Abstract
It is believed that short-chain fatty acids (SCFAs)—the bacterial metabolites produced by the digestion of dietary fiber—potentially contribute to the prevention of colitis. However, this beneficial effect has not been conclusively proven. We thus attempted to verify this beneficial effect by examining whether colitis can be caused or worsened by the deficiency in dietary fiber in mice. We found that dextran sodium sulfate (DSS)-induced colitis was accelerated under a fiber-deficient condition, but the fiber deficiency itself did not provoke colitis. Moreover, episodes of diarrhea and epithelium damage in the large intestine were found upon analysis 24 h after the intervention. Unfortunately, these symptoms and tissue damage could not be ameliorated by administering SCFAs. On the other hand, a fiber-deficient condition increased the population of Desulfovibrio spp. and decreased the population of Lactobaccillus spp. regardless of the presence or absence of DSS upon analysis 24 h after the intervention. These results suggest that a deficiency in dietary fiber makes the intestinal environment irritable to colitis-inducing stimuli within the short term. This change does not appear to be related to the presence of SCFAs, but to the alteration of microbiota. Hence, a regular intake of dietary fiber is strongly recommended to avoid colitis and preserve intestinal health.
1. Introduction
Dietary fiber consists of non-digestive polysaccharides and is typically classified into water soluble fiber and insoluble fiber [1]. Dietary fiber induces the secretion of water from the intestinal wall to the intestinal tract that prescribes a moderate softness to stool and prevents constipation [2]. In addition, the bacterial fermentation of dietary fiber results in short-chain fatty acids (SCFAs), which are recognized as beneficial metabolites [3]. Acetate, propionate and butyrate are the most abundant SCFAs, which provide intestinal epithelial cells with the energy necessary for their survival [4,5,6] and enhance the barrier function of intestinal epithelial cells [7,8] under a physiological condition.
Owing to these protective properties, dietary fiber is considered to have a suppressive effect on inflammatory bowel disease (IBD) [9]. Nevertheless, some patients with IBD are intolerant to fiber consumption due to the diminished fermentative activity of their intestinal bacteria [10]. In addition, the suppressive effect of dietary fiber is controversial in animal models of colitis [11,12]. Therefore, the beneficial effect of dietary fiber on IBD has not been confirmed.
A previous mouse study indicated that colitis could be worsened by fiber deficiency and that a decreased amount of intestinal SCFAs could be partially responsible for the aggravation of colitis [13]. However, much like the human gut microbiome, animal gut microbiomes vary across research groups as they are significantly affected by their breeding environments. Taking this into consideration, the aggravation of colitis and decease in SCFA levels should be validated in different facilities. There is also no certainty regarding when signs that indicate the exacerbation of colitis begin to occur in response to a dietary fiber deficiency. Furthermore, it is unclear whether SCFAs are indeed responsible for the worsening of colitis.
Therefore, a comprehensive analysis of the role of dietary fiber in colitis is necessary. In order to assess this, we examined whether a fiber-deficient condition induces or accelerates colitis, including an estimation of the onset. In addition, the relationship between the alternation in intestinal microbiota and the amount of SCFAs and degree of colitis was also evaluated.
2. Materials and Methods
2.1. Colitis-Inducing Procedure, Diet Feeding and Administration of SCFAs as Rescue Experiment
Seven-week-old male SPF C57BL/6N mice were purchased from SLC Japan Inc. (Shizuoka, Japan). All animals were maintained under a strict 12 h light cycle (lights on at 7:00 am and off at 19:00), including an acclimatization period for 1 week. After acclimatization, mice were randomly divided into four groups according to differences in diets (normal diet or fiber-free diet) and the presence or absence of 2% dextran sulfate sodium (DSS; MP Biomedicals, Inc., Tokyo, Japan), which was allowed to be consumed through drinking water ad libitum as a colitis inducer. The normal diet (ND) was the MF diet (Oriental Yeast Co., Ltd., Tokyo, Japan), which is commonly used as a standard diet for mouse breeding. The fiber-free diet (FF) was D18102201 (Research Diets, Inc., New Brunswick, NJ, USA), which was made similar to the way it was made in a previous study [14]. The basic components of the fiber-deficient diet are listed in Supplementary Table S1. To evaluate the severity of colitis, the DAI (disease activity index) score, an indicator of DSS enteritis, was calculated by summing up the scores of the following three parameters: stool consistency, blood in feces and weight loss (Supplementary Table S2) [15]. For rescue experiment, SCFA mixture (180 mM of sodium acetate, 60 mM of sodium propionate and 60 mM sodium butyrate, which are equivalent to the concentrations adopted in previous studies [13,16]) was added to drinking water, and mice were allowed to consume the water ad libitum. Successively, mice had free access to water which contained SCFAs, DSS and FF for 5 days. Then, the effect of SCFAs was evaluated by colitis-associated parameters as described above.
