Development of a Novel Emulsion Formulation of Trichoderma asperelloides PSU-P1 Conidia against Stem Canker on Dragon Fruit Caused by Neoscytalidium dimidiatum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Materials and Spore Suspension Preparation
2.2. Formulation Preparation
2.3. In Vivo Testing of Formulation
2.4. Viability of Trichoderma Conidia, Antifungal Ability of Formulation, and pH Evaluation
2.5. Statistical Analysis
3. Results
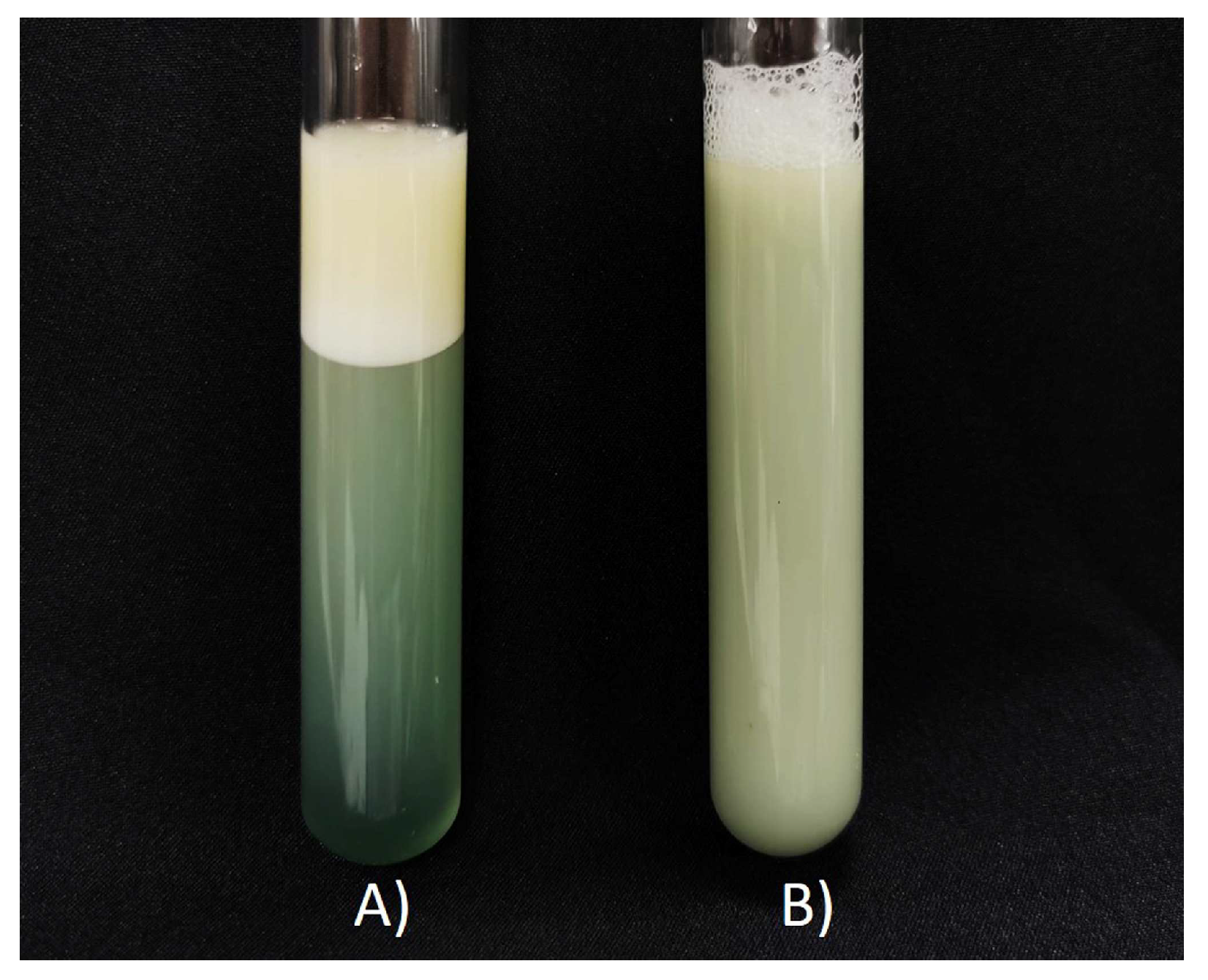
3.1. Selection of an Appropriate Formulation
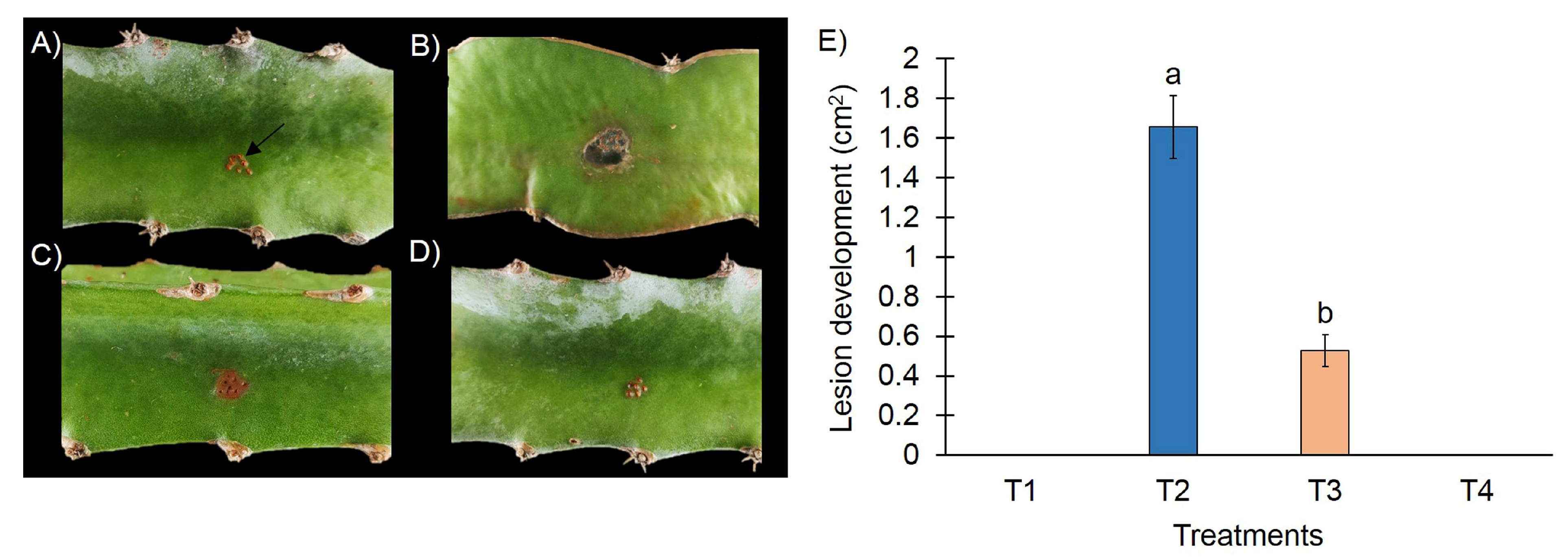
3.2. In Vivo Test of Formulation
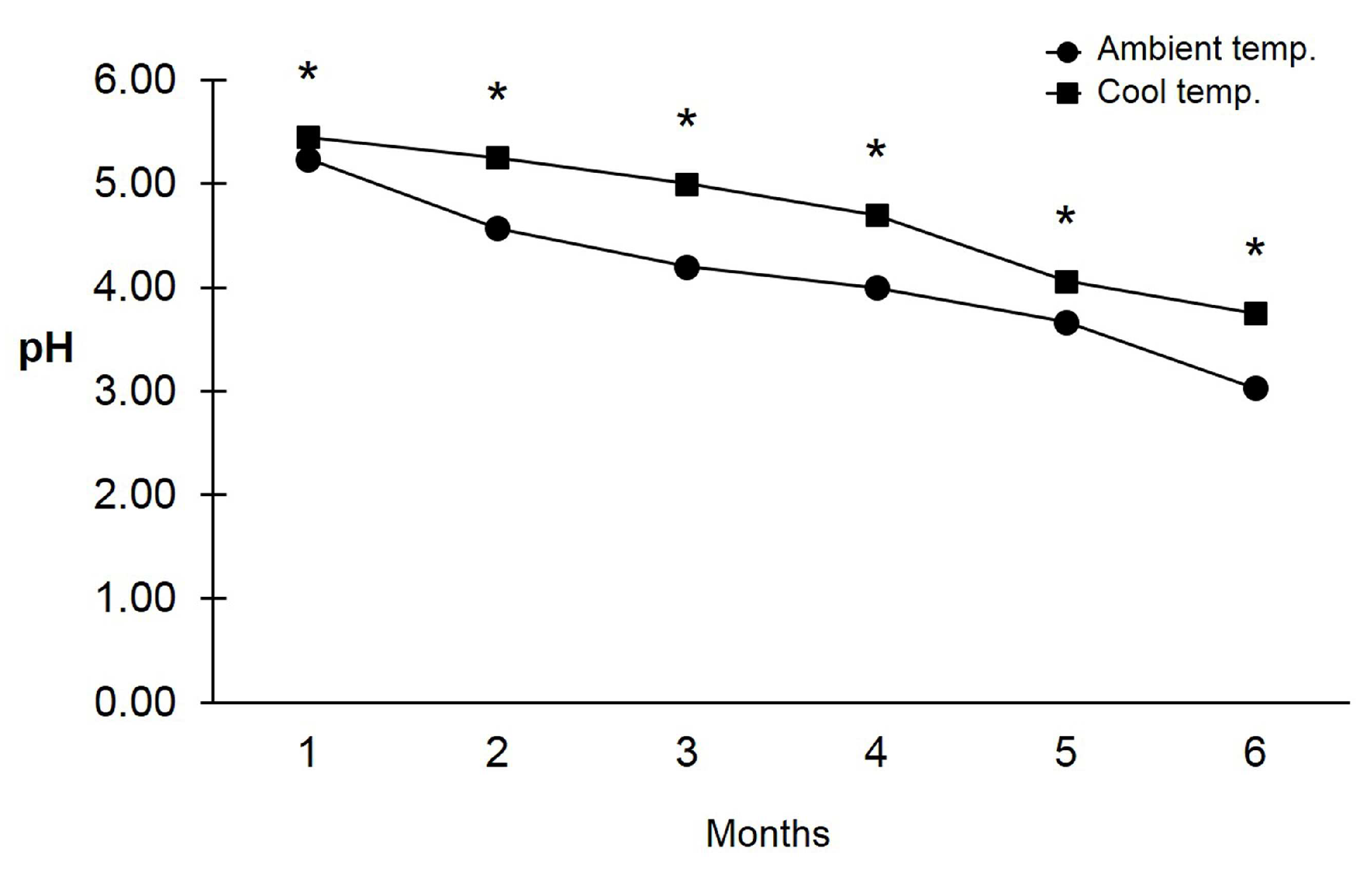
3.3. pH and Viability of Trichoderma asperelloides PSU-P1 Conidia in Formulation
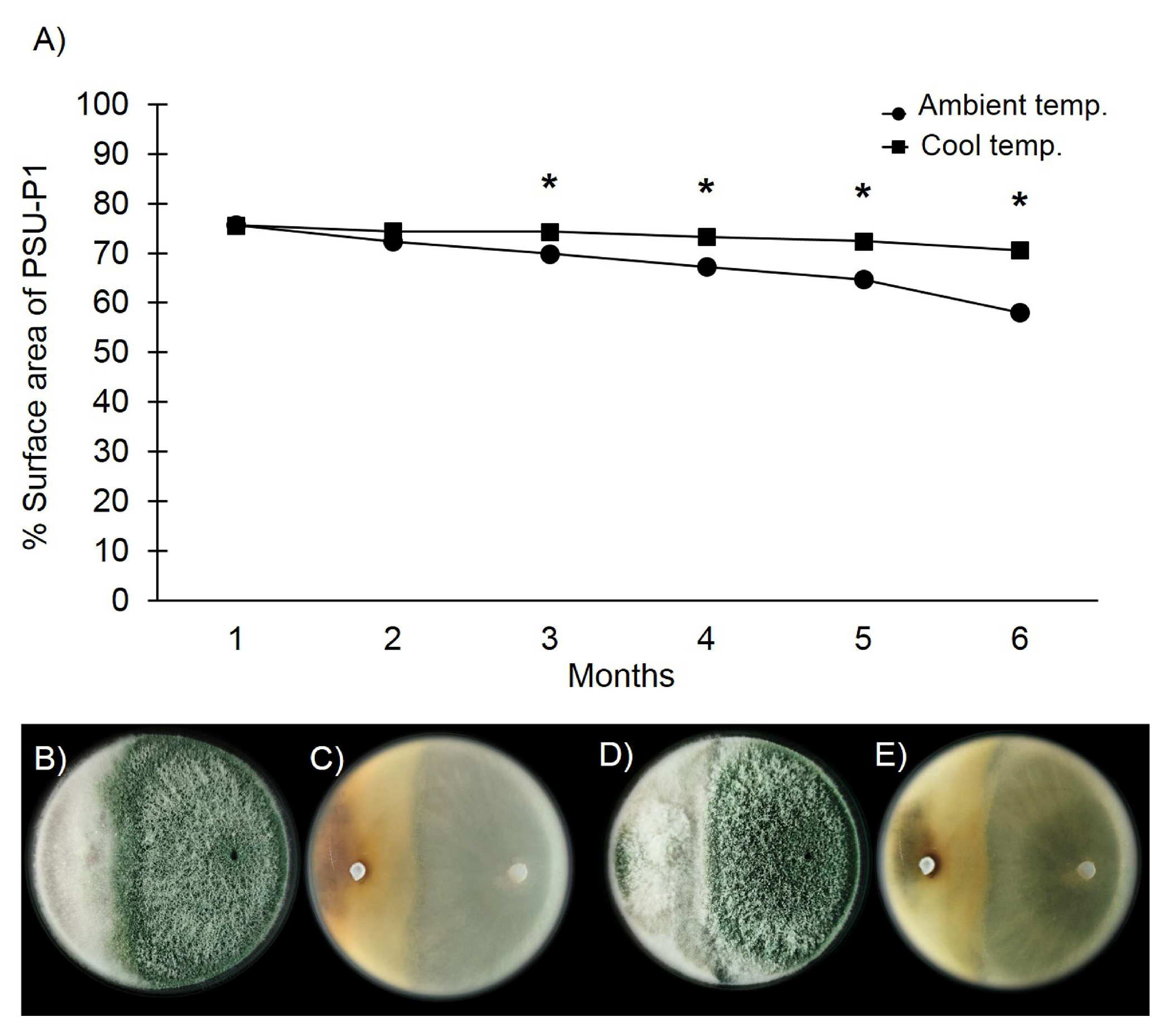
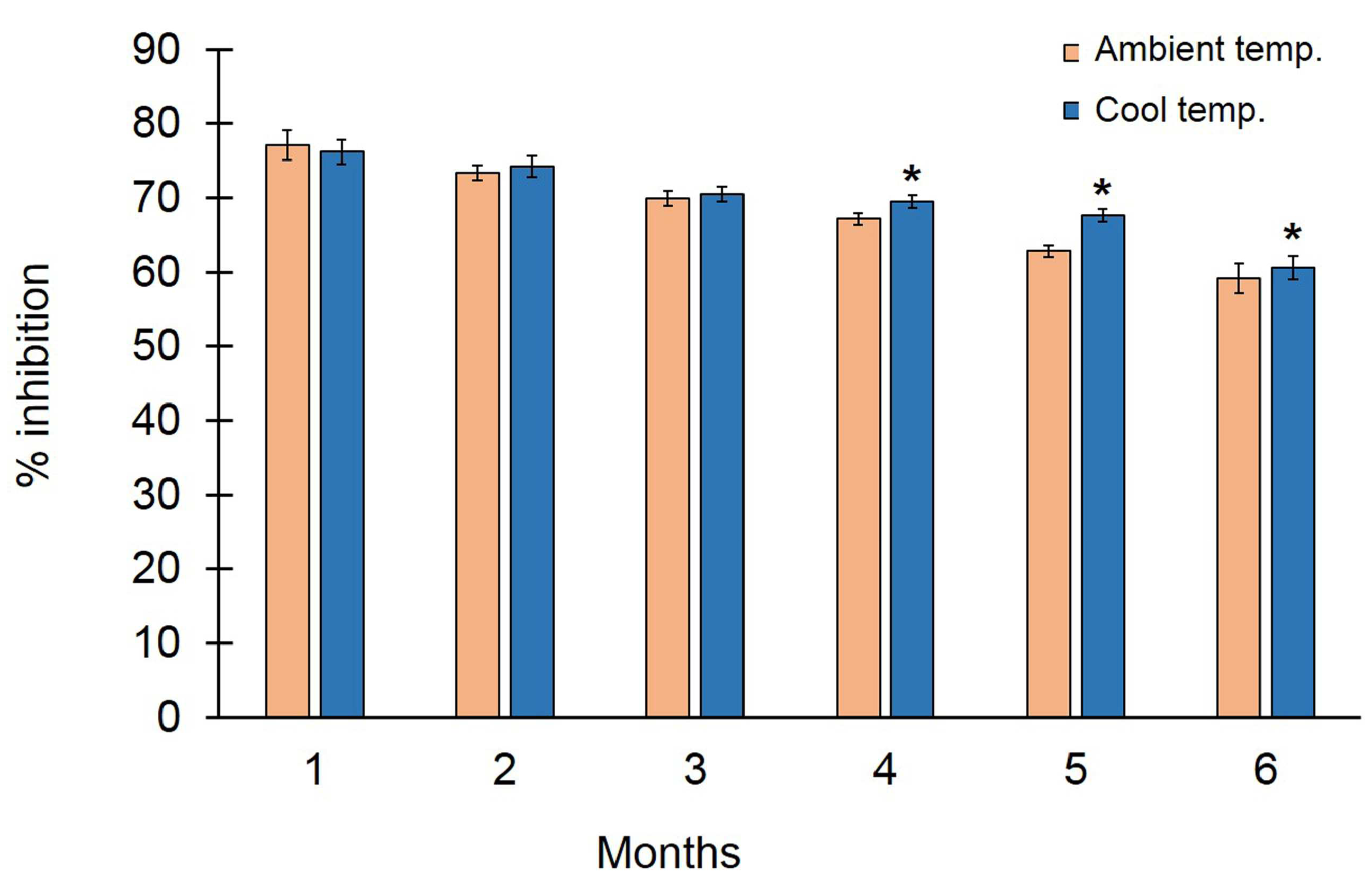
3.4. Antifungal Ability of Trichoderma asperelloides PSU-P1 Conidia in Formulation against Neoscytalidium dimidiatum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tseng, Y.-H.; Rouina, H.; Groten, K.; Rajani, P.; Furch, A.C.U.; Reichelt, M.; Baldwin, I.T.; Nataraja, K.N.; Uma Shaanker, R.; Oelmüller, R. An Endophytic Trichoderma strain promotes growth of its hosts and defends against pathogen attack. Front. Plant Sci. 2020, 11, 573670. [Google Scholar] [CrossRef]
- Phoka, N.; Pornsuriya, C.; Sunpapao, A. High-throughput sequencing provides insight into soil fungal community structure and diversity in plant protected areas of Songkhla zoo in southern Thailand. Chiang Mai J. Sci. 2022, 49, 524–537. [Google Scholar] [CrossRef]
