Abstract
In this study, we have evaluated the effects of previously characterized bacteriocins produced by E. faecium strains ST651ea, ST7119ea, and ST7319ea, against biofilm formation and biofilms formed by L. monocytogenes ATCC15313 and vancomycin-resistant E. faecium VRE19. The effects of bacteriocins on the biofilms formed by L. monocytogenes ATCC151313 were evaluated by crystal violet assay and further confirmed by quantifying viable cells and cell metabolic activities through flow cytometry and TTC assay, respectively, indicating that bacteriocin activities required to completely eradicate biofilms are at least 1600 AU mL−1, 3200 AU mL−1, and 6400 AU mL−1, respectively for each bacteriocin evaluated. Furthermore, bacteriocins ST651ea and ST7119ea require at least 6400 AU mL−1 to completely eradicate the viability of cells within the biofilms formed by E. faecium VRE19, while bacteriocin ST7319ea requires at least 12800 AU mL−1 to obtain the same observations. Assessment of synergistic activities between selected conventional antibiotics (ciprofloxacin and vancomycin) with these bacteriocins was carried out to evaluate their effects on biofilm formation and pre-formed biofilms of both test microorganisms. Results showed that higher concentrations are needed to completely eradicate metabolic activities of cells within pre-formed biofilms in contrast with the biofilm formation abilities of the strains. Furthermore, synergistic activities of bacteriocins with both ciprofloxacin and vancomycin are more evident against vancomycin-resistant E. faecium VRE19 rather than L. monocytogenes ATCC15313. These observations can be further explored for possible applications of these combinations of antibiotics as a possible treatment of clinically relevant pathogens.
1. Introduction
Biofilms are typically composed of either a homogeneous or mixture of different species/strains to form a structured multi-cellular community, enclosed in a complex matrix, that typically acts as a protective barrier to various antimicrobial substances [1,2]. Bacterial communities enclosed in this structure (biofilms) are usually comprised of highly dense cells within proximity, made up of combined live microorganisms, dead cells, and numerous biopolymers. Furthermore, complex chemical gradients and compositions are also found within these ecosystems. This enables microorganisms within the system to occur in a wide array of functional physiological states that allows them to survive the fluctuating conditions within the film. Thus, this serves as a deadlock environment for a high probability of interspecies or intraspecies genetic material exchange, which, in turn, results in the possible development of highly adaptive microorganisms such as antimicrobial-resistant strains [1,3,4]. Biofilm formation is regulated by intracellular signaling, through the release of specific metabolic products, that triggers a phenomenon called quorum sensing [5,6,7].
Bacterial biofilms formed by spoilage or food-borne pathogenic organisms within food systems have been one of the major problems faced by the industry [7]. This has also been discussed by Poulsen [8], including the various negative effects of biofilms in food processing involving engineering, health care, and food technological facets [9,10,11,12,13,14].
L. monocytogenes, a known food-borne pathogen that causes listeriosis, has been considered a primary safety concern in the food industry [15]. According to the regulations in the EU and the USA, zero tolerance for L. monocytogenes was granted by the food industry. This is due to their ability to possess various adaptive mechanisms to survive a wide range of environmental conditions, including adaptation to acidic and osmotic stress and psychotropic properties [16,17]. All these physiological characteristics enable this pathogen to survive multiple hurdles employed in the production of fresh produce and processed foods, a huge and profitable industry [18].
Although biofilm formation is not considered a primary virulence factor for L. monocytogenes, the capacity of any potentially pathogenic bacterium to form a biofilm exacerbates its ability for better survival in aberrant niches; which amplifies its ability to pose serious contamination and health-associated consequences. This can be attributed to the adaptive capabilities of microorganisms enclosed in this film to survive in extreme environments such as surfaces of fomites or the presence of disinfectants or antimicrobials, especially in clinical and food production, which uses these compounds frequently, consequently, facilitating the increase in the incidence of resistant pathogens selection and development [19,20,21].
The silent war against the continuous emergence of AMR or multidrug-resistant (MDR) microorganisms has been going on for decades. The frequent use and misuse of antibiotics drugs, which is amplified amidst the COVID-19 pandemic, as a consequence of reduced access to healthcare by sanitary restrictions, lockdowns, remote consultations, and not controlled antibiotic therapies at domicile located patience are only parts of the examples that can be responsible for the misuse of the antibiotics and can be factors, accelerated the increase and development and selective survival of these pathogens. Thakur et al. [15] have predicted that about 10 million AMR infection-associated deaths in the year 2050 will be recorded, surpassing deaths associated with cancer, measle, diarrheal diseases, and diabetes. Nosocomial infections associated with MDR have been high in immunocompromised individuals. One of which includes the emergence and increasing occurrence of vancomycin-resistant enterococci (VRE), especially in clinical settings. According to CDC (2019), enterococci infections have been a minor occurrence (<10%); however, the increased number of its nosocomial-associated infections caused WHO to elevate this pathogen on the pedestal along with Salmonella, Helicobacter pylori, and Staphylococcus aureus for their elucidation and discovery of alternative control agents [22,23]. Although enterococci are known to be a common member of the human microbiota, typically localized in the lower gastrointestinal tract of humans, in some cases, their occurrence in aberrant niches within the host poses a serious health problem. Some of the serious health association of these opportunistic pathogens includes infective endocarditis, urinary tract infections (UTI), rare cases of intra-abdominal infections and meningitis, and systemic infections such as bacteremia [24,25]. Another concern raised for this opportunistic pathogen is its ability to form biofilms in fomites, particularly in catheters, that have been noted to contribute to at least 25% of catheter-associated UTIs [26]. As aforementioned, although the capacity to form biofilms has not been of primary concern, it has an accumulative input on the possible threat it poses; thus, it was included in the considerations raised by the European Food Safety Authority for all safety assessments of various probiotic candidates under the enterococci group [27].
In the quest for finding naturally occurring alternatives to antibiotics, antimicrobial peptides or bacteriocins—small bioactive peptides that typically inhibits the growth of closely related microorganism—can be considered as a promising candidate [28,29]. Although an arsenal of antimicrobial by-products are produced by LAB, bacteriocins have been identified as stable and highly potent [29,30]. In addition, these antimicrobials have long been employed as naturally occurring preservatives in various fermented goods and are also employed in fresh produces and minimally processed foods [31]. Its use as an alternative for antibiotics and other commercial antimicrobials has long been rallied by various scientific groups and individuals [32,33,34]. However, its effect on the biofilms of pathogenic microorganisms has also gained the spotlight. This is due to its potency, nature, stability to different environmental factors and precision on its target spectra [28,35].
In a previous study [36], bacteriocinogenic strains of Enterococcus faecium ST651ea, ST7119ea, and ST7319ea were isolated from Korean traditional soybean paste and expressed bacteriocins were characterized. It was shown that bacteriocins ST651ea, ST7119ea, and ST7319ea were proteinaceous by nature, bioactive after exposure to a large range of temperatures, pH, and in the presence of chemicals commonly applied in protein purification processes and/or food industry [36]. Moreover, based on the sequence of amplicons generated after PCR targeting known enterocins genes and reconstructed amino acid sequences of produced putative enterocins, were concluded that E. faecium ST651ea, ST7119ea, and ST7319ea can be considered producers of modified enterocin A, B, and P [36].
Thus, this study aimed to evaluate the effects of previously characterized bacteriocins with potent inhibitory effects against Listeria spp. and VRE [36], against the biofilms formed by L. monocytogenes, and vancomycin-resistant Enterococcus faecium. Furthermore, the study also aimed to assess the possible synergistic activities of bacteriocin with ciprofloxacin, a wide-spectrum fluoroquinolone commonly used for UTI and renal infections, or vancomycin, one of the drugs commonly used to treat systemic infections, against biofilm formation and biofilms formed by both test microorganisms.
2. Materials and Methods
2.1. Bacteriocins Preparation
Previously isolated and characterized as bacteriocinogenic enterococci strains, E. faecium ST651ea, ST7119ea, and ST7319ea [36], deposited in the collection of HEM Pharma Ltd. (Suwon, Korea), were grown in MRS (Difco, Franklin Lakes, NJ, USA) for 18 h at 37 °C. Bacteriocins containing CFS were collected by centrifugation (4000× g at 4 °C, 30 min), filter sterilized (0.22 µm Sartorius Minstart syringe hydrophobic filters, Göttingen, Germany), and heat-treated (80 °C for 10 min) to inactivate potentially produced heat-labile antimicrobial proteins or extracellular proteolytic enzymes. As previously shown by Fugaban et al. [36], studied strains E. faecium ST651ea, ST7119ea, and ST7319ea produced bacteriocins, showed high similarity to enterocin A, B, and P, characterized as thermostable polypeptides. Semi-purification of the bacteriocins was carried out as previously described by Fugaban et al. [36]. The expressed bacteriocins by the studied strains were precipitated to obtain 60% protein saturation using ammonium sulfate from 500 mL of CFS-containing bacteriocins. Precipitated proteins were collected by centrifugation (20,000× g, 60 min, 4 °C), and the obtained pellets were re-suspended in 50 mL 25 mM potassium phosphate buffer, pH 6.5. Hydrophobic column chromatography (SepPakC18, Waters Millipore, Milford, MA, USA) was used to separate the precipitated proteins eluted with a step gradient from 20% to 80% iso-propanol in 25 mM phosphate buffer (pH 6.5). Obtained partially purified bacteriocins were stored at −20 °C and were used throughout the study. Bacteriocin activity was evaluated as previously described by Fugaban et al. [36]. Appropriate controls were applied to confirm that observed inhibition properties were consequences of the effect of bacteriocins and not of the applied in the purification process chemicals.
