Isolation and Characterization of Lactic Acid Bacteria from Cocoa Mucilage and Meat: Exploring Their Potential as Biopreservatives for Beef
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Isolation of Lactic Acid Bacteria (LAB)
2.2. Morphological Characterization
2.2.1. Morphological Analysis
2.2.2. Biochemical Characterization of Isolated Bacteria
2.2.3. Detection of Protease in the Bacteria under Study
2.2.4. Detection of CO2 in the Study Bacteria
2.2.5. Catalase Test by Slippage (Drop) Method
2.2.6. Gram Stain
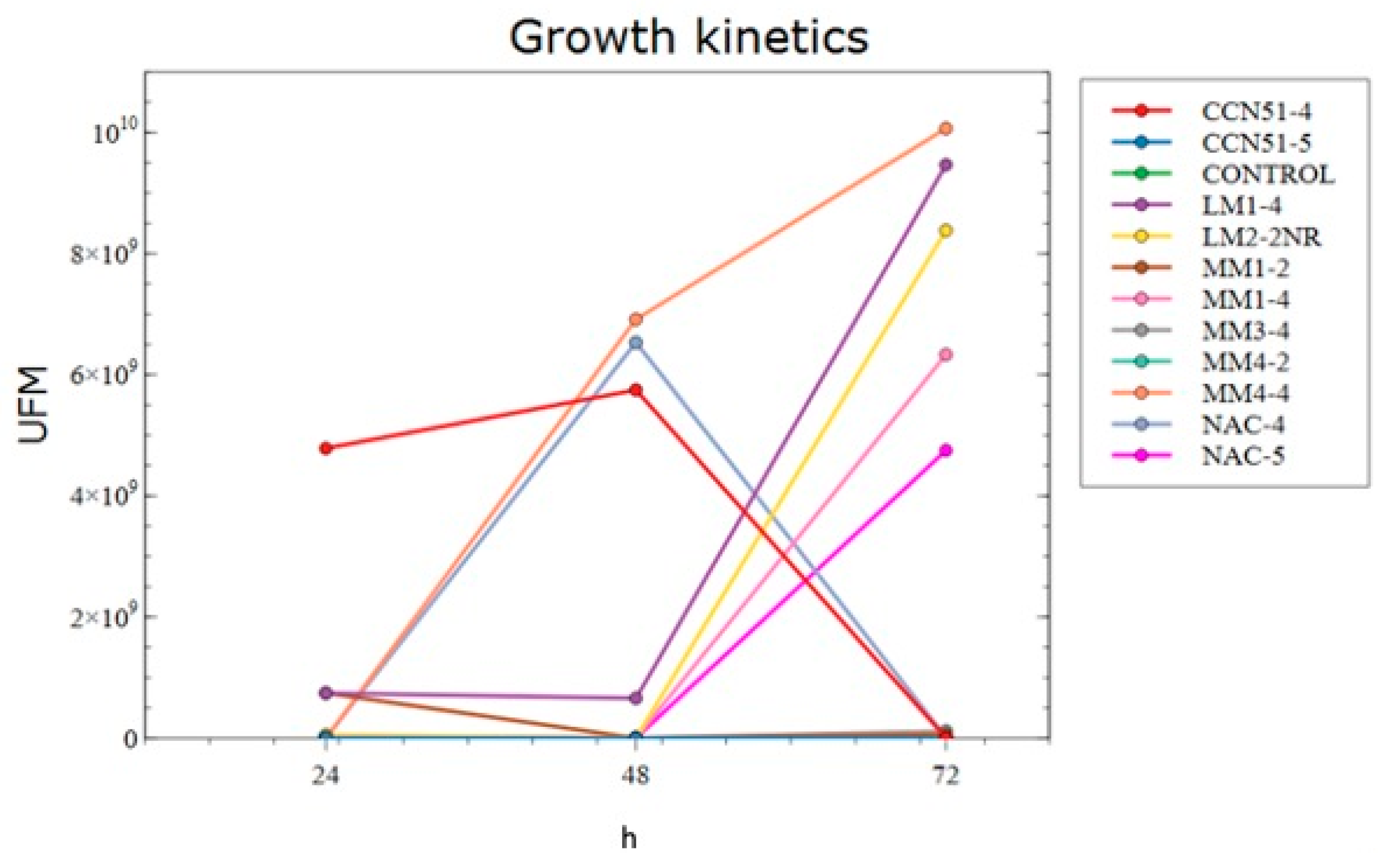
2.2.7. Growth Kinetics
2.2.8. Supernatant Antibiosis—Bacteria
2.2.9. Bacteria–Bacteria Antagonism
2.2.10. LAB Antagonism—Beef
2.3. Physicochemical Analysis
2.3.1. Humidity
2.3.2. Protein
2.3.3. Ash
2.3.4. Fat
2.3.5. Fiber
3. Results and Discussion
3.1. Isolation of Lactic Acid Bacteria (LAB)
3.2. Growth Kinetics
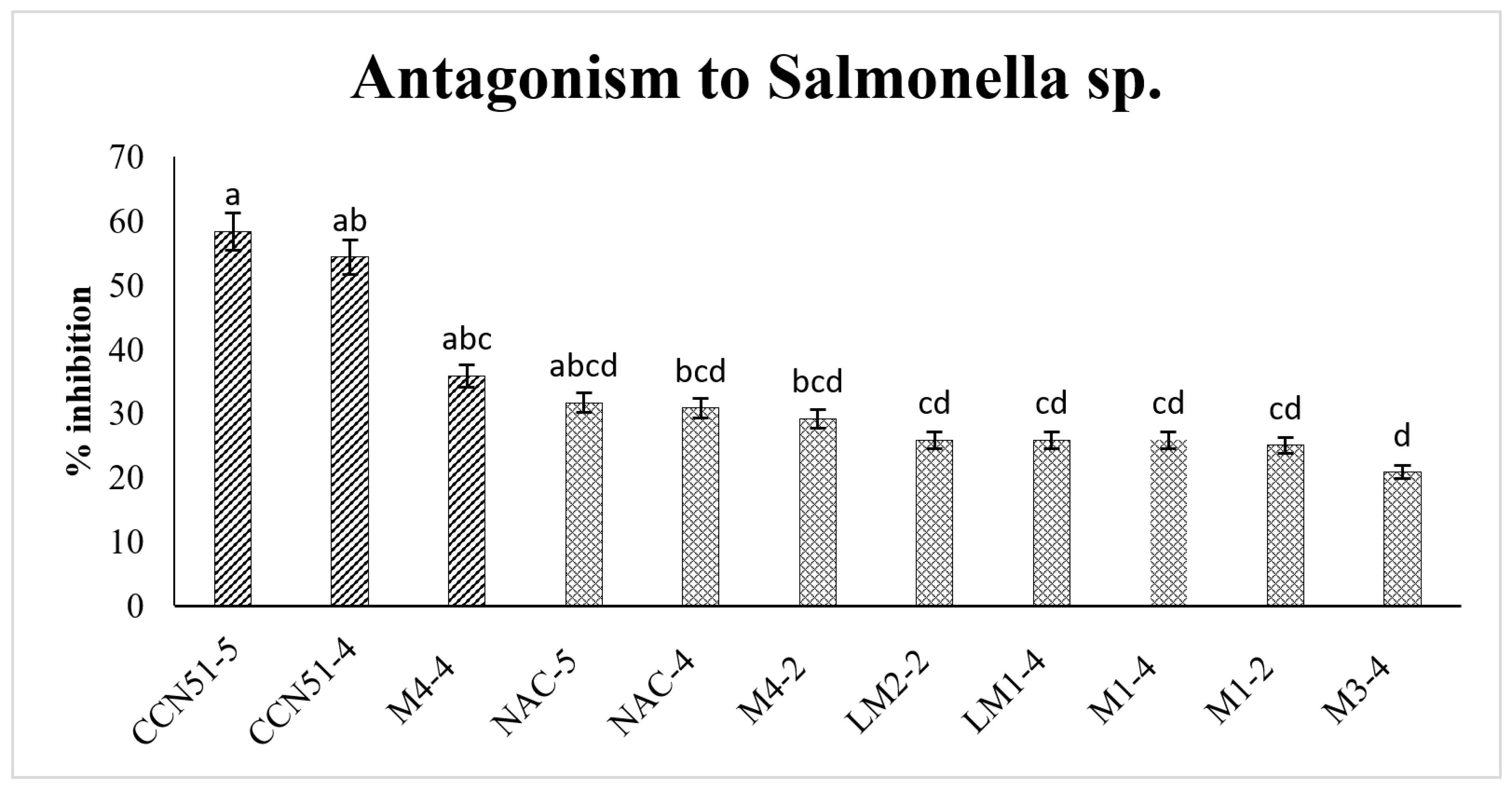
3.3. Antagonism of Lactic Acid Bacteria on Salmonella sp.
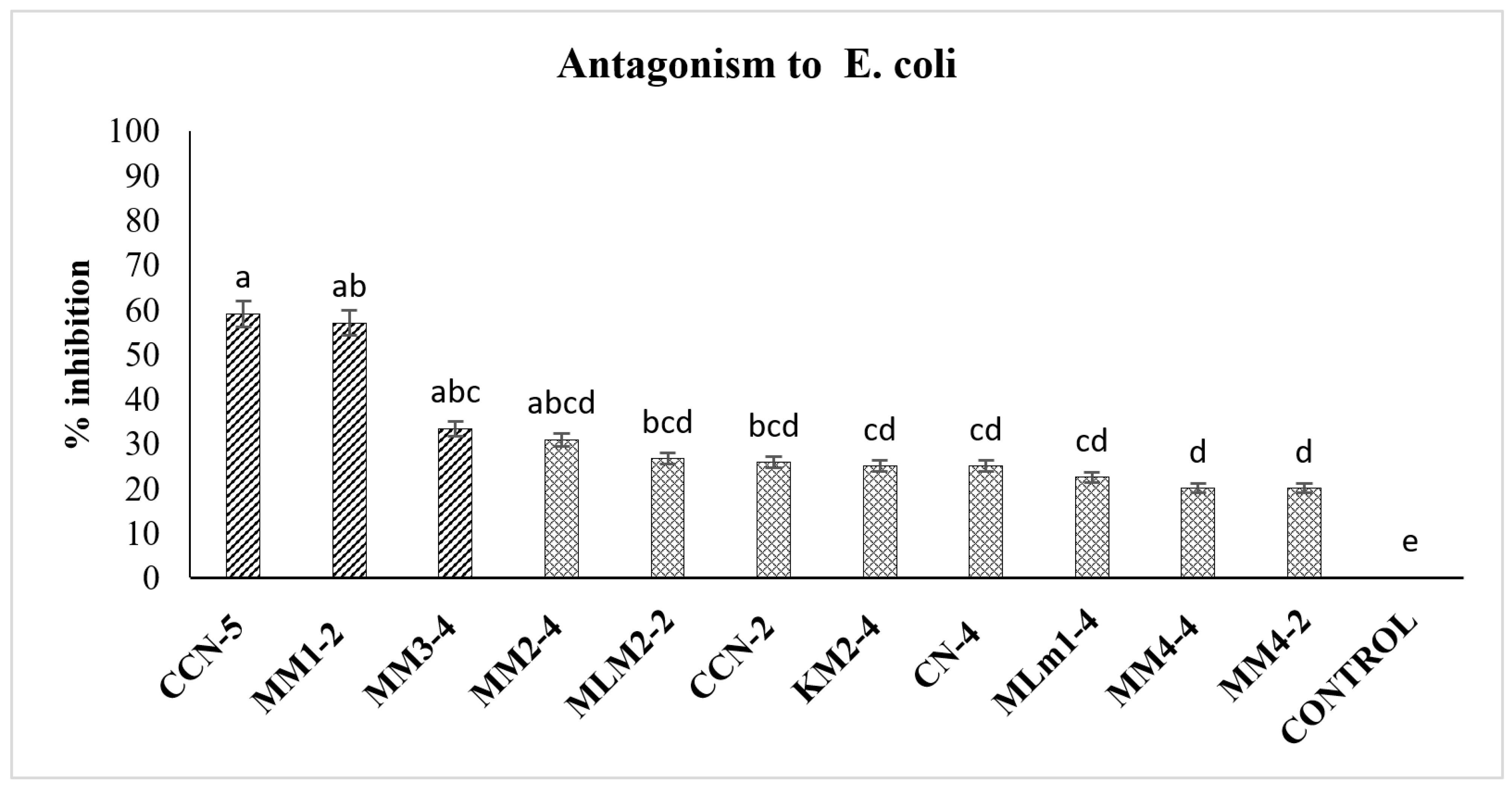
3.4. Antagonism of Lactic Acid Bacteria on E. coli
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conejo, Á. Evaluation of New Technologies in the Development of Healthy Meat Products; Universidad Politécnica de Madrid: Madrid, Spain, 2019. [Google Scholar]
- Morata, A. New Food Preservation Technologies, 2nd ed.; Editorial Cima Express: Madrid, Spain, 2010. [Google Scholar]
- Roncalés, P. Optimization of Packaging Systems and Food Preservation; Elsevier: Zaragoza, Spain, 2010. [Google Scholar]
- Sánchez Escalante, A.; Torrescano Urrutia, G.; Camou Arriola, J.; González Méndez, N.; Hernández Watanabe, G. Combined preservation systems to extend the shelf life of meat and meat products. Nacameh 2008, 2, 124–159. [Google Scholar] [CrossRef]
- Agudelo, N.; Torres, M.; Alvarez, C.; Vélez, L. Bacteriocins Produced by Lactic Acid Bacteria and Their Application in the Food Industry. Aliment. Hoy 2015, 23, 63–72. [Google Scholar]
- Altamarino, D.; Chavarría, M. Microbial Consortium Dose and Temperature Degree in the Useful Life of an Artisanal Sausage; Escuela Superior Politécnica Agropecuaria de Manabí Manuel Féliz López: Calceta, Ecuador, 2019. [Google Scholar]
- Fadda, S.; Chambon, C.; Champomier-Vergès, M.C.; Talon, R.; Vignolo, G. Lactobacillus role during conditioning of refrigerated and vacuum-packaged Argentinean meat. Meat Sci. 2018, 79, 603–610. [Google Scholar] [CrossRef] [PubMed]
- García Lacarra, T.; Martín de Santos, M.d.R.; Sanz Pérez, B.; Hernández, P. Extension of the shelf life of fresh meat I: Packaging in modified atmospheres and use of lactic acid bacteria and bacteriocins. Rev. Española Cienc. Tecnol. Aliment. 1995, 35, 1–18. [Google Scholar]
- Vásquez, M.S.M.; Suárez, M.H.; Montoya, O.I. Evaluation of bacteriocins as a protective medium for the biopreservation of meat under refrigeration. Rev. Chil. Nutr. 2009, 36, 228–238. [Google Scholar]
- Heredia, P.; Hernández, A.; González, A.; Vallejo, B. Bacteriocins of lactic acid bacteria: Mechanisms of action and antimicrobial activity against pathogens in cheeses. Interciencia 2017, 42, 340–346. [Google Scholar]
- Vásquez, M.S.M.; Suárez, M.H.; Zapata, B.S. Use of antimicrobial substances produced by lactic acid bacteria in meat preservation. Rev. Chil. Nutr. 2009, 36, 64–71. [Google Scholar]
