Lactic Bacteria with Plant-Growth-Promoting Properties in Potato
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Starch Agar
2.3. Cellulolytic Activity
2.4. Production of Indoleacetic Acid
2.5. P Quantification in Test Tubes
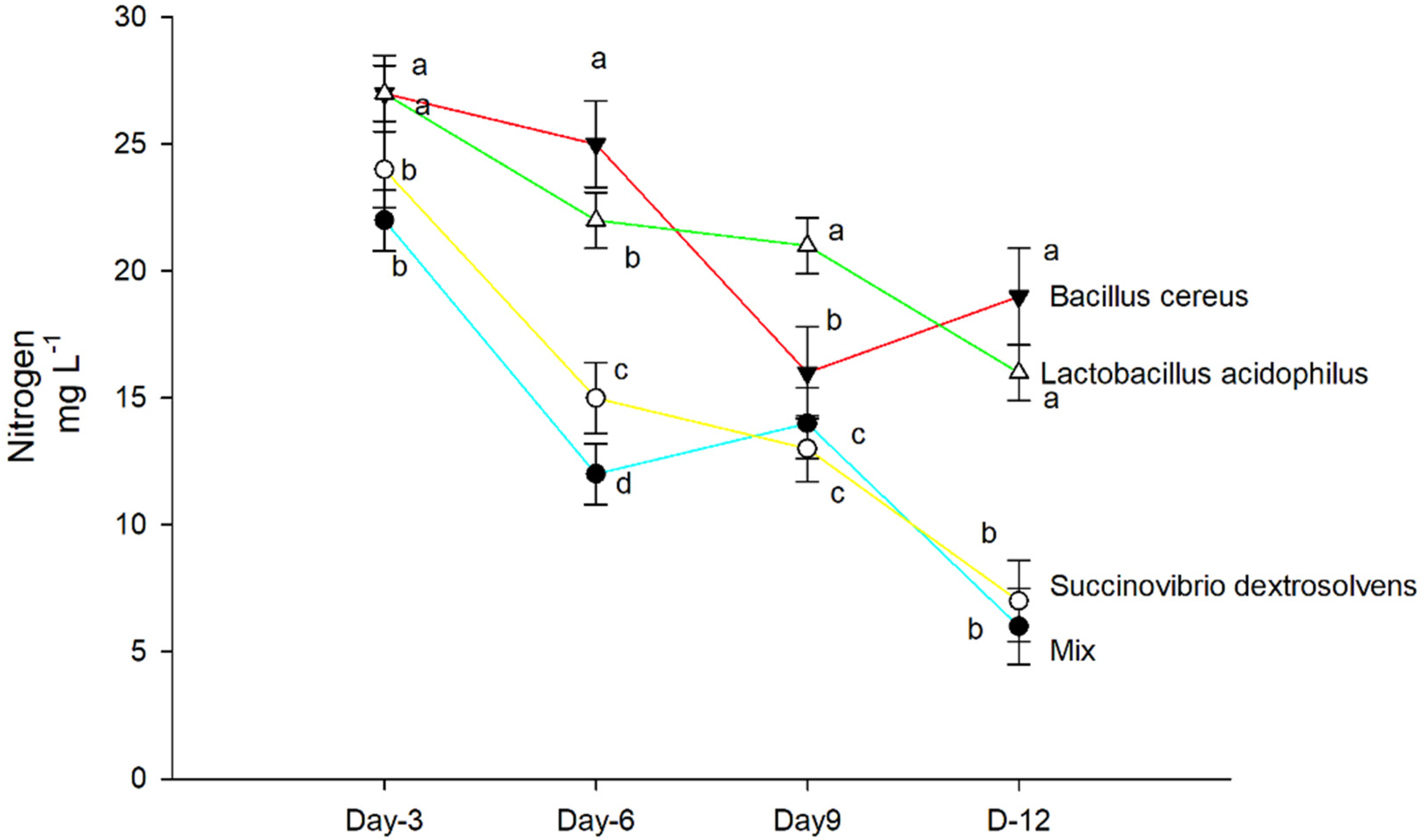
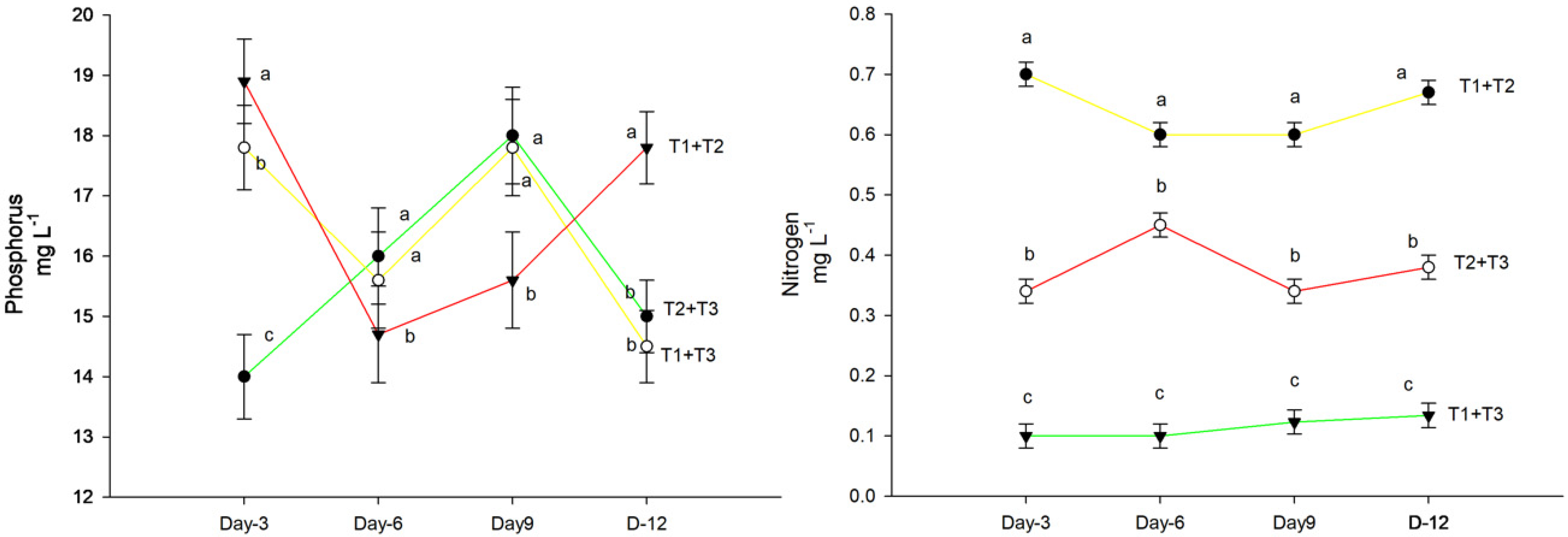
2.6. Nitrogen Quantification in Test Tubes
2.7. Planting
2.8. Inoculations
3. Evaluations in Potato Plants
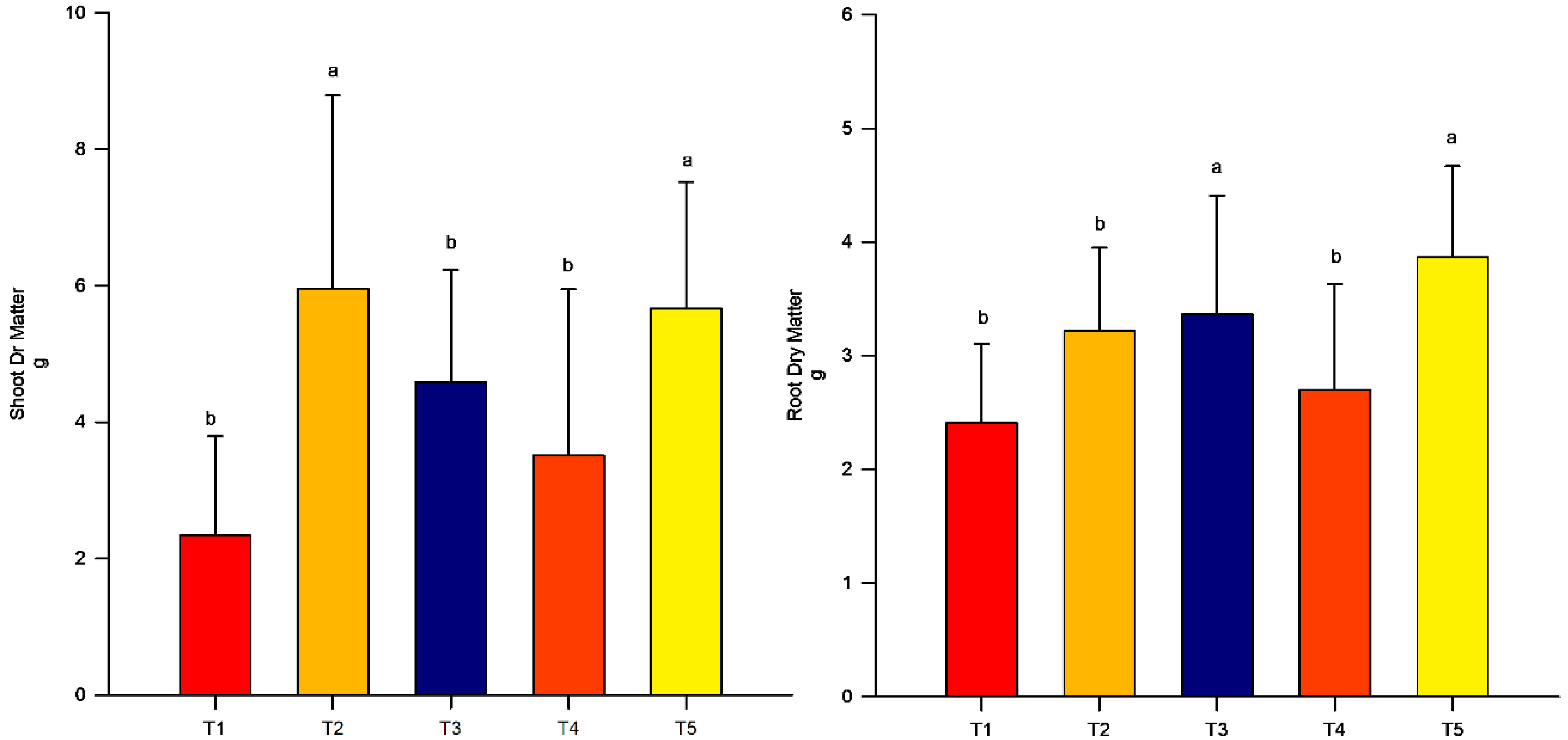
3.1. Dry Mass
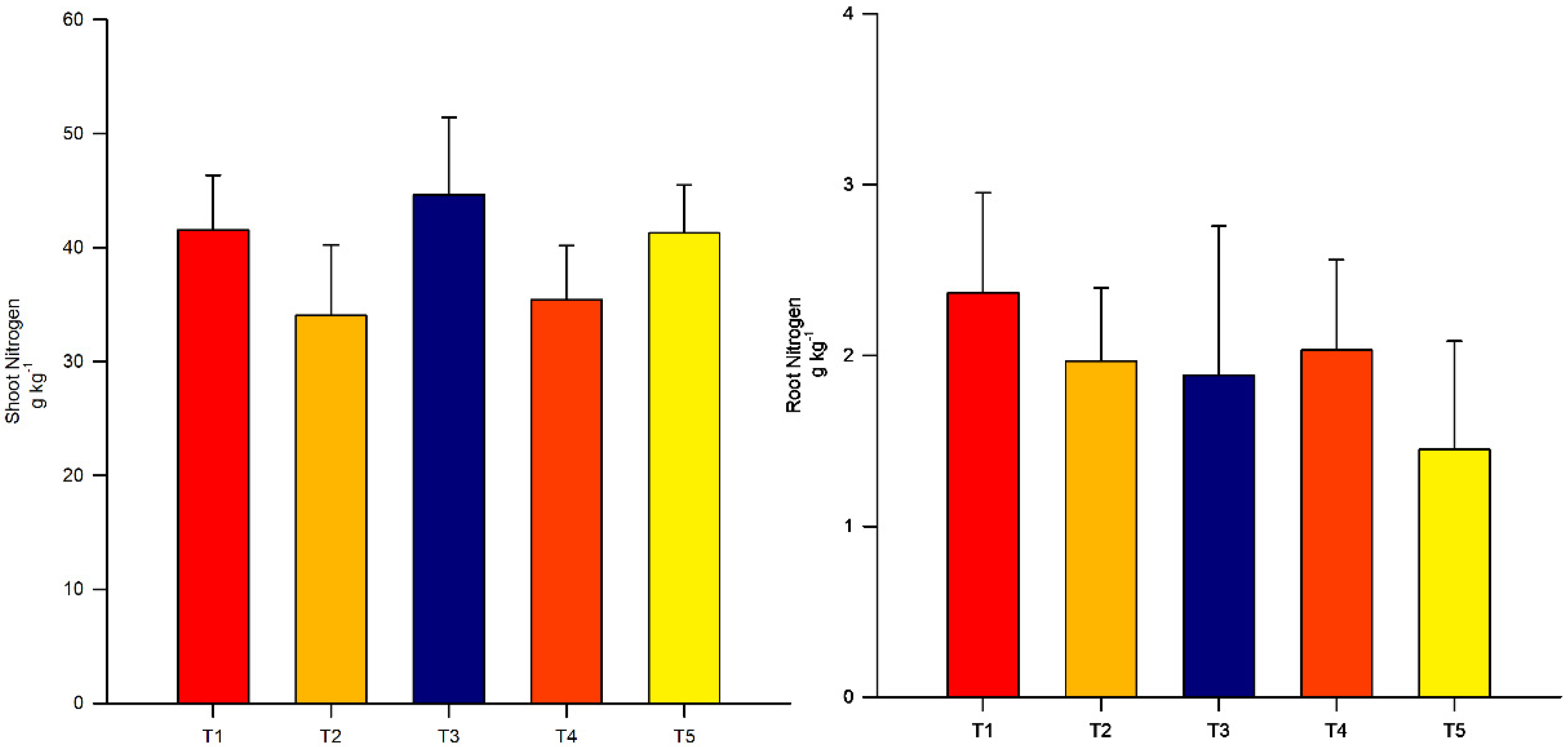
3.2. Nitrogen Concentration in Shoots and Roots
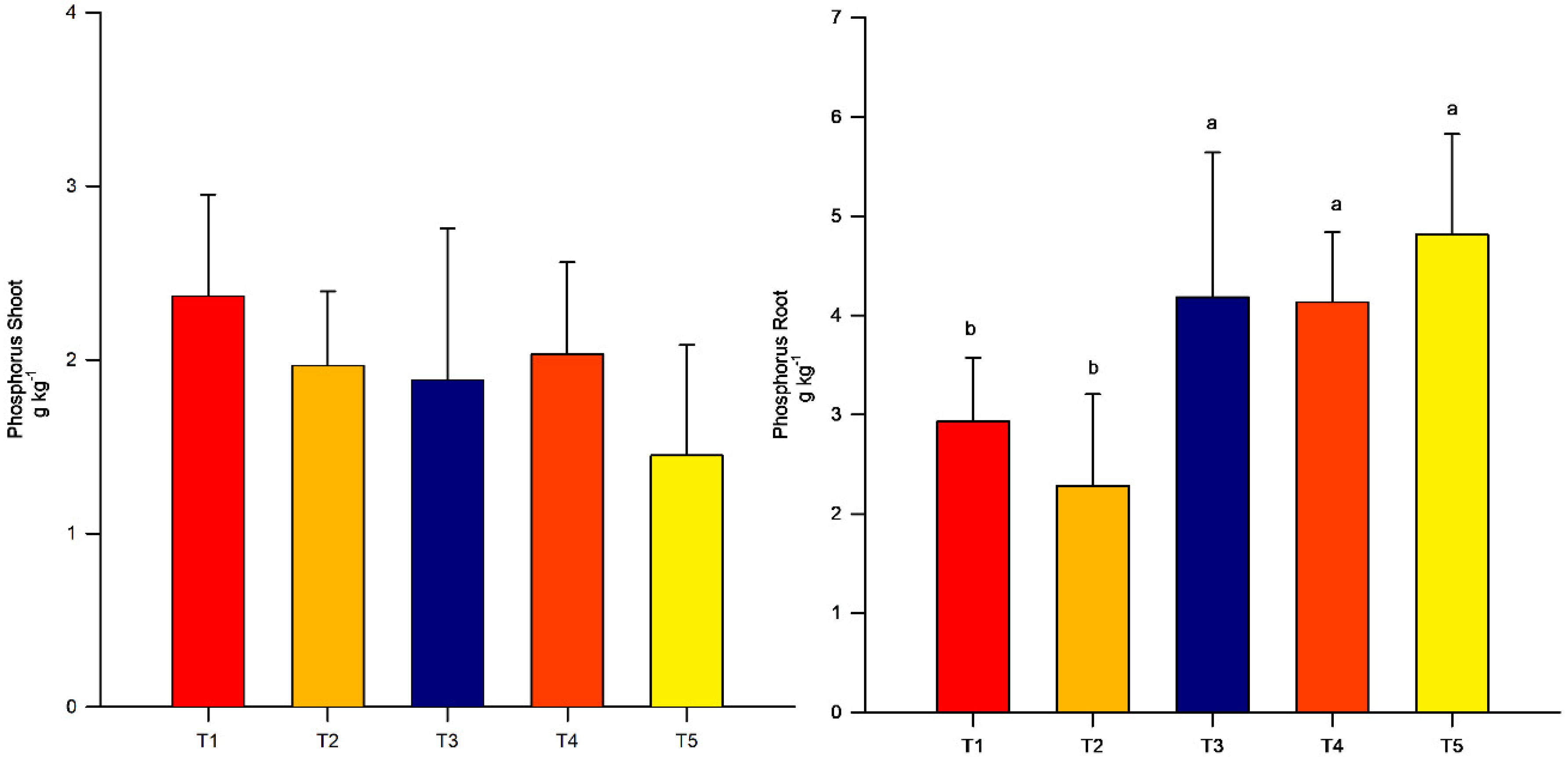
3.3. Shoot and Root Phosphorus Concentrations
3.4. Statistical Analysis
4. Results
Dry Matter
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, Y.; Fall, T.; Su, Z.; Bortolozo, F.; Mussoline, W.; England, G.; Dinkins, D.; Morgan, K.; Clark, M.; Liu, G. Effect of Phosphorus Fertilization on Yield of Chipping Potato Grown on High Legacy Phosphorus Soil. Agronomy 2022, 12, 812. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotox. