Abstract
Sugarcane mosaic virus (SCMV) (genus, Potyvirus; family, Potyviridae) is widespread, deleterious, and the most damaging pathogen of sugarcane (Saccharum officinarum L. and Saccharum spp.) that causes a substantial barrier to producing high sugarcane earnings. Sugarcane mosaic disease (SCMD) is caused by a single or compound infection of SCMV disseminated by several aphid vectors in a non-persistent manner. SCMV has flexuous filamentous particle of 700–750 nm long, which encapsidated in a positive-sense, single-stranded RNA molecule of 9575 nucleotides. RNA interference (RNAi)-mediated antiviral innate immunity is an evolutionarily conserved key biological process in eukaryotes and has evolved as an antiviral defense system to interfere with viral genomes for controlling infections in plants. The current study aims to analyze sugarcane (Saccharum officinarum L. and Saccharum spp.) locus-derived microRNAs (sof-miRNAs/ssp-miRNAs) with predicted potential for targeting the SCMV +ssRNA-encoded mRNAs, using a predictive approach that involves five algorithms. The ultimate goal of this research is to mobilize the in silico- predicted endogenous sof-miRNAs/ssp-miRNAs to experimentally trigger the catalytic RNAi pathway and generate sugarcane cultivars to evaluate the potential antiviral resistance surveillance ability and capacity for SCMV. Experimentally validated mature sugarcane (S. officinarum, 2n = 8X = 80) and (S. spp., 2n = 100–120) sof-miRNA/ssp-miRNA sequences (n = 28) were downloaded from the miRBase database and aligned with the SCMV genome (KY548506). Among the 28 targeted mature locus-derived sof-miRNAs/ssp-miRNAs evaluated, one sugarcane miRNA homolog, sof-miR159c, was identified to have a predicted miRNA binding site, at nucleotide position 3847 of the SCMV genome targeting CI ORF. To verify the accuracy of the target prediction accuracy and to determine whether the sugarcane sof-miRNA/ssp-miRNA could bind the predicted SCMV mRNA target(s), we constructed an integrated Circos plot. A genome-wide in silico-predicted miRNA-mediated target gene regulatory network was implicated to validate interactions necessary to warrant in vivo analysis. The current work provides valuable computational evidence for the generation of SCMV-resistant sugarcane cultivars.
1. Introduction
Sugarcane (Saccharum officinarum) is a prolific tropical and subtropical crop that is economically important, has a long life span, serves as a biofuel, is enriched with energy-rich roughage, and is also a source of agroindustrial residues [1,2,3]. The genome of octaploid sugarcane (S. officinarum) (2n = 80; x = 10) [4,5], also known as “noble” sugarcane, and the genome of sugarcane species and cultivars have been assembled, drafted, and resequenced [6,7,8,9,10,11]. Sugarcane mosaic virus (SCMV) is a highly transmissible and pathogenic potyvirus that causes sugarcane mosaic virus disease (SCMD) [12,13]. Potyviruses are spread by a common complex of sap-sucking vectors such as aphid species [14]. Innovative approaches are still needed to increase sugarcane productivity [15]. The genome of SCMV consists of a +ss RNA molecule with a length of 9575 nucleotides encoding a single large polyprotein. The genome polyprotein precursor was predicted to be cleaved resulting in ten functional proteins: P1, HC-Pro, P3, 6 K1, CI, 6 K2, VPg, NIa, Nib, and CP [16,17,18,19].
In plants, microRNAs (miRNA) are endogenously expressed small (19–25 nucleotides), evolutionarily conserved, non-coding (NC)-ss RNA molecules [20]. In higher plants, the biogenesis and transcription of the miRNA gene (MIR) is controlled by RNA polymerase II, which is then transcribed into single-standard polycistronic primary transcripts (pri-miRNAs). They control a variety of biological processes in plants by regulating gene expression, cell growth, development, differentiation, and host–virus interactions [21,22]. The miRNA-mediated RNAi is a post-transcriptional gene-silencing mechanism that provides antimicrobial innate immunity and regulates host–virus interactions to limit or inhibit viral infection [23].
Artificial miRNA-mediated (amiRNA) technology is an alternative, robust biotechnology based on engineering miRNA genes to control viral infections in plants [24]. RNAi-based amiRNA constructs have been used in research to induce antiviral resistance in plants against plant viruses such as tomato [25,26], cucumber [27], rice [28], and cotton [29]. Mature miRNAs in the sugarcane genome have been predicted, identified, isolated, analyzed, and validated to evaluate host–virus interactions and gene regulation, and they have been associated with abiotic and biotic stresses [30,31,32,33,34,35,36,37,38,39,40]. Recently, experimental validations of 35 conserved mature locus-derived high-confidence sof-miRNAs/ssp-miRNAs in the sugarcane genome and further depositions in the miRBase database were reported.
An integrative multi-network approach based on SCMV infection assessment was used to identify target binding sites of sugarcane genome-encoded sof-miRNAs/ssp-miRNAs in the SCMV genome. The identification of multiple host-derived miRNA binding sites in the SCMV genome for the creation of transgenic sugarcane varieties resistant to SCMV is the main objective of this study. In this study, several miRNA prediction tools were evaluated and used to identify microRNA–mRNA binding sites in the SCMV genome for use in developing transgenic or non-transgenic modified sugarcane plants with resistance to SCMV and, potentially, closely related potyviruses. Potential targets of the most promising sugarcane miRNAs for breeding were also of interest to better understand potyvirus–sugarcane plant interactions during infection. Until now, there have been no reports on the use of an amiRNA-based strategy to develop SCMV tolerance in sugarcane plants, based on the prediction of homologous amiRNAs for silencing SCMV. The predicted locus-derived sof-miRNAs/ssp-miRNAs in the sugarcane genome were further evaluated to understand the complex interactions between sugarcane host planta and SCMV potyviruses and to identify novel antiviral targets.
2. Materials and Methods
2.1. Sugarcane MicroRNAs and SCMV Genome Data Retrieval and Processing
Experimentally validated high-confidence mature sugarcane microRNAs (sof-miRNA156-sof-miR11892/ssp-miR156-ssp-1432) (Accession ID: MIMAT0001656-MIMAT0001671/MIMAT0020291-MIMAT0020290) and Saccharum sp.-microRNAs (ssp-miR166-ssp-miR1432) (Accession ID: MIMAT0030451-MIMAT0020290) (Table S1) were retrieved from the miRNA registry (miRBase, version 22) [41]. The full-length SCMV +ssRNA genome sequence (9575 bases) (Accession number KY548506) was acquired from the NCBI GenBank database [42].
2.2. Potential Targets of Sugarcane MicroRNAs in the SCMV Genome
The prediction of effective microRNA–mRNA binding sites is a first step toward understanding microRNA-regulated gene regulatory networks. The accuracy of miRNA target site prediction can be affected by several factors, such as the specificity and sensitivity of the algorithm, the choice of reference sequence, and the length of the target sequence. Various in silico methods for effective silencing have been developed for the computational prediction of miRNA–mRNA target sites. A computational approach refers to the use of multiple computational methods, algorithms, or tools to analyze and interpret biological data. This approach combines different types of publicly available in silico algorithms, including miRanda [43,44], RNA22 [45,46], TAPIR [47], psRNATarget [48,49], and RNAhybrid [50] (Table 1).

Table 1.
Different features and parameters of algorithms applied for miRNA target predictions.
2.3. miRanda
miRanda is one of the first miRNA target predictors, a highly versatile algorithm based on the seed-based interactions of miRNA target duplexes [43]. It was implemented as a standard tool to detect potential miRNA binding sites. RNA–RNA duplex dimerization and sequence complementarity are features considered by the miRanda algorithm. It considers the cross-species conservation of target sites, which distinguishes it from other algorithms [44]. The miRanda algorithm has been implemented in C, and its first version was published in 2003. The default parameters were selected for the analysis (Table 1).
2.4. RNA22
The RNA22 algorithm has a diverse, web-based application with implemented interactive exploration. It uses a pattern-recognition based approach to serve as a miRNA target discovery tool. It predicts statistically significant target patterns using maximum folding energy (MFE) [45,46]. Site complementarity and non-seed-based interactions are important features. Its prediction is also based on highly sensitive and significant target patterns. The default parameters were chosen (Table 1).
