Effects of Different Nutritional Zinc Forms on the Proliferation of Beneficial Commensal Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Test Compounds
2.3. MIC
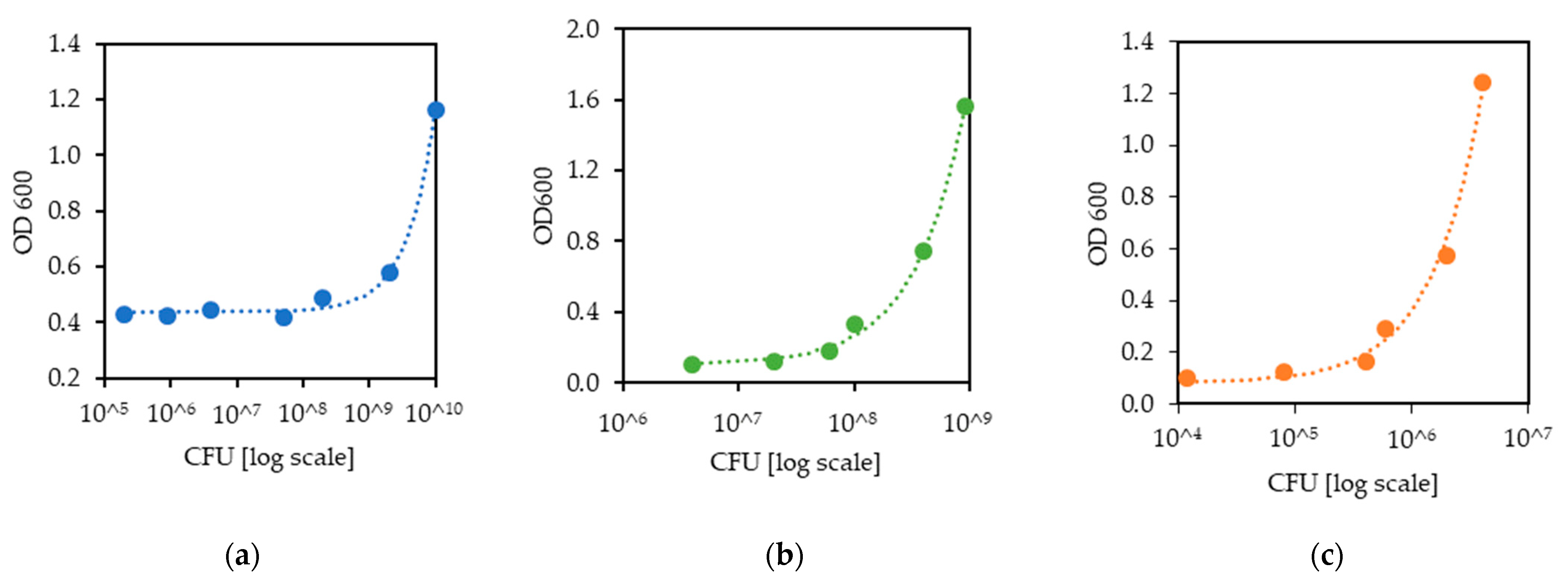
2.4. Correction between CFU and OD
2.5. Growth Kinetics
2.6. Statistical Analysis
3. Results
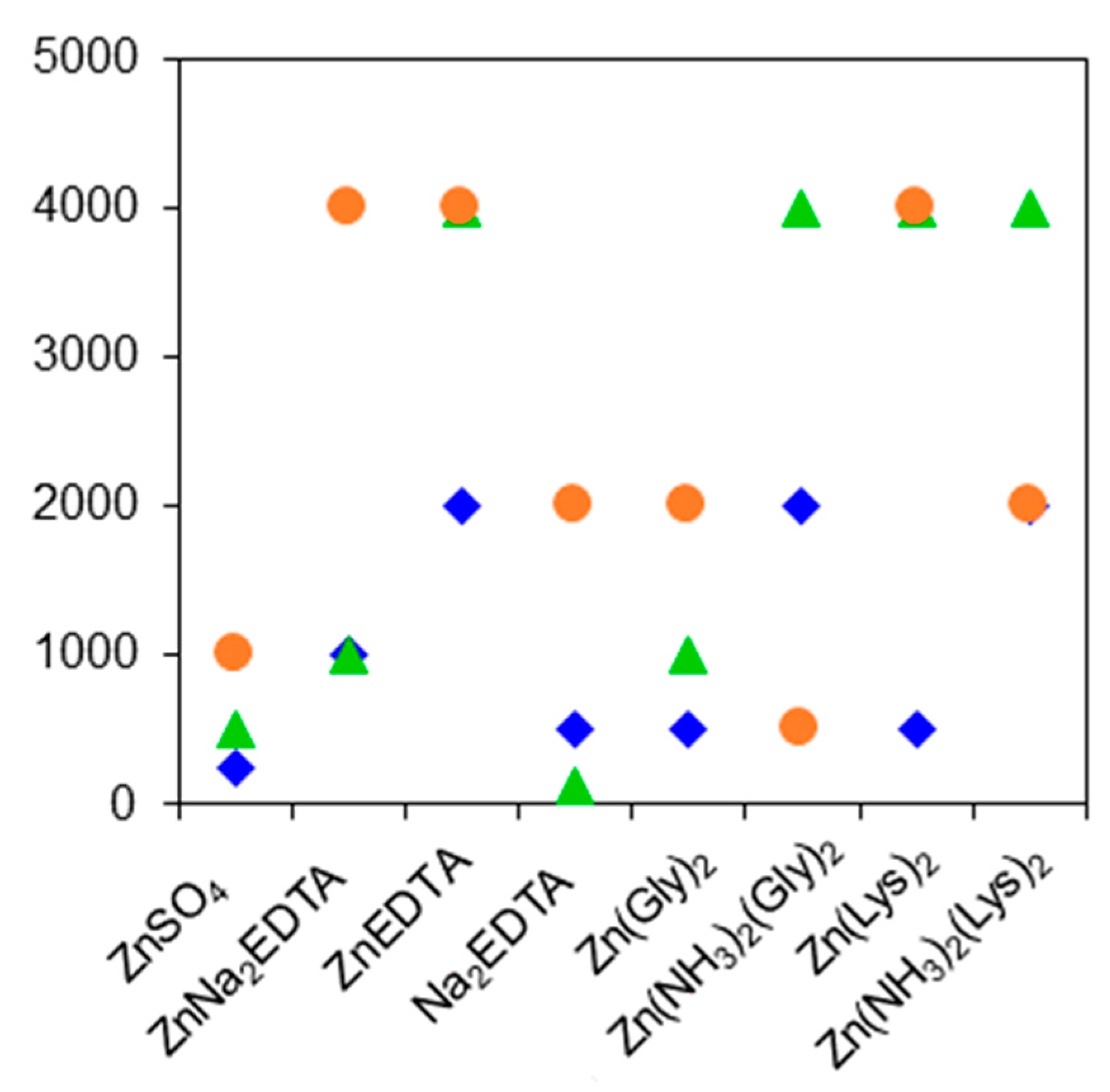
3.1. MIC
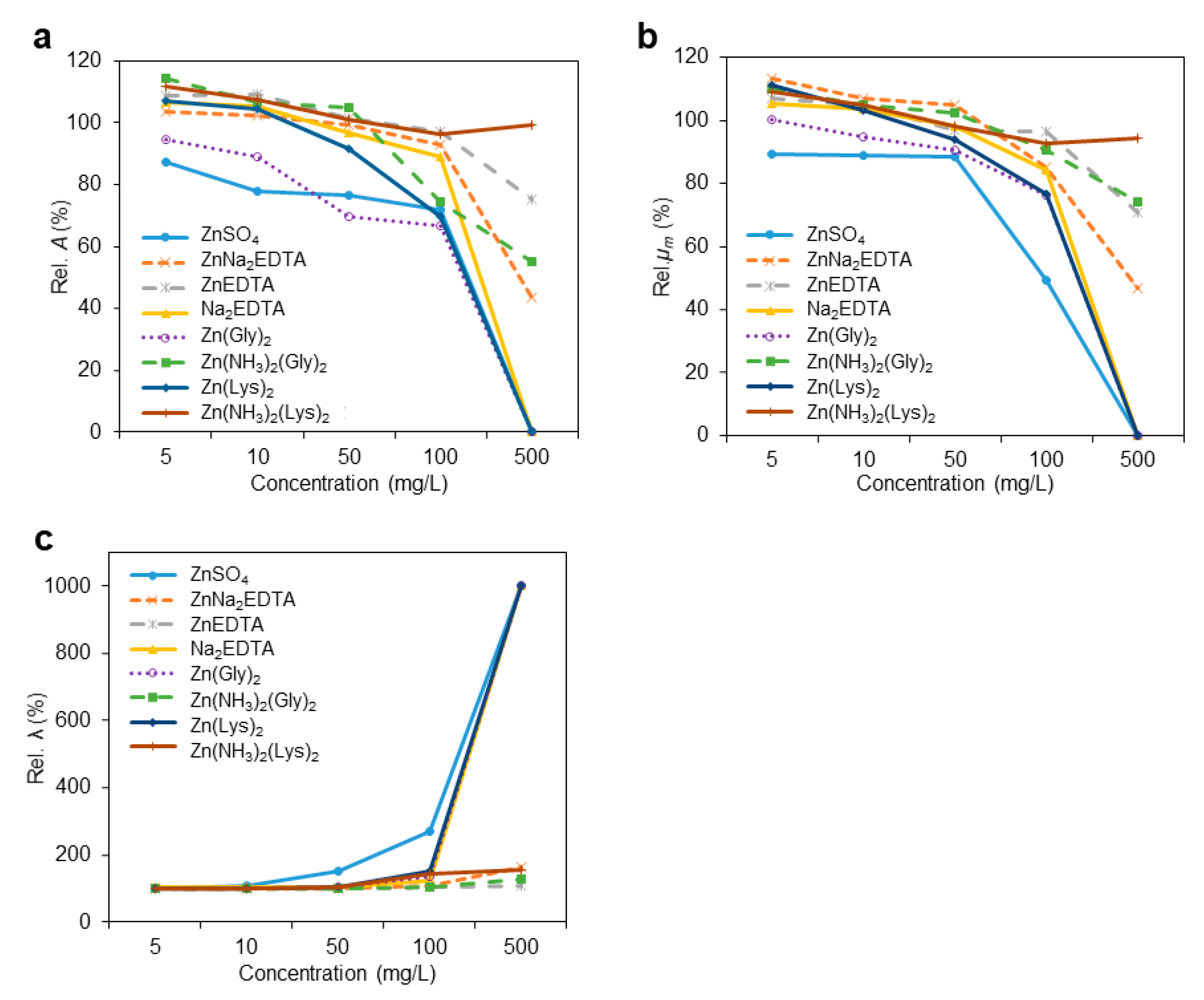
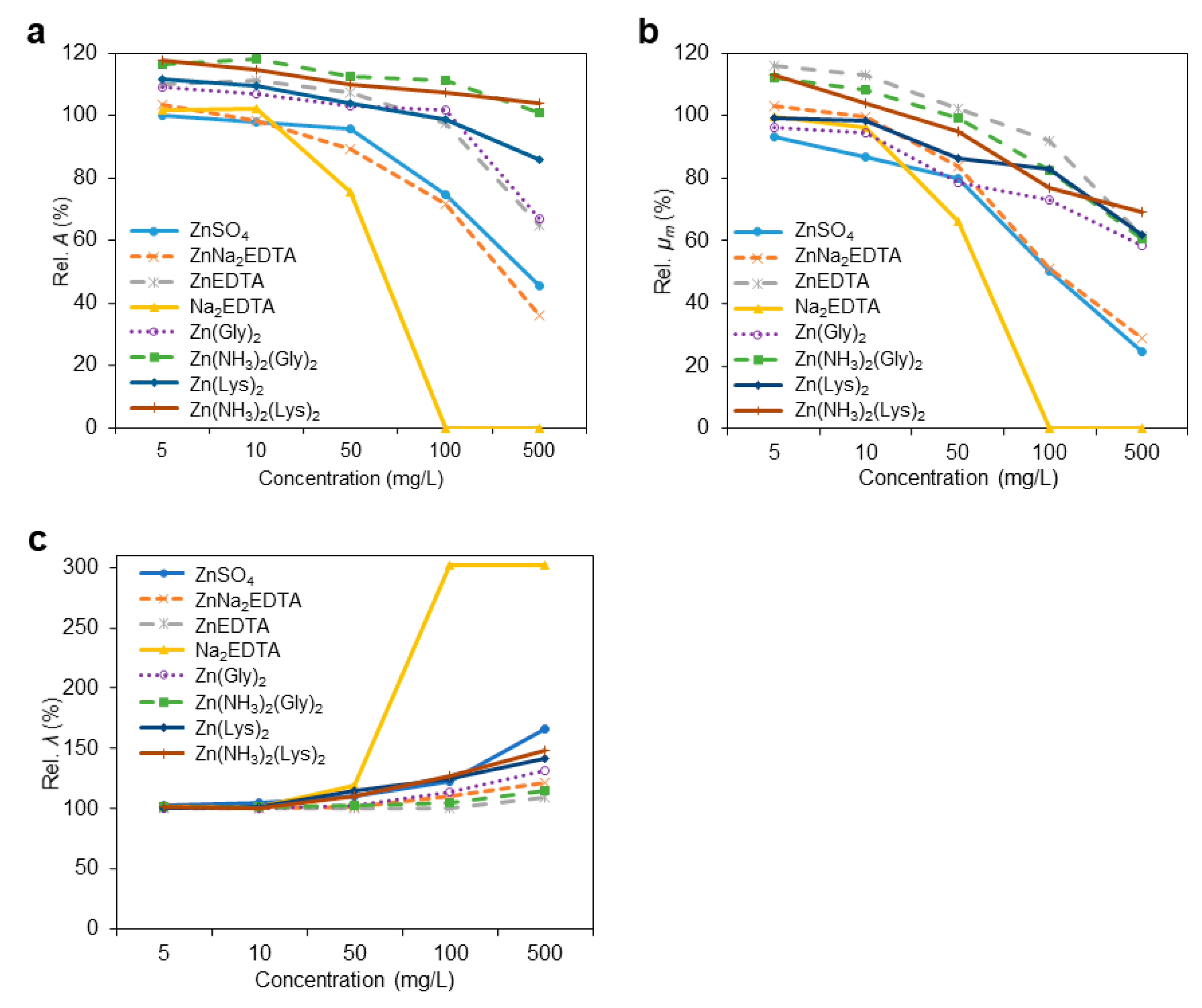
3.2. Growth Kinetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anders, J.L.; Moustafa, M.A.M.; Mohamed, W.M.A.; Hayakawa, T.; Nakao, R.; Koizumi, I. Comparing the gut microbiome along the gastrointestinal tract of three sympatric species of wild rodents. Sci. Rep. 2021, 11, 19929. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef]
- Tellez-Isaias, G.; Latorre, J.D. Editorial: Alternatives to Antimicrobial Growth Promoters and Their Impact in Gut Microbiota, Health and Disease: Volume II. Front. Vet. Sci. 2022, 9, 857583. [Google Scholar] [CrossRef] [PubMed]
- Neish, A.S. Microbes in gastrointestinal health and disease. Gastroenterology 2009, 136, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Leser, T.D.; Mølbak, L. Better living through microbial action: The benefits of the mammalian gastrointestinal microbiota on the host. Environ. Microbiol. 2009, 11, 2194–2206. [Google Scholar] [CrossRef]
- Ryan, M.; Schloter, M.; Berg, G.; Kostic, T.; Kinkel, L.; Eversole, K.; Macklin, J.; Schelkle, B.; Kazou, M.; Sarand, I.; et al. Development of Microbiome Biobanks-Challenges and Opportunities. Trends Microbiol. 2021, 29, 89–92. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. The Gut Microbiota Influenced by the Intake of Probiotics and Functional Foods with Prebiotics Can Sustain Wellness and Alleviate Certain Ailments like Gut-Inflammation and Colon-Cancer. Microorganisms 2022, 10, 665. [Google Scholar] [CrossRef]
- Blandino, G.; Inturri, R.; Lazzara, F.; Di Rosa, M.; Malaguarnera, L. Impact of gut microbiota on diabetes mellitus. Diabetes Metab. 2016, 42, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Schneiderhan, J.; Master-Hunter, T.; Locke, A. Targeting gut flora to treat and prevent disease. J. Fam. Pract. 2016, 65, 34–38. [Google Scholar] [PubMed]
