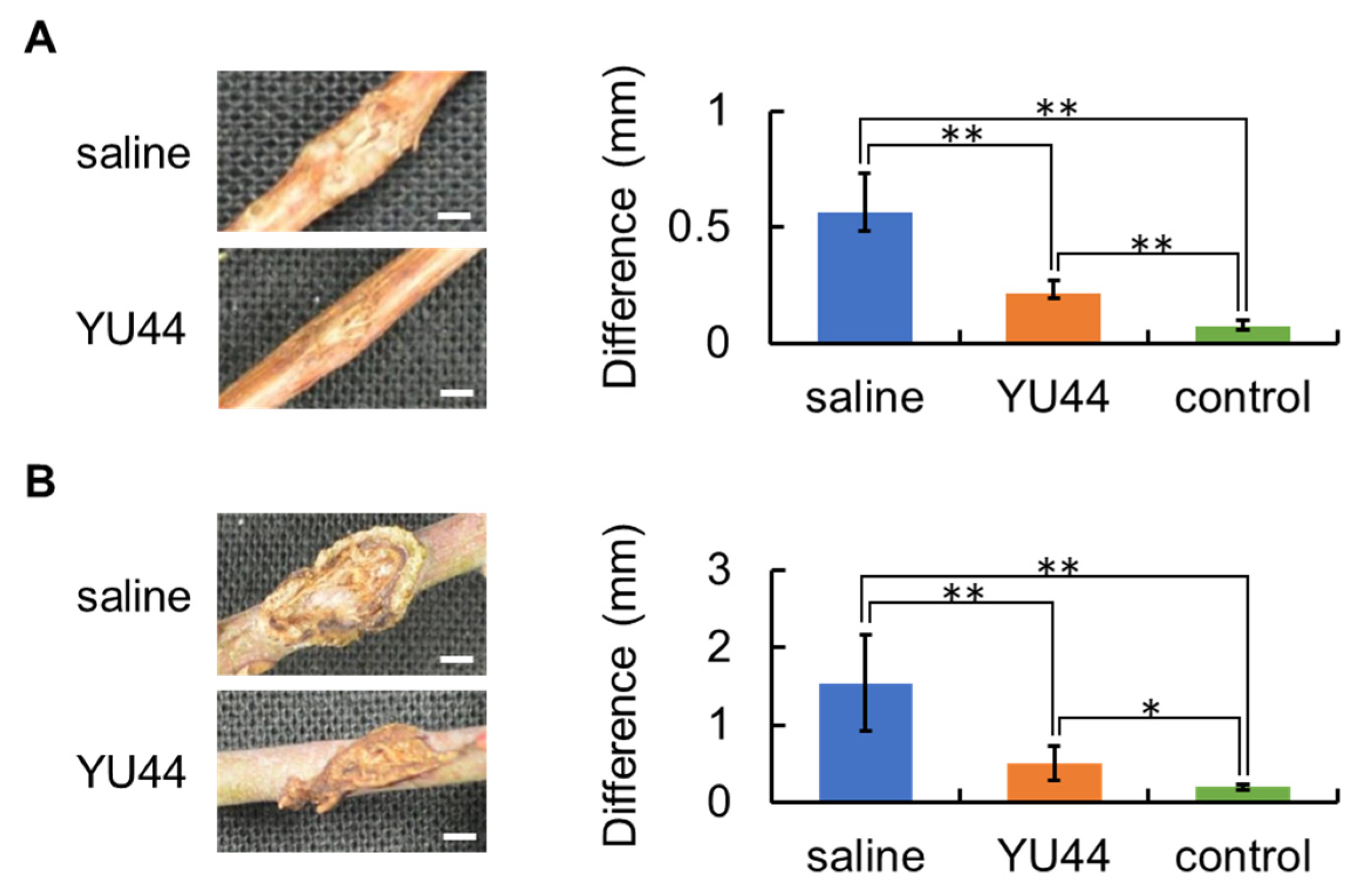
2.7. In Vivo Bioassay Using Grapevine and Rose Shoots
To determine whether YU44 exhibits antagonistic effects in plants, in vivo bioassays were performed using grapevine and rose seedlings. YU44, A. vitis octo2, and R. radiobacter Rr1 were cultured in SCD liquid medium. Each culture was centrifuged at 4000 rpm for 5 min, and the supernatant was discarded. The bacterial pellets were suspended in saline, and the bacterial suspensions were adjusted to an OD600 of 1.0 to 1.3.
