Abstract
The gut microbiota interacts with the host’s immune function, and evidence supports a relationship between the gut microbiota and age-related disease. Consumption of herbs and spices, which contain bioactive compounds such as polyphenols, is associated with gut microbiota characteristics that may act to prevent or manage age-related declines in health. This review evaluates the evidence describing the effect of herb/spice intake on the gut microbiota and health during aging. Commonly consumed herbs/spices, their impact on prominent gut bacteria phyla (Bacteriodetes, Firmicutes), and diseases of aging are highlighted. Studies in humans and animals are reviewed. Mechanisms of action are discussed, and future directions for research are proposed. Dietary enrichment with herbs and spices is a potential novel intervention for mitigating declines in physiological function with age.
1. Introduction
Herbs and spices are an integral part of our daily diet. From time immemorial, these have been used for seasoning foods and are still consumed for culinary purposes. They improve the taste, color, and flavor of food and beverages, making them more palatable [1]. In addition to their widespread role as condiments, they have also garnered attention for promoting health and reducing disease risk. They are enriched with health-promoting bioactive constituents, including polyphenols, terpenes, and alkaloids [2]. Mainly, these constituents exhibit antioxidant, antimicrobial, and anti-inflammatory activities and are also responsible for modulating the gut microbiota (GM) [3]. The GM comprises a highly heterogeneous community of microbes (prokaryotic bacteria and archaea, fungi, and eukaryotic parasites and viruses). Thus, the GM is considered important in its role to facilitate digestion and maintain host health and homeostasis. It regulates the host’s immune functions and is crucial in healthy aging and longevity. Hence, a right balance must exist for the GM to flourish, or its dysregulation may lead to improper bodily functions, ultimately giving rise to various age-related chronic diseases [4].
The diversity and profile of the GM are unique to each person and typically remain stable throughout life. Nonetheless, several factors can modulate the microbial constituents, such as genetic background, medication use, diet, and lifestyle [5]. Diet is especially a key factor in influencing the diversity of the GM, suggesting a close link between the food we eat and the microbial organisms in the gut [6]. Specific gut microbes are associated with specific foods and food groups in a way that the diet influences the health of the GM, and the microbial composition influences health outcomes [7]. For example, diets containing processed foods and red meat are associated with deleterious changes in the microbiota profile and increased disease risk [8,9,10]. Unhealthy microbes disrupt the host metabolism and trigger intestinal inflammation, leading to an increased risk of cardiac events, strokes, and type 2 diabetes. In contrast, diets high in fiber-rich vegetables, herbs and spices, and polyunsaturated foods such as nuts, fish, eggs, chia seeds, and sunflower seeds support healthy gut microbes with reduced risk of chronic diseases [11,12]. However, these connections are complex and specifically based on an individual’s dietary regimen.
Aging is marked by the deterioration of tissues and organs, accompanied by changes in the GM. The GM is emerging as a critical factor in the aging process. The composition of gut microbes gradually changes with age and impacts the innate immunity, risk of sarcopenia, and cognitive function [13]. Age-associated alterations in the GM contribute to increased susceptibility to multiple age-related chronic diseases, including cancer, cardiovascular diseases (CVD), rheumatoid arthritis, diabetes, neuroinflammation, and neurodegenerative diseases. Age-related alterations in the GM are influenced by physiological decline and individual factors, including diet, lifestyle, medication, and reduced social contact [14]. The relation between food and the GM is closely related to healthy aging and longevity. Therefore, dietary interventions that focus on restoring a healthy GM are promising therapeutics for the aging population.
Numerous advantages of a healthy diet on GM and health have been attributed to the anti-inflammatory, antioxidant, and antimicrobial effects facilitated by the metabolites of dietary fiber and phenolic compounds [15,16,17]. Protein, fats, digestible and non-digestible carbohydrates, probiotics, and dietary polyphenols all trigger changes in the GM with consequential impacts on the host’s immunological and metabolic indicators [13]. In this context, using herbs and spices is an age-old practice that is used even today to increase the intake of phytochemicals such as polyphenols [18]. Research indicates that certain species contain high concentrations of polyphenols, which have been associated with favorable effects on various health conditions, including certain cancers and chronic diseases such as cardiovascular disease, type II diabetes, and impaired cognitive function [19]. Only a small fraction of polyphenols, approximately 5–10% of the total intake, is absorbed in the small intestine, with the majority, about 90–95%, reaching the large intestine [20]. It is hypothesized that these polyphenols influence the composition of the gut microbiome by undergoing conversion into low-molecular-weight phenolic metabolites, thereby modulating metabolic pathways. This modulation leads to a significant increase in the abundance of beneficial bacteria such as Lactobacillus and Bifidobacterium, while inhibiting the growth of harmful microbiota such as Clostridium histolyticum and Clostridium perfringens, which are associated with inflammatory bowel disease and gastrointestinal disorders, respectively [21]. Furthermore, polyphenolic compounds have been observed to affect bacterial cell membranes, inhibiting the growth of Gram-negative Salmonella and Escherichia strains while not impacting Gram-positive lactic acid bacteria [22].