2.2. ELISA
Cytokines were measured using ELISA kits, which are described below, according to the manufacturer’s instructions: TNFα (Ref 88-7324-22), IL1-β (Ref 88-7013-22), IL-6 (Ref 88-7064-22) and IL-10 (Ref 88-7105-22). All kits were products of Thermo Fisher Scientific K.K. (Tokyo, Japan). Large intestines were homogenized with 1 mL of lysis buffer and according to the conventional method [15]. The homogenates were diluted three times with PBS, and the concentration of cytokines was measured. The expression levels of cytokines were determined by correcting for total protein levels of the tissues, which were measured using a kit formulation from Takara Bio (TaKaRa BCA Protein Assay Kit, Kusatsu, Japan).
2.3. Histopathology
Fresh large intestines were embedded into OCT compound without being fixed and were frozen with liquid nitrogen. The embedded tissue was sliced to a thickness of 10 µm and stained with hematoxylin and eosin (H&E) after adequate drying on a glass slide. Tissue sections were observed blindly and evaluated by histological scores according to scoring methods summarized in Supplementary Table S3 [15].
2.4. Quantification of SCFAs
Quantification was performed with LC/MS/MS (LCMS8030; Shimazu corporation, Kyoto, Japan) and a Mastro2™ C18 column (length; 2.0 mm × 150 mm, Particle size; 3 µm; Shimazu corporation) based on the method package for SCFAs (C146-2209A; Shimazu corporation).
2.5. Fecal Bacteria Analysis
Population of fecal bacteria was analyzed by new generation sequencing as using Miseq Illumina, San Diego, CA, USA) as done in previous study [17]. Breafly, bacterial DNA was extracted from stool with NucleoSpin DNA Stool kit (MACHEREY–NAGEL GmbH & Co. KG, Dueren, Germany) according to the manufacturer’s instructions. The V3–V4 region of bacterial 16S rRNA was amplified by PCR. After purified the amplicon, barcode sequences were added to label the samples and purified again. This DNA library was applied to Sequencing. Annotation and calculation of obtained sequences were processed by 16S Metagenomics Database Creator v1.0.0 (Illumina, San Diego, CA, USA).
2.6. RNA Extraction, and Microarray Analyses
For the comprehensive mRNA analysis of inflammatory mediators, microarray analysis was done. Two days after NDD or FFD intervention, the RNA was isolated from snap frozen large intestine using ISOGEN II (NIPPON GENE Co., Ltd, Toyama, Japan, cat.no. 311-07361). The RNA was extracted from three mice of each group. One point five micro gram of RNA was converted to cDNA using SuperScript IV VILO Master Mix with ezDNase enzyme (Invitrogen, Thermo. Fisher Scientific, Tokyo, Japan, cat.no. 11766050). Microarray analysis was outsourced to Kamakura Techno–Science, Inc. (Kamakura, Japan). They performed the analysis using the 3D-Gene® mRNA Oligo chip. In the analysis, the RNA of NDD group and FFD group was pooled, and the mRNA expression was compared. In addition, to exclude genes with extremely low expression levels, we conducted an analysis focusing on genes with a normalized intensity of 50 or higher.
2.7. Statistical Analyses
Statistical differences between four groups were determined using two-way ANOVA with Tukey’s test as host hoc analysis. Mann–Whitney U test was applied to differences between two groups. In case of analysis of population of bacteria, Tukey’s test was used as one-way ANOVA. Data were analyzed by SPSS software. Version 26. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Fiber-Deficient Diet Exacerbates Colitis-Associated Symptoms
We initially examined whether a fiber deficiency aggravates colitis. Mice were fed ND or FF for 5 days with or without DSS-administration (Figure 1A). We confirmed that the amount of food intake was comparable across all groups (Supplementary Figure S1) to eliminate the confounding effect due to malnutrition. The DAI scores increased consistently from day 1 to day 5 in both ND + DSS+ (NDD) group and FF + DSS+ (FFD) group, and the DAI scores of FFD group were higher than those of NDD group at every evaluation point (Figure 1B). In contrast, these scores did not increase at all in the ND + DSS− (ND) group and FF+ DSS− (FF) group (Figure 1B). Looking at the individual parameters that make up the DAI, the weight loss scores and the bloody stool scores of the FFD group consistently increased from day 2 to day 5 (Figure 1C,E), whereas episodes of diarrhea occurred from day 1 (Figure 1D). These changes were either absent or less severe in the NDD group. In addition, the onset of these symptoms in the NDD group was delayed compared to the FFD group (Figure 1C–E). Furthermore, we measured the lengths of large intestines, which are associated with the severity of DSS-induced colitis and ulcerative colitis [18]. The intestinal lengths of the FFD group were shorter than those of NDD group (Figure 1F,G) whereas FF itself did not affect them (Figure 1F,G).