2.5. TAPIR
The TAPIR algorithm is used to assess the seed-based interactions of plant miRNAs in the target sequence. It is a highly precise plant miRNA target prediction algorithm used to detect target binding sites in the target sequence. It is used to deliver precise miRNA target predictions, including target mimics, with FASTA and RNAhybrid search options [47]. The default parameters were chosen (Table 1).
2.6. psRNATarget
The psRNATarget algorithm is a highly sensitive, newly designed web-based tool developed for plant miRNA prediction. The target binding sites of plant miRNAs are predicted based on complementary scoring schema. The algorithm predicts the inhibition pattern of cleavage [48,49]. The default parameters were chosen (Table 1).
2.7. RNAhybrid
The RNAhybrid is a seed-based scanning algorithm based on intermolecular hybridization used to predict effective binding sites of miRNAs in the target sequence. It predicts target binding sites in a very easy and flexible manner [50]. It is an online available tool. It is used for the rapid prediction of miRNA targets based on the MFE hybridization of mRNAs and miRNAs. The default parameters were chosen (Table 1).
2.8. RNAfold
The RNAfold algorithm is available on a web server implemented in the ViennaRNA package [51].
2.9. Statistical Analysis
The miRNA–mRNA target prediction biological data were further processed. Graphical representations of the miRNA data were prepared using the R language [52].
3. Results
3.1. Prediction and Analyysis of Sugarcane MicroRNAs Targeting SCMV Genome
An integrative computational approach for identifying the possible interactions of high-confidence target sites of mature sugarcane miRNAs located in the SCMV positive-sense single-stranded (+ssRNA) genome from among the 28 sugarcane miRNAs (sof-miRNAs/ssp-miRNAs) revealed sof-miRNA/ssp-miRNA-derived MIR genes at a high proportion of sugarcane miRNA gene loci [33,53,54,55,56]. The predicted SCMV +ssRNA-encoded mRNA sequences were localized hypothetically by the sugarcane locus-derived sof-miRNA/ssp-miRNAs based on the miRanda algorithm predicted 19 miRNA-mRNA target pairs and RNA22: 15 sugarcane sof-miRNAs/ssp-miRNAs and 20 binding sites i. The TAPIR identified seven binding sites of mature sugarcane locus-derived sof-miRNA/ssp-miRNA target pairs. In total, 16 sugarcane miRNAs targeting 30 cleavable attachments sites in the SCMV genome were identified by the psRNATarget algorithm. RNAhybrid predicted 28 high-probability binding sites of sugarcane miRNAs in the SCMV genomic RNA sequence (Figure 1 and Figure 2, File S1, Table S2).
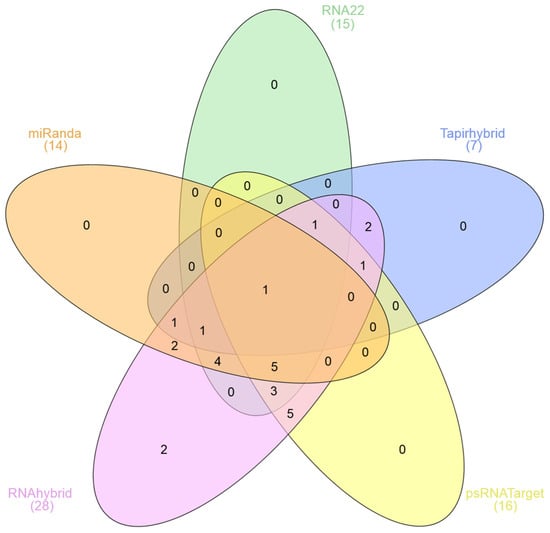
Figure 1.
Five-set Venn diagram representing mutually common binding sites of mature sugarcane miRNAs predicted to potentially target the SCMV genome. The in silico prediction was established using computational tools (miRanda, RNA22, TAPIR, psRNATarget, and RNAhybrid) to identify potential targets of sugarcane-encoded miRNAs. The areas of overlap among computational tools show miRNA binding sites. The high-order intersection of five algorithms revealed the most potent mature sugarcane miRNA―ssp-miR1444c-3p.
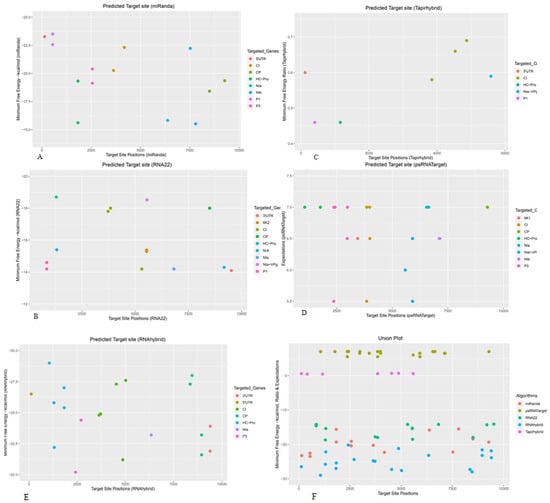
Figure 2.
Individual sugarcane sof-miRNAs/ssp-miRNAs and their predicted high-confidence binding sites in the SCMV genome were predicted based on the ‘five algorithms’ approach. (A) miRNA sites were detected by miRanda. (B) Several miRNA target sites were detected by RNA22. (C) TAPIR identified sugarcane miRNA binding sites. (D) psRNATarget predicted several binding sites of sugarcane miRNAs. (E) The prediction of miRNA sites by RNAhybrid. (F) Union plot representing all predicted binding sites detected by all the algorithms used. Multiple copies of miRNA target binding sites are represented by colored dots. Targeted genes of SCMV are indicated by different colors.
3.2. Sugarcane miRNAs Targeting P1
The potyviral first protease protein P1 was encoded by P1 ORF (149–847) (698 bases). The miRanda and RNA22 algorithms predicted the bindings of two sof-miRNAs: sof-miR168 (a, b) at nucleotide positions 547 and 846, respectively, as shown in (Figure 2A,B). The sof-miRNA168a was targeted at nucleotide position 406, using the TAPIR algorithm (Figure 2C). No sof-miRNA/ssp-miRNA was predicted to target the P1 region, by the psRNATarget and RNAhybrid algorithms (Figure 2D,E) (File S1) (Tables S2 and S3).
3.3. Sugarcane miRNAs Targeting HC-Pro
The HC-Pro ORF (848–2227 nucleotides) encodes. The miRanda and RNA22 algorithms predicted the target site of sof-miR168 (a, b) at nucleotide position 1827 and sof-miR168a at nucleotide position 1296 (Figure 2A,B).
TAPIR predicted the attachment site of sof-miRNA159e at locus 1159 (Figure 2C). The psRNATarget algorithm detected the binding of ssp-miR444 (a, b, c-3p) at nt positions 1058 and 1763 (Figure 2D). The RNAhybrid algorithm predicted seven sof-miRNAs/ssp-miRNAs: sof-miR159c, sof-miR168 (a, b), ssp-miR444 (a, b, c-3p), and ssp-miR1432 at nucleotide coordinates 1830, 1296, 1818, 1057, and 1316, respectively (Figure 2E, File S1, Tables S2 and S3).
3.4. Sugarcane miRNAs Targeting P3
The P3 ORF (2228–3268 nt) encodes a membrane-associated P3 protein which is required for SCMV genomic RNA replication, potential cell-to-cell spread (movement and transport) and is also responsible for determining host-range and symptoms [57,58,59]. The miRanda algorithm predicted the binding of sof-miRNAs: sof-miR168 (a, b) at nucleotide position 2562 (Figure 2A). No sof-miRNA/ssp-miRNA was predicted for targeting the P3 region by the RNA22 and TAPIR algorithms (Figure 2B,C). The potential target sites of sof-miR167 (a, b), sof-miR168a, ssp-miR437c, and ssp-miR444 (a, b, c-3p) at nucleotide positions 2427, 2971, 2981, and 2367, respectively, were detected by the psRNATarget algorithm (Figure 2D). In addition, RNAhybrid identified sugarcane sof-miRNAs/ssp-miRNAs; sof-miR167 (a, b) and ssp-miR437b at nucleotide positions 2699 and 2416, respectively (Figure 2E, File S1, Tables S2 and S3).