- Zhang, C.-I.; Zhang, B.; Yang, X.-Y.; Naicker, O.; Zhao, L. Brown rot disease caused by Trichoderma hamatum on the edible lily, Lilium leichtlinii var. maximowiczii. Chiang Mai J. Sci. 2022, 49, 1500–1508. [Google Scholar] [CrossRef]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar] [PubMed]
- Oszust, K.; Cybulska, J.; Frąc, M. How do Trichoderma genus fungi win a nutritional competition battle against soft fruit pathogens? A report on niche overlap nutritional potentiates. Int. J. Mol. Sci. 2020, 21, 4235. [Google Scholar] [CrossRef]
- Wonglom, P.; Daengsuwan, W.; Ito, S.; Sunpapao, A. Biological control of Sclerotium fruit rot of snake fruit and stem rot of lettuce by Trichoderma sp. T76-12/2 and the mechanism involved. Physiol. Mol. Plant Pathol. 2019, 107, 1–7. [Google Scholar] [CrossRef]
- Ruangwong, O.-U.; Pornsuriya, C.; Pitija, K.; Sunpapao, A. Biocontrol mechanisms of Trichoderma koningiopsis PSU3-2 against postharvest anthracnose of chili pepper. J. Fungi 2021, 7, 276. [Google Scholar] [CrossRef]
- Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.; Matsui, K.; Arikit, S.; Sunpapao, A. Role of volatiles from the endophytic fungus Trichoderma asperelloides PSU-P1 in biocontrol potential and in promoting the plant growth of Arabidopsis thaliana. J. Fungi 2020, 6, 341. [Google Scholar] [CrossRef]
- Intana, W.; Kheawleng, S.; Sunpapao, A. Trichoderma asperellum T76-14 released volatile organic compounds against postharvest fruit rot in muskmelons (Cucumis melo) caused by Fusarium incarnatum. J. Fungi 2021, 7, 46. [Google Scholar] [CrossRef]