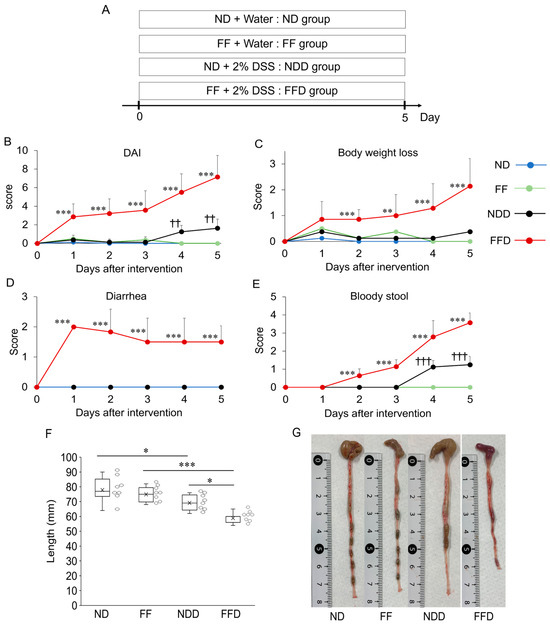
Figure 1.
Fiber deficiency exacerbates DSS-induced colitis symptoms. Experimental protocol for diet-feeding and DSS-administration (A). Macroscopic parameters for the evaluation of colitis—DAI scores (B), weight loss scores (C), diarrhea scores (D), and bloody stool scores (E)—were evaluated during feeding and DSS-administration. In addition, the length of large intestine (F) and typical macroscopic appearance (G) of dissected mice were also noted. Each group consists of 8 mice. Statistically significant differences are shown as follows: FFD group vs. other groups in case of (B–E): ** p < 0.01, *** p < 0.001; NDD group vs. ND and FF groups in case of (B–E): †† p < 0.01, ††† p < 0.001; Significant differences between indicated groups in case of (F): * p < 0.05, *** p < 0.001.
3.2. Fiber-Deficient Diet Enhances the Inflammatory Response in DSS-Induced Colitis
We subsequently evaluated myeloperoxidase (MPO) activity and histopathological changes in large intestine as direct indicators of the tissue damage. The MPO activity increased in the FFD group only (Figure 2A). The histopathological scores of the NDD group were higher than those of the ND group and FF group. However, the scores of the FFD group were far higher than those of other groups. As shown in Figure 2B, typical histological sections of the FFD group showed that destruction of villi was significantly severe, which was in stark contrast to that of the NDD group (Figure 2C). Besides, the ND group and the FF groups presented a normal intestinal structure, including an arranged epithelium and clear crypt contours without abnormal cell invasion inside of the villi and basal membrane (Figure 2C). In addition to MPO activity and histopathological changes, we further examined intestinal cytokine levels, which have been suggested to act as the mediators (TNF-α, IL1-β, IL-6) or the regulators (IL-10) of DSS-inducing colitis [15]. Interestingly, TNF-α, IL1-β, and IL-10 levels were higher than those of ND group. Moreover, in comparison to the NDD group, the FFD group showed higher IL-1β and IL-10 levels, but not TNF-α levels (Figure 2D).
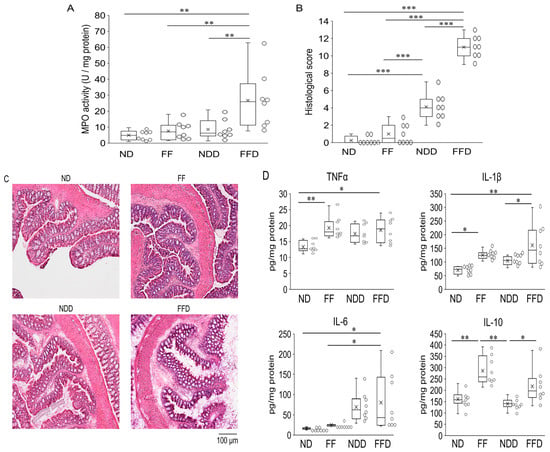
Figure 2.
Fiber deficiency enhances the inflammatory response in DSS-induced colitis. Colitis-associated parameters were evaluated at 5 days after intervention. MPO activity of large intestine, which was calculated as per milligram tissue protein (A), histological scores of large intestines (B), representative images of H&E-stained large intestine (C), inflammatory cytokine levels in the tissue of the large intestine (D). Each group consists of 8 mice. Statistical differences are shown as follows: * p < 0.01, ** p < 0.01, *** p < 0.001.
3.3. Fiber-Deficient Diet Reduces Fecal SCFAs Content
SCFAs are suggested to be protective factors against colitis since these molecules contribute to the maintenance of the intestinal epithelial barrier and the viability of its constituent cells. Hence, we quantified the amount of SCFAs in the cecum, where SCFAs produced. We focused on acetate, butyrate, and propionate specifically due to their predominant quantities. The quantities of all these SCFAs were significantly lower in the FF group and the FFD group compared to other groups, whereas the SCFAs quantities of the FF group and the FFD group were comparable (Figure 3A). There were no differences between the ND group and the NDD group also (Figure 3A). These results indicate that fiber deficiency itself affected the quantity of SCFAs. On the other hand, in both the FF group and the FFD group, the wet weight of cecum decreased to a similar extent in comparison to the ND group (Figure 3B). This result suggests that the total amount of SCFAs in stool decreased due to the absence of dietary fiber, regardless of DSS-administration.