- Ganatsios, V.; Nigam, P.; Plessas, S.; Terpou, A. Kefir as a Functional Beverage Gaining Momentum towards Its Health Promoting Attributes. Beverages 2021, 7, 48. [Google Scholar] [CrossRef]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.-L.; Kho, W.-L.; You, S.-H.; Yeh, R.-H.; Tang, S.-W.; Hsieh, C.-W. Effects of Bacillus subtilis var. natto and Saccharomyces cerevisiae mixed fermented feed on the enhanced growth performance of broilers. Poult. Sci. 2009, 88, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, M.K.P.; Nakandakare, I.B.; Terhune, J.S.; Wood, T.; Paiva, M.J.R. Dietary supplementation with Bacillus subtilis, Saccharomyces cerevisiae and Aspergillus oryzae enhance immunity and disease resistance against Aeromonas hydrophila and Streptococcus iniae infection in juvenile tilapia Oreochromis niloticus. Fish Shellfish Immunol. 2015, 43, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Darby, T.M.; Owens, J.; Saeedi, B.; Luo, L.; Matthews, J.D.; Robinson, B.S.; Naudin, C.; Jones, R.M. Lactococcus Lactis Subsp. cremoris Is an Efficacious Beneficial Bacterium that Limits Tissue Injury in the Intestine. iScience 2019, 12, 356–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lacerda, J.R.M.; Da Silva, T.F.; Vollú, R.E.; Marques, J.M.; Seldin, L. Generally recognized as safe (GRAS) Lactococcus lactis strains associated with Lippia sidoides Cham. are able to solubilize/mineralize phosphate. Springerplus 2016, 5, 828. [Google Scholar] [CrossRef] [Green Version]
- Elshaghabee, E.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, W.; Lv, Z.; Liu, D.; Guo, Y. Bacillus subtilis and yeast cell wall improve the intestinal health of broilers challenged by Clostridium perfringens. Br. Poult. Sci. 2017, 58, 635–643. [Google Scholar] [CrossRef]
- Granstad, S.; Kristoffersen, A.B.; Benestad, S.L.; Sjurseth, S.K.; David, B.; Sørensen, L.; Fjermedal, A.; Edvardsen, D.H.; Sanson, G.; Løvland, A.; et al. Effect of Feed Additives as Alternatives to In-feed Antimicrobials on Production Performance and Intestinal Clostridium perfringens Counts in Broiler Chickens. Animals 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobierecka, P.A.; Eolech, B.; eKsiążek, M.; Ederlatka, K.; Adamska, I.; Majewski, P.; Jagusztyn-Krynicka, E.; Wyszyńska, A.K. Cell Wall Anchoring of the Campylobacter Antigens to Lactococcus lactis. Front. Microbiol. 2016, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Jozic, D.; Bourenkow, G.; Bartunik, H.; Scholze, H.; Dive, V.; Henrich, B.; Huber, R.; Bode, W.; Maskos, K. Crystal structure of the dinuclear zinc aminopeptidase PepV from Lactobacillus delbrueckii unravels its preference for dipeptides. Structure 2002, 10, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-S.; Christensen, J.E.; Broadbent, J.R.; Steele, J.L. Identification and characterization of Lactobacillus helveticus PepO2, an endopeptidase with post-proline specificity. Appl. Environ. Microbiol. 