- Gams, W.; Bissett, J. Morphology and identification of Trichoderma. In Trichoderma and Gliocladium: Basic Biology, Taxonomy and Genetics; Harman, G.E., Kubicek, C.P., Eds.; Taylor and Francis: London, UK, 1998; pp. 3–34. [Google Scholar]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valero, J.R. Dissolved oxygen as principal parameter for conidia production of biocontrol fungi Trichoderma viride in non-Newtonian wastewater. J. Ind. Microbiol. Biotechnol. 2006, 33, 941–952. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Ann. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Kaewchai, S.; Soytong, K.; Hyde, K.D. Mycofungicides and fungal biofertilizers. Fungal Divers. 2009, 38, 25–50. [Google Scholar]
- Bashan, Y.; de Bashan, L.; Prabhu, S.; Hernandez, J.P. Advances in plant growth promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Doni, F.; Isahak, A.; Zain, C.; Ariffin, S.; Mohamad, W.; Yusoff, W. Formulation of Trichoderma sp. SL2 inoculants using different carriers for soil treatment in rice seedling growth. SpringerPlus 2014, 3, 532. [Google Scholar] [CrossRef]
- Jaafar, R.A.; Abdul Rahman, A.R.B.; Mahnood, N.Z.C.; Vasudevan, R. Proximate analysis of dragon fruit (Hylocereus polyrhizus). Am. J. Appl. Sci. 2009, 6, 1341–1346. [Google Scholar]
- Abirami, K.; Swain, S.; Baskaran, V.; Sakthivel, K.; Bommayasamy, N. Distinguishing three dragon fruit (Hylocereus spp.) species grown in Andaman and Nicobar Islands of India using morphological, biochemical and molecular traits. Sci. Rep. 2021, 11, 2894. [Google Scholar] [CrossRef]