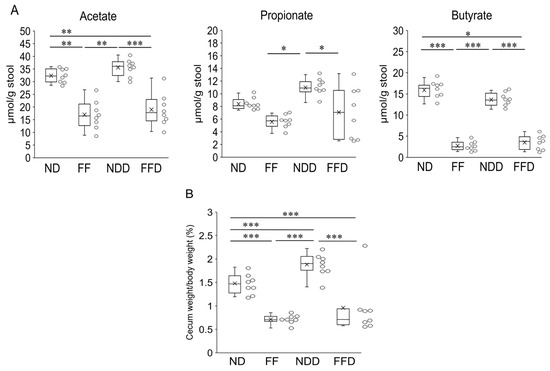
Figure 3.
Fiber deficiency reduced fecal SCFAs contents and cecum weights. Amount of acetate, propionate, and butyrate per gram of feces (A) and percentage of cecum weight per mouse body weight (B) at 5 days after the intervention. Each group consists of 8 mice. Statistical differences are shown as follows: * p < 0.05, ** p < 0.01, *** p < 0.001.
3.4. Fiber-Deficient Diet Accelerates DSS-Induced Colitis within 24 h with Decreased SCFAs Levels in Cecum
We speculated that the clear onset of colitis exacerbation would occur within a few days after the fiber deficiency since the diarrhea symptoms appeared about 24 h after the fiber deficiency. Therefore, we evaluated the colitis-associated parameters 1 day and 2 days after the FFD-intervention and compared the parameters with those of NDD group to confirm the onset of the exacerbation. The lengths of large intestine were shorter in the FFD group on both days (Figure 4A,B). In addition, the histological scores of FFD group were higher than those of NDD group (Figure 4D) with a mild disruption of the epithelium on both days (Figure 4E). These structural changes were either milder or absent in the NDD group (Figure 4D,E). The increased MPO activities were observed in FFD group at day 2 only (Figure 4C). In addition, the cytokine levels of TNF-α, IL1β, IL-6 and IL-10 were comparable between the NDD group and the FFD group on both days (Figure 4F). We further screened the inflammatory mediators by microarray analysis. The genes of IL-7, CCl19, CXCL9, CCL8, IL-18 and IL18-bp were upregulated and IL-17 rc gene was downregulated by FFD-intervention (Supplementary File S1, named as microarray analysis).
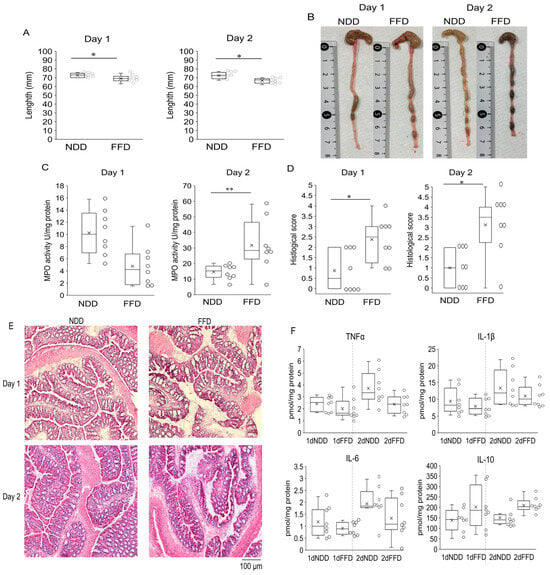
Figure 4.
Signs of colitis were observed from one day to two days after FFD-intervention. Length of large intestine (A) and the macroscopic appearance (B), MPO activity (C), histological scores (D), representative images of the H&E-staining (E), cytokine levels (F) in large intestine derived from in NDD group and FFD group. Each group consists of 8 mice. Statistical differences are shown as follows: * p < 0.05, ** p < 0.01.
In addition, we quantified SCFAs in the cecum and estimated the timing when these quantities decreased. The quantities of all three SCFAs and the cecum weight were lower in the FFD group than in the NDD group both 1 day and 2 days after the FFD-intervention (Figure 5A,B).
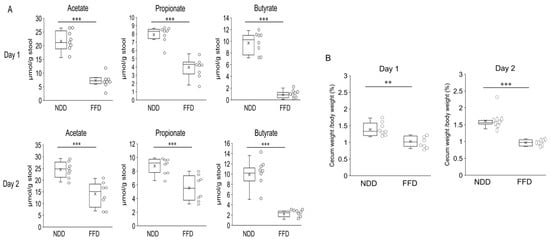
Figure 5.