Mainly, these polyphenolic compounds display prebiotic effects and modulate the GM to provide health benefits to the host [23]. Though the health-promoting impact of culinary herbs and spices through their antioxidant, antimicrobial, and anti-inflammatory properties are known, there are only a few studies describing the prebiotic effect of herbs and spices. The polyphenol compounds in herbs and spices are proposed to exert a prebiotic effect and modulate the gut microbial composition for health benefits (Figure 1).
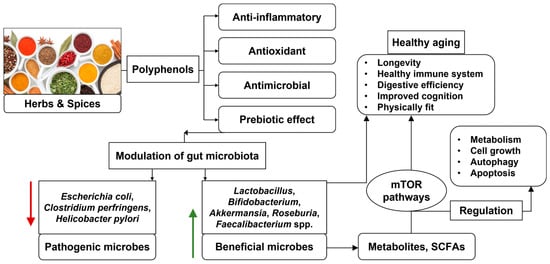
Figure 1.
Role of herbs and spices for modulation of gut microbiota for healthy aging (author).
2. Gut Microbiota
The human body coexists with a complex and dynamic population of microorganisms, mainly trillions of prokaryotic and eukaryotic microbes, and viruses. These microbial organisms colonize various parts of our body, such as the GI tract, skin, lungs, oral cavity, and vagina, and they play a crucial role in the host’s health and physiology [24]. However, the microbes that reside in the gut are considered the most significant in influencing the host’s health and diseases. The collection of the microbes that colonize the GI tract is referred to as the GM, and it has co-evolved with the host over millennia to establish a symbiotic relationship. The gut microbial community plays many beneficial roles in maintaining the host’s homeostasis and regulating health. The GM metabolizes food and has a principal role in harvesting energy, which represents immunity by protecting against pathogens [25].
The intricate interplay between the GM and the host is governed by the gut–brain axis (GBA). The gut–brain axis represents a complex bidirectional communication network between the GI tract and the central nervous system (CNS). It encompasses a sophisticated interplay involving communication pathways between the brain and gut through the CNS, enteric nervous system, vagus nerve, neuroendocrine system, and neuroimmune system. This extensive bidirectional network allows the brain to modulate gut functions such as immune activation, enteric reflex, and intestinal permeability and the gut to influence emotion, mood, cognition, and mental health [26]. More recently, the role of gut microbiota has emerged as a key component in regulating the gut–brain axis and the functions of the brain by secreting and releasing neurochemicals and metabolites such as neurotransmitters, neuropeptides, chemokines, short-chain fatty acids (SCFAs), polyamines, and branched-chain amino acids. Eventually, these components reach the brain through blood, nerve cells, and endocrine cells, and they have the potential to influence the brain activities and behavior of the immune cells [27].
Each individual possesses a unique GM profile adhering to specific roles and functions. The human GM typically comprises six main phyla, namely, Proteobacteria, Firmicutes, Actinobacteria, Fusobacteria, Verrucomicrobia, and Bacteroidetes. About 90% of the total gut bacteria consists of the members of the Firmicutes and Bacteroidetes phyla that are mostly found to inhabit the large intestine. The genera Bacillus, Lactobacillus, Enterococcus, Clostridium, and Ruminicoccus make up most of the Firmicutes phylum, whereas the Prevotella and Bacteroides genera highly constitute the Bacteroidetes phylum. In contrast, Actinobacteria is relatively less diverse and is mostly composed of microbes from the Bifidobacterium genus [23,28,29,30].
The GM regulates nutrient availability, thereby influencing the secretion of biologically active peptides from enteroendocrine cells, which in turn can impact the gut–brain axis [31]. The GM interacts with the host via a variety of microbial biomolecules such as SCFAs (acetate, propionate, and butyrate), tryptophan derivatives (indole and derivatives of indole, tryptamine, and indole propionic acid), polyamines (spermidine and derivatives, putrescine, and spermine), trimethylamine, choline, bile acids, and polyphenols [32,33]. These metabolites play an important role in modulating the functions of hosts and mainly rely on host nutritional signaling pathways such as insulin/insulin-like growth factor-1 signaling pathway and mechanistic or mammalian target of rapamycin (mTOR) pathways to affect the host’s health, lifespan, and gut dysbiosis [34,35]. Omega-3 fatty acids, fermented foods, probiotics, and polyphenol-rich foods such as herbs and spices may improve our gut health, benefiting the gut–brain axis and, ultimately, our health and the aging process.