- Wonglom, P.; Thithuan, N.; Bunjongsiri, P.; Sunpapao, A. Plant-Parasitic algae (Cephaleuros spp.) in Thailand, including four new records. Pacific Sci. 2018, 72, 363–371. [Google Scholar] [CrossRef]
- Hong, C.F.; Gazis, R.; Crane, J.H.; Zhang, S. Prevalence and epidemics of Neoscytalidium stem and fruit canker on pitahaya (Hylocereus spp.) in South Florida. Plant Dis. 2020, 104, 1433–1438. [Google Scholar] [CrossRef]
- Dy, K.S.; Wonglom, P.; Pornsuriya, C.; Sunpapao, A. Morphological, molecular identification and pathogenicity of Neoscytalidium dimidiatum causing stem canker of Hylocereus polyrhizus in southern Thailand. Plants 2022, 11, 504. [Google Scholar] [CrossRef]
- Thaochan, N.; Pornsuriya, C.; Chairin, T.; Sunpapao, A. Roles of systemic fungicide in antifungal activity and induced defense responses in rubber tree (Hevea brasiliensis) against leaf fall disease caused by Neopestalotiopsis cubana. Physiol. Molec. Plant Pathol. 2020, 111, 101511. [Google Scholar] [CrossRef]
- Lin, C.; Ni, H.; Huang, C.; Yang, H. Pathogen characterization and chemical control of pitaya stem canker disease. Australasian Plant Dis. Notes 2017, 10, 1–4. [Google Scholar]
- Yoon, M.Y.; Cha, B.; Kim, J.C. Recent trends in studies on botanical fungicides in agriculture. Plant Pathol. J. 2013, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, E.; Mousavi Moghadam, M.; Khanbabaei, R. The effect of fricyclazole on testosterone changes and testicular structure in mice. J. Babol. Univ. Med. Sci. 2015, 17, 43–49. [Google Scholar]
