Abstract
Alcoholism presents a significant health concern with notable socioeconomic implications. Alcohol withdrawal syndrome (AWS) can manifest when individuals cease or drastically reduce their alcohol consumption after prolonged use. Non-alcoholic fatty liver disease (NAFLD) is characterized by substantial lipid accumulation in the liver cells of individuals with no history of alcohol consumption. There is evidence suggesting an association between cognitive impairment and both conditions. This study aimed to evaluate cognitive impairment in patients with NAFLD and AWS using the Mini-Mental State Examination (MMSE). This study involved 120 patients admitted to two hospitals in Craiova, Romania. Results indicated that patients with NAFLD did not exhibit cognitive impairment as measured by MMSE (Mean = 29.27, SD = 0.785). Conversely, patients with AWS showed more pronounced cognitive dysfunction, with a mean MMSE score at admission of 16.60 ± 4.097 and 24.60 ± 2.832 after 2 weeks under treatment with Vitamins B1 and B6 and Cerebrolysin. Additionally, our findings suggested that cognitive dysfunction among alcohol consumers was correlated with the severity of clinical symptoms, as demonstrated by the severity of tremors in our study. The two-week period under treatment and alcohol withdrawal was insufficient for cognitive function to return to normal levels. Observational studies on longer periods of time are advised.
1. Introduction
Nowadays, alcohol is commonly associated with entertainment, with around one in three people worldwide being a consumer [1]. According to recent reports from Eurostat, in Europe, 1 in 12 persons is a regular consumer, and almost one out of five of these people has an episode of heavy drinking at least once a month [2]. Even though most of the statistics report men as being more avid consumers, recently, the gender gap has gently started to narrow [3].
Alcoholism poses a significant health concern with significant socioeconomic ramifications [4]. This condition accounts for 15–20% of psychiatric admissions and numerous medical, surgical and traumatic emergencies [5].
Among the most encountered irreversible effects of chronic alcohol consumption is cognitive dysfunction [6], which can be more and more observed in patients with no other neurological impairment and can be traced to chronic alcohol intake [7]. Executive functions [8], orientation skills [9] and memory are also affected [6]. Usually, by the time these patients seek medical attention, the cognitive impairments are already established [10,11] with proven effects upon all cognitive domains [12].
The development of multimodal neuroimaging systems have helped the medical community better observe the effects of alcohol consumption on the brain such as cortical shrinkage and white matter degradation, differences in brain activity during task processing, visual perception impairment, damaged cognitive control mechanisms and many others [13].
Some researchers investigated the effect of alcohol abstinence on the improvement of various components of cognitive function such as memory recovery, orientation and inhibition [14,15,16]. Regarding the duration of abstinence necessary for the normalization of cognition, there are not many authors that can agree on a timestamp [6], as some studies reported a significant improvement after two to four weeks [17], whilst others did not find any modification as late as the seventh week [18].
Alcohol withdrawal syndrome (AWS) can occur when patients stop drinking or drastically decrease their alcohol intake after long-term consumption [19]. The symptoms of withdrawal can range widely, from slight tremors to delirium tremens, a condition that causes seizures and, if left untreated, can be fatal [20].
Non-alcoholic fatty liver disease (NAFLD) is characterized by a significant lipid storage in the hepatocytes of patients with negative history of alcohol consumption [21,22]. About 30% individuals can suffer from NAFLD, which is the most common liver damage in the world [23]. Certain authors have observed that individuals diagnosed with NAFLD may exhibit subpar cognitive function across various domains such as general cognition and attention [24,25]. However, the literature presents inconclusive findings, as some studies fail to establish a clear association between NAFLD and cognitive impairments [26]. Introduction of the term metabolic-dysfunction-associated fatty liver disease (MAFLD) presents a recent development aimed at replacing the term NAFLD [27]. Unlike NAFLD, MAFLD does not necessitate excluding other causes of liver disease, such as excessive alcohol consumption or viral hepatitis [27,28]. NAFLD literally refers only to non-alcohol related hepatopathy and does not adequately explain the links with metabolic impairment and related cardiovascular risks [29].
Mini-Mental State Examination (MMSE) is a simple-to-use screening tool broadly used by medical professionals in their daily practice to assess a patient’s cognition [30], consisting of 11 questions that can be administered by healthcare practitioners [31].
Cerebrolysin is a medication that, for some time now, has been used in hospital settings to treat neurological problems such as strokes [32] and traumatic brain injuries [33], and it has captured the interest of the medical world for its beneficial effects [34]. It is a composed of low-molecular-weight peptides and amino acids [35], extracted from the braincells of a porcine [36]. There are some studies on animal models and observational studies on humans suggesting a beneficial effect of Cerebrolysin usage on cognition enhancement in patients with moderate to severe brain injuries [37]. Also, there are a few studies that determined that this compound possesses neurotrophic properties explained in Figure 1, promoting neuronal sprouting and enhancing neuronal survival and neurogenesis [38]. In 2019, a study on rats concluded that it has the potential to improve memory function in individuals with chronic alcoholism by reducing oxidative damage and inhibiting apoptosis in the hippocampus, indicating its potential as a treatment for cognitive impairment associated with alcohol abuse [39].
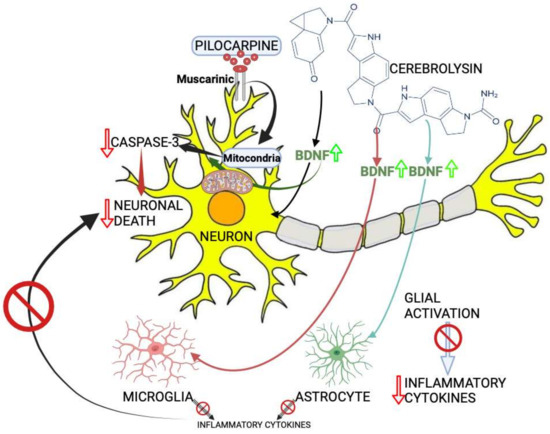
Figure 1.