3.5. Sugarcane miRNAs Targeting 6K1
The 6K1 ORF (3269–3469 nucleotides) encode a 6K1 protein that functions in viral genome replication. It mediates cell-to-cell movement, controlling defense mechanism and gene regulation. It is a key component of the 6K2-induced viral replication complex (VRC) and regulation [60,61]. The 6K1 had the least number of predicted sugarcane sof-miRNAs/ssp-miRNAs. The ssp-miR444c-3p was predicted to optimally target 6K1 at nucleotide position 3441, according to the psRNATarget algorithm (Figure 2D, File S1, Tables S2 and S3).
3.6. Sugarcane miRNAs Targeting CI
The CI ORF (3470–5383 nt) encodes a multifunctional cylindrical inclusion protein (CI) essential for ATP binding and RNA helicase activity [62,63,64]. CI was targeted by two miRNAs: sof-miR396 and ssp-miR166 at nt positions 3634 and 4178, respectively, as indicated by the miRanda algorithm (Figure 2A). The RNA22 algorithm predicted two miRNAs: sof-miR159c and ssp-miR444b at nt positions 3730 and 5311, respectively, (Figure 2B). In addition, TAPIR predicted three sugarcane miRNAs: sof-miR159c, ssp-miR437a, and ssp-miR1128 at nucleotide positions 3847, 4869, and 4534, respectively (Figure 2C). The psRNATarget algorithm identified seven miRNAs: sof-miR159 (a, b, c, d, e), ssp-miR444b, and ssp-miR1432 at nt positions 3847, 3992, and 3980, respectively (Figure 2D). Five miRNA-binding sites were detected by RNAhybrid: sof-miR396 (start site 5016), sof-miR408e (3633), ssp-miR166 (3714), ssp-miR437a (4868), and ssp-miR1128 (4533) (Figure 2E, File S1, Tables S2 and S3).
3.7. Sugarcane miRNAs Targeting 6K2
Potyvirus 6K2 (5384–5542 nt) encodes the multifunctional protein 6K2, induces the formation of RE-derived complexes, and develops resistance to drought [65,66]. The RNA22 algorithm identified five sugarcane sof-miRNAs: sof-miR408 (a, b, c, d, and e) at locus position 5538 (Figure 2B, File S1, Tables S2 and S3).
3.8. Sugarcane miRNAs Targeting NIa-VPg
Potyvirus NIa-VPg ORF (5543–6109 nt) encodes a viral genome-linked protein (VPg) that functions as a virulence determinant and genome translator [67,68,69,70,71]. It is also involved in replication, translation, and movement [72,73,74]. The RNA22 and TAPIR algorithms predicted the binding of ssp-miR444c-3p at locus position 5552 (Figure 2B,C). The psRNATarget algorithm predicted six miRNAs: sof-miR156, sof-miR159 (a, b, c, d), and ssp-miR444c-3p (Figure 2D). No sugarcane sof-miRNA/ssp-miRNA was predicted to target the NIa-VPg region using the RNAhybrid algorithm (Figure 2E, File S1, Tables S2 and S3).
3.9. Sugarcane miRNAs Targeting NIa
Potyvirus NIa ORF (6110–6835 nt) encodes nuclear inclusion protein a (NIa), which is involved in RNA binding and also interacts with NIb [75,76]. miRanda, RNA22, and RNAhybrid predicted the binding of only one sugarcane miRNA: ssp-miR528, sof-miR396, and ssp-miR827 at nucleotide positions 6376, 6821, and 6338, respectively (Figure 2A,B,E). The psRNATarget algorithm identified three sugarcane miRNAs: sof-miR408e and ssp-miR444 (a, b) at nucleotide positions 6544 and 6641, respectively (Figure 2D). No miRNA target pair was identified to target NIa by the TAPIR algorithm(Figure 2C, File S1, Tables S2 and S3).
3.10. Sugarcane miRNAs Targeting NIb
Potyvirus NIb ORF (6836–8398) encodes nuclear inclusion protein b (NIb), which is involved in translocation activity and also interacts with NIa [77]. It contains nuclear signals and is also referred to as RdRp [78]. The miRanda algorithm detected the binding of two sugarcane ssp-miRNAs: ssp-miR169 and ssp-miR1432 at nucleotide positions 7798 and 7523, respectively (Figure 2A). The psRNATarget algorithm predicted the binding of two sugarcane ssp-miRNAs: sof-miR396 and ssp-miR444b at nucleotide positions 7798 and 7523, respectively (Figure 2D). No miRNA-target pair was identified based on the RNA22, TAPIR, and RNAhybrid algorithms (Figure 2B,C,E, File S1, Tables S2 and S3).
3.10.1. Sugarcane miRNAs Targeting CP
Potyvirus CP ORF (8399–9337) encodes a multitasking coat protein (CP), which is involved in the development of virion assembly. The CP is involved in all steps of the potyviral life cycle [79,80,81]. The miRanda algorithm predicted the binding of three sugarcane ssp-miRNAs (ssp-miR444 (a, b, c-3p) start site 8501). ssp-miR444c-3p also targeted the CP region at nucleotide position 9268 (Figure 2A). The RNA22 algorithm predicted the binding of the ssp-miRNA444 family at nt positions 8502 and 9181 (Figure 2B). The psRNATarget algorithm predicted the binding of ssp-miR444c-3p at nt position 9282 (Figure 2D). Potential binding sites of sugarcane miRNAs, sof-miR159 (a, b, d, e), sof-miR408 (a, b, c, d), and ssp-miR169, were detected by the RNAhybrid algorithm at nucleotide positions 8953, 8355, and 8458, respectively (Figure 2E, File S1, Tables S2 and S3).
3.10.2. Sugarcane miRNAs Targeting UTR
The potyvirus 5′ untranslated region (5′ UTR) (1–148 nt) and 3′ UTR (9341–9575 nt) are involved in the replication and translational activities of the ORFs [82,83]. The sof-miR408 (a, b, c, d) was predicted to target the 5′ UTR at nt positions 139 by miRanda (Figure 2A). Similarly, ssp-miR528 was identified to target the 5′ UTR at nt position 122 by TAPIR and RNAhybrid (Figure 2C,E). RNA22 predicted the binding of sof-miR168 (a, b) at nt position 9520 in the 3′ UTR (Figure 2B). RNAhybrid predicted the binding of two sugarcane miRNAs in the 3′UTR: sof-miR156 and ssp-miR437c at nt positions 9402 and 9395, respectively (Figure 2E, File S1, Tables S2 and S3).
3.11. Identification of Consensual Sugarcane MicroRNAs
The present study was concluded based on the consensus of the genomic target binding sites of sugarcane miRNAs determined by different algorithms. Among them, we selected nine sugarcane miRNAs (sof-miR159c, sof-miR168a, ssp-miR437a, ssp-miR528, ssp-miR444 (a, b), ssp-miR444c-3p), (ssp-miR1128, and ssp-miR1432), which were based on the consensus genomic positions 3847 (target gene CI), 1296 (HC-Pro), 4869 (CI), 122 (5′ UTR), 8502/1058 (CP/HC-Pro), 5583 (NIa-VPg), 4534 (CI), and 1316 (HC-Pro) detected (Table 2 and Table 3). Of the nine consensus locus-derived sof-miRNAs/ssp-miRNAs in the sugarcane genome investigated in this study, only one sof-miRNA (sof-miR159c at nt position 3847 targeting CI) was identified by the union of genomic consensus positions by at least three algorithms (RNA22, TAPIR, and psRNATarget) (Figure 3, Table 2 and Table 3, File S1, Tables S2 and S3).

Table 2.
Predicted high-confidence binding sites of consensus sugarcane miRNAs targeting the SCMV genome detected by different computational algorithms.

Table 3.
Predicted consensus sugarcane-encoded miRNA target sites localized in the different target genes of SCMV-SO.