- Rees, H.J.; Drakulic, J.; Cromey, M.G.; Bailey, A.M.; Foster, G.D. Endophytic Trichoderma spp. can protect strawberry and privet plants from infection by the fungus Armillaria mellea. PLoS ONE 2022, 17, e0271622. [Google Scholar] [CrossRef] [PubMed]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Ruangwong, O.-U.; Wonglom, P.; Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.-I.; Sunpapao, A. Biological control activity of Trichoderma asperelloides PSU-P1 against gummy stem blight in muskmelon (Cucumis melo). Physiol. Mol. Plant Pathol. 2021, 115, 101663. [Google Scholar] [CrossRef]
- Intana, W.; Wonglom, P.; Suwannarach, N.; Sunpapao, A. Trichoderma asperelloides PSU-P1 induced expression of pathogenesis-related protein genes against gummy stem blight of muskmelon (Cucumis melo) in field evaluation. J. Fungi 2022, 8, 156. [Google Scholar] [CrossRef]
- yed-Ab-Rahman, S.F.; Carvalhais, L.C.; Omar, D. Development of plant-based emulsion formulation to control bacterial leaf blight and sheath brown rot of rice. Heliyon 2020, 6, e03151. [Google Scholar] [CrossRef]
- Batta, Y.A. Postharvest biological control of apple gray mold by Trichoderma harzianum Rifai formulated in an invert emulsion. Crop Prot. 2004, 23, 19–26. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, M.; Rani, A. Trichoderma: Mass production, formulation, quality control, delivery and its scope in commercialization in India for the management of plant disease. Afr. J. Agric. Res. 2014, 9, 3838–3852. [Google Scholar]