The mechanism proposed for Cerebrolysin effects on seizure-induced neuronal death. Increased brain-derived neurotrophic factor (BDNF) levels by Cerebrolysin can induce the suppression of glial cell activation, which is believed to take place after seizures induced by pilocarpine. A red cercle and a red arrow indicates inhibition, and the green arrow indicates enhancement. Created with BioRender.com.
The aim of our study was to repeatedly assess the cognitive function of chronic alcohol users at hospital admission and 2 weeks after they were abstinent and under supportive treatment in order to check if there were significant improvements. Also, we wanted to check if there was a cognitive impairment in patients diagnosed with NAFLD and to see if there are differences between the two groups.
2. Materials and Methods
2.1. Selection of Patients
For our analysis, we included patients hospitalized in Craiova’s Clinical Neuropsychiatry Hospital for acute AWS who had a hospitalization period of more than 14 consecutive days and patients diagnosed with NAFLD hospitalized in the Gastroenterology Department of the County Clinical Emergency Hospital of Craiova, Romania, for routine check-up. As there is no definitive evidence of cognitive alteration among patients with NAFLD [26,40], we will consider these patients as our control group.
We conducted a prospective study. The inclusion period lasted for 12 consecutive months, from January 2022 to December 2022.
Patients with autoimmune liver disorders, virus B and virus C chronic hepatitis, liver cirrhosis, hepatocellular carcinoma or other malignancies were excluded due to altered values of hepatic enzymes. Also, we excluded all patients with signs of infection due to possible misinterpretation of liver enzymes and, also, patients with an uncertain history of alcohol consumption.
All the patients, or where it was needed, a legal representative, signed the formal consent for taking part in this study.
The University of Medicine and Pharmacy of Craiova, Romania’s ethical committee approved the current study protocol, which complied with all Declaration of Helsinki criteria (no. 237/20 December 2021).
2.2. Clinical Evaluation
For the screening of cognitive function, we used the MMSE test which is a test most commonly used to detect and evaluate the progress of a cognitive disorder associated with neurodegenerative diseases [41]. It includes five sections: orientation, attention, concentration and calculation, memory and language. The administration of this simple, structured scale requires no more than 5–10 min. The maximum, total score is 30 points. The MMSE ranges were 24–30 for no cognitive impairment, 19–23 for mild, 10–18 for moderate and 0–9 for severe cognitive impairment [41,42,43,44]. MMSE was used at the time of admission in the clinics, and for the patients admitted with symptoms of acute alcohol withdrawal, a second evaluation was performed after two weeks. The evaluation was performed by S.M.
2.3. Definition of NAFLD and Ultrasound Assessment
NAFLD was defined by the presence of hepatic steatosis on ultrasound, excluding heavy drinking individuals. All patients were evaluated by ultrasound conducted by a gastroenterologist with approximately 35 years of experience in intra-abdominal ultrasound (IUS) utilizing a Hitachi Arietta V70 ultrasonography system (Hitachi Ltd., Tokyo, Japan) along with the convex transducer. Also, all the patients with NAFLD had an evaluation of liver fibrosis using FibroScan Mini+ 430 (Echosens, Paris). Patients were instructed to follow a fasting period of at least 6 to 8 h prior to the examination. The ultrasound assessment was focused on the liver of all patients hospitalized in the Gastroenterology Department of the County Clinical Emergency Hospital of Craiova, Romania. We have only used the definition of NAFLD and not MAFLD because the latter diagnosis applies to patients displaying both hepatic steatosis and any of the following metabolic conditions: overweight or obesity, diabetes mellitus or evidence of metabolic dysregulation in lean individuals, and does not exclude the use of alcohol [45].
2.4. Biological Analyses
From all the patients included in the study, a “fasting” blood sample was drawn by an experienced nurse from a peripheral vein by routine phlebotomy. Those samples were used to determine the complete blood count (CBC), erythrocyte sedimentation rate (ESR), C reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), all assessed by routine (automated) laboratory procedures. CRP, ESR and CBC were analyzed for all the patients considered for the study. Based on their abnormal values that were not in line with acute withdrawal syndrome and the NAFLD diagnosis, we excluded a number of 30 patients (9 from the AWS group and 21 from the NAFLD group) and referred them for further clinical and paraclinical testing.
2.5. Treatments
During their admission in the hospital, the AWS group patients were closely monitored by the medical and auxiliar staff. These patients had no access to alcoholic beverages or similar substances. The treatment they received included Cerebrolysin, which we determined in a previous experimental study to have protective effects on the brain cells [46], as well as Vitamin B6 and B1 infusion and symptomatics.
The NAFLD group did not require prolonged hospitalization as the patients were solely attending for a routine check-up and did not receive any medication.
2.6. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics Version 26 and Microsoft Excel 2021 (Microsoft Corp., Redmond, WA, USA). Unless noted otherwise, we show in figures and tables the mean value and standard deviation (SD). We considered there is a statistical significance if p < 0.05, p < 0.01 or p < 0.001.
3. Results
Our study included a total of 120 patients, with a preponderance of males, representing 74.16% of the participants. In AWS patients (study group), there was a predominance of males, representing 83.3% of the total, while in patients with NALFD (control group), the gender gap was not that well represented, with 53.3% of the total participants being females. More than half of the AWS patients were from a rural area, while in the NAFLD group, the balance was in favor of the urban area with 63.3% of patients living in the cities. The hospitalization period was longer for AWS patients than the control group (20.44 ± 14.59 days vs. 1.8 ± 0.95 day, with a minimum = 14 days and a maximum = 34 days vs. minimum = 1 day and a maximum = 4 days). All the patients from the NAFLD group had their liver fibrosis level assessed by fibroscan; all of them had no fibrosis with a mean of 4,6 KPa and a standard deviation of 1.2 KPa.
Table 1 shows the descriptive data about the patients included in the study.

Table 1.
General characteristics of patients.