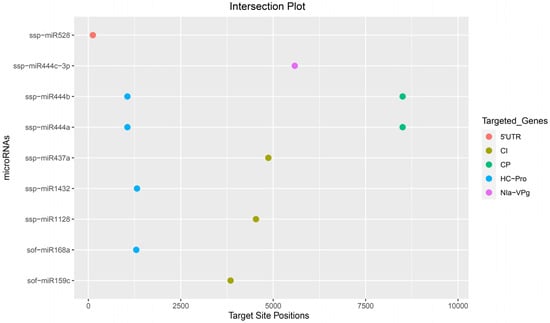
Figure 3.
Intersection plot shows the consensus high-confidence binding sites of mature sugarcane miRNAs predicted by at least two computational tools. The colored dots represent sugarcane miRNA binding sites targeting different genes of SCMV.
3.12. Identification of the miRNA–mRNA Regulatory Network
A Circos plot represents the predicted host–virus interactions of sugarcane miRNAs and SCMV target genes. A Circos plot was generated to visualize a comprehensive master miRNA regulatory network with novel antiviral targets (Figure 4). The generation of the miRNA–mRNA regulatory network was conducted using ‘Circos’ software [84].
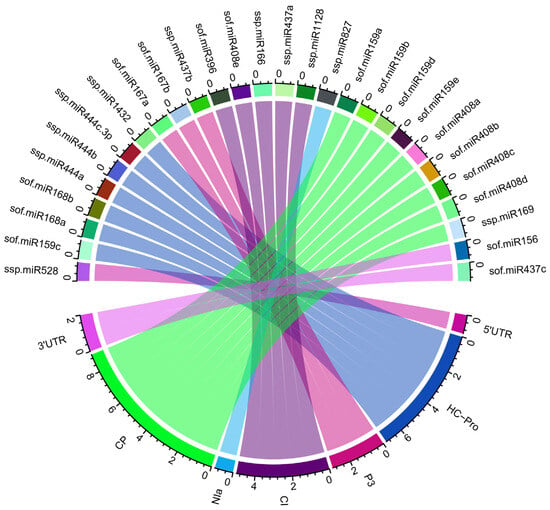
Figure 4.
Integrated Circos plot shows multiple targets of sugarcane-encoded miRNAs. The colored connection lines are targeted genes (ORFs) in the SCMV genome. Construction, exploration, target predictions, and interactions between the sugarcane miRNAs and SCMV genes are mapped.
3.13. RNA Secondary Structures
The computationally predicted locus-derived mature miRNAs in the sugarcane genome were analyzed by generating their secondary structures using the original precursor sequences. Pre-miRNA hairpin sequences were used for manual curation. The main parameters of the predicted stable secondary structures were evaluated (Table 4). The stable secondary structures of the potential consensus sugarcane precursor sequences were predicted by the RNAfold algorithm [51].

Table 4.
Features of the predicted precursors of sugarcane were determined.
4. Discussion
The SCMV is a monopartite potyvirus suspected as an etiological agent that has spread to Pakistan and China due to its high transmissibility and has become an increasingly potential long-lasting threat to sugarcane and maize production in the last two decades [13,17,85]. In our previous studies, we have investigated experimentally validated mature locus-derived microRNAs in the sugarcane genome, which were predicted to be targets of SCBGAV, SCYLV, and SCBV based on in silico criteria [37,38,39]. Several studies have identified complex host–virus interactions and have investigated miRNAs targeting plant viruses using an in silico approach [86,87,88,89,90,91,92]. miRNAs have emerged as novel endogenous targets for multiple levels of miRNA gene-level regulation [53,93,94]. Several studies have shown that the efficacy of amiRNA-based RNA interference leads to specific gene silencing in transgenic crops to reduce host plant virus infection [27,28,95,96,97]. In this computational research, mature sugarcane sof-miRNAs/ssp-miRNAs were aligned with the genomic sequence of the SCMV target to identify miRNA–mRNA binding sites hypothesized to understand complex host–virus specific interactions with the P1, HC-Pro, P3, 6K1, CI, 6K2, NIa-VPg, NIa-Pro, NIb, and CP of SCMV. The P1 is the least-conserved hypervariable that modulates host responses and is essential for the replication of the viral +ssRNA genome [98,99]. Host adaptation is a key process for virus genome evolution [100,101]. P1 is also related to virus–host adaptation [102]. The HC-Pro is a multifunctional, non-structural dimeric helper component proteinase. It has been reported as a viral suppressor. HC-Pro is required to enhance expression via the fusion of P1, symptom development, and viral replication [103,104,105,106,107,108]. Until now, the potential for exploiting the regulation of sugarcane genome-encoded miRNA to abate infection by SCMV has not been investigated as a strategy for developing tolerant or resistant sugarcane cultivars. The results of this study provide the first computationally based evaluation of mature locus-derived miRNAs in the sugarcane plant genome to enable the prediction of effective miRNA binding sites and provide new tools for better understanding the molecular and omic interactions between sugarcane plant host cells and SCMV-encoded mRNAs/protein.
Based on our findings, the SCMV genome (HC-Pro, CI, NIa-VPg, and CP) is susceptible to nine consensus sugarcane miRNAs. We found that nine miRNAs could theoretically originate from the sugarcane genome (Table 2 and Table 3). In silico tools, RNA22, TAPIR, and psRNATarget, identified a genomic consensus base pair complementarity in sof-miR159c at nucleotide position 3847 (Figure 2 and Table 2). The ssp-miR444c-3p was predicted by all five algorithms, making it the only unique sugarcane miRNA identified in this study (Figure 1 and Table S2). We identified the maximum folding energy of the consensus functional miRNA–mRNA target pair, which is −18.00 Kcal/mol, using RNA22. RNA22 is a highly sensitive algorithm that uses a pattern-based approach to target miRNAs. Using the psRNATarget algorithm, we estimated an expectation score of 5.50 for a consensus target pair (Table 2) [109]. The RNA22 and psRNATarget algorithms predicted target sites using a non-seed-based approach. Experimentally determining miRNA–mRNA interactions can be expensive and time-consuming; making the accurate computational prediction of miRNA targets a high priority. The limitations and bottlenecks of existing algorithms and approaches are interpreted using the union and intersection level of the predictions in this study. The miRNA targeting relies on the base pairing of miRNA–mRNA targets [110]. These results suggest that the predicted consensus miRNA–mRNA duplex represents a ‘true target’. Our results indicate that sugarcane miRNAs likely play a role in the interaction between host and virus. Our results highlight the interaction of SCMV ss-RNA with the sugarcane miRNA target interaction network.
Potyvirus cylindrical inclusion helicase (CI) is required for the initiation of the viral replication mechanism, cell-to-cell movement, and plant–host, protein–virus interactions [62,63,111]. Computational predictions and analyses revealed that the sugarcane consensus sof-miR159c is a high-confidence target site potentially targeting the CI ORF (Table 3). The conserved precursor MIR159 is considered to be controlled by plant growth and fertility [112]. In our previous study, the consensus sof-miR159e (Accession ID: MIMAT0001661), predicted to have an effective target binding site at nucleotide position 5535 in the SCBV genome, was identified as the most effective miRNA by the miRanda, RNA22, and RNAhybrid algorithms.
While miRNA–mRNA target pair interactions between locus-derived miRNAs in the sugarcane genome and SCMV have been determined, the development of amiRNA-based constructs and further transformations in sugarcane to control SCMV are not fully understood. We have performed a comprehensive analysis of SCMD-associated Potyvirus for the first time, which is a first step toward the development of miRNA-based antiviral therapy. An amiRNA construct relies on the high-level specificity of a nucleotide base pairing to control deleterious off-target effects. The small size of amiRNA is a unique feature for the development of a single gene expression vector to control multiple potyviruses in transgenic sugarcane. This approach offers specificity and sensitivity and complements existing molecular approaches for analyzing targets for SCMV disease abatement. A number of environmental concerns have been raised regarding the large-scale use of virus-resistant transgenic plants [113,114,115,116,117,118]. As amiRNAs have high specificity to the designed target gene, detrimental off-target effects can be minimized, permitting their silencing expression to be stably transmitted to future generations [119,120,121,122,123]. The results indicate that the use of in silico tools provides better results than a single algorithm when developing amiRNA-based mdm-miRNA therapeutics to target SCMV and other plant viruses as well. Despite the frequent use of RNAi in biology and agriculture, there are several drawbacks and challenges in designing efficient silencing constructs. Furthermore, the small size of amiRNA permits for the insertion of multiple and distinct amiRNAs within a single gene expression cassette, which can then be transformed to develop transgenic plant resistant to multiple viruses simultaneously [27,95,124]. The in silico analysis was designed for experimental validation to show whether these predicted miRNAs could make the plants resistant to SCMV. Future work will be focused on transiently expressing these miRNAs or injecting RNA hairpins in N. benthamiana to show its efficacy against SCMV.