2.2. Determination of Minimum Inhibitory Concentrations (MIC) of Antibiotics against Planktonic Cells of L. monocytogenes ATCC15313 and E. faecium VRE19
The MIC of antibiotics vancomycin (CheilJedang Pharma Co., Seoul, Korea) and ciprofloxacin (Sigma-Aldrich, St. Louis, MO, USA) were determined for L. monocytogenes ATCC15313 and E. faecium VRE19 (provided by prof. Kwak, Handong Global University, Pohang, Korea) via broth microdilution assay according to the recommendations of Clinical Laboratory Standards Institute (CLSI). Test organisms L. monocytogenes ATCC15313 and E. faecium VRE19 were grown in BHI for 18 h at 37 °C, and the cells were harvested (4000× g, 10 min), followed by cell washing cells twice using sterile 1× PBS (Lonza, Basel, Switzerland) before re-suspending in the same solution. Antibiotics used in the assay were prepared as suggested by the guidelines. For both antibiotics used in the assay, 256 µg mL−1 were used as the highest final concentration and were diluted in a two-fold manner. The antibiotics previously prepared were distributed in a 96-well flatbottom microplate (SPL Life Sciences, Pochon, Kyonggi-do, Korea) to a final volume of 60 µL and leaving the last two columns as controls (growth and sterility controls). Inoculum preparation was carried out by adjusting the harvested cells into 0.5 McFarland units (approximately 107 CFU mL−1) and distributed in the corresponding plates for each antibiotic. Plates were incubated for 18 h at 37 °C, and MIC, defined as the lowest antibiotic concentration that completely inhibits the growth of bacteria, was determined by visual assessment and confirmed by spectrophotometry (OD 600 nm).
2.3. Determination of Minimum Inhibitory Concentrations (MIC) of Bacteriocins against Planktonic Cells of Target Microorganisms
Activities of semi-purified bacteriocins ST651ea, ST7119ea, and ST7319ea were assessed as suggested by Todorov and Dicks [37] and Todorov et al. [38] against the planktonic cells of L. monocytogenes ATCC15313 and E. faecium VRE19. Sterile BHI were inoculated with 10% 18 h-old cultures of selected test organisms. Eighty microliters of prepared bacterial suspension were distributed to the first 11 columns of sterile 96-well microtiter plates. Different concentrations of semi-purified bacteriocins, on the other hand, were prepared in a two-fold dilution manner in a sterile 100 mM potassium phosphate buffer, pH 6.5. Equal amounts of corresponding bacteriocin dilutions were dispensed on the first 10 columns in the wells to obtain a 1:1 ratio of bacterial culture and bacteriocin. The untreated column was used as growth control, while sterile BHI added on the 12th column was used as sterility control. All setups were incubated at 37 °C for 18 h. The MIC was determined as the lowest concentration required to completely inhibit bacterial growth.
2.4. Molecular Detection of Vancomycin Resistance-Associated Genes of E. faecium VRE19
Clinical isolate E. faecium VRE19 was identified to be resistant to vancomycin based on the antibiogram profiling carried out through microbroth dilution assay and confirmed through ETEST® antibiotic strips (bioMérieux, Marcy-I’Étoile, France), was screened further for the presence of vancomycin resistance genes including vanA, vanB, vanC, vanD, vanE, and vanG. Bacterial cells of E. faecium VRE19, grown in 100 mL of BHI overnight at 37 °C, were used for the DNA isolation by applying ZR Fungal/Bacterial DNA Kit (Zymo Research, Irvine, CA, USA) carried out according to the manufacturer’s recommendations. The DNA concentration and purity were assessed using SPECTROS star Nano nanodrop (BMG LABTECH, Rotenberg, Germany) before the PCR assay, which was carried out as previously described by Fugaban et al. [36].
2.5. Biofilm Formation of L. monocytogenes ATCC15313 and E. faecium VRE19
The ability of L. monocytogenes ATCC15313 and E. faecium VRE19 was assessed as suggested by Doijad et al. [39] with some modifications. Briefly, 18 h-old cultures of respective strains were inoculated in a sterile BHI at a final cell concentration of ~105 CFU mL−1. One hundred and fifty microliters were transferred to the first 10 columns of sterile 96-well flatbottom microtiter plates (SPL Life Sciences), while the last column was added with sterile BHI only to serve as sterility control. Prepared plates were incubated at 37 °C for 24–36 h to allow the setups to form biofilms.
2.6. Quantification of Biofilms by Crystal Violet Assay
After allowing the biofilms to form crystal violet assay was carried out to quantify the biofilms as suggested by Todorov et al. [38] with some modifications. The assay was carried out by carefully discarding the cultures, followed by washing using 1× PBS. The attached biofilms were fixed with 120 µL of methanol for 15 min, and the excess was discarded. Subsequently, the plates were left to dry for an additional 10 min and stained with 120 µL of 1% (w/v) crystal violet for 15 min. The excess crystal violet was flushed out using distilled water, and plates were left to dry for 30 min. The adhered CV to the biofilms was extracted by 95% ethanol (v/v) and incubated for 15 min before absorbance reading at OD 550 nm (SPECTROStar). The biofilm formation ability of the test organisms used in this study was assessed based on the guidelines described by Stepanović et al. [40], and the statistical evaluation of significant differences among samples was carried out using t-test analysis (p < 0.05).
2.7. Quantification of Viable Cells from Bacteriocin-Treated Biofilms of L. monocytogenes ATCC15313 and E. faecium VRE19 by Flow Cytometry
The proportion of viable, damaged, and dead bacterial cells from bacteriocin-treated setups were quantified using a dye-exclusion assay with propidium iodide (PI). Biofilms were allowed to form in flatbottom 12-well sterile microtiter plates containing 1 mL of BHI inoculated with ~106 cells mL−1 for 24–36 h. Bacteriocins ST651ea, ST7119ea, and ST7319ea were prepared in aliquots of different concentrations using 100 mM phosphate buffer (pH 6.5). The liquid culture from the plates was discarded and added with 1 mL of previously prepared bacteriocin, whereas sterility control and growth control wells were added with sterile phosphate buffer. The biofilm challenge assay was carried out for 1 h.
Determination of viable bacterial cells was assessed using dye-exclusion assay with PI (Sigma-Aldrich, St. Louis, MO, USA) by flow cytometry was carried out as suggested by R&D systems (Sigma-Aldrich). Samples of 0.5 mL from each well were drawn, and cells were harvested by centrifugation at 10,000× g for 10 min. Obtained pellets were re-suspended in 1× staining buffer formulated with 1× PBS, 0.5% bovine serum albumin (BSA, Sigma-Aldrich), and 0.05% NaN3 (Sigma-Aldrich). Bacterial suspensions were stained with PI (final concentration of 30 µg mL−1) for 5 min in the dark. Sorting and quantification of cells were determined using Flow Cytometer ZE5 and analyzed using Everest software v 2.2.08.0 (Bio-Rad Laboratories, Hercules, CA, USA). Growth control and sterility control were included.
2.8. Determination of Metabolic Activity
Detection of microbial viability was carried out as suggested by Krajenc et al. [41] with modifications as follows. The pre-formed biofilm challenge was carried out as previously described, but instead of crystal violet staining, 100 µL of BHI supplemented with 0.1% of triphenyl tetrazolium chloride (TTC, Sigma Aldrich) was added to each well and incubated for 6 h at 37 °C. The medium was discarded, and metabolic activity was then assessed based on the development of red color, which denotes a successful extraction of formazan from the viable cells by adding 150 µL of 70:30 ethanol: acetone solution to each well and incubating it for 18 h at 37 °C. Complete abrogation of metabolic activities was used to determine and analyze the synergistic activities against test organisms and setups used.
2.9. Assessment of Synergistic Activities of Bacteriocins and Antibiotics against Biofilm Formation of L. monocytogenes ATCC15313 and E. faecium VRE19
The synergistic activities of each bacteriocin with either vancomycin or ciprofloxacin were assessed in a binary combinatorial effect using the MIC previously identified as baselines for the highest concentrations of combination cocktails. Each binary component antimicrobial cocktail was prepared using a 1:1 (v/v) ratio of designated bacteriocin and corresponding antibiotics of designated concentrations. All the bacteriocins studied were prepared in two-fold dilutions as previously described, whereas the antibiotics were prepared as described in the CLSI for the preparation of antibiotics for antimicrobial susceptibility testing (AST). BHI seeded with 18 h-old cultures of corresponding applied test organisms (L. monocytogenes ATCC15313 and E. faecium VRE19) were distributed individually in 96-well flatbottom sterile microtiter plates. Each well was added with 70 µL of test organisms, leaving the last two for sterility control and growth control. A total of 70 µL of previously prepared binary component antimicrobial cocktails of corresponding concentrations and ratios were dispensed accordingly. Plates were incubated for 36 h at 37 °C and quantified and analyzed as previously described. All setups were carried out in duplicates.
Synergistic activities were interpreted using the fractional inhibitory concentration (FIC) index as follows:
where A is the MIC inhibition of bacteriocin used in the setup, while B is the corresponding antibiotics used. Results were interpreted as suggested by Faleiro and Miguel [42], where indices ranging between 0 and 0.5 indicates synergistic activity in a two-component system; values ranging from 0.5 and 1.0 are considered to have an additive effect on bacterial inhibition, values between 1.01 and 2.0 indicative of indifference between two combined inhibitory substances, and values between 2.0 and 4.0 indicate antagonism.
2.10. Evaluation of Synergism of Bacteriocins and Antibiotics on the Biofilm Formed by L. monocytogenes ATCC15313 and E. faecium VRE19
The pre-formed biofilms of the test organisms assessed in this study were challenged using the same binary component antimicrobial cocktails as previously described. Formation of L. monocytogenes ATCC15313 and E. faecium VRE19 biofilms were carried out in 96-well flatbottom sterile microtiter plates using BHI seeded with 10% of each test organism. Each well was inoculated with 120 µL of appropriative bacterial suspension along with the growth control, while the same volume for BHI was used for the sterility control. Each corresponding setup was carried out in triplicates. All prepared biofilm plates were incubated for 36 h at 37 °C. Before the biofilm challenge, planktonic cells from the biofilm plates were removed by discarding the culture followed by washing the plates twice with sterile 1× PBS. Plates were left to dry for 15 min in a sterile environment. Bacteriocins of corresponding concentrations were prepared as previously described, and 100 µL of each corresponding treatment was distributed accordingly. Biofilm challenge assay was carried out for 2 h at 37 °C. Remnant biofilms after the assay were quantified as previously described. Synergy was assessed through calculated FIC values.