In order to see if there is a correlation between the hospitalization period and the MMSE score at admission for the AWS group, we used a Pearson correlation coefficient. There was a significant strong negative correlation between the two abovementioned variables, r(87) = −0.638, p < 0.0001. This shows that the lower the MMSE score was at admission, the longer the patients needed to be kept in the hospital, in order to be monitored and receive treatment. The ones with the highest scores were discharged faster. In Figure 2, we present these results in a scatterplot for a better and more accurate representation.
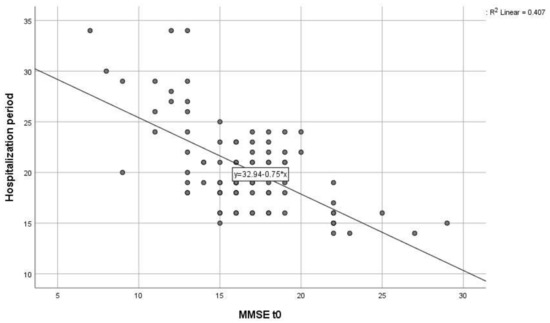
Figure 2.
Scatter plot of the correlation between MMSE score at hospital admission (MMSE t0) and hospitalization period for the AWS group.
We present in Table 2; Table 3 the descriptive statistics of both groups. We performed an Independent Samples t-test which compares the means of GGT between the NAFLD group and the AWS group. There was a significant difference in mean GGT between alcohol consumers and the NAFLD patients (t(19.733) = 6.717, p < 0.001). The average GGT for alcohol consumers was 200.8 U/L higher than the average GGT for non-consumers.

Table 2.
Descriptive statistics of the AWS group.

Table 3.
Descriptive statistics of the NAFLD group.
For an easier and better data analysis we split the AWS group into age groups, as follows:
- 20 to 29 years: 5 patients (5.6%);
- 30 to 39 years: 17 patients (18.9%);
- 40 to 49 years: 25 patients (27.8%);
- 50 to 59 years: 30 patients (33.3%);
- 60 to 69 years: 10 patients (11.1%);
- 70 to 79 years: 3 patients (3.3%).
A one-way ANOVA was performed to evaluate the relationship between patients’ MMSE score at both admission and after 2 weeks and the age category defined as before. The means and standard deviations are presented in Table 4 below.

Table 4.
Descriptive statistics of the MMSE at admission (MMSE t0) and MMSE after 2 weeks (MMSE t2) of the AWS group split according to age groups.
The ANOVA was not significant at the 0.05 level, F(5,84) = 0.772, p = 0.537 for the MMSE at admission and F(5,84) = 0.554, p = 0.735 for the MMSE after 2 weeks.
The control group, consisting of patients with NAFLD, did not have any subject with cognitive impairment measured by MMSE, with a minimum score of 28 and maximum score 30 (see also Table 3).
Mean MMSE score at admission in patients with AWS was 16.60 ± 4.097, compared to patients with NAFLD which was 29.27 ± 0.785. Also, a one-sample t-test was run to determine whether there is a difference in cognition measured using MMSE score between the control group and the alcohol consumers’ group after 2 weeks of withdrawal and treatment. Mean MMSE score at 2 weeks (M = 24.6, SD = 2.832) was lower than the MMSE score of the control group at admission which did not have any participants with abnormal results, a statistically significant mean difference of 4.667, 95% CI [4.011 to 5.322], t(115.87) = 14.095, p < 0.001. The distribution of MMSE score can be observed in Figure 3.
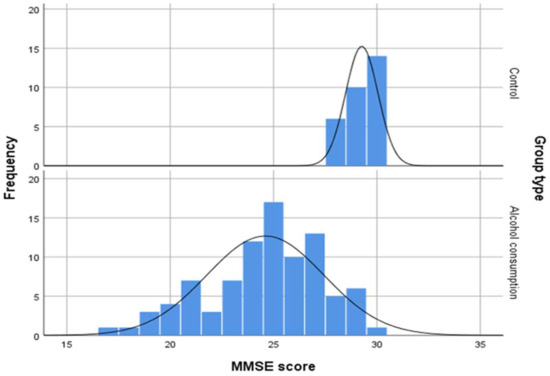
Figure 3.
Distribution of MMSE scores of control group at admission and MMSE score of the alcohol consumers’ group after 2 weeks of withdrawal and treatment.
In Figure 4 is presented the scatterplot of MMSE at admission and MMSE after 2 weeks of treatment which shows a strong, positive, linear association between the two. There are not many outliers in the data.
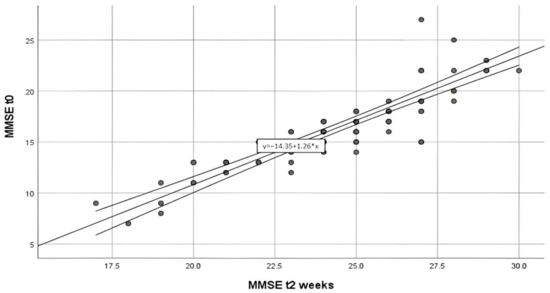
Figure 4.
Scatter plot of the association between MMSE score at admission in the hospital (MMSE at t0) and MMSE score after 2 weeks of treatment (MMSE t2 weeks).
A Pearson correlation coefficient was calculated to evaluate the relationship between MMSE at admission and MMSE after 2 weeks of hospitalization. There was a significant very strong positive relationship between the MMSE scores, r([88]) = [0.881], p = [<0.001].
A paired samples t-test was performed to evaluate whether there was a difference between the MMSE score at admission and MMSE score after 2 weeks of alcohol withdrawal and treatment (as mentioned before). The results indicated that the MMSE after 2 weeks of alcohol withdrawal and treatment (M = [24.60], SD = [2.832]) was significantly higher than the MMSE score at hospital admission (M = [16.60], SD = [4.097]), t([89]) = [36.349], p = [<0.001], showing a considerable improvement.
We analyzed the frequency of the tremor type in correlation with the severity of cognitive impairment as indicated by the MMSE score; the obtained results are presented in Figure 5 and Table 4.