2003, 69, 1276–1282. [Google Scholar] [CrossRef] [Green Version]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. Int. Rev. J. 2013, 4, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Capdevila, D.A.; Wang, J.; Giedroc, D.P. Bacterial Strategies to Maintain Zinc Metallostasis at the Host-Pathogen Interface. J. Biol. Chem. 2016, 291, 20858–20868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-X.; Tian, Q.-J.; Liang, S.-C.; Zhou, Y.-Y.; Zou, H.-C. Bioaccumulation of heavy metals by the dominant plants growing in Huayuan manganese and lead/zinc mineland, Xiangxi. Huan Jing Ke Xue 2012, 33, 2038–2045. [Google Scholar]
- Milani, N.; Sbardella, M.; Ikeda, N.; Arno, A.; Mascarenhas, B.; Miyada, V. Dietary zinc oxide nanoparticles as growth promoter for weanling pigs. Anim. Feed Sci. Technol. 2017, 227, 13–23. [Google Scholar] [CrossRef]
- Fontecha-Umaña, F.; Ríos-Castillo, A.G.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Antimicrobial Activity and Prevention of Bacterial Biofilm Formation of Silver and Zinc Oxide Nanoparticle-Containing Polyester Surfaces at Various Concentrations for Use. Foods 2020, 9, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surjawidjaja, J.E.; Hidayat, A.; Lesmana, M. Growth inhibition of enteric pathogens by zinc sulfate: An in vitro study. Med. Princ. Pract. 2004, 13, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Osinaga, P.W.; Grande, R.H.M.; Ballester, R.Y.; Simionato, M.R.L.; Rodrigues, C.R.M.D.; Muench, A. Zinc sulfate addition to glass-ionomer-based cements: Influence on physical and antibacterial properties, zinc and fluoride release. Dent. Mater. 2003, 19, 212–217. [Google Scholar] [CrossRef]
- Theophel, K.; Schacht, V.J.; Schlã¼Ter, M.; Schnell, S.; Stingu, C.-S.; Schaumann, R.; Bunge, M. The importance of growth kinetic analysis in determining bacterial susceptibility against antibiotics and silver nanoparticles. Front. Microbiol. 2014, 5, 544. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, F.; Ardestani, F.; Najafpour, G. Growth kinetic models of five species of Lactobacilli and lactose consumption in batch submerged culture. Braz. J. Microbiol. 2017, 48, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, M.D.; Rice, C.J.; Lucchini, S.; Pin, C.; Thompson, A.; Cameron, A.; Alston, M.; Stringer, M.F.; Betts, R.P.; Baranyi, J.; et al. Lag Phase is a Distinct Growth Phase that Prepares Bacteria for Exponential Growth and Involves Transient Metal Accumulation. J. Bacteriol. 2012, 194, 686–701. [Google Scholar] [CrossRef] [Green Version]