- Vallejo, C.; Vera, J.; Quintana, J.; Verdezoto, D.; Cajas, L.; Mendoza, T. Lactic acid bacteria present in the cocoa (Theobroma cacao L.) mucilage of two varieties. Rev. Investig. Talent. V 2018, 1, 59–68. [Google Scholar]
- Fiorentini, Â.M.; Sant’Anna, E.S.; Porto, A.C.S.; Mazo, J.Z.; Franco, B.D.G.M. Influence of bacteriocins produced by Lactobacillus plantarum BN in the shelf-life of refrigerated bovine meat. Braz. J. Microbiol. 2001, 32, 42–46. [Google Scholar] [CrossRef]
- Vanegas, M.; Ossa, J.; Gardeazábal, P.; Coral Durango, A. Lactic acid bacteria (LAB) as an alternative for the conservation of beef packed in modified atmosphere. Aliment. Hoy 2011, 20, 18–27. [Google Scholar]
- Singh, V.P. Recent approaches in food bio-preservation—A review. Open Vet. J. 2018, 8, 104–111. [Google Scholar] [CrossRef]
- Villa, K.; Chanci, I.; Wilches, L.; Cardona, J. Characterization of lactic acid bacteria metabolites and inhibitory effect of bacteriocins on pathogenic microorganisms in food: Systematic review of the literature, 2008–2012. Rev. Biosalud 2014, 13, 45–61. [Google Scholar]
- Guerrero, I.; Mendiolea, R.; Ponce, E.; Prado, A. Inoculation of lactic acid bacteria on meat surfaces as a means of decontamination in semitropical conditions. Meat Sci. 1995, 40, 397–411. [Google Scholar] [CrossRef]
- Daeschel, M.A. Applications of Bacteriocins in Food Systems. In Biotechnology and Food Safety; Butterworth-Heinemann: Oxford, UK, 1990; pp. 91–104. [Google Scholar]
- Monroy, D.; Castro, T.; Fernández, F.; Mayorga, L. Bacteriocins produced by probiotic bacteria. ContactoS 2009, 73, 63–72. [Google Scholar]
- Chen, H.; Hoover, D.G. Bacteriocins and Their Food Applications. Compr. Rev. Food Sci. Food Saf. 2003, 2, 82–100. [Google Scholar]
- Luzuriaga Peña, D.L. Extraccion y Aprovechamieno del Mucilago de Cacao (Theobroma cacao) Como Materia Prima en la Elaboracion de Vino. Undergraduate Thesis, Universidad Tecnológica Equinoccial, Quito, Ecuador, 2012. [Google Scholar]
- Albán, D. Use of Lactic Acid Bacteria from the Mucilage of National Cocoa (Theobroma cacao L.) for the Preservation of Beef. Ph.D. Thesis, Universidad Técnica Estatal de Quevedo, Quevedo, Ecuador, 2017. [Google Scholar]
- De Man, J.; Rogosa, D.; Sharpe, M. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar]
- Atlas, R. Handbook of Microbiological Media, 4th ed.; Taylor & Francis Group, LLC.: Washington, DC, USA, 2010. [Google Scholar]
- Vázquez, R.; Salvador, M.; Adriano, L.; DeGyves, G.; Vázquez, A. Use of starter culture of native lactic acid bacteria for producing an artisanal Mexican cheese safe and sensory acceptable. CyTA-J. Food 2018, 16, 460–468. [Google Scholar] [CrossRef]
- Phang, I.; San Chan, Y.; Wong, K.; Lau, S.Y. Isolation and characterization of urease-producing bacteria from tropical peat. Biocatal. Agric. Biotechnol. 2018, 13, 168–175. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.C.; Zhang, S.; Fu, Y.; Fan, X.; Patel, J.S.; Zhang, M. Characterization of phosphate-solubilizing bacteria isolated from calcareous soils. Appl. Soil Ecol. 2015, 96, 217–224. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Li, W. Effect of probiotic on larvae shrimp (Penaeus vannamei) based on water quality, survival rate and digestive enzyme activities. Aquaculture 2009, 287, 349–353. [Google Scholar] [CrossRef]