5. Conclusions and Future Directions
The SCMV, which infects sugarcane crops worldwide, is the most damaging potyvirus pathogen associated with an ongoing SCMD epidemic that reduces yield in all sugarcane cultivars cultivated in China. This study involved in silico tools and approaches to characterize the target binding sites of mature sugarcane locus-derived miRNAs in the SCMV genome. Among the 28 sugarcane miRNAs from the miRBase database, only one, sof-miRNA (sof-miR159c), was identified as the most effective, naturally occurring sof-miRNA biomolecule for targeting the SCMV genome (nucleotide 3847 onward), based on the consensus of multiple algorithms used herein. This approach offers specificity and sensitivity and complements existing molecular approaches for analyzing targets for SCMV disease abatement. The current focus of attention is the development of SCMV-resistant sugarcane plants that abate the effects of SCMD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres15010019/s1, Table S1: Mature sugarcane microRNA sequences used for the prediction of binding sites in the SCMV genome; Table S2: Identification of high-confidence binding sites of sugarcane miRNAs in the SCMV; Table S3: Gene-wise prediction; File S1: Prediction results of computational tools.
Author Contributions
M.A.A., W.W. and S.Z. conceived the study. All authors analyzed the computational data. M.A.A., H.G., Z.I., W.u.Z., H.M. and M.Z. made the graphs. M.A.A., W.W. and H.G. wrote the original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFD2301100), Central Public-Interest Scientific Institution Basal Research Fund (1630052023003) and Earmarked fund for Chinese Agriculture Research System (CARS-170301).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are available in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carvalho-Netto, O.V.; Bressiani, J.A.; Soriano, H.L.; Fiori, C.S.; Santos, J.M.; Barbosa, G.V.; Xavier, M.A.; Landell, M.G.; Pereira, G.A. The potential of the energy cane as the main biomass crop for the cellulosic industry. Chem. Biol. Technol. Agric. 2014, 1, 20. [Google Scholar] [CrossRef]
- Ahmed, A.; Dompreh, E.; Gasparatos, A. Human wellbeing outcomes of involvement in industrial crop production: Evidence from sugarcane, oil palm and jatropha sites in Ghana. PLoS ONE 2019, 14, e0215433. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F. Measuring convergence in the sugarcane industry in China’s Guangxi province. PLoS ONE 2021, 16, e0244617. [Google Scholar] [CrossRef] [PubMed]
- Piperidis, G.; Piperidis, N.; D’Hont, A. Molecular cytogenetic investigation of chromosome composition and transmission in sugarcane. Mol. Genet. Genom. 2010, 284, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, P.; Li, X.; Huang, Y.; Wang, Q.; Luo, L.; Jing, Y.; Liu, X.; Deng, Z.; Wu, J.; et al. Characterization of chromosome composition of sugarcane in nobilization by using genomic in situ hybridization. Mol. Cytogenet. 2018, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Acevedo, R.; de la Espina, S.M.D.; Jouve, N.; De La Torre, C. Genome remodelling in three modern S. officinarum× S. spontaneum sugarcane cultivars. J. Exp. Bot. 2004, 55, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Tang, H.; Zhang, Q.; Hua, X.; Ma, X.; Zhu, F.; Jones, T.; Zhu, X.; Bowers, J.; et al. Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneum L. Nat. Genet. 2018, 50, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Shearman, J.R.; Pootakham, W.; Sonthirod, C.; Naktang, C.; Yoocha, T.; Sangsrakru, D.; Jomchai, N.; Tongsima, S.; Piriyapongsa, J.; Ngamphiw, C.; et al. A draft chromosome-scale genome assembly of a commercial sugarcane. Sci. Rep. 2022, 12, 20474. [Google Scholar] [CrossRef]
- Yan, H.; Zhou, H.; Luo, H.; Fan, Y.; Zhou, Z.; Chen, R.; Luo, T.; Li, X.; Liu, X.; Li, Y.; et al. Characterization of full-length transcriptome in Saccharum officinarum and molecular insights into tiller development. BMC Plant Biol. 2021, 21, 228. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, R.; Han, S.; Li, Z.; Xiao, J.; Li, Y.; Wang, L.; Li, S. Transcriptome analysis of sugarcane young leaves and protoplasts after enzymatic digestion. Life 2022, 12, 1210. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Y.; Yao, J.; Yin, Z.; Wang, X.; Xu, L.; Que, Y.; Mo, P.; Liu, X. Characterization and Phylogenetic Analyses of the Complete Mitochondrial Genome of Sugarcane (Saccharum spp. Hybrids) Line A1. Diversity 2022, 14, 333. [Google Scholar] [CrossRef]
- Lu, G.; Wang, Z.; Xu, F.; Pan, Y.-B.; Grisham, M.P.; Xu, L. Sugarcane mosaic disease: Characteristics, identification and control. Microorganisms 2021, 9, 1984. [Google Scholar] [CrossRef] [PubMed]
- He, E.-Q.; Bao, W.-Q.; Sun, S.-R.; Hu, C.-Y.; Chen, J.-S.; Bi, Z.-W.; Xie, Y.; Lu, J.-J.; Gao, S.-J. Incidence and Distribution of Four Viruses Causing Diverse Mosaic Diseases of Sugarcane in China. Agronomy 2022, 12, 302. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Gautam, S.; Rasmussen, D.A.; Srinivasan, R. Aphid transmission of Potyvirus: The largest plant-infecting RNA virus genus. Viruses 2020, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Diniz, A.L.; Ferreira, S.S.; Ten-Caten, F.; Margarido, G.R.; Dos Santos, J.M.; Barbosa, G.V.d.S.; Carneiro, M.S.; Souza, G.M. Genomic resources for energy cane breeding in the post genomics era. Comput. Struct. Biotechnol. J. 2019, 17, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yan, Z.; Zhu, T.; Xu, X.; Li, X.-D.; Tian, Y. The complete genomic sequence of Sugarcane mosaic virus from Canna spp. in China. Virol. J. 2018, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, K.; Herath, V.; Ahmed, K.; Tahir, M.; Verchot, J. Genetic diversity and molecular evolution of sugarcane mosaic virus, comparing whole genome and coat protein sequence phylogenies. Arch. Virol. 2022, 167, 2239–2247. [Google Scholar] [CrossRef]
- He, Z.; Dong, Z.; Gan, H. Genetic changes and host adaptability in sugarcane mosaic virus based on complete genome sequences. Mol. Phylogenetics Evol. 2020, 149, 106848. [Google Scholar] [CrossRef]
- Xie, X.; Chen, W.; Fu, Q.; Zhang, P.; An, T.; Cui, A.; An, D. Molecular variability and distribution of Sugarcane mosaic virus in Shanxi, China. PLoS ONE 2016, 11, e0151549. [Google Scholar] [CrossRef]
- Finnegan, E.J.; Matzke, M.A. The small RNA world. J. Cell Sci. 2003, 116, 4689–4693. [Google Scholar] [CrossRef]
- Millar, A.A. The function of miRNAs in plants. Plants 2020, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Islam, W.; Waheed, A.; Idrees, A.; Rashid, J.; Zeng, F. Role of plant microRNAs and their corresponding pathways in fluctuating light conditions. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2022, 1870, 119304. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Chen, M.; Xiang, M.; Guo, Z. RNAi-based antiviral innate immunity in plants. Viruses 2022, 14, 432. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.W.; Falk, B.W. Artificial microRNA guide strand selection from duplexes with no mismatches shows a purine-rich preference for virus-and non-virus-based expression vectors in plants. Plant Biotechnol. J. 2022, 20, 1069. [Google Scholar] [CrossRef] [PubMed]