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Babalola, O.O.; Prigent-Combaret, C. Impacts of Microbial Inoculants on the Growth and Yield of Maize Plant. Open Agric. J. 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Aziz, U.; Rehmani, M.S.; Wang, L.; Luo, X.; Xian, B.; Wei, S.; Wang, G.; Shu, K. Toward a Molecular Understanding of Rhizosphere, Phyllosphere, and Spermosphere Interactions in Plant Growth and Stress Response. Crit. Rev. Plant Sci. 2022, 40, 479–500. [Google Scholar] [CrossRef]
- Lobo, L.L.B.; de Andrade da Silva, M.S.R.; Castellane, T.C.L.; Carvalho, R.F.; Rigobelo, E.C. Effect of Indole-3-Acetic Acid on Tomato Plant Growth. Microorganisms 2022, 10, 2212. [Google Scholar] [CrossRef]
- Hu, X.F.; Chen, J.S.; Guo, J.F. Two phosphate- and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J. Microbiol. Biotechnol. 2006, 22, 983–990. [Google Scholar] [CrossRef]
- Huang, P.; Xu, J.; Kloepper, J.W. Plant–microbe–soil fertility interaction impacts performance of a Bacillus-containing bioproduct on bell pepper. J. Basic Microbiol. 2020, 60, 27–36. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Ana, C.d.S.; Saveetha, K.; Everlon, C.R. Bacillus cereus, Lactobacillus acidophilus and Succinovibrio dextrinosolvens promoting the growth of maize and soybean plants. Afr. J. Microbiol. Res. 2020, 14, 189–197. [Google Scholar] [CrossRef]
- Ramachandra, M.; Crawford, D.L.; Pometto, A.L. Extracellular enzyme-activities during lignocellulose degradation by Streptomyces spp.—A comparative-study of wild-type and genetically manipulated strains. Appl. Environ. Microbiol. 1987, 53, 2754–2760. [Google Scholar] [CrossRef]
- Pupin, B.; Nahas, E. Impact of successive sugarcane harvests and trash management practices on soil microbiological properties. Soil Res. 2011, 49, 183–189. [Google Scholar] [CrossRef]
- Kuss, A.V.; Kuss, V.V.; Lovato, T.; Flôres, M.L. Nitrogen fixation and in vitro production of indolacetic acid by endophytic diazotrophic bacteria. Pesqui. Agropecuária Bras. 2007, 42, 1459–1465. [Google Scholar] [CrossRef]
- Bremmer, J.; Mulvaney, C. Salicylic Acid Thisolfate Modification of Kjeldahl Method to Include Nitrate and Nitrite. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1982, 2, 621–622. [Google Scholar]
- Haag, H.; Sarruge, J.; de Oliveira, G.; Dechen, A. Nutrição mineral do cajueiro (Anacardium occidentale L.): I-deficiência dos macronutrientes-nota prévia. An. Esc. Super. Agric. Luiz Queiroz 1975, 32, 185–190. [Google Scholar] [CrossRef]
- Barbosa, J.; Maldonado Júnior, W. AgroEstat: Sistema para análises estatísticas de ensaios agronômicos. Jaboticabal Fac. Ciências Agrárias Veterinárias Unesp 2010, 1, 1–296. [Google Scholar]
- Aljebourya, G.H.; Mahmouda, S.N. Evaluation of antagonistic potential of Lactobacillus isolates against phytopathogenic fungi and pathogenic bacteria in vitro. Syst. Rev. Pharm. 2020, 11, 1699–1703. [Google Scholar]
- Midolo, P.; Lambert, J.; Hull, R.; Luo, F.; Grayson, M. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J. Appl. Bacteriol. 1995, 79, 475–479. [Google Scholar] [CrossRef]