Three seedlings each of Pinot Noir and
Rosa canina were used. Two wounds were made between the nodes of the shoot using sterilized tweezers
Figure S1). A 20 μL volume of the YU44 suspension was inoculated into one wound using a micropipette. Saline was inoculated into the other wound as a control. After drying for 4 h at room temperature, 20 μL of
A. vitis octo2 or
R. radiobacter Rr1 was inoculated into each wound on the grapevine or rose shoots, respectively, using a micropipette. The inoculated sites were wrapped with Parafilm
®, and the seedlings were covered with plastic bags. After incubation at room temperature for 24 d for grapevine seedlings and 14 d for rose seedlings, the widths of the shoot and the galls were measured using ImageJ, as shown in
Figure S1. The suppressive activity of YU44 against each crown gall was evaluated by measuring the difference between gall and shoot widths at the inoculation sites.
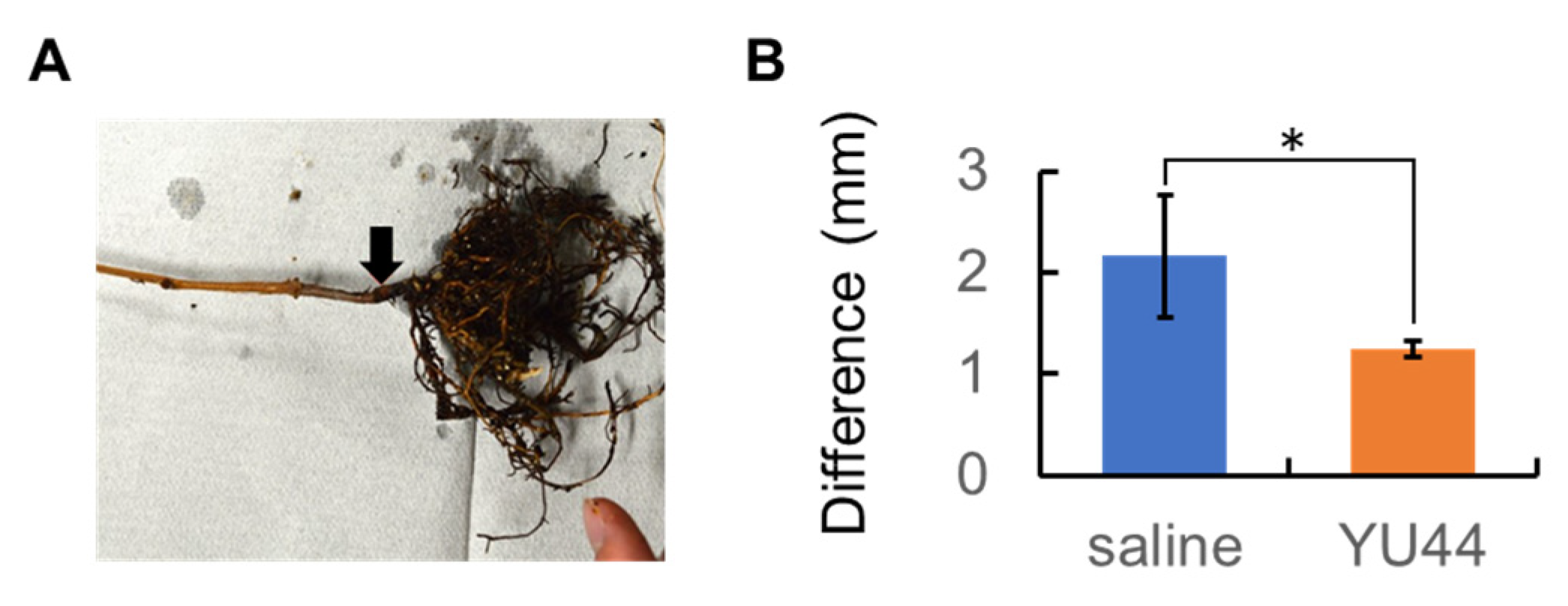
2.8. In Vivo Bioassay Using Grapevine Stem near the Base
The roots of three potted Cabernet Sauvignon seedlings were washed with sterile water and replanted in sterilized soil in new pots. Wounds were made on the stem near the base using sterilized tweezers (
Figure S1). A 50 mL volume of YU44 suspension, prepared as mentioned above, was sprayed onto the soil surface using a hand sprayer. Subsequently, 20 μL of
A vitis octo2 suspension, prepared as mentioned above, was inoculated into the wound using a microsyringe. The seedlings were covered with plastic bags. After incubation at room temperature for 81 d, the suppressive activity of YU44 against grapevine crown gall pathogens was evaluated by measuring the difference between gall and stem widths at the inoculation sites using ImageJ (
Figure S1).