- Al-Roshdi, M.R.; Ammara, U.; Khan, J.; Al-Sadi, A.M.; Shahid, M.S. Artificial microRNA-mediated resistance against Oman strain of tomato yellow leaf curl virus. Front. Plant Sci. 2023, 14, 1164921. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Prasad, M. Silencing AC1 of Tomato leaf curl virus using artificial microRNA confers resistance to leaf curl disease in transgenic tomato. Plant Cell Rep. 2020, 39, 1565–1579. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Liang, C.; Li, J.; Baker, B.; Luo, L. Polycistronic artificial microRNA-mediated resistance to cucumber green mottle mosaic virus in cucumber. Int. J. Mol. Sci. 2021, 22, 12237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yuan, Q.; Ai, X.; Chen, J.; Lu, Y.; Yan, F. Transgenic Rice Plants Expressing Artificial miRNA Targeting the Rice Stripe Virus MP Gene Are Highly Resistant to the Virus. Biology 2022, 11, 332. [Google Scholar] [CrossRef]
- Ali, I.; Amin, I.; Briddon, R.W.; Mansoor, S. Artificial microRNA-mediated resistance against the monopartite begomovirus Cotton leaf curl Burewala virus. Virol. J. 2013, 10, 231. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Su, Y.; Zou, J.; Wang, Z.; Xu, L.; Que, Y. miRNA alteration is an important mechanism in sugarcane response to low-temperature environment. BMC Genom. 2017, 18, 833. [Google Scholar] [CrossRef]
- Khan, M.S.; Khraiwesh, B.; Pugalenthi, G.; Gupta, R.S.; Singh, J.; Duttamajumder, S.K.; Kapur, R. Subtractive hybridization-mediated analysis of genes and in silico prediction of associated microRNAs under waterlogged conditions in sugarcane (Saccharum spp.). FEBS Open Bio 2014, 4, 533–541. [Google Scholar] [CrossRef]
- Ferreira, T.H.; Gentile, A.; Vilela, R.D.; Costa, G.G.L.; Dias, L.I.; Endres, L.; Menossi, M. microRNAs associated with drought response in the bioenergy crop sugarcane (Saccharum spp.). PLoS ONE 2012, 7, e46703. [Google Scholar] [CrossRef] [PubMed]
- Swapna, M.; Kumar, S. MicroRNAs and their regulatory role in sugarcane. Front. Plant Sci. 2017, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.R.; Kumar, R.; Chinnaswamy, A.; Karuppaiyan, R.; Kulshreshtha, N.; Ram, B. Current breeding and genomic approaches to enhance the cane and sugar productivity under abiotic stress conditions. 3 Biotech 2020, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.d.O.L.; Silva, R.G.d.; Nogueira, L.d.F.; Zingaretti, S.M. MicroRNAs regulate tolerance mechanisms in sugarcane (Saccharum spp.) under aluminum stress. Crop Breed. Appl. Biotechnol. 2021, 21, e34442115. [Google Scholar] [CrossRef]
- Wang, M.; Li, A.M.; Liao, F.; Qin, C.X.; Chen, Z.L.; Zhou, L.; Li, Y.R.; Li, X.F.; Lakshmanan, P.; Huang, D.L. Control of sucrose accumulation in sugarcane (Saccharum spp. hybrids) involves miRNA-mediated regulation of genes and transcription factors associated with sugar metabolism. GCB Bioenergy 2022, 14, 173–191. [Google Scholar] [CrossRef]
- Ashraf, F.; Ashraf, M.A.; Hu, X.; Zhang, S. A novel computational approach to the silencing of Sugarcane Bacilliform Guadeloupe A Virus determines potential host-derived MicroRNAs in sugarcane (Saccharum officinarum L.). PeerJ 2020, 8, e8359. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Ashraf, F.; Feng, X.; Hu, X.; Shen, L.; Khan, J.; Zhang, S. Potential targets for evaluation of sugarcane yellow leaf virus resistance in sugarcane cultivars: In silico sugarcane miRNA and target network prediction. Biotechnol. Biotechnol. Equip. 2021, 35, 1980–1991. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Feng, X.; Hu, X.; Ashraf, F.; Shen, L.; Iqbal, M.S.; Zhang, S. In silico identification of sugarcane (Saccharum officinarum L.) genome encoded microRNAs targeting sugarcane bacilliform virus. PLoS ONE 2022, 17, e0261807. [Google Scholar] [CrossRef]
- Zhang, N.; Feng, X.; Zeng, Q.; Lin, H.; Wu, Z.; Gao, X.; Huang, Y.; Wu, J.; Qi, Y. Integrated analysis of miRNAs associated with sugarcane responses to low-potassium stress. Front. Plant Sci. 2022, 12, 3050. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Bourexis, D.; Brister, J.R.; Canese, K.; Comeau, D.C.; Funk, K.; Kim, S.; Klimke, W.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2021, 49, D10. [Google Scholar] [CrossRef] [PubMed]
- Enright, A.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D. MicroRNA targets in Drosophila. Genome Biol. 2003, 4, R1. [Google Scholar] [CrossRef]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. Human microRNA targets. PLoS Biol. 2004, 2, e363. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.C.; Huynh, T.; Tay, Y.; Ang, Y.-S.; Tam, W.-L.; Thomson, A.M.; Lim, B.; Rigoutsos, I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell 2006, 126, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Loher, P.; Rigoutsos, I. Interactive exploration of RNA22 microRNA target predictions. Bioinformatics 2012, 28, 3322–3323. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, E.; He, Y.; Billiau, K.; Van de Peer, Y. TAPIR, a web server for the prediction of plant microRNA targets, including target mimics. Bioinformatics 2010, 26, 1566–1568. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server. Nucleic Acids Res. 2011, 39, W155–W159. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Krüger, J.; Rehmsmeier, M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006, 34, W451–W454. [Google Scholar] [CrossRef]
- Lorenz, R.; Bernhart, S.; Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P.; Hofacker, I. ViennaRNA package 2.0. Algorithms Mol. Biol. 2013, 6, 26. [Google Scholar] [CrossRef]
- Gandrud, C. Reproducible Research with R and RStudio; Chapman and Hall/CRC: Boca Raton, FL, USA, 2018. [Google Scholar]
- Bajczyk, M.; Jarmolowski, A.; Jozwiak, M.; Pacak, A.; Pietrykowska, H.; Sierocka, I.; Swida-Barteczka, A.; Szewc, L.; Szweykowska-Kulinska, Z. Recent Insights into Plant miRNA Biogenesis: Multiple Layers of miRNA Level Regulation. Plants 2023, 12, 342. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jia, T.; Chen, X. The ‘how’and ‘where’of plant micro RNA s. New Phytol. 2017, 216, 1002–1017. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Hu, B.; Zhang, C. microRNAs and their roles in plant development. Front. Plant Sci. 2022, 13, 824240. [Google Scholar] [CrossRef] [PubMed]
- Gramzow, L.; Lobbes, D.; Innard, N.; Theißen, G. Independent origin of MIRNA genes controlling homologous target genes by partial inverted duplication of antisense-transcribed sequences. Plant J. 2020, 101, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Shine, M.; Cui, X.; Chen, X.; Ma, N.; Kachroo, P.; Zhi, H.; Kachroo, A. The potyviral P3 protein targets eukaryotic elongation factor 1A to promote the unfolded protein response and viral pathogenesis. Plant Physiol. 2016, 172, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Jenner, C.E.; Wang, X.; Tomimura, K.; Ohshima, K.; Ponz, F.; Walsh, J.A. The dual role of the potyvirus P3 protein of Turnip mosaic virus as a symptom and avirulence determinant in brassicas. Mol. Plant-Microbe Interact. 2003, 16, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Liao, W.; Niu, H.; Cui, X.; Chen, X.; Zhi, H. Comprehensive analysis of soybean mosaic virus P3 protein interactors and hypersensitive response-like lesion-inducing protein function. Int. J. Mol. Sci. 2019, 20, 3388. [Google Scholar] [CrossRef]
- Bera, S.; Arena, G.D.; Ray, S.; Flannigan, S.; Casteel, C.L. The Potyviral Protein 6K1 Reduces Plant Proteases Activity during Turnip mosaic virus Infection. Viruses 2022, 14, 1341. [Google Scholar] [CrossRef]