- Peeran, M.F.; Nagendran, K.; Gandhi, K.; Raguchander, T.; Prabakar, K. Water in oil based PGPR formulation of Pseudomonas fluorescens (FP7) showed enhanced resistance against Colletotrichum musae. Croop Protec. 2014, 65, 186–193. [Google Scholar] [CrossRef]
- Mbarka, J.B.; Begoude, B.A.D.; Ambang, Z.; Meboma, M.; Kuaté, J.; Schiffers, B.; Ten Hoopen, G.M. A new oil-based formulation of Trichoderma asperellum for the biological control of cacao black pod disease caused by Phytophthora megakarya. Biol. Control 2014, 77, 15–22. [Google Scholar] [CrossRef]
- Domingues, M.V.P.F.; de Moura, K.E.; Salomão, D.; Elias, L.M.; Patricio, F.R.A. Effect of temperature on mycelial growth of Trichoderma, Sclerotinia minor and S. sclerotiorum, as well as on mycoparasitism. Summa Phytopathol. Botucatu 2016, 42, 222–227. [Google Scholar] [CrossRef]
- Trushina, N.; Levin, M.; Mukherjee, P.K.; Horwitz, B.A. PacC and pH-dependent transcriptome of the mycotrophic fungus Trichoderma virens. BMC Genom. 2013, 14, 138. [Google Scholar] [CrossRef]
- Singh, A.; Shahid, M.; Srivastava, M.; Pandey, S.; Sharma, A.; Kumar, V. Optimal physical parameters for growth of Trichoderma species at varying pH, temperature and agitation. Virol Mycol 2014, 3, 127. [Google Scholar]
- Larena, I.; De Cal, A.; Melgarejo, P. Effects of stabilizers on shelf-life of Epicoccum nigrum formulations and their relationship with biocontrol of postharvest brown rot by Monilinia of peaches. J. Appl. Microbiol. 2007, 102, 570–582. [Google Scholar] [CrossRef]
- Wijesinghe, C.J.; Wilson Wijeratnam, R.S.; Samarasekara, J.K.R.R.; Wijesundera, R.L.C. Development of a formulation of Trichoderma asperellum to control black rot disease on pineapple caused by (Thielaviopsis paradoxa). Crop Prot. 2011, 30, 300–306. [Google Scholar] [CrossRef]