Fecal SCFAs levels decreased 1 day and 2 days after FFD-intervention. Amount of acetate, propionate, and butyrate per gram of feces (A) and percentage of cecum weight per mouse body weight (B) after inducing fiber deficiency and DSS intervention. Each group consists of 8 mice. Statistical differences are shown as follows: ** p < 0.01, *** p < 0.001.
3.5. Short Chain Fatty Acids Does Not Ameliorate Colitis
A rescue experiment was done to examine whether the reduced quantities of SCFAs were responsible for the aggravation of the colitis. Even though SCFAs were administered prior to the FFD-intervention and were continued until the end of the experiment, the stool score (Figure 6A,B), MPO activity (Figure 6C) and histological changes (Figure 6D,E) were not ameliorated. Neither group displayed increases in body weight score. The body weight loss that occurred due to the FFD-intervention was relatively coincidental, but reproducible.
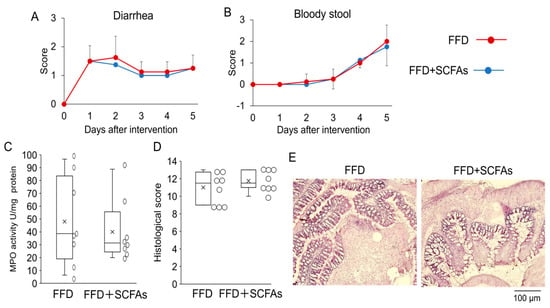
Figure 6.
Exacerbation of colitis was not ameliorated by the administration of SCFAs. Diarrhea scores (A), bloody stool scores (B), MPO activity (C), histological score (D) of large intestine were evaluated 5 days after the FFD intervention with or without SCFAs. The typical image of histology is shown as (E). There are no statistically significant differences in any parameters.
3.6. Fiber-Deficient Diet Changes Microbiota in the Short Term
Dietary fiber is not digested by host-derived enzyme and is considered to not interact with host cells, including epithelial cells. Based on these physiological properties of dietary fiber, it would affect intestinal bacterial growth and contribute to intestinal health by keeping the microbiota as healthy state. Conversely, disturbance in the gut microbiota likely to induce an irritable intestinal state in response to colitis-inducing stimuli. Therefore, we examined whether the fiber deficiency induces intestinal dysbiosis. We targeted the predominant bacteria which had 1% and more relative population in the microbiota. The fecal microbiota had been changed evidently 1 day after the fiber deficiency without DSS-administration (Figure 7A). In the comparison between the ND group and FF group, the population of almost all bacteria increased or decreased in the FF group (Figure 7A). The decreases in Barnesiella spp. and the increases in Bacteroides spp. were particularly drastic (Figure 7A). It was also noteworthy that Clostridium_XVa spp., Eisenbergiella spp., Lactobaccilus spp., Parabacteriodes spp., Allororevotella spp., and Desulfovibrio spp. were also affected by the fiber deficiency itself (Figure 7A). Apart from a few exceptions, similar tendencies were observed in the NDD group and the FFD group 1 day, 2 days, and 5 days after the intervention (Figure 7B–D).
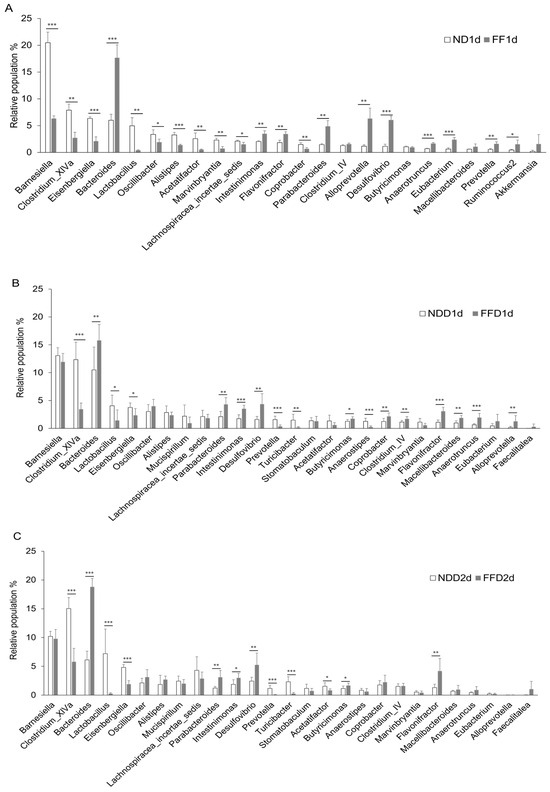
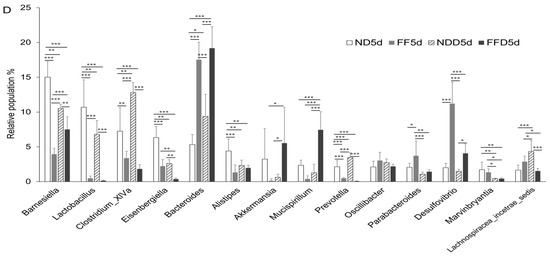
Figure 7.