- Linares, J. Selection of Lactic Acid Bacteria Isolated from Plant Sources for Their Antimicrobial Capacity and Their Use in Food Biopreservation. Ph.D. Thesis, Autonomous University of Chihuahua, Chihuahua, México, 2021. [Google Scholar]
- Jensen, J.; Jeppesen, E.; Olrik, K.; Kristensen, P. Impact of nutrients and physical factors on the shift from cyanobacterial to chlorophyte dominance in shallow Danish lakes. Can. J. Fish. Aquat. Sci. 1994, 51, 1692–1699. [Google Scholar] [CrossRef]
- MacFaddin, J. Biochemical Tests for the Identification of Clinically Important Bacteria; Editorial Médica Panamericana: Madrid, Spain, 2003. [Google Scholar]
- Liu, W.; Zhang, L.; Yi, H.; Shi, J.; Xue, C.; Li, H.; Jiao, Y.; Shigwedha, N.; Du, M.; Han, X. Qualitative detection of class IIa bacteriocinogenic lactic acid bacteria from traditional Chinese fermented food using a YGNGV-motif-based assay. J. Microbiol. Methods 2014, 100, 121–127. [Google Scholar] [CrossRef]
- Beveridge, T. Use of the Gram stain in microbiology. Biotech. Histochem. 2001, 76, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.J. Evaluation of the Growth Kinetics of PGPR and Its Antagonistic Activity Towards Meloidogyne Incognita “In Vitro”. Ph.D. Thesis, Universidad Técnica Estatal de Quevedo, Quevedo, Ecuador, 2018. [Google Scholar]
- Vargas Cedeño, G.E. Biodegradation of Polyethylene through the Use of Microorganisms. Bachelor’s Thesis, Universidad Técnica Estatal de Quevedo, Quevedo, Ecuador, 2020. [Google Scholar]
- Singh, A.; Maurya, S.; Singh, R.; Singh, U.P. Antibiotic potential of plant growth promoting rhizobacteria (PGPR) against Sclerotium rolfsii. Arch. Phytopathol. Plant Prot. 2012, 45, 1655–1662. [Google Scholar] [CrossRef]
- Rivera, J.; Villegas, A.; Miranda, L.; García, J. Identification of antagonistic acidolactic bacteria of Salmonella enterica var. Typhimurium isolated from artisanal cheese. Rev. Mex. Cienc. Agrícolas 2017, 8, 785–797. [Google Scholar] [CrossRef][Green Version]
- Katikou, P.; Ambrosiadis, I.; Georgantelis, D.; Koidis, P.; Georgakis, S.A. Effect of Lactobacillus-protective cultures with bacteriocin-like inhibitory substances’ producing ability on microbiological, chemical and sensory changes during storage of refrigerated vacuum-packaged sliced beef. J. Appl. Microbiol. 2005, 99, 1303–1313. [Google Scholar] [CrossRef]
- Reyes, N.; Mendieta, B. Determination of the Nutritional Value of Food; Universidad Nacional Agraria: Managua, Nicaragua, 2000. [Google Scholar]
- León, M.; Orduz, A.; Velandia, M. Physicochemical composition of sheep, chicken, beef and pork meat. @Limentech Food Technol. 2017, 15, 62–75. [Google Scholar] [CrossRef]
- Braña Varela, D.; Ramírez, E.; Rubio, M.d.l.S.; Sánchez, A.; Torrescano, G.; Arenas, M.; Partida, J.; Ponce, E.; Ríos, F. Manual of Quality Qnalysis in Meat Samples, 1st ed.; Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias: Ciudad de México, México, 2011. [Google Scholar]
- Niño de Polanía, L.; López, D.; Malagón, M. Meat: Manual of Analysis, 1st ed.; Hernández, C., Ed.; Ministerio de Salud: Bogotá, Colombia, 1995. [Google Scholar]