- Sharf, W.; Javaid, A.; Shoaib, A.; Khan, I.H. Induction of resistance in chili against Sclerotium rolfsii by plant-growth-promoting rhizobacteria and Anagallis arvensis. Egypt. J. Biol. Pest Control 2021, 31, 16. [Google Scholar] [CrossRef]
- Bhakta, J.; Munekage, Y.; Ohnishi, K.; Jana, B. Isolation and identification of cadmium-and lead-resistant lactic acid bacteria for application as metal removing probiotic. Int. J. Environ. Sci. Technol. 2012, 9, 433–440. [Google Scholar] [CrossRef]
- Pakdel, M.; Soleimanian-Zad, S.; Akbari-Alavijeh, S. Screening of lactic acid bacteria to detect potent biosorbents of lead and cadmium. Food Control 2019, 100, 144–150. [Google Scholar] [CrossRef]
- Li, X.; Ming, Q.; Cai, R.; Yue, T.; Yuan, Y.; Gao, Z.; Wang, Z. Biosorption of Cd2+ and Pb2+ from apple juice by the magnetic nanoparticles functionalized lactic acid bacteria cells. Food Control 2020, 109, 106916. [Google Scholar] [CrossRef]
- Laslo, V.; Pinzaru, S.C.; Zaguła, G.; Kluz, M.; Vicas, S.I.; Cavalu, S. Synergic effect of selenium nanoparticles and lactic acid bacteria in reduction cadmium toxicity. J. Mol. Struct. 2022, 1247, 131325. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Wang, P.; Zhang, H.; Ali, E.F.; Li, R.; Shaheen, S.M.; Zhang, Z. Lactic acid bacteria promoted soil quality and enhanced phytoextraction of Cd and Zn by mustard: A trial for bioengineering of toxic metal contaminated mining soils. Environ. Res. 2023, 216, 114646. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Najjar, A.A.; Alzahrani, S.O.; Alkhatib, F.M.; Shafi, M.E.; Selem, E.; Desoky, E.-S.M.; Fouda, S.E.; El-Tahan, A.M. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021, 28, 4461–4471. [Google Scholar] [CrossRef] [PubMed]
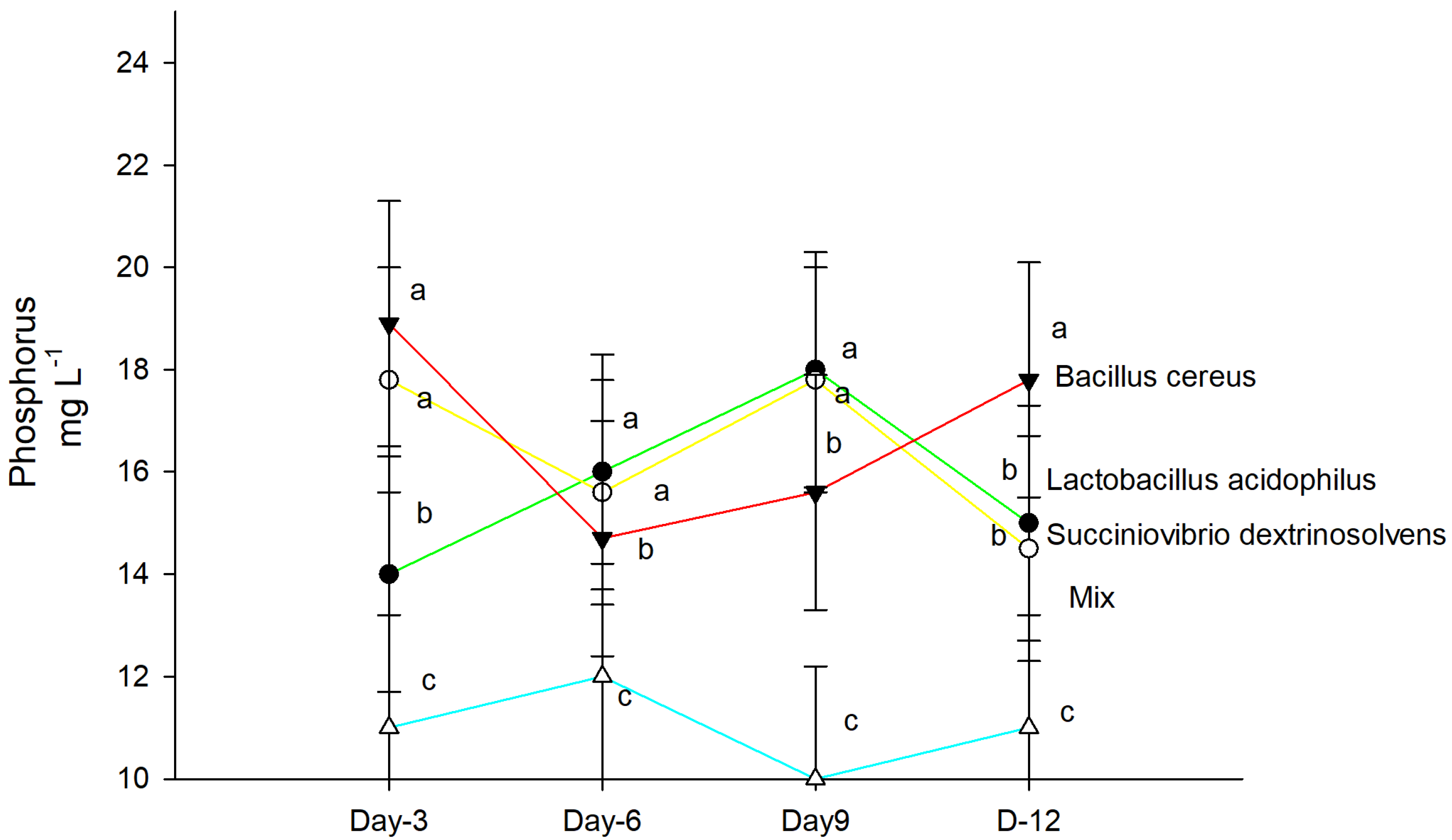
| Isolate | Siderophores | Amylolytic Activity | Cellulolytic Activity | IAA μg mL−1 | P Solubilization mg P L−1 | N Fixation mg N L−1 |
|---|---|---|---|---|---|---|
| B. cereus | - | + | - | 9.08 | 14.93 | 0.70 |
| S. dextrinosolvens | + | + | - | 10.25 | 41.38 | 0.42 |
| L. acidophilus | + | - | - | 7.25 | 5.58 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panetto, L.D.; Doria, J.