- Rudilla, H.; Merlos, A.; Sans-Serramitjana, E.; Fuste, E.; Sierra, J.M.; Zalacain, A.; Vinuesa, T.; Vinas, M. New and old tools to evaluate new antimicrobial peptides. AIMS Microbiol. 2018, 4, 522–540. [Google Scholar] [CrossRef] [PubMed]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalgaard, P.; Koutsoumanis, K. Comparison of maximum specific growth rates and lag times estimated from absorbance and viable count data by different mathematical models. J. Microbiol. Methods 2001, 43, 183–196. [Google Scholar] [CrossRef]
- Burdett, I.D.; Kirkwood, T.B.; Whalley, J.B. Growth kinetics of individual Bacillus subtilis cells and correlation with nucleoid extension. J. Bacteriol. 1986, 167, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivares-Marin, I.K.; González-Hernández, J.C.; Regalado-Gonzalez, C.; Madrigal-Perez, L.A. Saccharomyces cerevisiae Exponential Growth Kinetics in Batch Culture to Analyze Respiratory and Fermentative Metabolism. J. Vis. Exp. 2018, 139, e58192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Committee for Antimicrobial Susceptibility Testing of the European Society of Clinical, Microbiology; Infectious Diseases (ESCMID). EUCAST Definitive Document E.DEF 3.1, June 2000: Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howland, J.L. Biochemical Education. Short Protocols in Molecular Biology, Third Edition; Ausubel, F., Brent, E., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., Struhl, K., Eds.; John Wiley & Sons: New York, NY, USA, 1996; Volume 24, pp. 68, 836. [Google Scholar] [CrossRef]
- Larsen, N.; Boye, M.; Siegumfeldt, H.; Jakobsen, M. Differential expression of proteins and genes in the lag phase of Lactococcus lactis subsp. lactis grown in synthetic medium and reconstituted skim milk. Appl. Environ. Microbiol. 2006, 72, 1173–1179. [Google Scholar] [CrossRef] [Green Version]
- Gilpin, R.W.; Patterson, S.K.; A Knight, R. Quantitation of bacillus subtilis L-form growth parameters in batch culture. J. Bacteriol. 1981, 145, 651–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco, E.; Wang, S.; Sanet, M.; Fernández-Vázquez, J.; Jové, D.; Glaría, E.; Valledor, A.F.; O’Halloran, T.V.; Balsalobre, C. A new role for Zinc limitation in bacterial pathogenicity: Modulation of α-hemolysin from uropathogenic Escherichia coli. Sci. Rep. 2018, 8, 6535. [Google Scholar] [CrossRef] [Green Version]