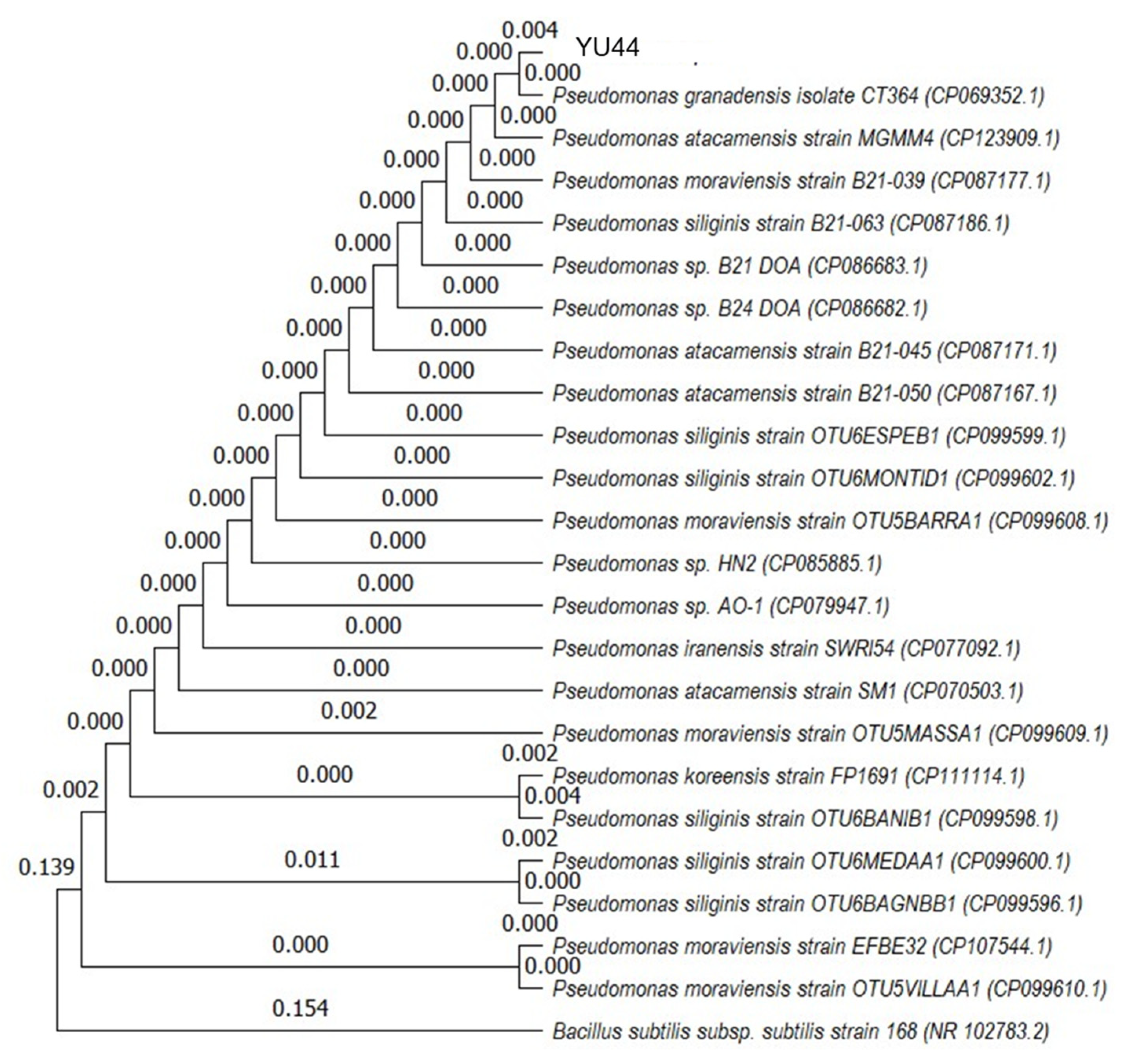
2.9. Identification of YU44 by 16S rDNA Sequence Analysis
Genomic DNA was extracted from a one-day SCD culture of YU44 using a DNeasy PowerSoil Kit (Qiagen, Hilden, Germany), in accordance with the manufacturer’s instructions. PCR conditions for amplifying partial 16S rDNA were as follows: after an initial incubation at 94 °C for 5 min, amplification was performed for 30 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min, followed by a final extension at 72 °C for 7 min. The nucleotide sequences of the primer set used were as follows: 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 519R (5′-GTATTACCGCGGCTGCTG-3′). The nucleotide sequence of the amplicon was analyzed using the dye terminator method and subjected to the Basic Local Alignment Search Tool (BLAST 2.17.0, NCBI). Phylogenetic analysis was performed using the partial 16S rDNA of YU44 and
Pseudomonas strains deposited in the NCBI database. The nucleotide sequences were subjected to the maximum likelihood method [
16] using Molecular Evolutionary Genetics Analysis software, MEGA12 (
www.megasoftware.net (accessed on 31 August 2025)). The partial 16S rDNA nucleotide sequence of YU44 was deposited in DDBJ/ENA/GenBank under accession no. LC891664.
2.10. Statistical Analysis
Data are presented as means ± standard deviations of biological replicates. Statistical analysis was performed using analysis of variance (ANOVA) followed by Student’s t-test in Excel Statistics software 2012 (Social Survey Research Information, Tokyo, Japan).
4. Discussion
In this study, we successfully identified