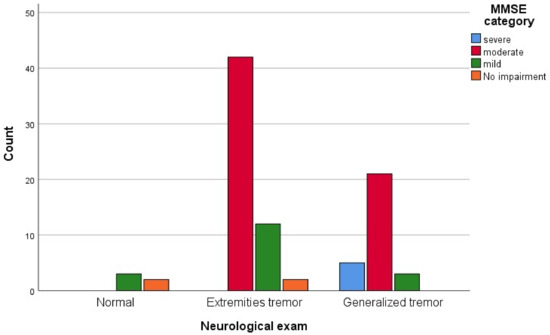
Figure 5.
Neurological exam dispersion according to the MMSE severity represented in a bar chart.
A chi-square test of independence was performed to evaluate the relationship between the neurological exam for which the results are described in Table 5 and MMSE score at admission as presented in Figure 6. The relationship between these variables was significant, χ2 [6], N = [30] = [6.718], p < [0.001]. Patients with a severe score of MMSE were more likely to have generalized tremors than the ones with no impairment identified by the MMSE score.

Table 5.
Neurological exam and MMSE category at admission in the AWS group.
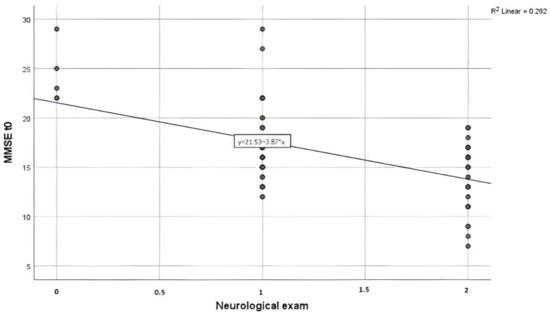
Figure 6.
Scatter plot of the association between MMSE at admission (MMSE t0) and the neurological exam for the AWS group. Nota bene: Neurological exam was coded as follows: 0—Normal, 1—Extremities tremor, 2—Generalized tremor.
No correlation was found between MMSE at admission and environment, gender or age group for the AWS group.
4. Discussion
Peer pressure, defined as the attempt to compel an individual to make a decision based on a norm, still plays an active role in the gender gap regarding alcohol consumption [47]. Traditional masculine norms often include beliefs such as the importance of being tough, aggressive, dominant and self-reliant [48] which are associated with the increased consumption of alcoholic beverages. Even if there is clear recent evidence that this difference between genders is becoming smaller [49], it is still a fact that worldwide, men are more likely associated not only with alcohol consumption but also with heavy drinking [50]. On top of that, the latest statistics show a high alcohol consumption in Eastern European countries which Romania is a part of [51]. The findings in our study endorse these reports as the group of alcohol consumers had a ratio of five men to one woman. However, we must admit that the results of our analysis show there is no correlation between the MMSE score of patients with acute symptoms of alcohol withdrawal and gender, even though there are some studies reporting a relationship between those variables [52].
High levels of GGT are traditionally linked with alcohol intake [53] and liver dysfunction [54]. According to Sueyoshi S et al., GGT serum profile determination could be used for the differential diagnosis of alcoholic-induced liver disease and NAFLD [55].
The updated concept and criteria of MAFLD could enable physicians to identify a larger cohort of patients at risk of adverse outcomes in clinical practice [45]. However, given the novelty of this concept, further investigation is necessary to evaluate its utility in clinical settings and especially in patients with cognitive disorders. As there are not many papers in the literature which tackle MAFLD and related cognitive impairment, we only refer to NAFLD [56,57]. Even if there is an elevation of the GGT, AST and ALT levels associated with excessive weight that, in time, can lead to NAFLD [58,59,60] and Metabolic syndrome [61], the GGT elevation in chronic alcohol consumption is far greater [62]. Our target group of alcohol consumers definitely had GGT levels superiorly increased in comparison to their counterparts. This supports the hypothesis of GGT being used as an accurate marker for alcohol consumption.
A meta-analysis in this area yielded diverse outcomes regarding the relationship between NAFLD and cognitive function, with only individuals with biopsy-proven liver fibrosis demonstrated to be having a confirmed association with cognitive dysfunction [24,25]. Regarding our study, we found no dysfunction in any of our patients diagnosed with NAFLD, even though they had no liver fibrosis assessed by FibroScan, but this can be a flaw due to the small size of our group with only 30 individuals.
In our study, we determined that even after 2 weeks of alcohol withdrawal and adequate treatment and under the careful supervision of medical professionals, in the cognitive assessment measured by the MMSE score, the patients from the AWS group performed poorly compared with the control group. A meta-analysis on the effect of alcohol on cognitive function showed that global cognitive dysfunction can be detected after weeks or even months of abstinence [12]. There should be more research conducted in order to determine a more accurate time period of recovery for patients with chronic alcohol consumption.
Even though after 2 weeks, the MMSE score of the AWS group was not comparable with that of the NAFLD group, a significant improvement in cognition was observed.
We can attribute a part of this improvement to the fact that patients with AWS received a treatment which consisted of a cocktail of Cerebrolysin and Vitamins B1 and B6. The results from our study are consistent with the ones found by other researchers, which supports the hypothesis that cognitive impairment among chronic alcohol consumers is a problem that can improve with alcohol withdrawal and adequate therapy. This topic of neurocognitive changes in neurological patients who received these drugs/supplements also chosen for our patients is a topic that has captivated the interest of many researchers. Some studies proved the beneficial effect of Vitamin B1 on cognitive recovery in elders [63,64] and the impact of Cerebrolysin in the recovery of patients after neurological damage [35,36,37,46]. According to a couple of studies from the current literature, nutritional techniques or supplements may improve cognitive function, despite many unknown biochemical mechanisms [65,66,67]. An important nutrient is creatine, which is used by the brain and muscle mass when consumption increases. The mechanisms of creatine action include fast energy provision by transferring the N-phosphoryl group from phosphocreatine to adenosine diphosphate, restoring adenosine triphosphate, and energy, which involves shifting the energy from the mitochondria to the cytosol [68]. Another micronutrient recently used in patients with cognitive disorders is beta-carotene, which acts like an antioxidant and has anti-inflammatory effects [69]. Further studies should be conducted in order to determine the mechanism of action and adequate duration of treatment.