- Apráez, J. Chemical Analysis of Animal Feed, 1st ed.; Editorial Universidad de Nariño: Nariño, Columbia, 2020. [Google Scholar]
- Dikeman, M.; Devine, C. Encyclopedia of Meat Sciences, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic Acid Bacteria as Antimicrobial Agents: Food Safety and Microbial Food Spoilage Prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Morrison, D.A. Phylogenetic tree-building. Int. J. Parasitol. 1996, 26, 589–617. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Falony, G.; Romanens, E.; Jimenez, J.C.; Amores, F.; Daniel, H.M.; De Vuyst, L. Species diversity, community dynamics, and metabolite kinetics of the microbiota associated with traditional Ecuadorian spontaneous cocoa bean fermentations. Appl. Environ. Microbiol. 2011, 77, 7698–7714. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Corzo, H.; Hernández, E.; González, A.; Giacomán, G. Characterization of lactic acid bacteria with antimicrobial activity isolated from cream cheese from Chiapas, Mexico. CienciaUAT 2021, 15, 144–155. [Google Scholar]
- De Vuyst, L.; Weckx, S. The cocoa bean fermentation process: From ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Crafack, M.; Mikkelsen, M.B.; Saerens, S.; Knudsen, M.; Blennow, A.; Lowor, S.; Takrama, J.; Swieg-ers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Influencing cocoa flavour using Pichia kluyveri and Kluyveromyces marxianus in a defined mixed starter culture for cocoa fermentation. Int. J. Food Microbiol. 2013, 167, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Meersman, E.; Steensels, J.; Mathawan, M.; Pieter-Jan, W.; Saels, V. Detailed Analysis of the Microbial Population in Malaysian Spontaneous Cocoa Pulp Fermentations Reveals a Core and Variable Microbiota. PLoS ONE 2013, 8, e81559. [Google Scholar] [CrossRef]
- Scarpari, L.M.; Meinhardt, L.W.; Maizzafera, P.; Pomella, A.W.V.; Schiavinato, M.A.; Cascardo, J.C.M.; Pereira, G.A.G. Biochemical changes during the development of witches’ broom: The most important disease of cocoa in Brazil caused by Crinipellis perniciosa. J. Exp. Bot. 2005, 56, 865–877. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Magalhães, K.T.; de Almeida, E.G.; da Silva Coelho, I.; Schwan, R.F. Spontaneous cocoa bean fermentation carried out in a novel-design stainless steel tank: Influence on the dynamics of microbial populations and physical-chemical properties. Int. J. Food Microbiol. 2013, 161, 121–133. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Da Cruz Pedrozo Miguel, M.G.; Lacerda Ramos, C.; Freitas Schwan, R. Microbiological and physicochemical characterization of small-scale cocoa fermentations and screening of yeast and bacterial strains to develop a defined starter culture. Appl. Environ. Microbiol. 2012, 78, 5395–5405. [Google Scholar] [CrossRef]
- Snauwaert, I.; Papalexandratou, Z.; De Vuyst, L.; Vandamme, P. Characterization of strains of Weissella fabalis sp. nov. and Fructobacillus tropaeoli from spontaneous cocoa bean fermentations. Int. J. Syst. Evol. Microbiol. 2013, 63, 1709–1716. [Google Scholar]
- Ardhana, M.M.; Fleet, G.H. The microbial ecology of cocoa bean fermentations in Indonesia. Int. J. Food Microbiol. 2003, 86, 87–99. [Google Scholar] [CrossRef]