3. Aging and Age-Related Changes in Gut Microbiota
Aging is a multifaceted biological phenomenon characterized by a gradual decline in the function and physiology of tissues and organs. It is linked to increased incidence of age-related co-morbidities such as cognitive decline and frailty and serves as a significant risk factor for the onset of multiple chronic diseases, including neurodegenerative diseases, cardiovascular diseases, metabolic diseases, immune dysfunction, and musculoskeletal diseases [36]. The mechanism underlying aging involves disturbances in proteostasis, mitochondrial dysfunction, altered nutrient sensing, telomere shortening, genomic instability, cellular senescence, depletion of stem cells, epigenetic modifications, disruptions in intercellular communication, and gut dysbiosis [37,38].
There is an increasing recognition that the dynamics and composition of the intestinal microbiota play an active role in altering the host’s health and healthy aging process. This concept is mainly associated with the generalization that a healthy older individual’s gut is characterized by the presence of a diverse microbial community, whereas low microbial diversity is associated with frailty and diseased states [39]. As bacterial colonization of the GI tract initiates at birth and evolves throughout life, the composition of the GM and its vitality highly depends on the host’s age. Age-related changes in the GM appear to negatively impact an aging individual’s health by increasing the risk of multiple pathologies [40]. With aging, gut microbial alterations include the loss of beneficial microbes and the accumulation of proinflammatory pathobionts. This condition is accelerated and further exacerbated by lifestyle factors such as the use of antibiotics, reduced mobility, and restricted diet [4]. Frail elderly people exhibit gut dysbiosis characterized by a significant decrease in the population of beneficial commensal bacteria, including Bifidobacterium, Akkermansia muciniphila, Lactobacillus, and SCFA-producing bacteria. Simultaneously, there is a notable rise in opportunistic and potentially proinflammatory commensal microbes from families such as Enterobacteriaceae. However, these observations are not correlative and do not apply to certain age groups from different geological locations or genetic backgrounds. Especially, the GM of centenarians and supercentenarians is enriched with beneficial commensals such as Bifidobacteria and Christensenella [28,41,42].
The GM can be regulated and modulated through appropriate measures, suggesting a potential target for aging interventions. Supplementation or improvements in habitual diet have the potential to modulate GM diversity and may help to address age-associated frailty and pathology. In this regard, herbs and spices at culinary doses have the ability to affect the composition of the GM and slow declining health. Thus, reducing age-associated physiological decline through a proper diet-based modulation of the microbiome can be one of the most promising research avenues for healthy aging.
4. Dietary Interventions for Modulation of Gut Microbiota
Diet is widely recognized as one of the most influential factors in shaping the composition of the gut microbiota. Food provides nutrients to our body and the substrate necessary for the growth and development of the GM, which interacts with the epithelial lining and mucosal immune system and maintains the host’s homeostasis. Thus, the food we eat shapes the GM’s structure, function, and composition and, eventually, the healthy aging process [43]. Primarily ‘animal-based’ or ‘plant-based’ diets lead to significant alterations in the gut microbiota in humans [44,45]. The GM ferments undigested dietary fibers, producing short-chain fatty acids that play crucial roles in enterocyte differentiation, growth arrest, and apoptosis. The primary SCFAs produced are acetic acid, propionic acid, butyric acid, and, in smaller amounts, lactic acid, 2-hydroxy propanoic acid, and valeric and caproic acids. Acetic and propionic acids are mainly absorbed from the colonic lumen, while butyric acid serves as the primary substrate of colonocytes. Pathologies such as inflammatory bowel disease, irritable bowel syndrome, cancer, and others are closely linked to SCFA production [46].
Research has demonstrated that spices and herbs such as rosemary, ginger, garlic, sage, turmeric, parsley, cloves, peppers, thyme, dill, saffron, fennel, and oregano, among others, are rich sources of antioxidants. They are enriched with phenolic and non-phenolic compounds and are mostly composed of luteolin, quercetin, curcumin, kaempferol, capsaicin, allicin, apigenin, and piperine and have been used as functional foods for various age-associated risk factors [2,47]. Table 1 summarizes recent human studies of the effects of herb/spice intake on the GM and health outcomes. A randomized, controlled feeding study supported that adding a high dose (6.6 g · d−1 · 2100 kcal−1) of mixed culinary herbs and spices improves cardiometabolic health [3]. In addition, multiple single-herb/spice (turmeric, curcumin, cinnamon) feeding and supplementation studies have been conducted showing positive effects on lipid metabolism, glucose homeostasis, vascular health, and markers of inflammation and oxidative stress [48]. Nonetheless, data on the dietary intake of herbs and spices are limited. Further studies, particularly longitudinal ones, are essential to explore the dietary intake of culinary herbs and spices. Few dietary assessment methodologies that capture herb and spice intake are currently available (citations as in future research directions) [49,50].