Tremors are one of the most encountered clinical findings in neurological disorders [70]. In patients with acute alcohol withdrawal, they are often present in different levels of intensity [71], as we also pointed out in our target group. A curious fact is that there was a strong correlation between the severity of the tremor and the MMSE score at admission, which proves, once again, the more severe the cognitive impairment is, the more obvious and louder the clinical symptoms are.
The fact that we only surveyed our patients for 2 weeks, which showed only a mild improvement, and did not continue to monitor them after is a flaw in our study design.
On the other hand, we also managed to confirm there is no cognitive impairment associated with NAFLD, as some studies reported [24,25]. We still have to mention that the patients included in our study with NAFLD were not severe cases, and we did not perform a liver biopsy in order to determine the extent of the illness.
The fact that we only surveyed our patients for 2 weeks, and did not continue to monitor them after, is a flaw in our study design, as we did not manage to observe the final outcome of the patients. Also, another limitation to be mentioned is the fact that our studied group was not big enough to draw a generally accepted conclusion, so we would like to encourage researchers to continue the research in this field. As we mentioned before, the men to women ratio is quite disproportionate, which can be partially attributed to the alcohol-consuming pattern of people in Romania. Also, another fact is that, in Romania, the man-to-woman ratio of alcohol consumers is more disproportionate in rural areas. However, we consider it necessary for more investigations to be carried out in this direction.
5. Conclusions
Through our study, we managed to conclude that GGT is a good marker for evaluating and differentiating alcohol consumers from non-consumers.
Also, we managed to identify the fact that among alcohol consumers, there are cognitive dysfunction that are associated with the severity of clinical symptomatology, represented in our study by tremors.
The two-week period under treatment and alcohol withdrawal was not enough for cognitive function to reach the normal limits, even though there were significant improvements in most of the patients. The mechanism of action of Cerebrolysin and Vitamins B1 and B6 should be better described. There is a need for more studies in this area, and they should be conducted for longer periods of time.
Even though no cognitive impairment among NAFLD patients was observed in our study, we would like to encourage more researchers to study this matter as these results are controversial.
Author Contributions
Conceptualization, S.M. and C.-M.I.; methodology, S.M.; software, M.-A.P.; validation; formal analysis, I.R.; investigation, S.M.; resources, S.M.; data curation, C.-M.I.; writing—original draft preparation, M.-A.P.; writing—review and editing, C.-M.I.; visualization, M.-A.P.; supervision, D.-N.F. and I.R.; project administration, I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The University of Medicine and Pharmacy of Craiova, Romania’s ethical committee approved the current study protocol (no. 237/20 December 2021), which complied with all Declaration of Helsinki criteria.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ilhan, M.N.; Yapar, D. Alcohol Consumption and Alcohol Policy. Turk. J. Med. Sci. 2020, 50, 1197–1202. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Alcohol Consumption. 2023. Available online: https://ourworldindata.org/alcohol-consumption (accessed on 16 February 2024).
- Fama, R. Alcohol’s Unique Effects on Cognition in Women: A 2020 (Re)View to Envision Future Research and Treatment. Alcohol Res. 2020, 40, 3. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.G.; Fullman, N.; Hawley, C.; Arian, N.; Zimsen, S.R.M.; Tymeson, H.D.; Venkateswaran, V.; Tapp, A.D.; Forouzanfar, M.H.; Salama, J.S.; et al. Alcohol Use and Burden for 195 Countries and Territories, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef]
- Eurostat Alcohol Consumption Statistics, 2021. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Alcohol_consumption_statistics#Frequency_of_alcohol_consumption (accessed on 15 February 2024).
- Pelletier, S.; Nalpas, B.; Alarcon, R.; Rigole, H.; Perney, P. Investigation of Cognitive Improvement in Alcohol-Dependent Inpatients Using the Montreal Cognitive Assessment (MoCA) Score. J. Addict. 2016, 2016, 1539096. [Google Scholar] [CrossRef]
- Bernardin, F.; Maheut-Bosser, A.; Paille, F. Cognitive Impairments in Alcohol-Dependent Subjects. Front. Psychiatry 2014, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Ihara, H. Group and Case Study of the Dysexecutive Syndrome in Alcoholism without Amnesia. J. Neurol. Neurosurg. Psychiatry 2000, 68, 731–737. [Google Scholar] [CrossRef]