- Camu, N.; De Winter, T.; Verbrugghe, K.; Cleenwerck, I.; Vandamme, P.; Takrama, J.S.; Vancanneyt, M.; De Vuyst, L. Dynamics and Biodiversity of Populations of Lactic Acid Bacteria and Acetic Acid Bacteria Involved in Spontaneous Heap Fermentation of Cocoa Beans in Ghana. Appl. Environ. Microbiol. 2007, 73, 1824. [Google Scholar] [CrossRef] [PubMed]
- Lagunes, S.; Loiseau, G.; Paredes, J.; Barel, M.; Guiraud, J. Study on the microflora and biochemistry of cocoa fermentation in the Dominican Republic. Int. J. Food Microbiol. 2007, 114, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.S.; Teniola, O.D.; Ban-Koffi, L.; Owusu, M.; Andersson, T.S.; Holzapfel, W.H. The microbiology of Ghanaian cocoa fermentations analysed using culture-dependent and culture-independent methods. Int. J. Food Microbiol. 2007, 114, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Kostinek, M.; Ban-Koffi, L.; Ottah-Atikpo, M.; Teniola, D.; Schillinger, U.; Holzapfel, W.H.; Franz, C.M.A.P. Diversity of predominant lactic acid bacteria associated with cocoa fermentation in Nigeria. Curr. Microbiol. 2008, 56, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Lefeber, T.; Janssens, M.; Camu, N.; De Vuyst, L. Kinetic analysis of strains of lactic acid bacteria and acetic acid bacteria in cocoa pulp simulation media toward development of a starter culture for cocoa bean fermentation. Appl. Environ. Microbiol. 2010, 76, 7708–7716. [Google Scholar] [CrossRef]
- Lozano, R.; Orellana, Z. Unidad Académica de Ciencias Administrativas y Proyecto de Grado Previo a la Obtención del Título de: Ingeniero en Contaduría Pública y Auditoría—CPA Título del Proyecto: “Estudio de Factibilidad Para la Instalación de una Fábrica de Plástico Dedicad”; Universidad Estatal de Milagro: Milagro, Ecuador, 2013. [Google Scholar]
- Papalexandratou, Z.; Camu, N.; Falony, G.; De Vuyst, L. Comparison of the bacterial species diversity of spontaneous cocoa bean fermentations carried out at selected farms in Ivory Coast and Brazil. Food Microbiol. 2011, 28, 964–973. [Google Scholar] [CrossRef]
- Mesa, Y.; Mas, S.; Anaya, M.; Cobo, H.; Díaz, M. Study of the behavior of Lactic Acid Bacteria (LAB) of the Bioyogur culture at different doses of magnetic treatment. Tecnol. Química 2016, 36, 439–456. [Google Scholar]
- Jurado, H.; Martínez, J.; Morillo, J.; Romero, D.; Orbes, A.; Mesías, L. Determination of fermentation kinetics in two probiotic media, in vitro performance tests and inhibition effect of Lactobacillus plantarum. Vet. Zootecnía 2011, 10, 23–41. [Google Scholar]
- Calderón, J. Adjustment of a Kinetic Model for the Growth of Lactobacillus acidophilus in the Fermentation of a Complex Substrate. Bachelor’s Thesis, Fundación Universidad de América, Bogotá, Columbia, 2017. [Google Scholar]
- Zamora Rodríguez, L. Isolation, Identification and Conservation of Cultures of Lactic Bacteria Antagonistic to the Contaminating Microbiota of Slaughterhouse Blood. Ph.D. Thesis, Universidad de Girona, Girona, Spain, 2003. [Google Scholar]
- Wirth, F. The pH is an important parameter related to the susceptibility of meat to deterioration and is used to decide on the type of processing to which the meat is going to be used. Fleischwirtsch. Español 1987, 1, 22–28. [Google Scholar]