- Cui, H.; Wang, A. Plum pox virus 6K1 protein is required for viral replication and targets the viral replication complex at the early stage of infection. J. Virol. 2016, 90, 5119–5131. [Google Scholar] [CrossRef]
- Deng, P.; Wu, Z.; Wang, A. The multifunctional protein CI of potyviruses plays interlinked and distinct roles in viral genome replication and intercellular movement. Virol. J. 2015, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Sorel, M.; García, J.A.; German-Retana, S. The Potyviridae cylindrical inclusion helicase: A key multipartner and multifunctional protein. Mol. Plant-Microbe Interact. 2014, 27, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, C.; Hong, J.; Xiong, R.; Kasschau, K.D.; Zhou, X.; Carrington, J.C.; Wang, A. Formation of complexes at plasmodesmata for potyvirus intercellular movement is mediated by the viral protein P3N-PIPO. PLoS Pathog. 2010, 6, e1000962. [Google Scholar] [CrossRef] [PubMed]
- Lõhmus, A.; Varjosalo, M.; Mäkinen, K. Protein composition of 6K2-induced membrane structures formed during Potato virus A infection. Mol. Plant Pathol. 2016, 17, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Nihranz, C.T.; Casteel, C.L. The potyviral protein 6K2 from Turnip mosaic virus increases plant resilience to drought. Mol. Plant-Microbe Interact. 2023, 36, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dong, M.; Cheng, G.; Liu, S.; Zhang, H.; Shang, H.; Zhou, Y.; Huang, G.; Zhang, M.; Wang, F.; et al. Selective interaction of sugarcane EIF4E with VPGS from sugarcane mosaic pathogens. Viruses 2021, 13, 518. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, I. The genome-linked protein VPg of vertebrate viruses—A multifaceted protein. Curr. Opin. Virol. 2011, 1, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, S.; Chatel, H.; Fortin, M.G.; Laliberté, J.-F. Interaction of the viral protein genome linked of turnip mosaic potyvirus with the translational Eukaryotic Initiation Factor (iso) 4E ofArabidopsis thalianaUsing the Yeast two-hybrid system. Virology 1997, 234, 84–92. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.C.; Volpon, L.; Rahardjo, A.K.; Osborne, M.J.; Culjkovic-Kraljacic, B.; Trahan, C.; Oeffinger, M.; Kwok, B.H.; Borden, K.L. Structural studies of the eIF4E–VPg complex reveal a direct competition for capped RNA: Implications for translation. Proc. Natl. Acad. Sci. USA 2019, 116, 24056–24065. [Google Scholar] [CrossRef]
- Płochocka, D.; Wełnicki, M.; Zielenkiewicz, P.; Ostoja-Zagorski, W. Three-dimensional model of the potyviral genome-linked protein. Proc. Natl. Acad. Sci. USA 1996, 93, 12150–12154. [Google Scholar] [CrossRef]
- Puustinen, P.; Makinen, K. Uridylylation of the potyvirus VPg by viral replicase NIb correlates with the nucleotide binding capacity of VPg. J. Biol. Chem. 2004, 279, 38103–38110. [Google Scholar] [CrossRef] [PubMed]
- Eskelin, K.; Hafrén, A.; Rantalainen, K.I.; Mäkinen, K. Potyviral VPg enhances viral RNA translation and inhibits reporter mRNA translation in planta. J. Virol. 2011, 85, 9210–9221. [Google Scholar] [CrossRef] [PubMed]
- Schaad, M.C.; Lellis, A.D.; Carrington, J.C. VPg of tobacco etch potyvirus is a host genotype-specific determinant for long-distance movement. J. Virol. 1997, 71, 8624–8631. [Google Scholar] [CrossRef] [PubMed]
- Daròs, J.-A.; Carrington, J.C. RNA binding activity of NIa proteinase of tobacco etch potyvirus. Virology 1997, 237, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.; Rodrigo, G.; Aragonés, V.; Ruiz, M.; Lodewijk, I.; Fernández, U.; Elena, S.F.; Daròs, J.-A. Interaction network of tobacco etch potyvirus NIa protein with the host proteome during infection. BMC Genom. 2016, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Valdez, P.; Olvera, R.E.; Carrington, J.C. Functions of the tobacco etch virus RNA polymerase (NIb): Subcellular transport and protein-protein interaction with VPg/proteinase (NIa). J. Virol. 1997, 71, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.; Eskelin, K.; Lohmus, A.; Mäkinen, K. Molecular and cellular mechanisms underlying potyvirus infection. J. Gen. Virol. 2014, 95, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Lõhmus, A.; Dutta, P.; Pollari, M.; Mäkinen, K. Interplay of HCPro and CP in the Regulation of Potato Virus A RNA Expression and Encapsidation. Viruses 2022, 14, 1233. [Google Scholar] [CrossRef] [PubMed]
- Lindenau, S.; Winter, S.; Margaria, P. The amino-proximal region of the coat protein of cucumber vein yellowing virus (family Potyviridae) affects the infection process and whitefly transmission. Plants 2021, 10, 2771. [Google Scholar] [CrossRef]
- Martínez-Turiño, S.; García, J.A. Potyviral coat protein and genomic RNA: A striking partnership leading virion assembly and more. Adv. Virus Res. 2020, 108, 165–211. [Google Scholar]
- Zhang, J.; Roberts, R.; Rakotondrafara, A.M. The role of the 5′ untranslated regions of Potyviridae in translation. Virus Res. 2015, 206, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Zhang, J.; Mayberry, L.K.; Tatineni, S.; Browning, K.S.; Rakotondrafara, A.M. A unique 5′ translation element discovered in triticum mosaic virus. J. Virol. 2015, 89, 12427–12440. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, R.; Zhou, T.; Fan, Z. Genetic diversity and population structure of Sugarcane mosaic virus. Virus Res. 2013, 171, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Tariq, H.K.; Hu, X.-W.; Khan, J.; Zou, Z. Computational Biology and Machine Learning Approaches Identify Rubber Tree (Hevea brasiliensis Muell. Arg.) Genome Encoded MicroRNAs Targeting Rubber Tree Virus 1. Appl. Sci. 2022, 12, 12908. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Ali, B.; Brown, J.K.; Shahid, I.; Yu, N. In Silico Identification of Cassava Genome-Encoded MicroRNAs with Predicted Potential for Targeting the ICMV-Kerala Begomoviral Pathogen of Cassava. Viruses 2023, 15, 486. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.N.; Rashid, S.; Iqbal, M.S.; Jamal, A.; Khalid, S.; Shamim, Z. In Silico prediction of potential mirnas to target zymv in cucumis melo. Pak. J. Bot 2022, 54, 1319–1325. [Google Scholar]
- Jabbar, B.; Iqbal, M.S.; Batcho, A.A.; Nasir, I.A.; Rashid, B.; Husnain, T.; Henry, R.J. Target prediction of candidate miRNAs from Oryza sativa for silencing the RYMV genome. Comput. Biol. Chem. 2019, 83, 107127. [Google Scholar] [CrossRef]
- Akhter, Y.; Khan, J.A. Genome wide identification of cotton (Gossypium hirsutum)-encoded microRNA targets against Cotton leaf curl Burewala virus. Gene 2018, 638, 60–65. [Google Scholar]
- Iqbal, M.S.; Jabbar, B.; Sharif, M.N.; Ali, Q.; Husnain, T.; Nasir, I.A. In silico MCMV silencing concludes potential host-derived miRNAs in maize. Front. Plant Sci. 2017, 8, 372. [Google Scholar] [CrossRef]
- Gaafar, Y.Z.A.; Ziebell, H. Novel targets for engineering Physostegia chlorotic mottle and tomato brown rugose fruit virus-resistant tomatoes: In silico prediction of tomato microRNA targets. PeerJ 2020, 8, e10096. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Yang, Y.; Schmid, M.; Wang, Y. miRNA mediated regulation and interaction between plants and pathogens. Int. J. Mol. Sci. 2021, 22, 2913. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, A.A.; Tenkegna, T.A. The role of miRNA in plant–virus interaction: A review. Mol. Biol. Rep. 2021, 48, 2853–2861. [Google Scholar] [CrossRef] [PubMed]