3. Results
3.1. MIC of Antimicrobials Used
Bacteriocins produced by E. faecium strains ST651ea, ST7119ea, and ST7319ea were obtained from CFS obtained after cultivation in MRS for 24 h at 37 °C and precipitation with ammonium sulfate (60% saturation). After chromatography on SepPakC18, fractions eluted with 60% isopropanol in 25 mM phosphate buffer (pH 6.5) presented the highest bacteriocin activity. Taking into consideration levels of bacteriocin activity and color of fractions eluted with 40%, 60%, and 80% isopropanol in 25 mM phosphate buffer (pH 6.5), fraction 60% isopropanol was selected for further application. The detection of the minimum inhibitory concentration of semi-purified bacteriocins produced by E. faecium strains ST651ea, ST7119ea, and ST7319ea, previously characterized by Fugaban et al. [36], were further assessed for their potential to inhibit the growth of biofilms. In this study, confirmation of MIC of planktonic cells of both test organisms were conducted in liquid culture as suggested by Todorov et al. [38]. Recorded activities against the planktonic cells of L. monocytogenes bacteriocins needed to completely inhibit the growth of L. monocytogenes ATCC15313 were 1600 AU mL−1, 3200 AU mL−1, 3200 AU mL−1, respectively for semi-purified bacteriocins ST651ea, ST7119ea, and ST7319ea. While MIC recorded for E. faecium VRE19 were 1600 AU mL−1, 3200 AU mL−1, and 6400 AU mL−1, accordingly. These recorded activities are used as a reference point for the identification of the minimum inhibitory concentration for the bacteriocins studied against the planktonic cells of L. monocytogenes ATCC15313 and E. faecium VRE19.
On the other hand, MIC for ciprofloxacin and vancomycin were quantified using microbroth dilution. MIC of ciprofloxacin against L. monocytogenes ATCC15313 and E. faecium VRE19 were 512 mg L−1 and 128 mg L−1, respectively. While vancomycin, a glycopeptide antibiotic, requires at least 64 mg L−1 against L. monocytogenes ATCC15313 and 128 mg L−1 for E. faecium VRE19 to completely inhibit the growth of their planktonic cells.
3.2. Molecular Detection of Vancomycin Resistance-Associated Genes in E. faecium VRE19
Confirmation of the phenotypic vancomycin-resistance previously observed on the test organism E. faecium VRE19 has been carried out through a PCR-based approach. Results indicated that E. faecium VRE19 has vancomycin resistance coded by vanA and vanB genes. Phenotypic demonstration of this resistance was found to be survival of resistant enterococci at high concentrations of vancomycin (≤250 mg L−1). In this study, previous MIC detection assays confirm the phenotypic manifestation of this observation.
3.3. Biofilm Inhibition by Partially Purified Bacteriocins ST651ea, ST7119ea, and ST7319ea
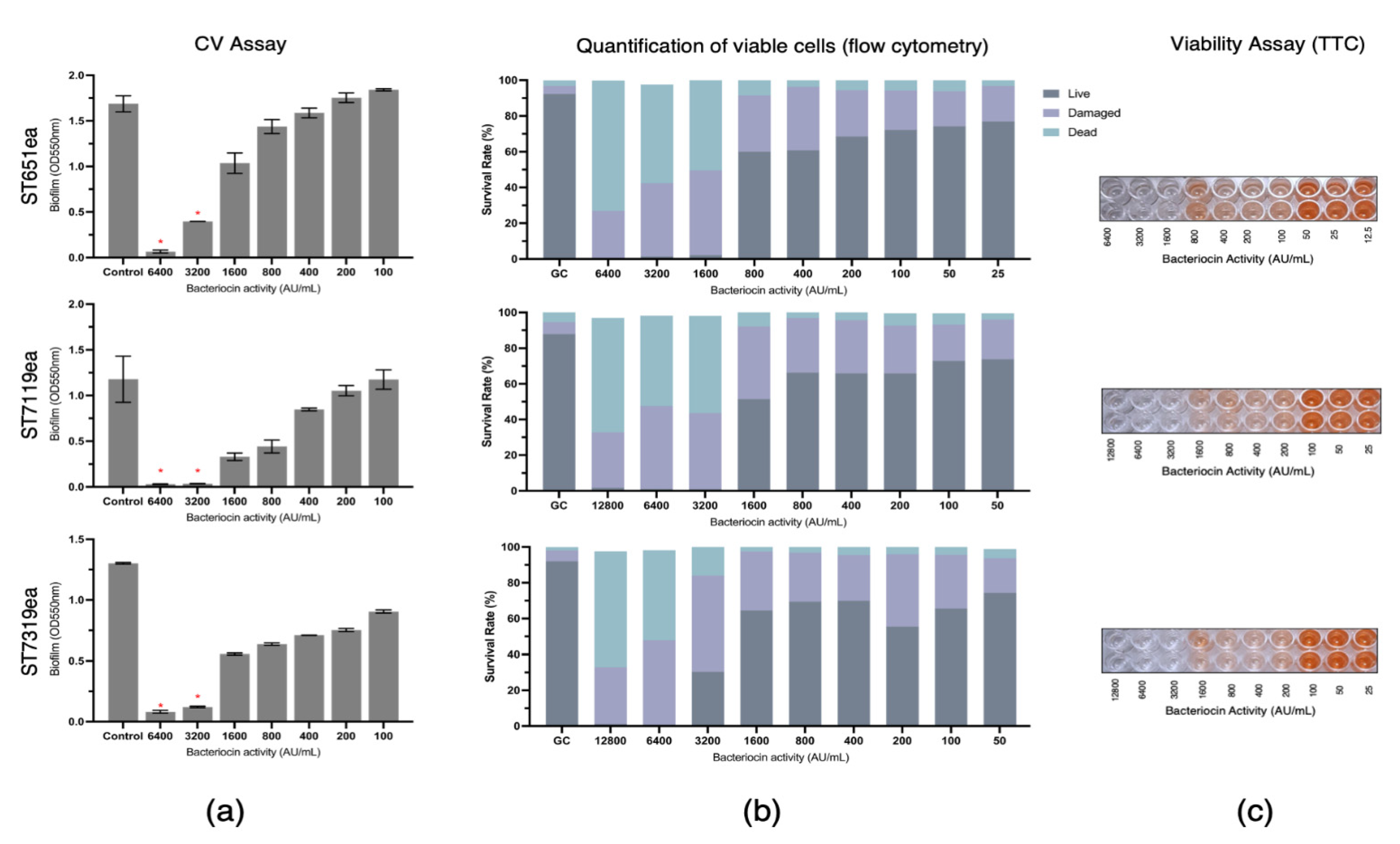
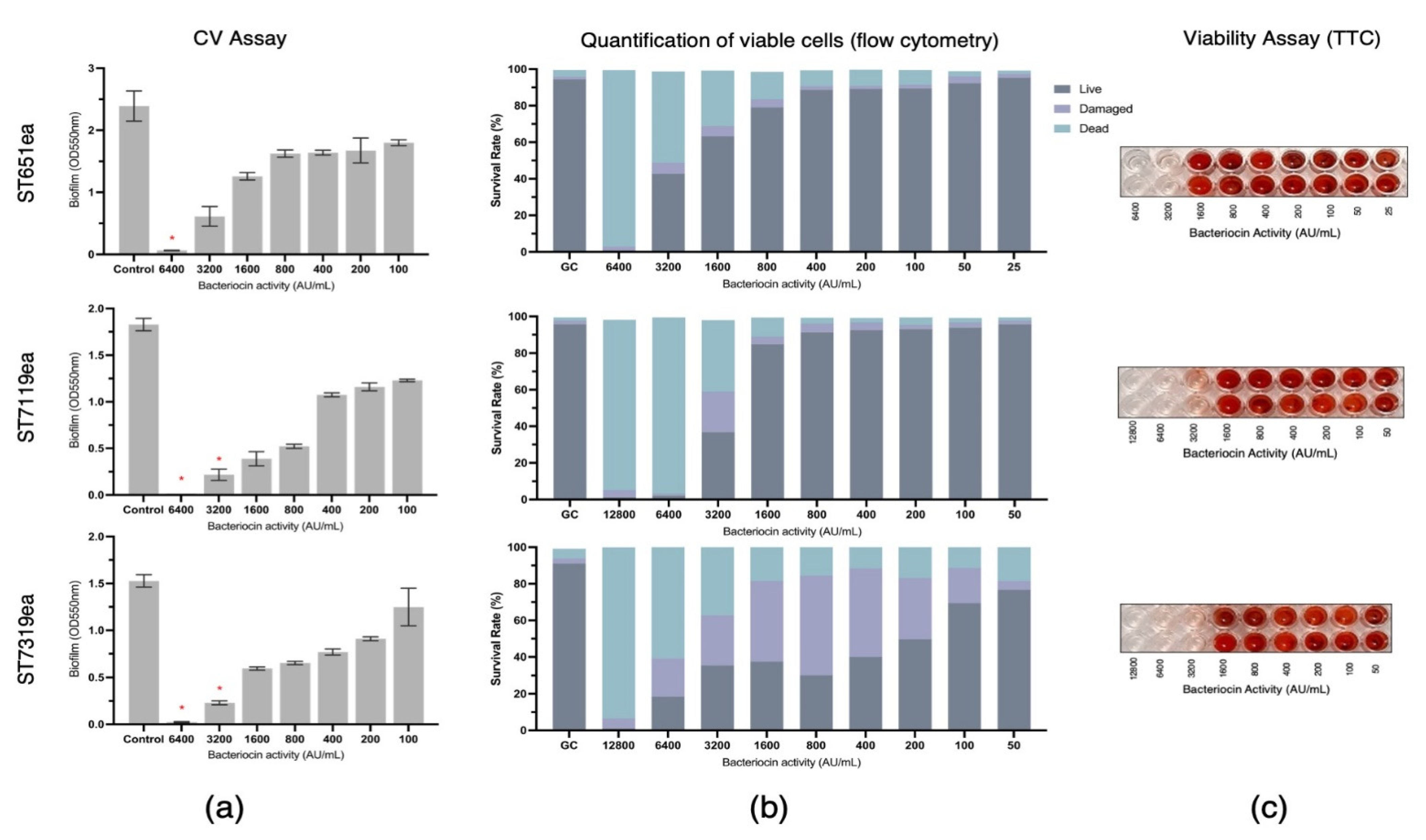
The biofilm eradication activities of partially purified bacteriocins produced by strains E. faecium ST651ea, ST7119ea, and ST7319ea were assessed by challenging the pre-formed biofilms of L. monocytogenes ATCC15313 and E. faecium VRE19 for 1 h. Following CV staining and absorbance reading at 550 nm, a significant reduction (p < 0.05) of biofilm mass was observed with the treatment of at least 3200 AU mL−1 for all bacteriocins evaluated against biofilm formed by L. monocytogenes ATCC15313, while this is the same minimum concentration required for both bacteriocin ST7119ea and ST7319ea against the biofilms formed by E. faecium VRE19, ST651ea (Figure 1a and Figure 2a) requires two-fold higher to significantly destroy the biofilms formed by this microorganism. The last observations agree with the fact that MICs for bacteriocins produced by E. faecium ST651ea, ST7119ea, and ST7319ea was 6400 AU mL−1, 6400 AU mL−1, and 12,800 AU mL−1, respectively.