- Fernández Yanza, M.A.; Morocho Quichimbo, B.J. Determination of Lactic Acid Bacteria and Identification of Sources of Contamination in Finished Meat Products Vacuum-Packed. Master’s Thesis, Universidad de Cuenca, Cuenca, Ecuador, 2020. [Google Scholar]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef]
- Borrás, L.; Valiño, E.; Rodríguez, C. Microbial preparation with lactic acid activity as a biological accelerator in fermentation processes for animal feed. Cienc. Agric. 2017, 14, 7–13. [Google Scholar]
- Mendoza, T. Elaboration of Cream Cheese with Lactic Bacteria from Cocoa Mucilage (Theobroma cacao L.) Fine Aroma. Bachelor’s Thesis, Universidad Técnica Estatal de Quevedo, Quevedo, Ecuador, 2017. [Google Scholar]
- Mirkovic, N.; Polovic, N.; Vukotic, G.; Jovcic, B.; Miljkovic, M.; Radulovic, Z.; Diep, D.; Kojic, M. Lactococcus lactis LMG2081 Produces Two Bacteriocins, a Nonlantibiotic and a Novel Lantibiotic. Appl. Environ. Microbiol. 2016, 82, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Castellano, P.; Belfiore, C.; Fadda, S.; Vignolo, G. A review of bacteriocinogenic lactic acid bacteria used as bioprotective cultures in fresh meat produced in Argentina. Meat Sci. 2008, 79, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Feiner, G. Chapter 39—The microbiology of specific bacteria. In Meat Products Handbook; Elsevier: Amsterdam, The Netherlands, 2006; pp. 670–672. [Google Scholar]
- Moscoso, J. Biopreservation of Raw Sausages through the Use of Staphylococcus carnosus and Lactobacillus plantarum as Protective Cultures. Master’s Thesis, Universidad del Azuay, Cuenca, Spain, 2017. [Google Scholar]
- Hovhannisyan, H.; Goodarzi, A.; Barseghyan, A. Antimicrobial substances production at refrigeration temperatures by Lactobacillus 52 delbrueckii MH10: A Candidate for food biopreservation. Int. J. Nutr. Food Sci. 2016, 5, 184. [Google Scholar]
- Jones, R.J.; Hussein, H.M.; Zagorec, M.; Brightwell, G.; Tagg, J.R. Isolation of lactic acid bacteria with inhibitory activity against pathogens and spoilage organisms associated with fresh meat. Food Microbiol. 2008, 25, 228–234. [Google Scholar] [CrossRef]
- Gutierrez Ramírez, L.A.; Campuzano Montoya, O.I.; Villadiego Ruiz, O.S. Evaluation of the bactericidal potential of extracts of lactic acid bacteria extracts on the in vitro growth of E. coli, Salmonella sp. and Listeria monocytogenes. Rev. CENIC Cienc. Biológicas 2005, 36, 1–25. [Google Scholar]
- Suárez, H.; Francisco, A.; Beirão, L. Influence of Bacteriocins produced by Lactobacillus plantarum LPBM10 on the shelf life of Cachama Piaractus brachypomus × Colossoma macropomum hybrid fillets vacuum packed. VITAE 2008, 15, 32–40. [Google Scholar]
- Bhattacharya, D.; Nanda, P.K.; Pateiro, M.; Lorenzo, J.M.; Dhar, P.; Das, A.K. Lactic acid bacteria and bacteriocins: Novel biotechnological approach for biopreservation of meat and meat products. Microorganisms 2022, 10, 2058. [Google Scholar] [CrossRef]
- Hernández, S. Lactic Acid Bacteria as Biopreservatives for Beef; Universidad Autónoma de Chapingo: Chapingo, Mexico, 2019. [Google Scholar]
- Hernández, S.; Miranda, L.; Maldonado, E.; Alarcón, B. Inhibition of Salmonella and E. coli on ground beef by lactic acid bacteria. Univ. Autónoma Chapingo 2019, 1, 39–52. [Google Scholar]