- Petchthai, U.; Yee, C.S.L.; Wong, S.-M. Resistance to CymMV and ORSV in artificial microRNA transgenic Nicotiana benthamiana plants. Sci. Rep. 2018, 8, 9958. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wang, J.-J.; Zhao, J.-H.; Fang, Y.-Y.; He, X.-F.; Guo, H.-S.; Duan, C.-G. A Brassica miRNA regulates plant growth and immunity through distinct modes of action. Mol. Plant 2020, 13, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Motawaa, M.; Wang, Q.; Zhang, X.; Khalid, A.; Cai, X.; Li, F. Simple webserver-facilitated method to design and synthesize artificial miRNA gene and its application in engineering viral resistance. Plants 2022, 11, 2125. [Google Scholar] [CrossRef]
- Valli, A.; Lopez-Moya, J.J.; Garcia, J.A. Recombination and gene duplication in the evolutionary diversification of P1 proteins in the family Potyviridae. J. Gen. Virol. 2007, 88, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Pasin, F.; Simón-Mateo, C.; García, J.A. The hypervariable amino-terminus of P1 protease modulates potyviral replication and host defense responses. PLoS Pathog. 2014, 10, e1003985. [Google Scholar] [CrossRef]
- LaTourrette, K.; Garcia-Ruiz, H. Determinants of Virus Variation, Evolution, and Host Adaptation. Pathogens 2022, 11, 1039. [Google Scholar] [CrossRef]
- Elena, S.F. Local adaptation of plant viruses: Lessons from experimental evolution. Mol. Ecol. 2017, 26, 1711–1719. [Google Scholar] [CrossRef]
- Tatineni, S.; Qu, F.; Li, R.; Morris, T.J.; French, R. Triticum mosaic poacevirus enlists P1 rather than HC-Pro to suppress RNA silencing-mediated host defense. Virology 2012, 433, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Kasschau, K.D.; Carrington, J.C. Long-distance movement and replication maintenance functions correlate with silencing suppression activity of potyviral HC-Pro. Virology 2001, 285, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Atreya, C.; Atreya, P.; Thornbury, D.; Pirone, T. Site-directed mutations in the potyvirus HC-Pro gene affect helper component activity, virus accumulation, and symptom expression in infected tobacco plants. Virology 1992, 191, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Del Toro, F.; Makki, M.; Tenllado, F.; Canto, T. Adaptation of a Potyvirus Chimera Increases Its Virulence in a Compatible Host through Changes in HCPro. Plants 2022, 11, 2262. [Google Scholar] [CrossRef] [PubMed]
- Valli, A.A.; Gallo, A.; Rodamilans, B.; López-Moya, J.J.; García, J.A. The HCPro from the Potyviridae family: An enviable multitasking Helper Component that every virus would like to have. Mol. Plant Pathol. 2018, 19, 744–763. [Google Scholar] [CrossRef] [PubMed]
- Sanobar, N.; Lin, P.-C.; Pan, Z.-J.; Fang, R.-Y.; Tjita, V.; Chen, F.-F.; Wang, H.-C.; Tsai, H.-L.; Wu, S.-H.; Shen, T.-L.; et al. Investigating the viral suppressor HC-pro inhibiting small rna methylation through functional comparison of HEN1 in angiosperm and bryophyte. Viruses 2021, 13, 1837. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Pollari, M.; Varjosalo, M.; Mäkinen, K. Association of host protein VARICOSE with HCPro within a multiprotein complex is crucial for RNA silencing suppression, translation, encapsidation and systemic spread of potato virus A infection. PLoS Pathog. 2020, 16, e1008956. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.S.; Bahmanpour, Z.; Daneshmandpour, Y.; Roudbari, F.; Sheervalilou, R.; Kazeminasab, S.; Emamalizadeh, B. An updated overview and classification of bioinformatics tools for MicroRNA analysis, which one to choose? Comput. Biol. Med. 2021, 134, 104544. [Google Scholar] [CrossRef]
- Chipman, L.B.; Pasquinelli, A.E. miRNA targeting: Growing beyond the seed. Trends Genet. 2019, 35, 215–222. [Google Scholar] [CrossRef]
- Movahed, N.; Patarroyo, C.; Sun, J.; Vali, H.; Laliberté, J.-F.; Zheng, H. Cylindrical inclusion protein of Turnip mosaic virus serves as a docking point for the intercellular movement of viral replication vesicles. Plant Physiol. 2017, 175, 1732–1744. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Ding, G.; Jin, Y. Evolution of MIR159/319 microRNA genes and their post-transcriptional regulatory link to siRNA pathways. BMC Evol. Biol. 2011, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Tepfer, M. Risk assessment of virus-resistant transgenic plants. Annu. Rev. Phytopathol. 2002, 40, 467–491. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Nawaz, M.A.; Kouretas, D.; Balias, G.; Savolainen, K.; Tutelyan, V.A.; Golokhvast, K.S.; Lee, J.D.; Yang, S.H.; Chung, G. Environmental impacts of genetically modified plants: A review. Environ. Res. 2017, 156, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, J.; Dietz-Pfeilstetter, A.; Hartung, F.; Kohl, C.; Romeis, J.; Sprink, T. Risk assessment and regulation of plants modified by modern biotechniques: Current status and future challenges. Annu. Rev. Plant Biol. 2019, 70, 699–726. [Google Scholar] [CrossRef] [PubMed]
- Bauer-Panskus, A.; Miyazaki, J.; Kawall, K.; Then, C. Risk assessment of genetically engineered plants that can persist and propagate in the environment. Environ. Sci. Eur. 2020, 32, 32. [Google Scholar] [CrossRef]
- Hokanson, K.E.; Ellstrand, N.C.; Dixon, A.G.; Kulembeka, H.P.; Olsen, K.M.; Raybould, A. Risk assessment of gene flow from genetically engineered virus resistant cassava to wild relatives in Africa: An expert panel report. Transgenic Res. 2016, 25, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Tepfer, M.; Jacquemond, M.; García-Arenal, F. A critical evaluation of whether recombination in virus-resistant transgenic plants will lead to the emergence of novel viral diseases. New Phytol. 2015, 207, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Ossowski, S.; Schwab, R.; Weigel, D. Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J. 2008, 53, 674–690. [Google Scholar] [CrossRef]
- Teotia, S.; Wang, X.; Zhou, N.; Wang, M.; Liu, H.; Qin, J.; Han, D.; Li, C.; Li, C.E.; Pan, S.; et al. A high-efficiency gene silencing in plants using two-hit asymmetrical artificial MicroRNAs. Plant Biotechnol. J. 2023, 21, 1799–1811. [Google Scholar] [CrossRef]
- Carbonell, A.; Fahlgren, N.; Mitchell, S.; Cox, K.L., Jr.; Reilly, K.C.; Mockler, T.C.; Carrington, J.C. Highly specific gene silencing in a monocot species by artificial micro RNA s derived from chimeric mi RNA precursors. Plant J. 2015, 82, 1061–1075. [Google Scholar] [CrossRef]
- Niu, Q.-W.; Lin, S.-S.; Reyes, J.L.; Chen, K.-C.; Wu, H.-W.; Yeh, S.-D.; Chua, N.-H. Expression of artificial microRNAs in transgenic Arabidopsis thaliana confers virus resistance. Nat. Biotechnol. 2006, 24, 1420–1428. [Google Scholar] [CrossRef]
- Cisneros, A.E.; Martín-García, T.; Primc, A.; Kuziuta, W.; Sánchez-Vicente, J.; Aragonés, V.; Daròs, J.-A.; Carbonell, A. Transgene-free, virus-based gene silencing in plants by artificial microRNAs derived from minimal precursors. Nucleic Acids Res. 2023, 51, 10719–10736. [Google Scholar] [CrossRef]
- Khalid, A.; Zhang, X.; Ji, H.; Yasir, M.; Farooq, T.; Dai, X.; Li, F. Large Artificial microRNA Cluster Genes Confer Effective Resistance against Multiple Tomato Yellow Leaf Curl Viruses in Transgenic Tomato. Plants 2023, 12, 2179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).