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; de Andrade, L.A.; Rigobelo, E.C. Lactic Bacteria with Plant-Growth-Promoting Properties in Potato. Microbiol. Res. 2023, 14, 279-288. https://doi.org/10.3390/microbiolres14010022
Panetto LD, Doria J, Santos CHB, Frezarin ET, Sales LR, de Andrade LA, Rigobelo EC. Lactic Bacteria with Plant-Growth-Promoting Properties in Potato. Microbiology Research. 2023; 14(1):279-288. https://doi.org/10.3390/microbiolres14010022
Chicago/Turabian StylePanetto, Lilian Dutra, Joyce Doria, Carlos Henrique Barbosa Santos, Edvan Teciano Frezarin, Luziane Ramos Sales, Luana Alves de Andrade, and Everlon Cid Rigobelo. 2023. "Lactic Bacteria with Plant-Growth-Promoting Properties in Potato" Microbiology Research 14, no. 1: 279-288. https://doi.org/10.3390/microbiolres14010022
APA StylePanetto, L. D., Doria, J., Santos, C. H. B., Frezarin, E. T., Sales, L. R., de Andrade, L. A., & Rigobelo, E. C. (2023). Lactic Bacteria with Plant-Growth-Promoting Properties in Potato. Microbiology Research, 14(1), 279-288. https://doi.org/10.3390/microbiolres14010022