- Alvarado, C.; García, B.; Martin, S.; Regalado, C. Food-associated lactic acid bacteria with antimicrobial potential from traditional Mexican foods. Rev. Latinoam. Microbiol. 2006, 48, 260–268. [Google Scholar]
- Simova, E.D.; Beshkova, D.M.; Angelov, M.P.; Dimitrov, Z.P. Bacteriocin production by strain Lactobacillus delbrueckii ssp. bulgaricus BB18 during continuous prefermentation of yogurt starter culture and subsequent batch coagulation of milk. J. Ind. Microbiol. Biotechnol. 2008, 35, 559–567. [Google Scholar] [PubMed]
- Senne, M.M.; Gilliland, S.E. Antagonistic action of cells of Lactobacillus delbrueckii subsp. lactis against pathogenic and spoilage microorganisms in fresh meat systems. J. Food Prot. 2003, 66, 418–425. [Google Scholar] [PubMed]
- Watson, J.A.; Schubert, J. Action of hydrogen peroxide on growth inhibition of Salmonella typhimurium. J. Gen. Microbiol. 1969, 57, 25–34. [Google Scholar] [CrossRef][Green Version]
- Finn, G.J.; Condon, S. Regulation of catalase synthesis in Salmonella typhimurium. J. Bacteriol. 1975, 123, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, T.J.; Slater, E.; McAlpine, K.; Rowbury, R.J.; Gilbert, R.J. Salmonella enteritidis phage type 4 isolates more tolerant of heat, acid, or hydrogen peroxide also survive longer on surfaces. Appl. Environ. Microbiol. 1995, 61, 3161–3164. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, R.; Lindblad, M. Inactivation of Escherichia coli, Listeria monocytogenes and Yersinia enterocolitica in fermented sausages during maturation/storage. Int. J. Food Microbiol. 2009, 129, 59–67. [Google Scholar] [CrossRef]
- Grande, M.J.; Lucas, R.; Abriouel, H.; Ben Omar, N.; Maqueda, M.; Martínez, M.; Martínez, M.; Valdivia, E.; Gálvez, A. Control of Alicyclobacillus acidoterrestris in fruit juices by enterocin AS-48. Int. J. Food Microbiol. 2005, 104, 289–297. [Google Scholar] [CrossRef]
- González, B.E.; Gómez, M.; Jiménez, Z. Bacteriocinas de probióticos. RESPYN Rev. Salud Pública Nutr. 2003, 4, 1–9. [Google Scholar]
- Klaenhammer, T.R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85. [Google Scholar] [CrossRef]
- Bemena, L.D.; Mohamed, L.A.; Fernandes, A.M.; Lee, B. Applications of bacteriocins in food, livestock health and medicine. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 924–949. [Google Scholar]
- Nogales, S.; Bressan, M.C.; Vaz, A.P.; Delgado, J.V.; Camacho, M.E. Physical-chemical study of the meat of the Marismeña bovine breed in different finishing systems. Arch. Zootec. 2011, 60, 453–456. [Google Scholar]
- Farfán, N.; Juarez, D.; Rossi, A.; Sammán, N. Chemical composition of Creole cattle meat. Arch. Latinoam. Nutr. 2000, 50, 400–404. [Google Scholar] [PubMed]
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, M.; Jami, M.; Kneifel, W.; Domig, K.J. Antimicrobial activity and partial characterization of bacteriocins produced by lactobacilli isolated from Sturgeon fish. Food Control 2013, 2, 379–385. [Google Scholar] [CrossRef]
- Vatanyoopaisarn, S.; Prapatsornwattana, K.; Kuhakongkeat, T.; Phalakornkule, C. Potential use of lactic acid bacteria with bacteriocin-like activity against Staphylococcus aureus as dual starter cultures in Thai fermented sausage “Sai Krok Prew”. Int. Food Res. J. 2011, 18, 697–704. [Google Scholar]