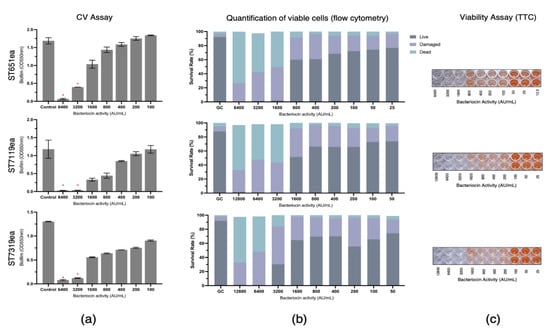
Figure 1.
Eradication of Listeria monocytogenes ATCC14313 biofilm by semi-purified bacteriocins ST651ea, ST7119ea, and ST7319ea after 1 h challenge. Biofilms were quantified by (a) crystal violet assay (significant changes in biofilms after challenge were indicated by * at p ≤ 0.05); (b) quantification of live, dead, and damaged cells through flow cytometry; and confirmation of (c) cell viability using TTC.
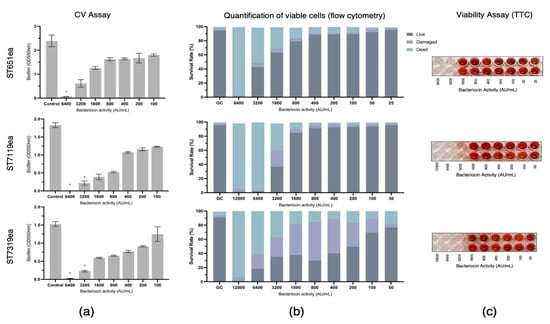
Figure 2.
Eradication of Enterococcus faecium VRE19 biofilm by semi-purified bacteriocins ST651ea, ST7119ea, and ST7319ea after 1 h challenge. Biofilms were quantified by (a) crystal violet assay (significant changes in biofilms after challenge were indicated by * at p ≤ 0.05); (b) quantification of live, dead, and damaged cells through flow cytometry; and confirmation of (c) cell viability using TTC.
Additionally, quantification of the rates of viable/live, dead, and damaged cells within the bacteriocin-treated biofilms was carried out after 1 h challenge showing that the minimum concentration needed for the bacteriocins evaluated to completely damage or kill the cells within the biofilms formed by L. monocytogenes is 1600 AU mL−1, 3200 AU mL−1, and 6400 AU mL−1, for bacteriocins ST651ea, ST7119ea, and ST7319ea, respectively. On the other hand, two-fold higher is required for bacteriocins ST651ea and ST7119ea to obtain the same effects against the VRE biofilm, while it requires a minimum of 12,800 AU mL−1 to eliminate the viability of the cells within the biofilm based on this assay (Figure 1b and Figure 2b). Similar results were observed when viable cells were visualized by TTC experimental approach (Figure 1c and Figure 2c) for L. monocytogenes ATCC15313 and E. faecium VRE19, respectively.
3.4. Assessment of Synergism of Bacteriocins and Antibiotics against Biofilm Formation of L. monocytogenes ATCC15313 and E. faecium VRE19
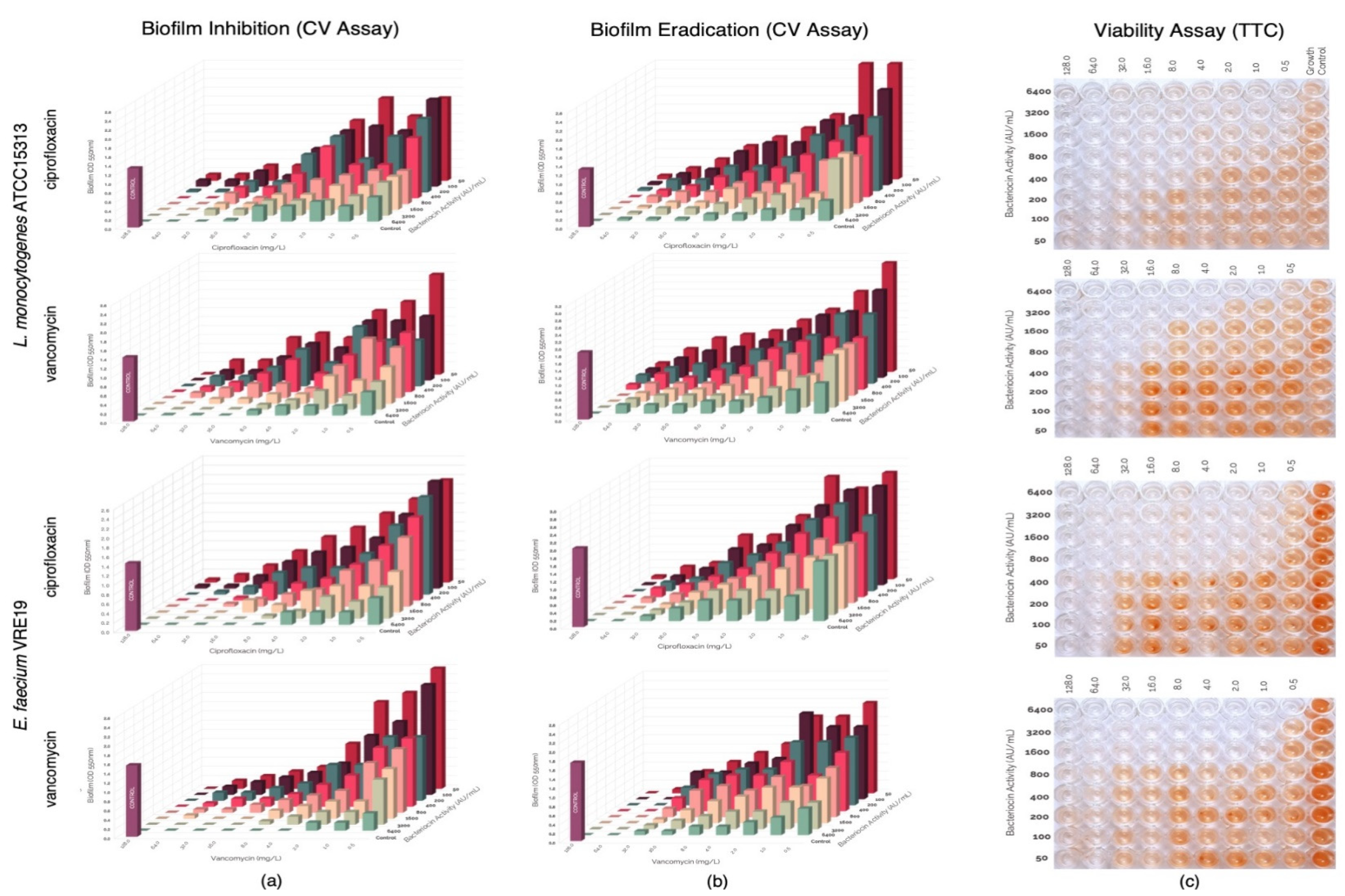
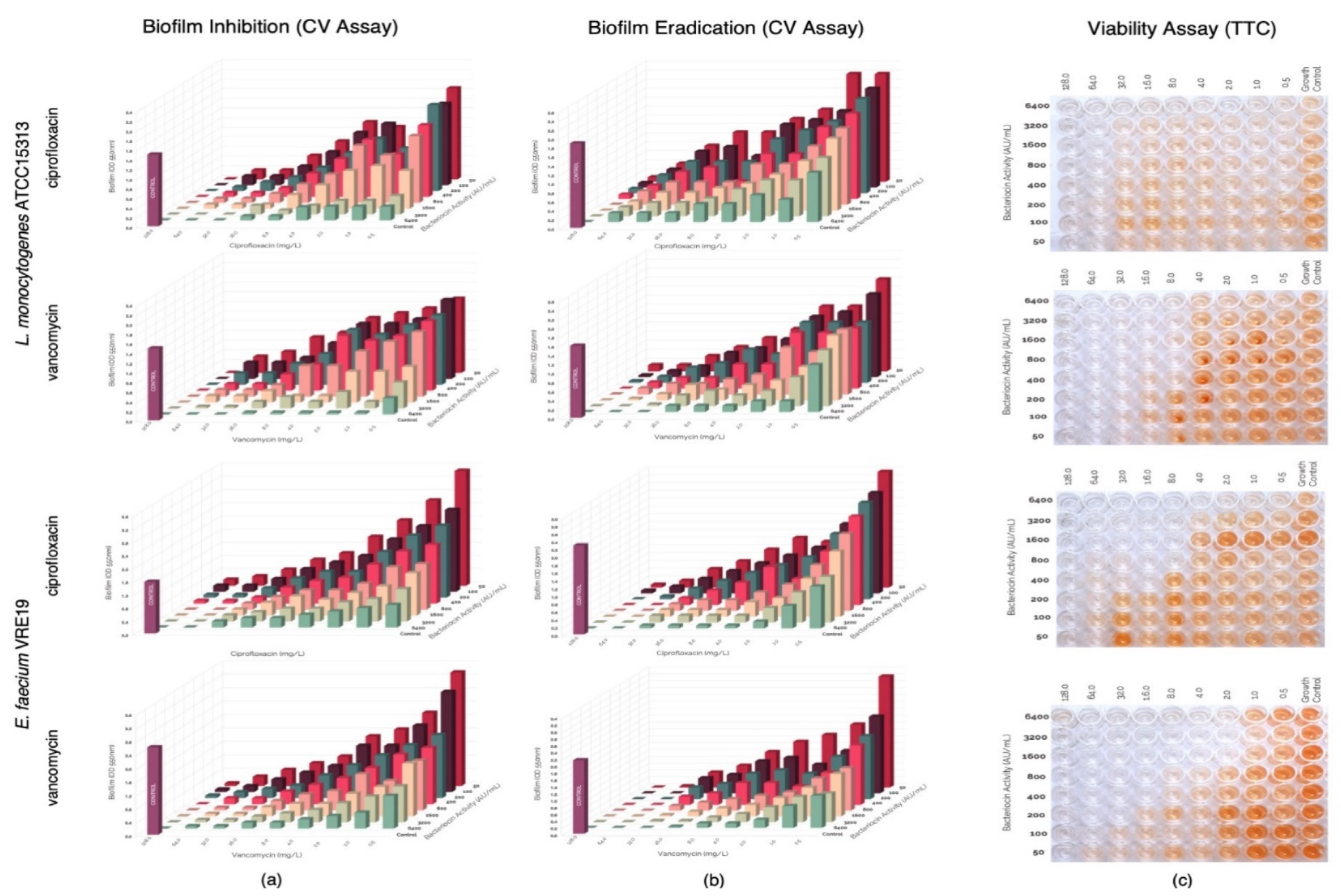
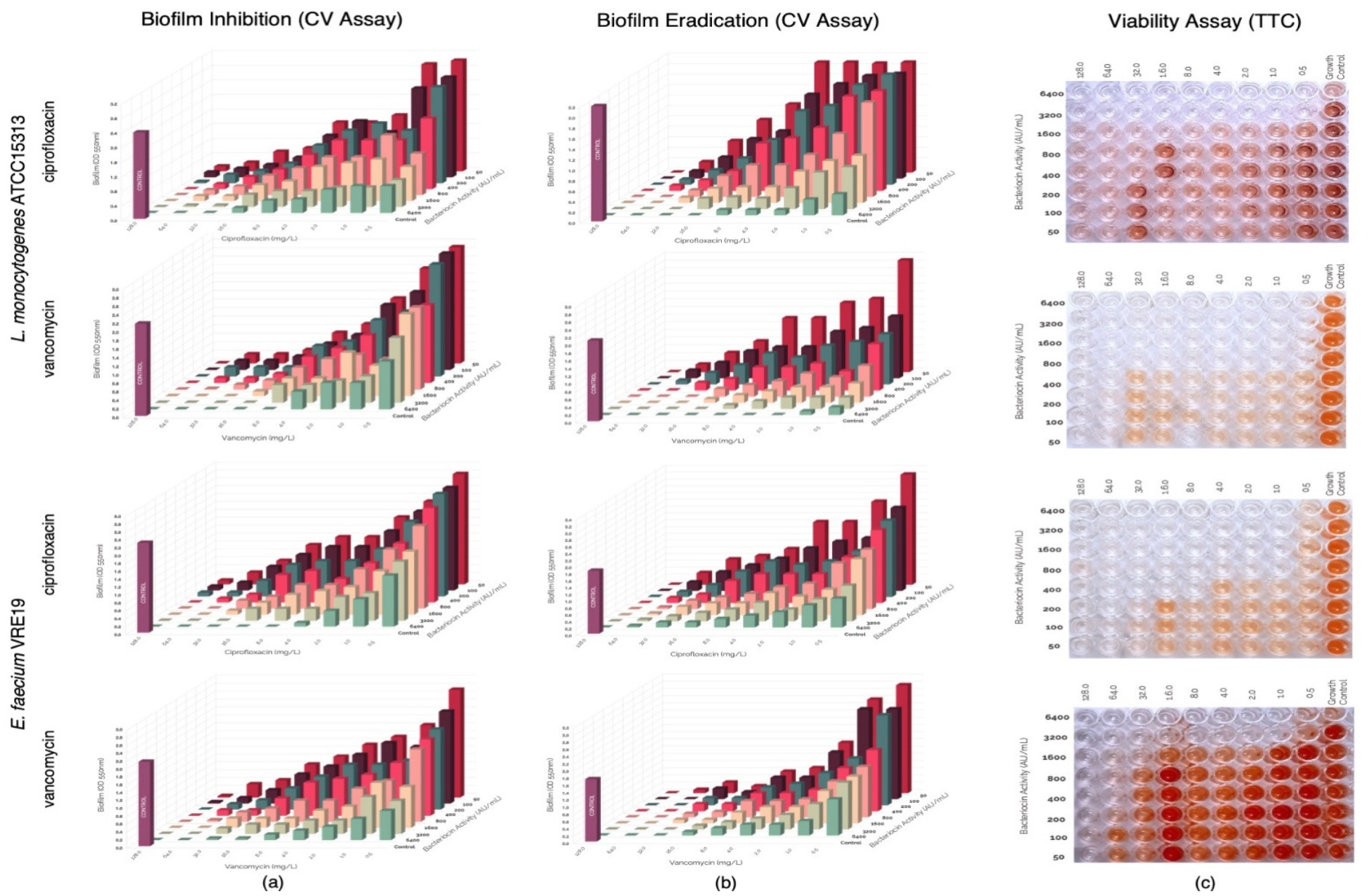
In this study, evaluation of the possible synergism between the bacteriocins produced by E. faecium ST651ea, ST7119ea, or ST7319ea with vancomycin or ciprofloxacin for their ability to inhibit the formation of biofilms of L. monocytogenes ATCC15313 and E. faecium VRE19 in vitro. Results showed that synergistic activities were demonstrated by all bacteriocins individually paired with ciprofloxacin against both test microorganisms (Table 1) (topographic presentation of biofilm formed after 36 h shown in Figure 3a, Figure 4a and Figure 5a) using the guidelines for combinations of antimicrobial substances. Conversely, the effect of vancomycin