- Brugger, D.; Windisch, W.M. Strategies and challenges to increase the precision in feeding zinc to monogastric livestock. Anim. Nutr. 2017, 3, 103–108. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sales, J. Effects of pharmacological concentrations of dietary zinc oxide on growth of post-weaning pigs: A meta-analysis. Biol. Trace Elem. Res. 2013, 152, 343–349. [Google Scholar] [CrossRef]
- Davin, R.; Manzanilla, E.; Klasing, K.; Pérez, J. Effect of weaning and in-feed high doses of zinc oxide on zinc levels in different body compartments of piglets. J. Anim. Physiol. Anim. Nutr. 2013, 97 (Suppl. S1), 6–12. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, J.; Vahjen, W. In vitro antibacterial activity of zinc oxide on a broad range of reference strains of intestinal origin. Vet. Microbiol. 2012, 160, 251–255. [Google Scholar] [CrossRef]
- Starke, I.C.; Pieper, R.; Neumann, K.; Zentek, J.; Vahjen, W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol. Ecol. 2014, 87, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, E.G.; Albayrak, S. Determination of the effect of gentamicin against staphylococcus aureus by using microbroth kinetic system. Biology 2009, 23, 110–114. [Google Scholar]
- Hulankova, R. The Influence of Liquid Medium Choice in Determination of Minimum Inhibitory Concentration of Essential Oils against Pathogenic Bacteria. Antibiotics 2022, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Spears, J.W.; Schlegel, P.; Seal, M.C.; Lloyd, K.E. Bioavailability of zinc from zinc sulfate and different organic zinc sources and their effects on ruminal volatile fatty acid proportions. Livest. Prod. Sci. 2004, 90, 211–217. [Google Scholar] [CrossRef]
- Alimohamady, R.; Aliarabi, H.; Bruckmaier, R.M.; Christensen, R.G. Effect of Different Sources of Supplemental Zinc on Performance, Nutrient Digestibility, and Antioxidant Enzyme Activities in Lambs. Biol. Trace Elem. Res. 2019, 189, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Van Heugten, E.; Spears, J.W.; Kegley, E.B.; Ward, J.D.; Qureshi, M.A. Effects of organic forms of zinc on growth performance, tissue zinc distribution, and immune response of weanling pigs. J. Anim. Sci. 2003, 81, 2063–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieper, R.; Dadi, T.H.; Pieper, L.; Vahjen, W.; Franke, A.; Reinert, K.; Zentek, J. Concentration and chemical form of dietary zinc shape the porcine colon microbiome, its functional capacity and antibiotic resistance gene repertoire. ISME J. 2020, 14, 2783–2793. [Google Scholar] [CrossRef]