Pseudomonas sp. YU44 from our established soil bacterial library as a promising biocontrol agent with broad-spectrum antagonistic activity against crown gall pathogens affecting both grapevine and rose. This discovery addresses a critical gap in current disease management strategies, where effective tools for controlling bacterial crown gall diseases remain limited compared to those for controlling fungal diseases [
4]. The antagonistic activity of YU44 is mediated by the secreted bioactive compounds, as evidenced by the inhibitory effect of the cell-free culture filtrate against crown gall pathogen growth. The comparative analysis with carbenicillin provides important context for the practical potential of YU44. For example, compared with the effects of 1 mg/mL carbenicillin on
Agrobacterium tumefaciens growth, timentin at 0.15 mg/mL showed similar efficacy [
17]. The equivalent efficacy against
A. vitis octo2 (comparable to 0.03 mg/mL carbenicillin) and
R. radiobacter Rr1 (comparable to 1 mg/mL carbenicillin) suggests that YU44 produces bioactive compounds with differential activity against these pathogens. Although it should be noted that an in vivo bioassay with carbenicillin was not conducted in the present study, this differential activity may reflect variations in cell wall composition, efflux mechanisms, or target sensitivity between the two bacterial species. In addition, although bacteriocins cannot be excluded as potential contributors to antimicrobial activity, several other well-established biocontrol mechanisms in
Pseudomonas spp., including siderophore-mediated iron competition, production of antimicrobial metabolites such as phenazines, pyrrolnitrin, or lipopeptides, and induction of host defense responses [
18], should also be considered as possible mechanisms underlying YU44 activity. Future studies are also needed to identify the secreted bioactive compounds.