Table 1.
Summary of effects of herbs and spices on gut microbiota profile and physiological outcomes in humans.
5. mTOR Signaling Pathway and Gut Microbiota
Nutrition influences the expression of genes and metabolism of the host and GM through numerous signaling pathways. These signaling processes are mostly controlled by the mammalian target of rapamycin (mTOR) pathways [36]. mTOR is a serine/threonine protein kinase that comprises two structurally similar but functionally different protein complexes, mTOR complex 1 and mTOR complex 2. These complex structures regulate the various cellular activities. mTOR serves as a critical mediator that detects alterations in the cellular environment and governs a multitude of intracellular activities essential for cell function and adaptation. These intracellular activities include transcription, translation, cell growth, and cytoskeletal organization. mTOR primarily regulates cell growth, metabolism, autophagy, and apoptosis by promoting protein synthesis in response to nutritional availability and growth factors. As a result, dysregulation of the mTOR signaling pathway has been linked with a wide range of human diseases such as cancer, diabetes, heart disease, and neurological disorders [58,59].
An increasing number of studies have linked intestinal microorganisms and their complex metabolites with host metabolic and immune responses via mTOR signaling pathways. Gut microbes can directly and indirectly stimulate mTOR through alterations in their abundance or metabolites, predominantly SCFAs. By integrating intra- and extracellular signals, mTOR can profoundly impact disease development. The GM and its metabolites regulate various host functions, including autophagy, fatty acid metabolism, oxidative phosphorylation, and immune response [60,61]. Dysregulation of these pathways is associated with various age-related health disorders involving the mTOR signaling pathway. Recent studies have identified mTOR as a novel target for curcumin, highlighting the potential beneficial effects of natural compounds and nutraceuticals in regulating the mTOR signaling pathway in various diseases [62].
These herbs and spices not only influence the mTOR pathway but also serve as therapeutic agents. For instance, rhein, derived from rhubarb, aids in treating UC by reducing Enterobacteriaceae and Turicibacter levels, as well as inhibiting the PI3K/AKT/mTOR pathway, thereby decreasing pro-inflammatory cytokines [63]. Similarly, curcumin acts as a therapeutic agent in treating hepatocellular carcinoma by suppressing mTOR activity, increasing the presence of Helicobacteraceae, and promoting apoptosis [64]. Furthermore, the therapeutic compound resveratrol, a specific mTOR complex inhibitor, alters GM composition by reducing Lactococcus and Clostridium XI, thereby preventing glucose intolerance and fat accumulation in mice fed a high-fat diet (HFD) [65].
6. Prebiotic Potential of Herbs and Spices
Traditional medicines have recognized the use of herbs and spices for their digestive stimulant properties [66]. The ability of herbs and spices to influence the digestive process is demonstrated by the enhanced production of digestive enzymes and prebiotic effects to modulate the functions of intestinal flora. Culinary herbs and spices are rich sources of antioxidants. They have the potential to impact health through antioxidant and antimicrobial properties. Additionally, they may also promote microbial modulation by fostering the growth of beneficial gut bacteria [53]. Herbs and spices, when consumed at culinary doses, display prebiotic activity. Prebiotics are the components that the GM selectively uses to promote host health. They contribute to a healthier GM composition by promoting the growth of beneficial bacteria, such as Bifidobacteria spp. and Lactobacilli spp., and suppressing the growth of pathogenic bacteria [52].
Petersen et al. (2020, 2021) [3,51] reported increases in α-diversity in association with reductions in blood pressure in adults fed a mixture of herbs and spices over 4 weeks. Lu et al., 2019 [53] showed changes in 26 microbial operational taxonomic units in participants taking 5 g mixed-spice capsules daily for 2 weeks. Further, in an observational study, Vita et al. (2022, 2024) observed significant beneficial changes in the GM profile in association with higher intakes of herbs/spices and polyphenols [51,53,56,57]. The fermentation of prebiotics by gut microbiota results in the production of SCFAs, including lactic acid, butyric acid, and propionic acid. As SCFAs play a crucial role in maintaining gut health, the alteration in SCFA levels has been associated with the development of age-related pathologies such as cardio-metabolic disorders [67].
The International Scientific Association of Probiotics and Prebiotics defines a prebiotic as a substrate that is selectively used by microbes to benefit the host’s health [54]. The most common prebiotics are fructo-oligosaccharides, trans-galacto-oligosaccharides, and galacto-oligosaccharides. Although carbohydrates are more likely than other micronutrients to fulfill the prebiotic characteristics, certain compounds that are not carbohydrates, such as polyphenols, are also classified as prebiotics [68].