Fiber-deficient diet affected intestinal microbiota in the short term. Each figure shows the relative population of bacteria in cecum. ND group and FF group at 1 day (A), NDD group and FFD group at 1 day (B), NDD group and FFD group 2 days (C) after the intervention. ND, FF, NDD and FFD groups at 5 days after the intervention (D). Each group consists of 4 mice in figure (A). Number of mice in each group is 8 in other figures. Statistical differences are shown as follows: * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Discussion
As is well-known, DSS-induced colitis mimics ulcerative colitis and the severity can be adjusted by the concentration of DSS as well as duration of the administration. In our present study, we adopted 2% DSS for 5 days as an experimental condition, under which mild colitis occurs with mild blood stool, light damage to epithelium, and sparse inflammatory cell infiltration when mice are fed conventional diet [14,19]. These symptoms were similarly observed in our NDD group. Our histopathological observation showed that the entire villi, including the epithelium, was severely damaged by FFD-intervention for 5 days. On the contrary, fiber-deficient condition alone did not provoke the disruption of epithelium structure, diarrhea, and blood stool. These results indicate that a fiber deficiency accelerates DSS-induced colitis whereas the colitis was not provoked by the fiber deficiency alone.
Sulfate residues of DSS destabilize the mucus layer and increase intestinal permeability [20], meaning that DSS can disrupt the epithelial barrier. Consistent with this physical property, DSS causes the destruction of the intestinal epithelium, which in turn triggers the infiltration of macrophages and neutrophils, leading to the development of colitis [21,22,23]. In our study, diarrhea and epithelial damage were observed in the FFD group 1 day after DSS-administration, indicating that a vulnerability to colitis-inducing stimuli had been acquired within 24 h after the induction of fiber deficiency. The early occurrence of these colitis-associated phenotypes implies that many DSS molecules had already reached the epithelium within 24 h after the FFD-intervention. Besides, the onsets of bloody stool and increased MPO activity occurred after the diarrhea episode. Viewing the early diarrhea episode as a defensive response to wash DSS away from the intestinal tract, the destruction of the epithelium and the activation of immune response were triggered beyond the capacity of the defensive response probably.
We measured the cytokines which were associated with DSS-induced colitis in a previous study [15]. Interleukin-1β potentially acts as a mediating factor in the exacerbation of colitis since IL-1β levels were increased by the FFD-intervention for 5 days. However, IL-1β is not involved in the onset of the exacerbation since the increased levels of IL-1β had not been observed 2 days after the FFD-intervention. On the other hand, both TNFα and IL-1β increased by the FF-feeding for 5 days, implying that the irritable tendency was induced by the fiber deficiency alone although no infiltration of cells and no disruption of tissue was observed. Besides, our microarray analysis revealed that the FFD-intervention affected the expression of multiple genes although it is difficult to give reasonable interpretation for all the genes. Among the upregulated genes, CCL8 is reported as an accelerating factor of DSS-induced colitis by inducing recruitment of monocyte [24], that is potentially affect the acceleration of the colitis in our present study. On the other hand, upregulation of IL-18 and its related gene, that would be compensatory defense response since a previous study using animal colitis model has been reported that IL-18 and its signal suppress the colitis [13]. The function of other upregulated genes on DSS-induced colitis have not been clarified yet. Besides, IL-17 rc were found as a downregulated gene whereas IL-17 gene was not affected by FFD-intervention. Interleukin 17 rc is the receptor for IL-17F, that would promote DSS-induced colitis in previous study although precise mechanism is unknown [25]. Therefore, the decreased interleukin 17 rc without alternation of IL-17 gene suggests that the involvement of IL-17 to the worsening colitis would be small or limited.
In our present study, a deficiency of dietary fiber markedly decreased the SCFAs quantity. It is noteworthy that the degrees of the decreases in SCFAs levels were comparable from 1 day to 5 days after the fiber deficiency, regardless of presence or absence of DSS. These results indicate that the production of SCFAs were distinctly affected in a very short time. In previous studies, decreased SCFAs levels in stool were found to be associated with the worsening of colitis, and the administration of SCFAs ameliorated colitis in an animal model [13,26]. These findings suggest that the rapid decreases in stool SCFAs levels could be responsible for the exacerbation of DSS-induced colitis. Nevertheless, our rescue experiment failed to suppress the magnitude of colitis. A possible reason is the differences in the onset and duration of the administration of SCFAs. Previous studies succeeded to ameliorate the colitis in the animal models by administering SCFAs for several weeks before colitis induction and continued the administration during induction of colitis [13,16]. Under such experimental conditions, Tregs were induced prior to induction of colitis and contributed to suppression of colitis [16]. In our study, we began the administration of SCFAs 2 days before the FFD-intervention. Therefore, the mere compensation of lacking SCFAs itself would not be effective enough to inhibit worsening of the colitis, especially over a short period. In addition, the FF group and the FFD group showed increased IL-10 levels in large intestine, suggesting that fiber deficiency did not impair the immunoregulation function. Rather, this function would be enhanced. Therefore, the worsening colitis appears to be independent of the physiological activity of SCFAs in our present study. The possible cause for the quick degradation of SCFAs is the alteration in microbiota. We observed drastic changes in the bacterial population due to fiber deficiency. In relation to SCFAs, Clostridium_XIVa spp. potentially affected the decreased amount of SCFAs since this Clostridium_XIVa spp. is a major SCFAs-producing strain [27]. In our current study, the population of Clostridium_XIVa spp. was decreased in the FF group and the FFD group, suggesting that fiber-deficient condition would suppress the growth of Clostridium_XIVa and brought about the decrease in SCFAs production.