can be seen to demonstrate synergism when paired with bacteriocins ST651ea, ST7119ea, or ST7319a against E. faecium VRE19 (Table 1); while only ST651ea worked in synergy with ciprofloxacin to inhibit the formation of L. monocytogenes ATCC15313 biofilm. The combinations of bacteriocins ST7119ea or ST7319ea with ciprofloxacin showed an additive effect instead against the formation of Listeria monocytogenes ATCC15313 biofilm in this assay (Table 1). Topographic presentation analysis of biofilm formation of both test organisms assessed was demonstrated in Figure 4a and Figure 5a. Identifying that some of the combinations of bacteriocins and antibiotics work synergistically against the formation of biofilms of both test organisms noting a significant reduction in the concentrations required for the inhibition of biofilm formation compared to the individual inhibitory activities recorded for each antimicrobial. General observations indicate that combinations of bacteriocins and ciprofloxacin have synergistic activity in the inhibition of L. monocytogenes ATCC15313, while the combination of bacteriocins and vancomycin had synergistic activities against E. faecium VRE19 biofilm formation.

Table 1.
The ≤MIC95 values of ciprofloxacin, vancomycin, and bacteriocins ST651ea, ST7119ea, and ST7319ea and the FIC indices calculated based on two-component antimicrobials (antibiotic-bacteriocin).
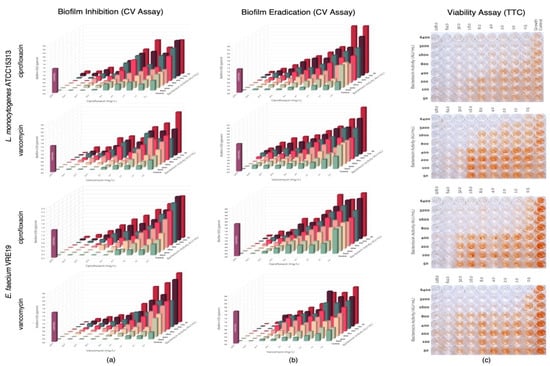
Figure 3.
Evaluation of synergy between semi-purified bacteriocin ST651ea and antibiotics (ciprofloxacin or vancomycin) on the (a) biofilm formation and (b) biofilm eradication of Listeria monocytogenes ATCC15313 and Eenterococcus faecium VRE19 as demonstrated on the topographic analysis biofilms by crystal violet assay; and (c) confirmation of cell viability were carried out using TTC assay.
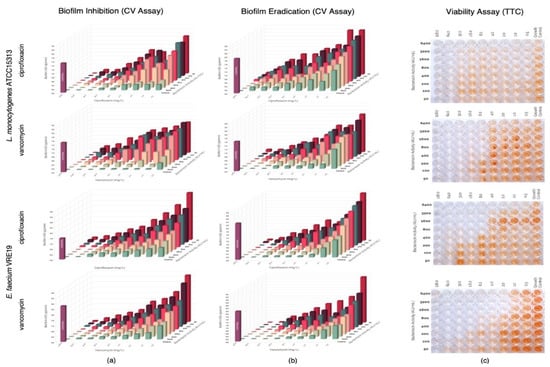
Figure 4.
Evaluation of synergy between semi-purified bacteriocin ST7119ea and antibiotics (ciprofloxacin or vancomycin) on the (a) biofilm formation and (b) biofilm eradication of Listeria monocytogenes ATCC15313 and Enterococcus faecium VRE19 as demonstrated on the topographic analysis biofilms by crystal violet assay; and (c) confirmation of cell viability were carried out using TTC assay.
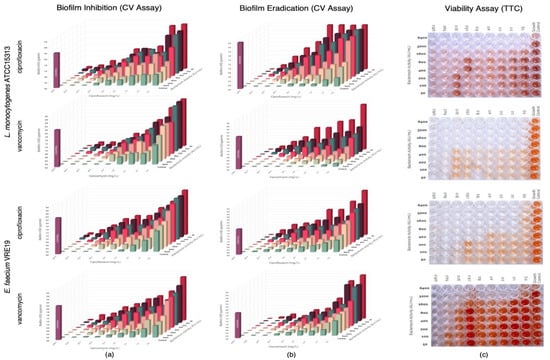
Figure 5.
Evaluation of synergy between semi-purified bacteriocin ST7319ea and antibiotics (ciprofloxacin or vancomycin) on the (a) biofilm formation and (b) biofilm eradication of Listeria monocytogenes ATCC15313 and Enterococcus faecium VRE19 as demonstrated on the topographic analysis biofilms by crystal violet assay; and (c) confirmation of cell viability were carried out using TTC assay.