Spices are rich in polyphenols. These compounds have low bioavailability and are extensively metabolized in the large intestine, favoring interactions with the GM. Interestingly, there is a two-way connection in which dietary polyphenols modulate the GM and the GM modulates the activity of polyphenolic compounds to exert biological effects. Such bidirectional association can regulate the host’s metabolic activity, specifically for converting these components into easily digestible metabolites and subsequent absorption to perform specific functions that affect the host’s health [69]. The bidirectional interaction between the dietary polyphenols and the gut microbes is essential for maintaining the host’s homeostasis and is fundamental for the symbiotic relationship between the host and gut microbes [70].
Polyphenols are present in many dietary sources, such as fruits, vegetables, berries, cocoa, herbs, and spices. Secondary metabolites of polyphenol catabolism exert their beneficial effects as prebiotic substrates by favoring the growth of beneficial gut microbial families, for instance, Bifidobacteriaceae and Lactobacillaceae, and lowering the number of harmful bacteria such as Helicobacter pylori, Clostridium perfringens, and Escherichia coli [71]. Dietary polyphenols obtained from fruits, berries, and teas have been investigated for clinical studies. Mainly anthocyanins, ellagic acid, catechins, and proanthocyanidins derived from fruits have been reported to support the growth of beneficial bacteria such as Bifidobacterium, Lactobacillus, Akkermansia, Faecalibacterium, and Roseburia spp., consequently increasing the production of SCFAs to promote the host’s health [17,72].
In vitro and in vivo studies have demonstrated that water-alcoholic extracts of herbs Ocimum sanctum, and Zingiber officinale exhibit high prebiotic activity. In comparison to the standard prebiotic, FOS, the extracts of these plants supported the in vitro growth of beneficial microbes such as Lactobacillus rhamnosus and Bifidobacterium infantis. Even at low concentrations of 5 mg/L, the extracts were highly effective and better than fructo-oligosaccharides in promoting the abundance of these microbes. In contrast, the hydro-alcoholic extract of Piper nigrum showed prebiotic activity similar to the standard prebiotic fructo-oligosaccharides [73]. Likewise, in vivo analysis of the extracts of the same plant species on healthy SD rats for 30 days showed profuse growth of Bifidobacterium and Lactobacillus and inhibited growth of harmful microbes such as Firmicutes and Bacteroides spp. In these studies, 100, 500, and 850 mg/kg-Bw of hydroalcoholic extracts of Piper nigrum, Zingiber officinale, and Ocimum sanctum, respectively, were used against 5 g/kg body weight of the standard prebiotic, fructo-oligosaccharides [74].
These polyphenolic compounds possess properties that support the growth and activity of beneficial gut bacteria, contributing to improved gut health and overall well-being. Their ability to selectively stimulate the growth of beneficial microbes while inhibiting the proliferation of harmful bacteria underscores their potential as valuable prebiotic agents [72]. Polyphenols such as gallic acid, apigenin, kaempferol, quercetin, luteolin, caffeic acid, vanillic acid, p-coumaric acid, chlorogenic acid, protocatechuic acid, 3-hydroxy benzaldehyde, and ferulic acid were identified in these plant species (Piper nigrum, Zingiber officinale, and Ocimum sanctum) [73,74].
Likewise, aqueous extract of culinary spices, including Mediterranean oregano, black pepper, rosemary, cayenne pepper, ginger, cinnamon, and turmeric, displayed prebiotic-like activity. In vitro studies showed that the aqueous extracts of all plant extracts (concentrations ranging from 4.5 mg/mL to 9.0 mg/mL) except turmeric promoted the growth of Bifidobacterium spp. and Lactobacillus spp. and suppressed the growth of selected Ruminococcus spp. The aqueous extracts of turmeric, cinnamon, rosemary, and oregano were found to be active against selected Fusobacterium spp. and Clostridium spp. [55]. This suggests the potential role of spices in the regulation of the GM for the promotion of gastrointestinal health.
In human subjects, mixed spices at culinary doses affect GM composition, indicating a significant prebiotic effect. The effect of five different mixed spices (capsules containing 5 g of cinnamon, oregano, ginger, rosemary, black pepper, and cayenne pepper) consumed daily in dietary doses found that up to 26 operational taxonomic units (OTUs) were modulated as compared with the control group consuming a maltodextrin placebo capsule [52]. Therefore, polyphenols found in culinary herbs and spices likely have a significant role in modulating the metabolism of the gut communities, which may contribute to optimal digestive function, improved digestive efficiency, and healthy aging.