- Paau, A.S. Formulation of beneficial organisms applied to soil. In Formulation of Microbial Biopesticides: Beneficial Microorganisms, Nematodes and Seed Treatments; Burges, H.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 235–254. [Google Scholar]
- Jin, X.; Custis, D. Microencapsulating aerial conidia of Trichoderma harzianum through spray drying at elevated temperatures. Biol. Cont. 2011, 56, 202–206. [Google Scholar] [CrossRef]

| Form. 1 | Ingredients (mL) | ||||||
|---|---|---|---|---|---|---|---|
| Coconut Oil | Palm Oil | Soybean Oil | DW | Tween 20 | Tween 80 | Spore Suspension | |
| 1 | 40 | - | - | 40 | 20 | - | X 2 |
| 2 | 40 | - | - | 40 | - | 20 | X |
| 3 | 20 | - | - | 60 | 20 | - | X |
| 4 | 20 | - | - | 60 | - | 20 | X |
| 5 | - | 40 | - | 40 | 20 | - | X |
| 6 | - | 40 | - | 40 | - | 20 | X |
| 7 | - | 20 | - | 60 | 20 | - | X |
| 8 | - | 20 | - | 60 | - | 20 | X |
| 9 | - | - | 40 | 40 | 20 | - | X |
| 10 | - | - | 40 | 40 | - | 20 | X |
| 11 | - | - | 20 | 60 | 20 | - | X |
| 12 | - | - | 20 | 60 | - | 20 | X |
| Form. 1 | Ingredient | ||||
|---|---|---|---|---|---|
| Selected Oil (mL) | DW (mL) | Dextrose (g) | Tween 20 (mL) | Spore Suspension (×108 Conidia/mL) | |
| 1 | 40 | 40 | 5 | 20 | X 2 |
| 2 | 40 | 40 | - | 20 | X |
| 3 | 45 | 45 | 5 | 10 | X |
| 4 | 45 | 45 | - | 10 | X |
| 5 | 30 | 60 | 5 | 10 | X |
| 6 | 30 | 60 | - | 10 | X |
| Form. 1 | Ratio of Coconut Oil:DW:Tween 20 (mL) | Dextrose (g) | Mixing Time (min.) |