3.5. Assessment of Synergism of Bacteriocins and Antibiotics against Pre-Formed Biofilms of L. monocytogenes ATCC15313 and E. faecium VRE19
Pre-formed biofilms were treated with antimicrobials combinations composed of either bacteriocins ST651ea, ST7119ea, or ST7319ea and vancomycin or ciprofloxacin. After the challenge, topographic residual biofilms were quantified by crystal violet biofilm staining assay while simultaneously monitoring the cellular metabolism of the residual biofilms in a parallel setup. The topographic representation of the biofilm formation results after the challenge is presented in Figure 3b, Figure 4b and Figure 5b. Observations on the activities of the antimicrobial combinations showed a decreased effect against the biofilms formed by both test organisms based on the FIC indices shown in Table 1. Biofilms known to provide a protective layer for these microorganisms play as adaptive and defense mechanisms against the antimicrobials employed. The topographic visualized levels of activities (Figure 3b, Figure 4b and Figure 5b) of combinations of bacteriocins and antibiotics against both test organisms and their corresponding FIC indices were calculated and presented in Table 1, demonstrating that higher amounts of each component for the majority of the combinations are needed to eradicate the previously formed biofilms relative to the concentrations needed to inhibit the biofilm formation of both test microorganisms. FIC indices showed that combinations of bacteriocins with ciprofloxacin majorly demonstrated an additive effect on the pre-formed biofilms of both test organisms, while synergistic activities were noted when bacteriocins were combined with vancomycin against E. faecium VRE19 but not against L. monocytogenes ATCC15313 (Table 1). The viability, measured by TTC assay of the residual biofilms formed by L. monocytogenes ATCC15313 or E. faecium VRE19 coinciding with the previous results (Figure 3c, Figure 4c and Figure 5c).
The fractional inhibitory concentration (FIC) index for two-component antibacterial compounds was interpreted as follows: values of ≤0.5, Synergism; >0.5–1.0, Additive effect; <2.0, Indifference; and ≥2.0–4, Antagonism.
4. Discussion
Bacteriocins produced by E. faecium ST651ea, ST7119ea, and ST7319ea, previously characterized by Fugaban et al. [36], were further assessed in this study for their potential activities against biofilms formed by L. monocytogenes and vancomycin-resistant enterococci. It has been reported that E. faecium ST651ea harbors genes coding for enterocins B and P, while both E. faecium ST7119ea and ST7319ea have genes for enterocin A and B [36]. Based on obtained nucleic acid sequenced targeting genes associated with the production of enterocins A, B, and P, recorded in E. faecium 651ea, ST7119ea, and ST7319ea, respectively, the putative amino acid sequences were reconstructed, and some mutations in the protein structure were observed [36]. Moreover, based on the comparative analysis of the spectrum of activity of the bacteriocins expressed by E. faecium 651ea, ST7119ea, and ST7319ea along with additional physiological and biochemical properties of studied bacteriocins, it was suggested that most probably they belong to the class IIa [36]. Moreover, it has been mentioned by Nes et al. [43,44] that majority of the known bacteriocins produced by Enterococcus spp. belong to class I (lantibiotics) and II bacteriocins (small unmodified peptides), whose mode of action is cell lysis [45,46,47]. Target molecules, such as lipid II for L. monocytogenes or the sugar permease systems found on the surface of target microorganisms, serve as the docking point for bacteriocins [30,32,43]. These modifications in the functionality of these docking molecules by the bacteriocins cause disturbance in the integrity of the cell membrane, thereby leading to intracellular component leakage, which eventually leads to the death of the target cell.
In this study, bacteriocins produced by E. faecium ST651ea, ST7119ea, and ST7319ea were partially purified by ammonium sulfate precipitation (60% protein saturation) obtained at 60% isopropanol in 100 mM phosphate buffer (pH 6.5) in a step-gradient elution assay were previously quantified against L. monocytogenes. Application of the bacteriocins as a crude extract, partially purified preparations, or pure (homogeneous) protein is strictly dependent on the experimental model. Purification is a costly procedure, and normally pure bacteriocins are applied in analytical procedures or medical applications. For most food-associated experiments and/or sanitization purposes, a crude extract or partially purified bacteriocins are typically applied. The previously identified MICs coincide with the ≤MIC95 of bacteriocins measured in this study, which was used for subsequent evaluations. Furthermore, these current data further strengthen the findings from the study as matching observations were demonstrated through the inhibitory kinetics of the assessed bacteriocins against actively growing cells of target microorganisms sampled after 3, 6, 9, and 24 h of incubation [36].
The MICs of two selected antibiotics, ciprofloxacin and vancomycin, were also determined against the planktonic cells of both test organisms used. Ciprofloxacin, a known fluoroquinolone antibiotic, has been used as the benchmark in quantifying and comparing the efficacy of newly discovered or elucidated fluoroquinolones [48]. It has been employed as a treatment across a wide range of pathogenic microorganisms, including infection-causing members of Enterobacteriaceae, Neisseria-associated meningococcal infections, and Pseudomonas infections, among others. Additionally, it has also been used as a common drug to treat UTI and renal infections [48,49,50], although, in some cases, it has been demonstrated that the occurrence of ciprofloxacin-resistant L. monocytogenes typically has a range of around 30–35% of all the strains evaluated [51]. Additionally, it has demonstrated that an inherent adaptive system is expressed by L. monocytogenes when exposed to disinfectant benzalkonium for an extended time, consequently resulting in resistance to ciprofloxacin [4,24,52,53]. On the other side, ciprofloxacin is primarily administered as a treatment for uncomplicated UTI infections only. Although ciprofloxacin is not considered a primary drug for enterococcal-associated UTIs due to its modest activity against this pathogen, it still demonstrated successful employment as a treatment. Perry et al. [54] stated that higher concentrations of ciprofloxacin are needed to assess the sensitivity of enterococci to this drug (5 µg per disc instead of 1 µg). Thus, in this study, we have evaluated the minimum inhibitory concentration of ciprofloxacin against L. monocytogenes ATCC15313 and E. faecium VRE19 independently through microbroth dilution.
Vancomycin, a tricyclic glycopeptide antibiotic that was initially isolated from Streptococcus orientalis, whose mechanism of action involves interference in the early stage of cell wall synthesis [55,56]. This glycopeptide antibiotic is typically administered intravenously due to its low absorption by oral intake. Furthermore, vancomycin has been used as one of the “last resort” drugs for the treatment of severe systemic infections caused by multi-drug-resistant Gram-positive bacteria. However, the exorbitant usage of this antibiotic has led development and occurrence of vancomycin-resistant enterococci and staphylococci [57,58] which pose a serious threat in medical practice. However, the occurrence of antibiotic resistance from this group is not unusual, noting that inherent resistance against vast groups of antibiotics was observed, especially against β-lactams (cephalosporins and penicillins), fluoroquinolones, clindamycin, and in low concentrations of aminoglycosides [59,60,61]. In this study, MIC of vancomycin against planktonic cells of L. monocytogenes ATCC15313 and E. faecium VRE19 were determined as previously described noting that a minimum of 64 mg L−1 and 128 mg L−1 are needed to completely inhibit the growth of each respective test organism. This further confirms that E. faecium VRE19, indeed, is resistant to vancomycin based on the cut-offs suggested by both CLSI and EFSA. All values measured against planktonic cells of both test microorganisms were used as the basis of all succeeding experiments.
To secure the integrity of the succeeding assays, confirmation of the presence of antibiotic resistance genes harbored by E. faecium VRE19 was carried out, identifying the presence of vanA and vanB genes. The selective pressure in the occurrence of VRE by excessive vancomycin treatment has caused the rise of different genotypic classifications of resistance to this drug. These include resistance phenotypes van A, B, C, D, E, and G. Plasmid-associated resistance has been elucidated to be responsible for vanA and vanB resistances, but the distinction between the two includes co-resistance to teicoplanin as characterized only for vanA phenotypes due to the associated modifications in the N-acetylmuramic acid (NAM on the vancomycin-resistant E. faecium and E. faecalis [62,63]. VanB phenotype, which is typically characterized by its high resistance to vancomycin (≤250 mg/L), is usually located in a plasmid, which increases the threat it poses regarding the transfer of resistance genes. On the other hand, vanC and vanD resistance-associated genes are all chromosomally located and non-transferrable, manifested by low resistance to vancomycin (16–32 mg L−1). Although these are still considered to be low concentrations of vancomycin, other factors such as the occurrence of pathogenicity-associated insertion sites glean the occurrence of these genes negatively; thus, its surveillance is of importance [64,65,66]. Additionally, vanE and vanG are both characterized by non-transferrable genes and are also characterized by resistance to low concentrations of vancomycin [67].
The biofilm inhibition and eradication capacities of the semi-purified enterocins produced by E. faecium strains ST651ea, ST7119ea, and ST7319ea were evaluated in two different assays as shown in Figure 1a and Figure 2a and further confirmed for the retention of bioactivity after treatment through triphenyl tetrazolium chloride (TTC) (Figure 1c and Figure 2c) and flow cytometry (Figure 1b and Figure 2b) assay. The observations support the hypothesis that higher concentrations of antimicrobials are needed to destroy or kill microorganisms protected within biofilms [1,2]. In addition, a study conducted by Pérez-Ibarreche et al. [68] on bioengineered nisin with activity against S. uberis biofilms also demonstrated the same patterns of increased concentrations of bacteriocins are needed against biofilms vs. planktonic cell counterparts. Furthermore, these similar observations were noted in the treatment of planktonic cells and biofilms of Pseudomonas aeruginosa with chemical disinfectants and antibiotics, as demonstrated by [69]. Although these results are promising, the use of high concentrations of antimicrobials, including bacteriocins, may lead to the development of resistance to these antimicrobial peptides [70].