7. Future Research Direction
Experimental short-term human studies support the beneficial effects of spices on outcomes relevant to aging, such as inhibiting colorectal neoplasm growth and reducing 24 h ambulatory blood pressure, high-fat-diet-induced lipemia, and biomarkers of oxidative stress [75,76,77]; however, long-term epidemiological studies that evaluate habitual intakes of herbs and spices and health are lacking. This is partly due to the difficulty measuring herbs and spice intakes, which are typically small in amount and variable across days. Only two validation studies of herb and spice intake questionnaires have been published [49,50]. In order to advance the understanding of the role of culinary herbs and spices in optimizing healthy aging, it is crucial to conduct repeated and longitudinal monitoring of dietary intakes and health outcomes in large cohorts of men and women. National cohort studies such as the U.S. National Health and Nutrition Examination Survey (NHANES) would be ideal contexts for probing for herb and spice intakes within the dietary assessment component. These measurements could be analyzed with data quantifying the markers of herb/spice absorption, fecal microbiota profiles [78], and incidence of disease [79]. Currently, the NHANES collects data on dietary supplement use but not culinary herbs and spice consumption [80]. In addition, the GM has a significant role in maintaining our health. Modulation of the GM composition with the use of prebiotics, probiotics, and microbiota transplant is emerging as a new technique in developing personalized medicine for treating various diseases [28,81]. In this regard, the present state of knowledge on the role of herbs and spices as prebiotics should be expanded. The comprehensive understanding of the ability of specific metabolites from herbs and spices to regulate the GM composition will potentially aid in the development of novel drugs for disease treatment.
8. Conclusions
This review described evidence supporting a significant relationship between herb and spice consumption, the GM composition and function, and host health, particularly in the context of aging. The rich content of polyphenols in herbs and spices appears to be a main factor in modulating the GM and its production of beneficial metabolites that possess anti-inflammatory, antioxidant bioactivity. Age-related chronic disease is characterized by systemic inflammation and oxidative stress, which may be effectively managed by the inclusion of herbs and spices in the diet. We strongly encourage continued research in this area, specifically in longitudinal aging studies that utilize validated methods for quantifying herb and spice intake.
Author Contributions
Conceptualization, S.P.; methodology, S.P. and K.S.; software, N.B.; validation, S.P., K.S. and C.B.; formal analysis, J.O.-R. and N.B.; writing—original draft preparation, S.P.; writing—review and editing, C.B. and J.O.-R.; supervision, K.S.; project administration, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zachariah, T.J.; Leela, N.K. Spices: Secondary Metabolites and Medicinal Properties. In Indian Spices: The Legacy, Production and Processing of India’s Treasured Export; Sharangi, A.B., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 277–316. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Anderson, S.; See, J.R.C.; Leister, J.; Kris-Etherton, P.M.; Lamendella, R. Herbs and Spices Modulate Gut Bacterial Composition in Adults at Risk for CVD: Results of a Prespecified Exploratory Analysis from a Randomized, Crossover, Controlled-Feeding Study. J. Nutr. 2022, 152, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Walter, J.; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802.e5. [Google Scholar] [CrossRef]
- Chassaing, B.; Vijay-Kumar, M.; Gewirtz, A.T. How Diet Can Impact Gut Microbiota to Promote or Endanger Health. Curr. Opin. Gastroenterol. 2017, 33, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Shan, K.; Xie, Y.; Zhang, G.; An, Q.; Yu, X.; Zhou, G.; Li, C. Body Weight Index Indicates the Responses of the Fecal Microbiota, Metabolome and Proteome to Beef/Chicken-Based Diet Alterations in Chinese Volunteers. npj Biofilms Microbiomes 2022, 8, 56. [Google Scholar] [CrossRef]
- Sasso, A.; Latella, G. Role of Heme Iron in the Association between Red Meat Consumption and Colorectal Cancer. Nutr. Cancer 2018, 70, 1173–1183. [Google Scholar] [CrossRef]