- Green, A.; Garrick, T.; Sheedy, D.; Blake, H.; Shores, E.A.; Harper, C. The Effect of Moderate to Heavy Alcohol Consumption on Neuropsychological Performance as Measured by the Repeatable Battery for the Assessment of Neuropsychological Status. Alcohol. Clin. Exp. Res. 2010, 34, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Sterling, S.A.; Palzes, V.A.; Lu, Y.; Kline-Simon, A.H.; Parthasarathy, S.; Ross, T.; Elson, J.; Weisner, C.; Maxim, C.; Chi, F.W. Associations Between Medical Conditions and Alcohol Consumption Levels in an Adult Primary Care Population. JAMA Netw. Open 2020, 3, e204687. [Google Scholar] [CrossRef]
- Bruijnen, C.J.W.H.; Dijkstra, B.A.G.; Walvoort, S.J.W.; Markus, W.; VanDerNagel, J.E.L.; Kessels, R.P.C.; DE Jong, C.A.J. Prevalence of Cognitive Impairment in Patients with Substance Use Disorder. Drug Alcohol Rev. 2019, 38, 435–442. [Google Scholar] [CrossRef]
- Stavro, K.; Pelletier, J.; Potvin, S. Widespread and Sustained Cognitive Deficits in Alcoholism: A Meta-analysis. Addict. Biol. 2013, 18, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Schulte, T.; Oberlin, B.G.; Kareken, D.A.; Marinkovic, K.; Müller-Oehring, E.M.; Meyerhoff, D.J.; Tapert, S. How Acute and Chronic Alcohol Consumption Affects Brain Networks: Insights from Multimodal Neuroimaging. Alcohol. Clin. Exp. Res. 2012, 36, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Janke van Holst, R.; Schilt, T. Drug-Related Decrease in Neuropsychological Functions of Abstinent Drug Users. Curr. Drug Abus. Rev. 2011, 4, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Loeber, S.; Duka, T.; Welzel Marquez, H.; Nakovics, H.; Heinz, A.; Mann, K.; Flor, H. Effects of Repeated Withdrawal from Alcohol on Recovery of Cognitive Impairment under Abstinence and Rate of Relapse. Alcohol Alcohol. 2010, 45, 541–547. [Google Scholar] [CrossRef]
- Fein, G.; McGillivray, S. Cognitive Performance in Long-Term Abstinent Elderly Alcoholics. Alcohol. Clin. Exp. Res. 2007, 31, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Kish, G.B.; Hagen, J.M.; Woody, M.M.; Harvey, H.L. Alcoholics’ Recovery from Cerebral Impairment as a Function of Duration of Abstinence. J. Clin. Psychol. 1980, 36, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Yohman, J.R.; Parsons, O.A.; Leber, W.R. Lack of Recovery in Male Alcoholics’ Neuropsychological Performance One Year after Treatment. Alcohol. Clin. Exp. Res. 1985, 9, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Day, E.; Daly, C. Clinical Management of the Alcohol Withdrawal Syndrome. Addiction 2022, 117, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Jesse, S.; Bråthen, G.; Ferrara, M.; Keindl, M.; Ben-Menachem, E.; Tanasescu, R.; Brodtkorb, E.; Hillbom, M.; Leone, M.A.; Ludolph, A.C. Alcohol Withdrawal Syndrome: Mechanisms, Manifestations, and Management. Acta Neurol. Scand. 2017, 135, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Băloşeanu, C.L.; Streba, C.T.; Vere, C.C.; Comănescu, V.; Rogoveanu, I. Association between Liver Histology, Carotid Ultrasonography and Retinal Vascular Changes in Patients with Nonalcoholic Fatty Liver Disease (NAFLD). Rom. J. Morphol. Embryol. 2012, 53, 609–614. [Google Scholar]
- Stepan, M.D.; Vintilescu, Ș.B.; Streață, I.; Podeanu, M.A.; Florescu, D.N. The Role of Vitamin D in Obese Children with Non-Alcoholic Fatty Liver Disease and Associated Metabolic Syndrome. Nutrients 2023, 15, 2113. [Google Scholar] [CrossRef]
- Kim, D.; Cholankeril, G.; Loomba, R.; Ahmed, A. Prevalence of Fatty Liver Disease and Fibrosis Detected by Transient Elastography in Adults in the United States, 2017–2018. Clin. Gastroenterol. Hepatol. 2021, 19, 1499–1501.e2. [Google Scholar] [CrossRef] [PubMed]
- Kjærgaard, K.; Mikkelsen, A.C.D.; Wernberg, C.W.; Grønkjær, L.L.; Eriksen, P.L.; Damholdt, M.F.; Mookerjee, R.P.; Vilstrup, H.; Lauridsen, M.M.; Thomsen, K.L. Cognitive Dysfunction in Non-Alcoholic Fatty Liver Disease—Current Knowledge, Mechanisms and Perspectives. J. Clin. Med. 2021, 10, 673. [Google Scholar] [CrossRef]
- Weinstein, A.A.; de Avila, L.; Paik, J.; Golabi, P.; Escheik, C.; Gerber, L.; Younossi, Z.M. Cognitive Performance in Individuals with Non-Alcoholic Fatty Liver Disease and/or Type 2 Diabetes Mellitus. Psychosomatics 2018, 59, 567–574. [Google Scholar] [CrossRef] [PubMed]
- George, E.S.; Sood, S.; Daly, R.M.; Tan, S.-Y. Is There an Association between Non-Alcoholic Fatty Liver Disease and Cognitive Function? A Systematic Review. BMC Geriatr. 2022, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.-F.; Schattenberg, J.M.; et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Andreetto, L.; D’Ardes, D.; Cocco, G.; Rossi, I.; Vicari, S.; Schiavone, C.; Cipollone, F.; Guagnano, M.T. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines 2023, 11, 883. [Google Scholar] [CrossRef]
- Peng, H.; Pan, L.; Ran, S.; Wang, M.; Huang, S.; Zhao, M.; Cao, Z.; Yao, Z.; Xu, L.; Yang, Q.; et al. Prediction of MAFLD and NAFLD Using Different Screening Indexes: A Cross-Sectional Study in U.S. Adults. Front. Endocrinol. 2023, 14, 1083032. [Google Scholar] [CrossRef] [PubMed]
- Patten, S.B.; Fick, G.H. Clinical Interpretation of the Mini-Mental State. Gen. Hosp. Psychiatry 1993, 15, 254–259. [Google Scholar] [CrossRef]