With the continuous development of antimicrobial-resistant pathogens, bacteriocin-based treatments or methods of control against various pathogens have been rallying for the past decades [28,30,32,43]. Furthermore, the increasing occurrence and persistence of “superbugs” in the clinical setting and the threats they pose amidst the current COVID-19 pandemic that drastically increased the consumption of various antibiotics now act as a selective pressure for the dominance of these pathogens [71,72]. Furthermore, O’Toole [71] also mentioned increased occurrence and outbreaks of extended-spectrum β-lactamase-producing Kl. pneumoniae, metallo-β-lactamase-producing carbapenem-resistant Enterobacterales, carbapenem-resistant A. baumannii, and vancomycin-resistant enterococci, which are clinically acquired, are now an alarming concern worldwide. Therefore, it is imperative to find solutions to these arising concerns with the use of different possible alternatives from conventional antibiotics, including bacteriocins are antimicrobial peptides produced by various microorganisms. Furthermore, these antimicrobials are particularly distinctive from antibiotics due to their narrower spectrum and lack of elaborate modifications in their peptide sequences [28,30,32,43,44]. Furthermore, these antimicrobial peptides have been in the spotlight, particularly those produced by lactic acid bacteria. This is due to the associated safety status of these microorganisms. Aside from this, the specificity of bacteriocins against their targets in comparison with antibiotics can be used as a key tool for targeted infection treatment. Although, handling and purification of these naturally occurring antimicrobials are still part of the challenge that needs development in this field. Likewise, their applications, although majorly assessed against planktonic cells of food-contaminants, still need further evaluation to assess in which other ways we can employ and advance these antimicrobial peptides as an important tool in both the clinical setting and the food industries. In this study, evaluation of possible synergistic activities across bacteriocins in combination with either ciprofloxacin or vancomycin was evaluated as demonstrated in Figure 3, Figure 4 and Figure 5. along with the quantification of the effects of their combinations quantified through FIC indices as shown in Table 1, identifying those combinations of vancomycin with bacteriocins work synergistically on the eradication of vancomycin-resistant E. faecium VRE19. This re-sensitization phenomenon can be attributed to various factors, for one, the different mechanisms of action of the two antimicrobial compounds used in the cocktails, the antibiotic and bacteriocin. The resistance mechanisms of vancomycin on VRE have been identified to be associated with alteration of peptidoglycan structure resulting in to decrease in binding, thus limiting its ability to carry out its function [73]. On the other hand, Diep et al. [45] hypothesized that enterocins, which primarily cause membrane perforation, use Man-PTS as a docking molecule, which has been supported by Barraza [74] in their study, may aid in the exacerbation of activities of vancomycin in the VRE cells. Synergistic activities of bacteriocin and antibiotics have also been demonstrated by Singh et al. [75] using nisin and β-lactam antibiotics as an adjunct treatment for MDR Salmonella enterica, whose mechanism of synergy was associated with the different mechanisms of action of antimicrobials used. On the other hand, most of the setup for vancomycin in combination with the bacteriocins do not result in synergism but only demonstrate additive functionalities.
The application of a combination between bacteriocins and antibiotics in the process of control of biofilms was previously suggested and explored [21,28,34,38]. Application of antimicrobials with different or same mode of actions has his arguments for the better success of control of biofilm-associated pathogens. On one side, antibiotics, such as vancomycin and bacteriocins from class IIa, are known to use the same receptor, lipid II, in the interaction between antimicrobials and target cells [30,32,43]. In these processes, both antimicrobials (antibiotic and bacteriocin) may have an extended effect on the target pathogens. Moreover, it was previously shown that when applied in high concentrations, nisin can act bactericidal even if lipid II receptor was not biologically available [30,32,43]. Thus, it can be an argument to suggest that in combined application between vancomycin and studied bacteriocins is a possibility that the applied antibiotic is targeting the test pathogens via lipid II receptor; however, bacteriocins were interfering with the target cells via different mechanisms.
When ciprofloxacin was applied, the opposite scenario was realized in the inhibition of the target pathogens. Ciprofloxacin is classified as a bactericidal antibiotic, part of the fluoroquinolone drug class. His mode of action was associated with inhibition of DNA replication by interfering with bacterial DNA topoisomerase and DNA-gyrase [76]. In this way of application, most probably applied bacteriocins were responsible for pore formation as a consequence of the interactions with lipid II and facilitating the effect of the ciprofloxacin to perform his bactericidal effect.
5. Conclusions
The use and application of bacteriocins as a promising alternative to conventional antibiotics have been proposed by various scientists for decades. The elucidation of their function and possible applications is now eyed as a possible solution to the alarming emergence of AMR/MDR pathogens. In this study, we have evaluated the possible use of bacteriocins in combination with selected conventional antibiotics as a treatment against biofilms formed by L. monocytogenes and vancomycin-resistant E. faecium, food-borne and clinically significant pathogens, respectively. Findings showed that combinations of naturally occurring antimicrobial peptides produced by beneficial enterococci with conventional antibiotics have more notable effects against both planktonic and biofilms of vancomycin-resistant E. faecium, although higher concentrations of both bacteriocins conventional antimicrobials are needed to completely eradicate functional or abolish metabolically active cells. This perspective can be further explored as an alternative way of addressing the current issues of increasing infections associated with AMR pathogens, but the use of high concentrations of antimicrobials, may it be bacteriocins or conventional antibiotics, intended for any application should be regarded carefully and regulated, especially as a bane, acting as another layer of selective pressure for development of new resistant strains, rather than a boon on this current issue.
Author Contributions
Concept: S.D.T.; Experimental work: J.I.I.F. and J.E.V.B.; Funds: W.H.H. and S.D.T.; Writing of the manuscript: J.I.I.F.; Corrections and editing: W.H.H. and S.D.T. All authors have read and agreed to the published version of the manuscript.
Funding
Grant from the National Research Foundation (NRF) funded by the Ministry of Science & ICT (NRF-2018M3A9F3021964), Seoul, Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data related to this work is available upon request.
Acknowledgments
To Handong Global University, Pohang, Korea, for providing the research facilities.
Conflicts of Interest
The authors declare no conflict to interest.
References
- Balcázar, J.L.; Subirats, J.; Borrego, C.M. The role of biofilms as environmental reservoirs of antibiotic resistance. Front. Microbiol. 2015, 6, 1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, S.C.; Yang, L.; Biofilms, I. Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Boca Raton, FL, USA, 2016; pp. 407–415. [Google Scholar]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Ghoush, M.H.A.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of antibiotic resistance in Listeria monocytogenes isolated from food products: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diggle, S.P.; Williams, P. Quorum sensing. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: Boca Raton, FL, USA, 2013; pp. 25–27. [Google Scholar]
- Frederix, M.; Downie, J.A. Quorum Sensing: Regulating the Regulators; Poole, R.K., Ed.; Academic Press: Boca Raton, FL, USA, 2011; Volume 58, pp. 23–80. [Google Scholar]
- Houdt, R.V.; Michiels, C.W. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, L.V. Microbial biofilm in food processing. LWT—Food Sci. Technol. 1999, 32, 321–326. [Google Scholar] [CrossRef]
- Fagerlund, A.; Langsrud, S.; Møretrø, T. Microbial diversity and ecology of biofilms in food industry environments associated with Listeria monocytogenes persistence. Curr. Opin. Food Sci. 2021, 37, 171–178. [Google Scholar] [CrossRef]
- Ranieri, M.R.; Whitchurch, C.B.; Burrows, L.L. Mechanisms of biofilm stimulation by subinhibitory concentrations of antimicrobials. Curr. Opin. Microbiol. 2018, 45, 164–169. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Lee Wong, A.C. Biofilms in food processing environments. J. Dairy Sci. 1998, 81, 2765–2770. [Google Scholar] [CrossRef]
- Patil, A.; Banerji, R.; Kanojiya, P.; Saroj, S.D. Foodborne ESKAPE biofilms and antimicrobial resistance: Lessons learned from clinical isolates. Pathol. Glob. Health 2021, 115, 339–356. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Thakur, M.; Asrani, R.K.; Patial, V. Listeria monocytogenes: A food-borne pathogen. In Foodborne Diseases; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Boca Raton, FL, USA, 2018; pp. 157–192. [Google Scholar]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Pearson, L.J.; Marth, E.H. Listeria monocytogenes—Threat to a safe food supply: A Review. J. Dairy Sci. 1990, 73, 912–928. [Google Scholar] [CrossRef]
- Alzamora, S.M.; López-Malo, A.; Tapia, M.S.; Welti-Chanes, J. Minimally processed foods. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Boca Raton, FL, USA, 2016; pp. 767–771. [Google Scholar]
- Kumar, A.; Jha, A. Drug development strategies. In Anticandidal Agents; Kumar, A., Jha, A., Eds.; Academic Press: Boca Raton, FL, USA, 2017; pp. 63–71. [Google Scholar]
- Møretrø, T.; Langsrud, S. Listeria monocytogenes: Biofilm formation and persistence in food-processing environments. Biofilms 2004, 1, 107–121. [Google Scholar] [CrossRef]
- Oloketuyi, S.F.; Khan, F. Inhibition strategies of Listeria monocytogenes biofilms—Current knowledge and future outlooks. J. Basic Microbiol. 2017, 57, 728–743. [Google Scholar] [CrossRef]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti-Infect. Ther. 2014, 12, 1221–12236. [Google Scholar] [CrossRef]