- Lin, S.; Lin, X.; Yang, Y.; Li, F.; Luo, L. Comparison of chelated zinc and zinc sulfate as zinc sources for growth and immune response of shrimp (Litopenaeus vannamei). Aquaculture 2013, 406–407, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Morowitz, M.J.; Carlisle, E.; Alverdy, J.C. Contributions of intestinal bacteria to nutrition and metabolism in the critically Ill. Surg. Clin. North Am. 2011, 91, 771–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celi, P.; Cowieson, A.; Fru-Nji, F.; Steinert, R.; Kluenter, A.-M.; Verlhac, V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Hsueh, Y.-H.; Ke, W.-J.; Hsieh, C.-T.; Lin, K.-S.; Tzou, D.-Y.; Chiang, C.-L. ZnO Nanoparticles Affect Bacillus subtilis Cell Growth and Biofilm Formation. PLoS ONE 2015, 10, e0128457. [Google Scholar] [CrossRef] [Green Version]
- Damaskos, D.; Kolios, G. Probiotics and prebiotics in inflammatory bowel disease: Microflora ‘on the scope’. Br. J. Clin. Pharmacol. 2008, 65, 453–467. [Google Scholar] [CrossRef] [Green Version]
- Wijburg, O.L.; Uren, T.K.; Simpfendorfer, K.; Johansen, F.-E.; Brandtzaeg, P.; Strugnell, R. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J. Exp. Med. 2006, 203, 21–26. [Google Scholar] [CrossRef] [PubMed]

| Microorganism (M) | B. subtilis | L. lactis | S. cerevisiae | SEM | p Value | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds (C) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | M | C | M*C | |
| 5 mg/L | ||||||||||||||||||||||||||||
| 1 Rel. A, % | 87.5 *i | 103.7 *ef | 108.7 *d | 106.8 *de | 94.8 *h | 114.2 *b | 107.2 *de | 111.6 *c | 100.1 g | 103.4 f | 110.8 *cd | 101.6 fg | 109.0 *d | 116.1 *ab | 111.3 *cd | 117.6 *a | 113.6 *bc | 113.3 *bc | 112.2 *cd | 112.6 *cd | 105.7 *ef | 107.5 *e | 101.4 g | 105.7 *ef | 0.8692 | <0.0001 | <0.0001 | <0.0001 |
| Rel. μm, % | 89.3 *f | 113.1 *b | 107.0 *bcd | 105.1 cd | 100.2 cde | 109.9 *bc | 111.1 *b | 109.0 *bc | 93.6 *f | 103.0 de | 116.1 *a | 99.9 e | 96.2 ef | 112.4 *a | 99.3 de | 113.0 *ab | 102.1 cde | 103.4 cde | 106.7 *cd | 102.2 cde | 102.8 cde | 101.9 cde | 103.9 cde | 104.3 cd | 1.6138 | 0.0264 | <0.0001 | <0.0001 |
| Rel. λ, % | 99.9 b | 99.0 b | 100.3 b | 102.2 a | 101.2 ab | 100.0 b | 100.9 ab | 99.7 b | 102.4 a | 101.0 ab | 99.9 b | 101.3 ab | 100.5 b | 101.2 ab | 100.8 ab | 101.1 ab | 100.8 ab | 99.8 b | 100.6 b | 101.6 ab | 101.3 ab | 101.7 ab | 101.5 ab | 101.0 ab | 0.5620 | 0.0392 | 0.0105 | 0.0809 |
| 10 mg/L | ||||||||||||||||||||||||||||