Microbiota is presently recognized as a factor that accounts for intestinal barrier integrity, thereby influencing the onset and the exacerbation of colitis. It is well known that dietary fiber is consumed by bacteria as nutrients. Therefore, dietary fiber could affect the composition ratio of the bacteria that make up the microbiota by affecting the growth of bacteria [28,29,30]. In our present study, the fiber deficient condition resulted in significant changes in various population of fecal bacteria within few days. Especially, the increase in Desulfovibrio spp. is interesting. As the name indicates, Desulfovibrio spp. is sulfate reducing bacteria and produce hydrogen sulfide, which is able to induce the distraction of epithelial cells as well as upregulation of proteolysis-inducing genes in colonocytes at higher concentration (around 20 mM) [31,32]. In addition, the vesicles of the outer membrane are also likely to destroy the epithelial barrier [33]. Therefore, increased Desulfovibrio spp. is likely to induce epithelial vulnerability to DSS-stimulation. Furthermore, Desulfovibrio spp. is abundant in IBD patients [34,35] and positively correlates with the disease activity [36]. Conversely, there have been multiple reports indicating a decrease in the population of Desulfovibrio spp. alongside the resolution of colitis in animal models [37,38,39]. These positive correlations of abundance of Desulfovibrio spp. and the severity of the colitis also support the possibility that Desulfovibrio spp. contributes to the worsening of colitis.
Besides, decreased in the population of Lactibacillus spp. may be involved in the acceleration of the colitis. Multiple Lactibacillus species have been found to ameliorate DSS-induced colitis [40,41]. The population of Lactobacillus spp.in the FF group and FFD group were found to be lower than those of ND group and NDD group. Combined with the increase in Desulfovibrio spp., this may have accelerated the formation of unhealthy microbiota which contribute to intestinal tract irritability.
Our experiments had a few limitations. Although we identified bacteria as genus, it is difficult to identify those species in comprehensive meta-analysis of bacteria. Therefore, further experiments such as fecal microbiota transplantation (FMT) are necessary to verify whether the alteration of microbiota is responsible for the aggravation of the colitis. However, FMT itself has certain technical limitations as an experimental tool. When administered orally, bacterial quantity is lost significantly owing to gastric juice or non-specific adhesion. In addition, our preliminary experiments revealed that when liquid was administered intrarectally, it was impossible to deliver the solution beyond the point where the feces were located. Furthermore, the use of antibiotics prior to FMT also would be a confounding factor since it could induce a toxic effect on the cecum. Given these limitations, it is difficult to artificially reproduce gut microbiota under a physiological condition. Recently, a prototypic coculture system which can evaluate the interaction of bacteria and epithelia cells has been developed commercially. Unfortunately, we could not use it in our study since it is still too expensive and has not been authorized as an experimental method yet. The popularization of such methods would enable the uncovering of relevant factors of interest in the future.
5. Conclusions
In any case, fiber-deficient condition remarkably caused the exacerbation of the colitis which is similar to ulcerative colitis. In addition, the exacerbation occurred in the short term. These results suggest that lacking dietary fiber could be risk factor of colitis even a brief periods of the deficiency. Although the effects of SCFAs is dispensable, altered microbiota induced by fiber-deficiency is the most important candidate of this phenomenon. Hence, consistent intake of dietary fiber would be very important to prevent colitis as well as keeping intestinal health well.
The highlight of this study as follows.
- Fiber-deficient condition aggravated DSS-induced colitis with decreases in the amounts of fecal SCFAs.
- The acceleration of the colitis was not rescued by supplementation of SCFAs.
- Fiber-deficient condition resulted in decreased Lactobacillus population and increased Desulfovibrio population in stool, that may potential enhancing factor of epithelial damage and accelerated the colitis consequently.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/gastroent15030052/s1, Figure S1: Intake of the diet; Table S1: Composition of FF; Table S2: Scoring criteria of disease activity index (DAI); Table S3. Scoring criteria of histopathology; Supplementary File S1: microarray analysis.