|---|---|---|---|
| 1 | 40:40:20 | 5 | 3.21 ± 0.02 d |
| 2 | 40:40:20 | - | 2.29 ± 0.06 c |
| 3 | 45:45:10 | 5 | 3.58 ± 0.01 e |
| 4 | 45:45:10 | - | 2.25 ± 0.02 c |
| 5 | 30:60:10 | 5 | 1.25 ± 0.06 b |
| 6 | 30:60:10 | - | 1.14 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intana, W.; Wonglom, P.; Dy, K.S.; Sunpapao, A. Development of a Novel Emulsion Formulation of Trichoderma asperelloides PSU-P1 Conidia against Stem Canker on Dragon Fruit Caused by Neoscytalidium dimidiatum. Microbiol. Res. 2023, 14, 1139-1149. https://doi.org/10.3390/microbiolres14030076
Intana W, Wonglom P, Dy KS, Sunpapao A. Development of a Novel Emulsion Formulation of Trichoderma asperelloides PSU-P1 Conidia against Stem Canker on Dragon Fruit Caused by Neoscytalidium dimidiatum. Microbiology Research. 2023; 14(3):1139-1149. https://doi.org/10.3390/microbiolres14030076
Chicago/Turabian StyleIntana, Warin, Prisana Wonglom, Kim Sreang Dy, and Anurag Sunpapao. 2023. "Development of a Novel Emulsion Formulation of Trichoderma asperelloides PSU-P1 Conidia against Stem Canker on Dragon Fruit Caused by Neoscytalidium dimidiatum" Microbiology Research 14, no. 3: 1139-1149. https://doi.org/10.3390/microbiolres14030076
APA StyleIntana, W., Wonglom, P., Dy, K. S., & Sunpapao, A. (2023). Development of a Novel Emulsion Formulation of Trichoderma asperelloides PSU-P1 Conidia against Stem Canker on Dragon Fruit Caused by Neoscytalidium dimidiatum. Microbiology Research, 14(3), 1139-1149. https://doi.org/10.3390/microbiolres14030076