- Gluhm, S.; Goldstein, J.; Loc, K.; Colt, A.; Liew, C.; Van Corey-Bloom, J. Cognitive Performance on the Mini-Mental State Examination and the Montreal Cognitive Assessment Across the Healthy Adult Lifespan. Cogn. Behav. Neurol. 2013, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Anandan, P.; Rengarajan, S.; Venkatachalam, S.; Pattabi, S.; Jones, S.; Prabhu, K.; Krishna, V.; Prasanth, K. Neuroprotection by Cerebrolysin and Citicoline Through the Upregulation of Brain-Derived Neurotrophic Factor (BDNF) Expression in the Affected Neural Cells: A Preliminary Clue Obtained through an In Vitro Study. Cureus 2024, 16, e54665. [Google Scholar] [CrossRef]
- Jarosz, K.; Kojder, K.; Skonieczna-Żydecka, K.; Andrzejewska, A.; Sołek-Pastuszka, J.; Jurczak, A. The Effects of Neuromonitoring and Cerebrolysin Administration on Outcomes in Patients with Traumatic Brain Injury-An Interventional Pilot Study. J. Clin. Med. 2024, 13, 353. [Google Scholar] [CrossRef]
- Kang, D.H.; Choi, B.Y.; Lee, S.H.; Kho, A.R.; Jeong, J.H.; Hong, D.K.; Kang, B.S.; Park, M.K.; Song, H.K.; Choi, H.C.; et al. Effects of Cerebrolysin on Hippocampal Neuronal Death After Pilocarpine-Induced Seizure. Front. Neurosci. 2020, 14, 568813. [Google Scholar] [CrossRef] [PubMed]
- Morega, S.; Gresita, A.; Mitran, S.I.; Musat, M.I.; Boboc, I.K.S.; Gheorman, V.; Udristoiu, I.; Albu, C.V.; Streba, C.T.; Catalin, B.; et al. Cerebrolysin Use in Patients with Liver Damage—A Translational Study. Life 2022, 12, 1791. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, L.E.; Abakumova, T.; Nurkhametova, D.; Ivanchenko, K. Cerebrolysin for Acute Ischaemic Stroke. Cochrane Database Syst. Rev. 2023, 10, CD007026. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Salinas, P.; Muñoz, D.; Olivares, S.; González, J.; Sáez, V.; Romero, V. A Retrospective Study of Cerebrolysin in Patients with Moderate to Severe Traumatic Brain Injury: Cognitive and Functional Outcomes. J. Med. Life 2023, 16, 1017–1021. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, M.; Lin, L.; Wang, J.; Sun-Waterhouse, D.; Dong, Y.; Zhuang, M.; Su, G. Identification of Antioxidative Peptides from Defatted Walnut Meal Hydrolysate with Potential for Improving Learning and Memory. Food Res. Int. 2015, 78, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Vaghef, L.; Farajdokht, F.; Erfani, M.; Majdi, A.; Sadigh-Eteghad, S.; Karimi, P.; Sandoghchian Shotorbani, S.; Seyedi Vafaee, M.; Mahmoudi, J. Cerebrolysin Attenuates Ethanol-Induced Spatial Memory Impairments through Inhibition of Hippocampal Oxidative Stress and Apoptotic Cell Death in Rats. Alcohol 2019, 79, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Petta, S.; Casuccio, A.; Maida, C.; Corte, V.D.; Daidone, M.; Di Raimondo, D.; Pecoraro, R.; Fonte, R.; Cirrincione, A.; et al. Reactive Hyperemia Index (RHI) and Cognitive Performance Indexes Are Associated with Histologic Markers of Liver Disease in Subjects with Non-Alcoholic Fatty Liver Disease (NAFLD): A Case Control Study. Cardiovasc. Diabetol. 2018, 17, 28. [Google Scholar] [CrossRef]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-Based Norms for the Mini-Mental State Examination by Age and Educational Level. JAMA 1993, 269, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.L.; D’Agostino, R.B.; Wolf, P.A. Norms for the Mini-Mental State Examination. JAMA 1993, 270, 2178. [Google Scholar]
- Ghai, P.; Magan, D.; Aneja, J.; Sharma, H.; Choudhary, A. Effect of Alcohol-Dependence on Cognitive Performance in Middle-Aged Men: Preliminary Results. Indian. J. Physiol. Pharmacol. 2023, 67, 303–309. [Google Scholar] [CrossRef]
- Perneczky, R.; Wagenpfeil, S.; Komossa, K.; Grimmer, T.; Diehl, J.; Kurz, A. Mapping Scores Onto Stages: Mini-Mental State Examination and Clinical Dementia Rating. Am. J. Geriatr. Psychiatry 2006, 14, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tao, X.; Zeng, M.; Mi, Y.; Xu, L. Clinical and Histological Features under Different Nomenclatures of Fatty Liver Disease: NAFLD, MAFLD, MASLD and MetALD. J. Hepatol. 2024, 80, e64–e66. [Google Scholar] [CrossRef] [PubMed]
- Morega, S.; Cătălin, B.; Simionescu, C.E.; Sapalidis, K.; Rogoveanu, I. Cerebrolysin Prevents Brain Injury in a Mouse Model of Liver Damage. Brain Sci. 2021, 11, 1622. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.; Larsen, J.; Catterall, E.; Moss, A.C.; Dombrowski, S.U. Peer Pressure and Alcohol Consumption in Adults Living in the UK: A Systematic Qualitative Review. BMC Public Health 2020, 20, 1014. [Google Scholar] [CrossRef] [PubMed]
- Mahalik, J.R.; Locke, B.D.; Ludlow, L.H.; Diemer, M.A.; Scott, R.P.J.; Gottfried, M.; Freitas, G. Development of the Conformity to Masculine Norms Inventory. Psychol. Men. Masc. 2003, 4, 3–25. [Google Scholar] [CrossRef]
- White, A. Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol Res. 2020, 40, 1. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.M.J.; Asharani, P.V.; Abdin, E.; Shahwan, S.; Zhang, Y.; Sambasivam, R.; Vaingankar, J.A.; Ma, S.; Chong, S.A.; Subramaniam, M. Gender Differences in Alcohol Use: A Nationwide Study in a Multiethnic Population. Int. J. Ment. Health Addict. 2022. [Google Scholar] [CrossRef]
- Nasui, B.A.; Popa, M.; Buzoianu, A.D.; Pop, A.L.; Varlas, V.N.; Armean, S.M.; Popescu, C.A. Alcohol Consumption and Behavioral Consequences in Romanian Medical University Students. Int. J. Environ. Res. Public. Health 2021, 18, 7531. [Google Scholar] [CrossRef] [PubMed]