- Atzeni, A.; Martínez, M.Á.; Babio, N.; Konstanti, P.; Tinahones, F.J.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Moreno-Indias, I.; et al. Association between Ultra-Processed Food Consumption and Gut Microbiota in Senior Subjects with Overweight/Obesity and Metabolic Syndrome. Front. Nutr. 2022, 9, 976547. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Khine, W.W.T.; Haldar, S.; De Loi, S.; Lee, Y.-K. A Single Serving of Mixed Spices Alters Gut Microflora Composition: A Dose–Response Randomised Trial. Sci. Rep. 2021, 11, 11264. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the Human Gut Microbiota by Phenolics and Phenolic Fiber-rich Foods. Comp. Rev. Food Sci. Food Safe 2020, 19, 1268–1298. [Google Scholar] [CrossRef]
- Awika, J.M.; Rose, D.J.; Simsek, S. Complementary Effects of Cereal and Pulse Polyphenols and Dietary Fiber on Chronic Inflammation and Gut Health. Food Funct. 2018, 9, 1389–1409. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Yadav, S.S. A review on health benefits of phenolics derived from dietary spices. Curr. Res. Food Sci. 2022, 5, 1508–1523. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E. (Ed.) Flavonoids and Related Compounds; Oxidative stress and disease; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-138-19941-5. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Kleessen, B.; Kroesen, A.J.; Buhr, H.J.; Blaut, M. Mucosal and Invading Bacteria in Patients with Inflammatory Bowel Disease Compared with Controls. Scand. J. Gastroenterol. 2002, 37, 1034–1041. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kahkonen, M.; Heinonen, M.; Maatta-Riihinen, K.; Oksman-Caldentey, K.-M. Berry Phenolics Selectively Inhibit the Growth of Intestinal Pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Ahlawat, S.; Asha; Sharma, K.K. Gut–organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, Y.; Zhao, J.; Chen, W.; Lu, W. Achieving healthy aging through gut microbiota-directed dietary intervention: Focusing on microbial biomarkers and host mechanisms. J. Adv. Res. 2024; in press. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Mitsuyama, E.; Yoshida, K.; Odamaki, T.; Xiao, J. Enriched metabolites that potentially promote age-associated diseases in subjects with an elderly-type gut microbiota. Gut Microbes 2021, 13, 1865705. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Molinero, N.; Antón-Fernández, A.; Hernández, F.; Ávila, J.; Bartolomé, B.; Moreno-Arribas, M.V. Gut Microbiota, an Additional Hallmark of Human Aging and Neurodegeneration. Neuroscience 2023, 518, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Valdés-Varela, L.; González, S.; Gueimonde, M.; De Los Reyes-Gavilán, C.G. Nutrition and the gut microbiome in the elderly. Gut Microbes 2017, 8, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Fouhy, F.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C.; Cotter, P.D. Composition of the early intestinal microbiota: Knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut Microbes 2012, 3, 203–220. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat—The Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- Di Rosa, C.; Di Francesco, L.; Spiezia, C.; Khazrai, Y.M. Effects of Animal and Vegetable Proteins on Gut Microbiota in Subjects with Overweight or Obesity. Nutrients 2023, 15, 2675. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.A. Health Benefits of Culinary Herbs and Spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef]
- Gupta, K.; Testa, H.; Greenwood, T.; Kostek, M.; Haushalter, K.; Kris-Etherton, P.M.; Petersen, K.S. The Effect of Herbs and Spices on Risk Factors for Cardiometabolic Diseases: A Review of Human Clinical Trials. Nutr. Rev. 2022, 80, 400–427. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Blomhoff, R.; Andersen, L.F. Intakes of culinary herbs and spices from a food frequency questionnaire evaluated against 28-days estimated records. Nutr. J. 2011, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Blanton, C. Relative Validity of an Online Herb and Spice Consumption Questionnaire. Int. J. Environ. Res. Public Health 2020, 17, 2757. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Davis, K.M.; Rogers, C.J.; Proctor, D.N.; West, S.G.; Kris-Etherton, P.M. Herbs and Spices at a Relatively High Culinary Dosage Improves 24-Hour Ambulatory Blood Pressure in Adults at Risk of Cardiometabolic Diseases: A Randomized, Crossover, Controlled-Feeding Study. Am. J. Clin. Nutr. 2021, 114, 1936–1948. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Rodionov, D.A.; Iablokov, S.N.; Pung, M.A.; Chopra, D.; Mills, P.J.; Peterson, S.N. Prebiotic Potential of Culinary Spices Used to Support Digestion and Bioabsorption. Evid.-Based Complement. Altern. Med. 2019, 2019, 8973704. [Google Scholar] [CrossRef]