| Rel. A, % | 77.8 *i | 102.4 ef | 109.3 *cd | 105.2 *de | 88.9 *h | 106.7 *de | 104.6 *e | 107.5 *d | 97.8 g | 98.0 g | 111.1 *c | 102.1 f | 106.5 *de | 117.8 *a | 109.5 *cd | 114.6 *b | 113.7 *bc | 112.8 *c | 110.5 *c | 111.4 *c | 100.0 g | 99.5 g | 100.4 g | 106.5 *de | 0.8229 | <0.0001 | <0.0001 | <0.0001 |
| Rel. μm, % | 89.0 *e | 107.0 *bc | 104.7 bc | 103.5 c | 94.9 d | 104.8 bc | 103.2 c | 104.8 bc | 87.1 *e | 99.9 cd | 113.3 *a | 96.5 d | 94.7 d | 108.3 *b | 98.3 d | 104.0 bc | 101.1 cd | 102.3 cd | 98.4 d | 99.0 cd | 95.1 d | 98.2 d | 98.0 d | 104.6 bc | 1.6139 | 0.0636 | <0.0001 | <0.0001 |
| Rel. λ, % | 108.1 *a | 99.8 c | 101.1 bc | 103.4 b | 100.6 c | 100.4 c | 100.2 c | 100.0 c | 104.3 *b | 100.7 c | 100.8 c | 101.4 bc | 100.4 c | 101.0 c | 101.5 bc | 100.8 c | 100.4 c | 99.9 c | 100.8 bc | 101.2 bc | 101.4 bc | 101.2 bc | 101.3 bc | 102.1 bc | 0.5523 | 0.0520 | <0.0001 | <0.0001 |
| 50 mg/L | ||||||||||||||||||||||||||||
| Rel. A, % | 76.6 *j | 99.2 f | 101.6 e | 96.6 g | 69.6 *k | 104.7 *d | 91.7 *h | 101.0 ef | 95.7 *g | 89.0 *i | 107.0 *c | 75.5 *j | 102.9 de | 112.5 *a | 103.7 *d | 109.6 *b | 112.6 *ab | 109.2 *bc | 111.1 *b | 113.2 *a | 99.2 fg | 98.5 fg | 99.9 fg | 105.4 *d | 0.6911 | <0.0001 | <0.0001 | <0.0001 |
| Rel. μm, % | 88.3 *d | 104.9 a | 96.7 bc | 98.2 bc | 90.4 *d | 102.4 ab | 94.0 cd | 98.1 bc | 80.3 *ef | 83.9 *e | 102.4 ab | 66.4 *g | 78.8 *f | 99.6 b | 86.5 *de | 95.1 c | 94.0 *cd | 102.5 ab | 100.1 ab | 104.2 a | 97.0 bc | 98.2 bc | 99.1 bc | 101.1 ab | 1.5130 | <0.0001 | <0.0001 | <0.0001 |
| Rel. λ, % | 150.5 *a | 100.4 f | 101.6 ef | 104.6 *e | 103.6 ef | 100.9 f | 104.3 *ef | 104.1 *ef | 110.6 *d | 101.7 f | 100.5 f | 119.6 *b | 102.5 ef | 102.1 ef | 114.7 *c | 110.0 *d | 99.8 f | 100.1 f | 101.4 ef | 107.6 *de | 101.5 ef | 101.7 ef | 101.2 ef | 101.4 ef | 1.1722 | <0.0001 | <0.0001 | <0.0001 |
| 100 mg/L | ||||||||||||||||||||||||||||
| Rel. A, % | 71.8 *j | 92.8 *fg | 97.3 e | 89.2 *h | 66.9 *k | 74.3 *i | 69.8 *j | 96.2 *fg | 74.3 *i | 71.7 *j | 97.1 e | 0.0 *l | 101.7 d | 111.0 *a | 98.6 e | 107.2 *b | 106.1 *c | 104.6 *cd | 100.9 de | 91.9 *gh | 99.0 e | 95.3 f | 98.2 e | 100.8 de | 0.7979 | <0.0001 | <0.0001 | <0.0001 |
| Rel. μm, % | 49.4 *f | 84.9 *c | 96.5 ab | 84.1 *c | 76.1 *d | 90.4 *bc | 76.8 *d | 92.7 *b | 50.5 *f | 51.2 *f | 92.0 *b | 0.0 *g | 73.3 *d | 82.4 *c | 83.2 *c | 77.0 *d | 90.9 *b | 85.4 *c | 92.1 *b | 86.6 *c | 90.8 *b | 65.6 *e | 95.9 ab | 100.1 a | 1.5349 | <0.0001 | <0.0001 | <0.0001 |