- Watfa, G.; Husson, N.; Buatois, S.; Laurain, M.C.; Miget, P.; Benetos, A. Study of Mini-Mental State Exam Evolution in Community-Dwelling Subjects Aged over 60 Years without Dementia. J. Nutr. Health Aging 2011, 15, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, I.; Zwolak, A.; Szczyrek, M.; Wawryniuk, A.; Skrzydło-Radomańska, B.; Daniluk, J. Biomarkers of Alcohol Misuse: Recent Advances and Future Prospects. Prz. Gastroenterol. 2016, 11, 78–89. [Google Scholar] [CrossRef]
- Tynjälä, J.; Kangastupa, P.; Laatikainen, T.; Aalto, M.; Niemelä, O. Effect of Age and Gender on the Relationship between Alcohol Consumption and Serum GGT: Time to Recalibrate Goals for Normal Ranges. Alcohol. Alcohol. 2012, 47, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, S.; Sawai, S.; Satoh, M.; Seimiya, M.; Sogawa, K.; Fukumura, A.; Tsutsumi, M.; Nomura, F. Fractionation of Gamma-Glutamyltransferase in Patients with Nonalcoholic Fatty Liver Disease and Alcoholic Liver Disease. World J. Hepatol. 2016, 8, 1610. [Google Scholar] [CrossRef]
- Pipitone, R.M.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Tulone, A.; Pennisi, G.; Grimaudo, S.; Petta, S. MAFLD: A Multisystem Disease. Ther. Adv. Endocrinol. Metab. 2023, 14, 204201882211455. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; He, R.; Jiang, H.; Wu, J.; Xi, Z.; He, K.; Liu, Y.; Zhou, T.; Feng, M.; Wan, P.; et al. Association between Metabolic Dysfunction-Associated Fatty Liver Disease and Cognitive Impairment. J. Clin. Transl. Hepatol. 2022, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Jalili, V.; Poorahmadi, Z.; Hasanpour Ardekanizadeh, N.; Gholamalizadeh, M.; Ajami, M.; Houshiarrad, A.; Hajipour, A.; Shafie, F.; Alizadeh, A.; Mokhtari, Z.; et al. The Association between Obesity with Serum Levels of Liver Enzymes, Alanine Aminotransferase, Aspartate Aminotransferase, Alkaline Phosphatase and Gamma-glutamyl Transferase in Adult Women. Endocrinol. Diabetes Metab. 2022, 5, e367. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Chan, C.; Lee, H.-W.; Huang, C.; Chen, Y.-J.; Liu, P.-C.; Lu, S.-N.; Chuang, W.-L.; Huang, J.-F.; Yu, M.-L.; et al. Influence of Nonalcoholic Fatty Liver Disease with Increased Liver Enzyme Levels on the Risk of Cirrhosis and Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2023, 21, 960–969.e1. [Google Scholar] [CrossRef]
- Fujii, H.; Doi, H.; Ko, T.; Fukuma, T.; Kadono, T.; Asaeda, K.; Kobayashi, R.; Nakano, T.; Doi, T.; Nakatsugawa, Y.; et al. Frequently Abnormal Serum Gamma-Glutamyl Transferase Activity Is Associated with Future Development of Fatty Liver: A Retrospective Cohort Study. BMC Gastroenterol. 2020, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, X.; Jiang, Z.; Zhang, K.; Zhang, M.; Li, Y.; Zhao, X.; Xiong, H. Liver Enzymes and Metabolic Syndrome: A Large-Scale Case-Control Study. Oncotarget 2015, 6, 26782–26788. [Google Scholar] [CrossRef] [PubMed]
- Alatalo, P.; Koivisto, H.; Puukka, K.; Hietala, J.; Anttila, P.; Bloigu, R.; Niemela, O. Biomarkers of Liver Status in Heavy Drinkers, Moderate Drinkers and Abstainers. Alcohol Alcohol. 2009, 44, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wang, H.; Li, C.; Shi, J.; Yong, F.; Jia, H. Association between Dietary Vitamin B1 Intake and Cognitive Function among Older Adults: A Cross-Sectional Study. J. Transl. Med. 2024, 22, 165. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.; Jaqua, E.; Nguyen, V.; Clay, J. B Vitamins: Functions and Uses in Medicine. Perm. J. 2022, 26, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Lee, J.; Shin, Y.-I.; Ko, M.-H.; Kim, D.Y.; Sohn, M.K.; Kim, J.; Kim, Y.-H. Cerebrolysin Combined with Rehabilitation Enhances Motor Recovery and Prevents Neural Network Degeneration in Ischemic Stroke Patients with Severe Motor Deficits. J. Pers. Med. 2021, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine Supplementation and Brain Health. Nutrients 2021, 13, 586. [Google Scholar] [CrossRef]
- Godwin Elechi, J.O.; Abrego Guandique, D.M.; Cannataro, R. Creatine in Cognitive Performance: A Commentary. Curr. Mol. Pharmacol. 2024, 17, e18761429272915. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.T.; Kim, E.H.; Faull, R.L.; Christie, D.L.; Waldvogel, H.J. Dissociated Expression of Mitochondrial and Cytosolic Creatine Kinases in the Human Brain: A New Perspective on the Role of Creatine in Brain Energy Metabolism. J. Cereb. Blood Flow. Metab. 2013, 33, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Abrego-Guandique, D.M.; Bonet, M.L.; Caroleo, M.C.; Cannataro, R.; Tucci, P.; Ribot, J.; Cione, E. The Effect of Beta-Carotene on Cognitive Function: A Systematic Review. Brain Sci. 2023, 13, 1468. [Google Scholar] [CrossRef] [PubMed]
- Lenka, A.; Jankovic, J. Tremor Syndromes: An Updated Review. Front. Neurol. 2021, 12, 684835. [Google Scholar] [CrossRef] [PubMed]
- Canver, B.R.; Newman, R.K.; Gomez, A.E. Alcohol Withdrawal Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441882/ (accessed on 15 March 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).