- Lu, Q.-Y.; Rasmussen, A.M.; Yang, J.; Lee, R.-P.; Huang, J.; Shao, P.; Carpenter, C.L.; Gilbuena, I.; Thames, G.; Henning, S.M.; et al. Mixed Spices at Culinary Doses Have Prebiotic Effects in Healthy Adults: A Pilot Study. Nutrients 2019, 11, 1425. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Summanen, P.H.; Lee, R.; Huang, J.; Henning, S.M.; Heber, D.; Finegold, S.M.; Li, Z. Prebiotic Potential and Chemical Composition of Seven Culinary Spice Extracts. J. Food Sci. 2017, 82, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Vita, A.A.; McClure, R.; Farris, Y.; Danczak, R.; Gundersen, A.; Zwickey, H.; Bradley, R. Associations between Frequency of Culinary Herb Use and Gut Microbiota. Nutrients 2022, 14, 1981. [Google Scholar] [CrossRef]
- Vita, A.A.; Roberts, K.M.; Gundersen, A.; Farris, Y.; Zwickey, H.; Bradley, R.; Weir, T.L. Relationships between Habitual Polyphenol Consumption and Gut Microbiota in the INCLD Health Cohort. Nutrients 2024, 16, 773. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Mannick, J.B.; Lamming, D.W. Targeting the biology of aging with mTOR inhibitors. Nat. Aging 2023, 3, 642–660. [Google Scholar] [CrossRef] [PubMed]
- Beevers, C.; Zhou, H.; Huang, S. Hitting the Golden TORget: Curcumin’s Effects on mTOR Signaling. Anti-Cancer Agents Med. Chem. 2013, 13, 988–994. [Google Scholar] [CrossRef]
- Dong, L.; Du, H.; Zhang, M.; Xu, H.; Pu, X.; Chen, Q.; Luo, R.; Hu, Y.; Wang, Y.; Tu, H.; et al. Anti-inflammatory Effect of Rhein on Ulcerative Colitis via Inhibiting PI3K /Akt/ mTOR Signaling Pathway and Regulating Gut Microbiota. Phytother. Res. 2022, 36, 2081–2094. [Google Scholar] [CrossRef]
- Jin, M.; Kong, L.; Han, Y.; Zhang, S. Gut Microbiota Enhances the Chemosensitivity of Hepatocellular Carcinoma to 5-fluorouracil in Vivo by Increasing Curcumin Bioavailability. Phytother. Res. 2021, 35, 5823–5837. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-J.; Lee, J.; Shin, N.-R.; Kim, M.-S.; Hyun, D.-W.; Yun, J.-H.; Kim, P.S.; Whon, T.W.; Bae, J.-W. Chronic Repression of mTOR Complex 2 Induces Changes in the Gut Microbiota of Diet-Induced Obese Mice. Sci. Rep. 2016, 6, 30887. [Google Scholar] [CrossRef] [PubMed]
- Fifi, A.; Axelrod, C.; Chakraborty, P.; Saps, M. Herbs and Spices in the Treatment of Functional Gastrointestinal Disorders: A Review of Clinical Trials. Nutrients 2018, 10, 1715. [Google Scholar] [CrossRef] [PubMed]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Pasinetti, G.M. The Gut Microbiota Links Dietary Polyphenols With Management of Psychiatric Mood Disorders. Front. Neurosci. 2019, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Narendra Babu, K.; Hemalatha, R.; Satyanarayana, U.; Shujauddin, M.; Himaja, N.; Bhaskarachary, K.; Dinesh Kumar, B. Phytochemicals, polyphenols, prebiotic effect of Ocimum sanctum, Zingiber officinale, Piper nigrum extracts. J. Herb. Med. 2018, 13, 42–51. [Google Scholar] [CrossRef]
- Kondapalli, N.B.; Hemalatha, R.; Uppala, S.; Yathapu, S.R.; Mohammed, S.; Venkata Surekha, M.; Rajendran, A.; Bharadwaj, D.K. Ocimum sanctum, Zingiber officinale, and Piper nigrum extracts and their effects on gut microbiota modulations (prebiotic potential), basal inflammatory markers and lipid levels: Oral supplementation study in healthy rats. Pharm. Biol. 2022, 60, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L.; et al. Phase IIa Clinical Trial of Curcumin for the Prevention of Colorectal Neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Henning, S.M.; Zhang, Y.; Zerlin, A.; Li, L.; Gao, K.; Lee, R.-P.; Karp, H.; Thames, G.; Bowerman, S.; et al. Antioxidant-rich spice added to hamburger meat during cooking results in reduced meat, plasma, and urine malondialdehyde concentrations. Am. J. Clin. Nutr. 2010, 91, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- McCrea, C.E.; West, S.G.; Kris-Etherton, P.M.; Lambert, J.D.; Gaugler, T.L.; Teeter, D.L.; Sauder, K.A.; Gu, Y.; Glisan, S.L.; Skulas-Ray, A.C. Effects of culinary spices and psychological stress on postprandial lipemia and lipase activity: Results of a randomized crossover study and in vitro experiments. J. Transl. Med. 2015, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.M.; Rolfe, V.; Walton, G.E.; Gibson, G.R. Gut microbial modulation by culinary herbs and spices. Food Chem. 2023, 409, 135286. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Dietary (accessed on 3 February 2024).
- Huang, G.; Khan, R.; Zheng, Y.; Lee, P.C.; Li, Q.; Khan, I. Exploring the role of gut microbiota in advancing personalized medicine. Front. Microbiol. 2023, 14, 1274925. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).