- Arrioja, D.; Mani, E.; Palou, E.; López, A. Antimicrobial activity and storage stability of cell-free supernatants from lactic acid bacteria and their applications with fresh beef. Food Control 2020, 115, 107286. [Google Scholar] [CrossRef]
- Larraín Prieto, R.; Bello Pérez, E.V. Composition of National Bovine Meat; Fundación para la Innovación Agraria (FIA): Santiago de Chile, Chile, 2013. [Google Scholar]


| Strains | Biochemical Tests | Colonial Morphology | ||||||
|---|---|---|---|---|---|---|---|---|
| Catalase | Urease | Protease | CO2 | Gram Stain | Form | Elevation | Margin | |
| KLM2-4 | + | − | − | ++ | − | Circular | High | Whole |
| KLM4 | − | − | + | ++ | + | |||
| KLM1-4 | + | − | + | + | − | |||
| MM4-2 | − | − | + | ++ | + | |||
| KLM4-4 | + | − | + | ++ | − | |||
| MM3-4 | − | − | − | ++ | + | |||
| MM1-2 | − | − | − | + | + | |||
| KM3-4 | + | − | − | + | − | |||
| KM4-4 | + | − | − | ++ | − | |||
| KM2-4 | − | − | − | ++ | + | |||
| KLm3-4 | + | − | + | ++ | − | |||
| KM1-6 | + | − | + | ++ | − | |||
| MM4-4 | − | − | − | ++ | + | |||
| KLM2-6 | + | − | + | ++ | − | |||
| MM4-4 | − | − | − | ++ | + | |||
| KM4-2 | + | − | + | ++ | − | |||
| CN-4 | − | − | − | + | + | |||
| CN-3 | − | − | − | ++ | + | |||
| KM4-6 | + | − | + | ++ | − | |||
| MM2-4 | − | − | − | ++ | + | |||
| KLM1-2 | + | − | + | ++ | − | |||
| CCN-2 | − | − | − | + | + | |||
| CCN-5 | − | − | − | − | + | |||
| KM1-4 | + | − | − | ++ | − | |||
| MLm2-2 | − | − | + | ++ | + | |||
| KM3-6 | + | − | + | ++ | − | |||
| MLM-4 | − | − | − | ++ | + | |||
| MLM2-2 | − | − | + | + | + | |||
| MLm1-4 | − | + | − | ++ | + | |||
| CN-2 | − | ++ | − | ++ | + | |||
| KLm1-6 | + | + | + | ++ | − | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morante-Carriel, L.; Abasolo, F.; Bastidas-Caldes, C.; Paz, E.A.; Huaquipán, R.; Díaz, R.; Valdes, M.; Cancino, D.; Sepúlveda, N.; Quiñones, J. Isolation and Characterization of Lactic Acid Bacteria from Cocoa Mucilage and Meat: Exploring Their Potential as Biopreservatives for Beef. Microbiol. Res. 2023, 14, 1150-1167. https://doi.org/10.3390/microbiolres14030077
Morante-Carriel L, Abasolo F, Bastidas-Caldes C, Paz EA, Huaquipán R, Díaz R, Valdes M, Cancino D, Sepúlveda N, Quiñones J. Isolation and Characterization of Lactic Acid Bacteria from Cocoa Mucilage and Meat: Exploring Their Potential as Biopreservatives for Beef. Microbiology Research. 2023; 14(3):1150-1167. https://doi.org/10.3390/microbiolres14030077
Chicago/Turabian StyleMorante-Carriel, Laura, Fernando Abasolo, Carlos Bastidas-Caldes, Erwin A. Paz, Rodrigo Huaquipán, Rommy Díaz, Marco Valdes, David Cancino, Néstor Sepúlveda, and John Quiñones. 2023. "Isolation and Characterization of Lactic Acid Bacteria from Cocoa Mucilage and Meat: Exploring Their Potential as Biopreservatives for Beef" Microbiology Research 14, no. 3: 1150-1167. https://doi.org/10.3390/microbiolres14030077
APA StyleMorante-Carriel, L., Abasolo, F., Bastidas-Caldes, C., Paz, E. A., Huaquipán, R., Díaz, R., Valdes, M., Cancino, D., Sepúlveda, N., & Quiñones, J. (2023). Isolation and Characterization of Lactic Acid Bacteria from Cocoa Mucilage and Meat: Exploring Their Potential as Biopreservatives for Beef. Microbiology Research, 14(3), 1150-1167. https://doi.org/10.3390/microbiolres14030077





_MARCO_VALDES.jpg)