| Rel. λ, % | 269.5 *b | 107.3 *k | 104.3 *kl | 123.3 *g | 133.6 *e | 103.4 l | 150.8 *c | 144.5 *d | 122.5 *g | 110.6 *j | 100.7 l | 301.5 *a | 114.1 *i | 104.6 kl | 125.0 *fg | 126.8 *f | 117.0 *hi | 116.0 *hi | 103.6 *l | 134.2 *e | 126.4 *f | 117.5 *h | 124.6 *fg | 120.8 *g | 1.0996 | <0.0001 | <0.0001 | <0.0001 |
| 500 mg/L | ||||||||||||||||||||||||||||
| Rel. A, % | 0.0 *k | 43.5 *i | 75.1 *e | 0.0 *k | 0.0 *k | 55.3 *h | 0.0 *k | 99.2 b | 45.2 *i | 36.0 *j | 64.5 *g | 0.0 *k | 66.8 *fg | 100.9 ab | 85.7 *d | 103.5 *a | 68.5 *f | 57.7 *h | 100.7 b | 46.2 *i | 96.5 *c | 0.0 *k | 94.9 *c | 96.7 *c | 0.9675 | <0.0001 | <0.0001 | <0.0001 |
| Rel. μm, % | 0.0 *l | 46.7 *g | 70.9 *de | 0.0 *l | 0.0 *l | 74.2 *d | 0.0 *l | 94.3 b | 24.6 *i | 28.9 *h | 61.6 *f | 0.0 *l | 58.5 *f | 60.7 *f | 62.1 *f | 69.2 *e | 7.4 *k | 74.8 *d | 88.5 *c | 18.3 *j | 66.2 *e | 0.0 *l | 99.1 a | 100.6 a | 1.1913 | <0.0001 | <0.0001 | <0.0001 |
| Rel. λ, % | 1003.2 *a | 161.9 *l | 107.3 *t | 1003.2 *a | 1003.2 *a | 128.4 q | 1003.2 *a | 156.4 *m | 165.6 *k | 121.1 *r | 109.0 *t | 301.5 *c | 131.6 *p | 114.8 s | 141.7 *o | 147.8 *n | 182.7 *j | 186.7 *i | 108.3 *t | 211.5 *d | 205.1 *e | 579.3 *b | 192.0 *f | 156.9 *m | 0.9927 | <0.0001 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnar-Nagy, V.; Tso, K.-H.; Hall, J.W.; Tellez-Isaias, G.; Hernandez-Velasco, X.; Layton, S.; Bata, Z. Effects of Different Nutritional Zinc Forms on the Proliferation of Beneficial Commensal Microorganisms. Microbiol. Res. 2022, 13, 500-513. https://doi.org/10.3390/microbiolres13030034
Molnar-Nagy V, Tso K-H, Hall JW, Tellez-Isaias G, Hernandez-Velasco X, Layton S, Bata Z. Effects of Different Nutritional Zinc Forms on the Proliferation of Beneficial Commensal Microorganisms. Microbiology Research. 2022; 13(3):500-513. https://doi.org/10.3390/microbiolres13030034
Chicago/Turabian StyleMolnar-Nagy, Viviana, Ko-Hua Tso, Jeffrey W. Hall, Guillermo Tellez-Isaias, Xochitl Hernandez-Velasco, Sherry Layton, and Zsofia Bata. 2022. "Effects of Different Nutritional Zinc Forms on the Proliferation of Beneficial Commensal Microorganisms" Microbiology Research 13, no. 3: 500-513. https://doi.org/10.3390/microbiolres13030034
APA StyleMolnar-Nagy, V., Tso, K.-H., Hall, J. W., Tellez-Isaias, G., Hernandez-Velasco, X., Layton, S., & Bata, Z. (2022). Effects of Different Nutritional Zinc Forms on the Proliferation of Beneficial Commensal Microorganisms. Microbiology Research, 13(3), 500-513. https://doi.org/10.3390/microbiolres13030034