- Miller, W.R.; Murray, B.E.; Rice, L.B.; Arias, C.A. Vancomycin-resistant enterococci: Therapeutic challenges in the 21st century. Infect. Dis. Clin. N. Am. 2016, 30, 415–439. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, T.; Xu, P.; Xu, X.; Ji, S.; Gao, W.; Shi, L. Role of efflux pumps in the in vitro development of ciprofloxacin resistance in Listeria monocytogenes. Front. Microbiol. 2018, 9, 2350. [Google Scholar] [CrossRef]
- Said, M.S.; Tirthani, E.; Lesho, E. Enterococcus infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Chng, J.-H.; Chong, K.K.L.; Lam, L.N.; Wong, J.J.; Kline, K.A. Biofilm-associated infection by enterococci. Nat. Rev. Microbiol. 2019, 17, 82–94. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.d.L.B.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G. Guidance on the assessment of the safety of feed additives for the target species. EFSA J. 2017, 15, e05021. [Google Scholar]
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28. [Google Scholar] [CrossRef]
- Drider, D.; Fimland, G.; Héchard, Y.; McMullen, L.M.; Prévost, H. The continuing story of Class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 2006, 70, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacterial lantibiotics: Strategies to improve therapeutic potential. Curr. Protein Pept. Sci. 2005, 6, 61–75. [Google Scholar] [CrossRef]
- Joerger, R.D. Alternatives to antibiotics: Bacteriocins, antimicrobial peptides, and bacteriophages. Poultry Sci. 2003, 82, 640–647. [Google Scholar] [CrossRef]
- Leite, E.L.; Oliveira, A.F.D., Jr.; Carmo, F.L.R.D.; Berkova, N.; Barh, D.; Ghosh, P.; Azevedo, V. Bacteriocins as an alternative in the treatment of infections by Staphylococcus aureus. An. Acad. Bras. Ciências 2020, 92, e20201216. [Google Scholar] [CrossRef]
- Melander, R.J.; Zurawski, D.V.; Melander, C. Narrow-spectrum antibacterial agents. MedChemComm 2018, 9, 12–21. [Google Scholar] [CrossRef]
- Fugaban, J.I.I.; Vazquez Bucheli, J.E.; Holzapfel, W.H.; Todorov, S.D. Characterization of partially purified bacteriocins produced by Enterococcus faecium strains isolated from soybean paste active against Listeria spp. and vancomycin-resistant enterococci. Microorganisms 2021, 9, 1085. [Google Scholar] [CrossRef]
- Todorov, S.D.; Dicks, L.M.T. Evaluation of lactic acid bacteria from kefir, molasses and olive brine as possible probiotics based on physiological properties. Ann. Microbiol. 2008, 58, 661–670. [Google Scholar] [CrossRef]
- Todorov, S.D.; de Paula, O.A.L.; Camargo, A.C.; Lopes, D.A.; Nero, L.A. Combined effect of bacteriocin produced by Lactobacillus plantarum ST8SH and vancomycin, propolis or EDTA for controlling biofilm development by Listeria monocytogenes. Rev. Arg. Microbiol. 2018, 50, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Doijad, S.P.; Barbuddhe, S.B.; Garg, S.; Poharkar, K.V.; Kalorey, D.R.; Kurkure, N.V.; Rawool, D.B.; Chakraborty, T. Biofilm-forming abilities of Listeria monocytogenes serotypes isolated from different sources. PLoS ONE 2015, 10, e0137046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Kranjec, C.; Ovchinnikov, K.V.; Grønseth, T.; Ebineshan, K.; Srikantam, A.; Diep, D.B. A bacteriocin-based antimicrobial formulation to effectively disrupt the cell viability of methicillin-resistant Staphylococcus aureus (MRSA) biofilms. NPJ Biofilms Microbiomes 2020, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, M.L.; Miguel, M.G. Use of essential oils and their components against multidrug-resistant bacteria. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Rai, M.K., Kon, K.V., Eds.; Academic Press: Boca Raton, FL, USA, 2013; pp. 65–94. [Google Scholar]
- Nes, I.F.; Diep, D.B.; Ike, Y. Enterococcal bacteriocins and antimicrobial proteins that contribute to niche control. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar., N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Nes, I.F.; Diep, D.B.; Hvarstein, L.S.; Brurberg, M.B.; Eijsink, V.; Holo, H. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek 1996, 70, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Diep, D.B.; Skaugen, M.; Salehian, Z.; Holo, H.; Nes, I.F. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc. Nat. Acad. Sci. USA 2007, 104, 2384–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pag, U.; Sahl, H.-G. Multiple activities in lantibiotics—Models for the design of novel antibiotics? Curr. Pharm. Des. 2002, 8, 815–833. [Google Scholar] [CrossRef]
- Rea, M.C.; Ross, R.P.; Cotter, P.D.; Hill, C. Classification of bacteriocins from Gram-positive bacteria. In Prokaryotic Antimicrobial Peptides: From Genes to Applications; Drider, D., Rebuffat, S., Eds.; Springer: New York, NY, USA, 2011; pp. 29–53. [Google Scholar]
- LeBel, M. Ciprofloxacin: Chemistry, mechanism of action, resistance, antimicrobial spectrum, pharmacokinetics, clinical trials, and adverse reactions. J. Hum. Pharmacol. Drug Ther. 1988, 8, 3–30. [Google Scholar] [CrossRef]
- Brown, P.D. Ciprofloxacin for the management of urinary tract infection. Women’s Health 2006, 2, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Sanders, C.C. Ciprofloxacin: In vitro activity, mechanism of action, and resistance. Rev. Infect. Dis. 1988, 10, 516–527. [Google Scholar] [CrossRef]
- Lyon, S.A.; Berrang, M.E.; Fedorka-Cray, P.J.; Fletcher, D.L.; Meinersmann, R.J. Antimicrobial resistance of Listeria monocytogenes isolated from a poultry further processing plant. Foodborne Pathog. Dis. 2008, 5, 253–259. [Google Scholar] [CrossRef]
- Noll, M.; Kleta, S.; Al Dahouk, S. Antibiotic susceptibility of 259 Listeria monocytogenes strains isolated from food, food-processing plants and human samples in Germany. J. Infect. Public Health 2018, 11, 572–577. [Google Scholar] [CrossRef]
- Rakic-Martinez, M.; Drevets, D.A.; Dutta, V.; Katic, V.; Kathariou, S. Listeria monocytogenes strains selected on ciprofloxacin or the disinfectant benzalkonium chloride exhibit reduced susceptibility to ciprofloxacin, gentamicin, benzalkonium chloride, and other toxic compounds. Appl. Environ. Microbiol. 2011, 77, 8714–8721. [Google Scholar] [CrossRef] [Green Version]
- Perry, J.D.; Ford, M.; Gould, F.K. Susceptibility of enterococci to ciprofloxacin. J. Antimicrob. Chemother. 1994, 34, 297–298. [Google Scholar] [CrossRef]
- Bruniera, F.R.; Ferreira, F.M.; Saviolli, L.R.M.; Bacci, M.R.; Feder, D.; Pedreira, M.D.L.G.; Peterlini, M.A.S.; Azzalis, L.A.; Junqueira, V.B.C.; Fonseca, F.L.A. The use of vancomycin with its therapeutic and adverse effects: A review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 694–700. [Google Scholar]
- Scholar, E. Vancomycin. In XPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–6. [Google Scholar]
- Boneca, I.G.; Chiosis, G. Vancomycin resistance: Occurrence, mechanisms, and strategies to combat it. Expert Opin. Ther. Targets 2003, 7, 311–328. [Google Scholar] [CrossRef]
- Mühlberg, E.; Umstätter, F.; Kleist, C.; Domhan, C.; Mier, W.; Uhl, P. Renaissance of vancomycin: Approaches for breaking antibiotic resistance in multidrug-resistant bacteria. Can. J. Microbiol. 2020, 66, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Cetinkaya, Y.; Falk, P.; Mayhall, C.G. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 2000, 13, 686–707. [Google Scholar] [CrossRef]
- Franz, C.M.A.P. Enterococci in foods—A conundrum for food safety. Int. J. Food Microbiol. 2003, 88, 105–122. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; Holzapfel, W.H.; Stiles, M.E. Enterococci at the crossroads of food safety? Int. J. Food Microbiol. 1999, 4, 1–24. [Google Scholar] [CrossRef]
- Coombs, G.W.; Pearson, J.C.; Daley, D.A.; Le, T.; Robinson, O.J.; Gottlieb, T.; Howden, B.P.; Johnson, P.D.R.; Bennett, C.M.; Stinear, T.P.; et al. Molecular epidemiology of enterococcal bacteremia in Australia. J. Clin. Microbiol. 2014, 52, 897–905. [Google Scholar] [CrossRef] [Green Version]
- Faron, M.L.; Ledeboer, N.A.; Buchan, B.W. Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant Enterococcus in the health care setting. J. Clin. Microbiol. 2016, 54, 2436–2447. [Google Scholar] [CrossRef] [Green Version]
- Courvalin, P. Vancomycin resistance in Gram-positive cocci. Clin. Infect. Dis. 2006, 42, S25–S34. [Google Scholar] [CrossRef]
- Leavis, H.L.; Willems, R.J.L.; Wamel, W.J.B.; Schuren, F.H.; Caspers, M.P.M.; Bonten, M.J.M. Insertion sequence–driven diversification creates a globally dispersed emerging multiresistant subspecies of E. faecium. PLoS Path 2007, 3, e7. [Google Scholar] [CrossRef] [PubMed]
- Tendolkar, P.M.; Baghdayan, A.S.; Gilmore, M.S.; Shankar, N. Enterococcal surface protein, esp, enhances biofilm formation by Enterococcus faecalis. Infect. Immun. 2004, 72, 6032–6039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, F.; Lv, X.; Duan, D.; Wang, L.; Huang, J. Characterization of a linezolid- and vancomycin-resistant Streptococcus suis isolate that harbors optrA and vanG operons. Front. Microbiol. 2019, 10, 2026. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ibarreche, M.; Field, D.; Ross, R.P.; Hill, C. A bioengineered nisin derivative to control Streptococcus uberis biofilms. Appl. Environ. Microbiol. 2021, 87, e0039121. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Jensen, P.Ø.; Burmølle, M.; Hentzer, M.; Haagensen, J.A.J.; Hougen, H.P.; Calum, H.; Madsen, K.G.; Moser, C.; Molin, S.; et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 2005, 151, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Kjos, M.; Nes, I.F.; Diep, D.B. Mechanisms of resistance to bacteriocins targeting the mannose phosphotransferase system. Appl. Environ. Microbiol. 2011, 77, 3335–3342. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, R.F. The interface between COVID-19 and bacterial healthcare-associated infections. Clin. Microbiol. Infect. 2021, 27, 1772–1776. [Google Scholar] [CrossRef]
- Pelfrene, E.; Botgros, R.; Cavaleri, M. Antimicrobial multidrug resistance in the era of COVID-19: A forgotten plight? Antimicrob. Resist. Infect. Control 2021, 10, 21. [Google Scholar] [CrossRef]
- Pingitore, E.V.; Todorov, S.D.; Sesma, F.; Franco, B.D.G.M. Application of bacteriocinogenic Enterococcus mundtii CRL35 and Enterococcus faecium ST88Ch in the control of Listeria monocytogenes in fresh Minas cheese. Food Microbiol. 2012, 32, 38–47. [Google Scholar] [CrossRef]
- Barraza, D.E.; Ríos Colombo, N.S.; Galván, A.E.; Acuña, L.; Minahk, C.J.; Bellomio, A.; Chalón, M.C. New insights into enterocin CRL35: Mechanism of action and immunity revealed by heterologous expression in Escherichia coli. Mol. Microbiol. 2017, 105, 922–933. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Prakash, P.; Achra, A.; Singh, G.P.; Das, A.; Singh, R.L. Standardization and classification of in vitro biofilm formation by clinical isolates of Staphylococcus aureus. J. Glob. Infect. Dis. 2017, 9, 93–101. [Google Scholar]
- Campoli-Richards, D.M.; Monk, J.P.; Price, A.; Benfield, P.; Todd, P.A.; Ward, A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1988, 35, 373–447. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).