The transition from in vitro to in vivo efficacy represents a critical validation step for biocontrol agents [
19]. In view of its potential use against soil-borne crown gall pathogens, application to the soil and the basal stem zone is considered appropriate. Because YU44 was originally isolated from soil, it is likely to be well adapted to the soil environment. Nevertheless, issues related to colony establishment and persistence in the rhizosphere must be carefully considered for its effective use as a biocontrol agent. This concern is consistent with a previous report, which emphasizes that the efficacy of microbial antagonists depends not only on their antagonistic activity but also on their ability to establish stable populations in soil ecosystems and cropping systems [
20]. Considering the practical application for controlling soil-borne crown gall disease, application to the soil and the basal stem region appears to be the most appropriate approach. In this context, the finding that soil-surface application of YU44 suppressed grapevine crown gall disease constitutes a significant achievement. To further evaluate the potential of YU44 against grapevine and rose crown gall pathogens, large-scale field trials will be required. In such trials, it will be essential to assess not only the disease control efficacy of YU44 but also its survival and persistence under field conditions. In addition, it will be important to investigate whether YU44 is able to migrate within grapevine and rose plants, as has been reported for non-pathogenic
A. vitis ARK-1, which has been shown to migrate within plants [
21].
Several limitations of this study warrant consideration. First, whereas YU44 demonstrated clear biocontrol activity, complete disease suppression was not achieved in our assays. As with other antagonistic microorganisms or commercially available microbial pesticides, this partial efficacy is common among biocontrol agents, but can be improved by optimizing application timing, concentration, or formulation approaches [
7]. Second, this study was performed under controlled laboratory conditions. Field trials under diverse environmental conditions will be essential to validate the consistency and reliability of the biocontrol efficacy of YU44. Such factors as soil pH, moisture, temperature fluctuations, and microbial community interactions may significantly influence its performance in commercial vineyards [
22]. Furthermore, biosafety and potential non-target effects should be carefully considered before field application.
Pseudomonas are generally regarded as environmentally compatible biocontrol agents, but future studies should evaluate the ecological safety of YU44, including its interactions with beneficial soil microorganisms and its potential effects on non-target plant species. Third, the bioactive compounds in YU44 were not identified. Although our culture filtrate experiments confirmed the secretion of bioactive compounds, the chemical characterization of these compounds would facilitate understanding of the mode of action and the potential for resistance development of the compounds. The potential phytotoxic effects of the YU44 culture filtrate also remain to be determined. However, in the inoculation assays conducted with live YU44 cells, neither grapevine nor rose plants exhibited any visible phytotoxic symptoms such as leaf wilting, chlorosis, or necrosis, suggesting that YU44 is unlikely to be harmful to host plant tissues under the tested conditions. Recent studies have highlighted the role of phage tail-like bacteriocins, known as tailocins, in interbacterial antagonism. For example, rhizoviticin, an F-type tailocin identified in
A. vitis VAR03-1, was shown to be a key determinant of its biocontrol activity against crown gall, with loss of rhizoviticin leading to a significant reduction in antitumorigenic activity in plants [
23]. Future chemical and genetic analyses of YU44 will be essential in determining whether bioactive compounds, including tailocin-like compounds, contribute to its biocontrol efficacy.
5. Conclusions
The identification and characterization of Pseudomonas sp. YU44 represent a significant step forward in the adaptive management of crown gall diseases under climate change. YU44 may offer clear environmental benefits over chemical pesticides, including greater ecological compatibility, lower risk of resistance development, and minimal impact on beneficial soil microorganisms, supporting sustainable viticulture. Our results showed that YU44 exhibits broad-spectrum antagonistic activity against multiple crown gall pathogens through its secreted bioactive compounds, and that soil application is a practical delivery method. Rather than replacing existing tools, YU44 can complement resistant rootstocks, sanitation practices, and cultivation methods, especially in nurseries with diverse host species. Although complete disease suppression was not achieved, substantial reductions in severity highlight the potential of YU44 as a viable, environmentally friendly component of integrated disease management. Future work should focus on field validation, bioactive compound identification, formulation optimization, and safety assessment to facilitate practical implementation and commercialization.