Author Contributions
Conceptualization, S.K. (Shoma Kanda) and H.U.; methodology, S.K. (Shoma Kanda); software, H.U.; validation, S.K. (Shoma Kanda) and H.U.; formal analysis, S.K. (Shoma Kanda) and T.N.; investigation, S.K. (Sonoko Karino), H.U., T.O. and K.W.; resources, H.U.; data curation, T.O.; writing—original draft preparation, S.K. (Shoma Kanda); writing—review and editing, H.U., T.O., K.W., T.Y. and K.N.; visualization, S.K. (Shoma Kanda) and H.U.; supervision, T.Y. and K.N.; project administration, H.U. and K.W.; funding acquisition, H.U., S.K. (Shoma Kanda) and H.U. are equal contributed authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, Grant Number 23K10958.
Institutional Review Board Statement
All experiments with animals in this study were approved by the Animal Care and Use Committee of Shimane University. (Approval code: IZ4-36; Approval date: 24 November 2022) and complied with the ARRIVE guidelines.
Informed Consent Statement
Not applicable.
Data Availability Statement
Our research data would be available on demand on each occasion.
Acknowledgments
There are no special thanks other than authors and funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Commission, C.A. Guidelines on Nutrition Labelling CXG 2-1985; FAO: Rome, Italy, 2021. [Google Scholar]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; O’Connor, A.L.; Becker, S.L.; Patel, R.K.; Martindale, R.G.; Tsikitis, V.L. Gut microbial metabolites and its impact on human health. Ann. Gastroenterol. 2023, 36, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Roediger, W.E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 1982, 83, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Marsman, K.E.; McBurney, M.I. Dietary fiber increases oxidative metabolism in colonocytes but not in distal small intestinal enterocytes isolated from rats. J. Nutr. 1995, 125, 273–282. [Google Scholar] [PubMed]
- Clausen, M.R.; Mortensen, P.B. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut 1995, 37, 684–689. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshida, S.; Hara, H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 2008, 100, 297–305. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Vegh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 3356–3366. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, H.K.; Bording-Jorgensen, M.; Santer, D.M.; Zhang, Z.; Valcheva, R.; Rieger, A.M.; Sung-Ho Kim, J.; Dijk, S.I.; Mahmood, R.; Ogungbola, O.; et al. Unfermented beta-fructan Fibers Fuel Inflammation in Select Inflammatory Bowel Disease Patients. Gastroenterology 2023, 164, 228–240. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Huang, S.; Wu, Z.; Pang, J.; Wu, Y.; Wang, J.; Han, D. Resistant Maltodextrin Alleviates Dextran Sulfate Sodium-Induced Intestinal Inflammatory Injury by Increasing Butyric Acid to Inhibit Proinflammatory Cytokine Levels. BioMed Res. Int. 2020, 2020, 7694734. [Google Scholar] [CrossRef]
- Miles, J.P.; Zou, J.; Kumar, M.V.; Pellizzon, M.; Ulman, E.; Ricci, M.; Gewirtz, A.T.; Chassaing, B. Supplementation of Low- and High-fat Diets with Fermentable Fiber Exacerbates Severity of DSS-induced Acute Colitis. Inflamm. Bowel Dis. 2017, 23, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Kim, J.J.; Shajib, M.S.; Manocha, M.M.; Khan, W.I. Investigating Intestinal Inflammation in DSS-induced Model of IBD. J. Vis. Exp. 2012, 3678. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Takata, K.; Yamada, D.; Usuda, H.; Wada, K.; Tada, M.; Mishima, Y.; Ishihara, S.; Horie, S.; Saitoh, A.; et al. Juvenile social defeat stress exposure favors in later onset of irritable bowel syndrome-like symptoms in male mice. Sci. Rep. 2021, 11, 16276. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, S.; McPheeters, G.; Svaninger, G.; Oresland, T.; Hultén, L. Small bowel length in inflammatory bowel disease. Int. J. Color. Dis. 1997, 12, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Bamba, S.; Andoh, A.; Ban, H.; Imaeda, H.; Aomatsu, T.; Kobori, A.; Mochizuki, Y.; Shioya, M.; Nishimura, T.; Inatomi, O.; et al. The severity of dextran sodium sulfate-induced colitis can differ between dextran sodium sulfate preparations of the same molecular weight range. Dig. Dis. Sci. 2012, 57, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Gustafsson, J.K.; Holmen-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjovall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Asano, K.; Takahashi, N.; Ushiki, M.; Monya, M.; Aihara, F.; Kuboki, E.; Moriyama, S.; Iida, M.; Kitamura, H.; Qiu, C.H.; et al. Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat. Commun. 2015, 6, 7802. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, D.; Jawale, C.; Li, Y.; Biswas, P.S.; McGeachy, M.J.; Gaffen, S.L. Divergent functions of IL-17-family cytokines in DSS colitis: Insights from a naturally-occurring human mutation in IL-17F. Cytokine 2021, 148, 155715. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Prame Kumar, K.; Wen, S.W.; Shim, R.; Wanrooy, B.J.; Stanley, D.; Moore, R.J.; Van, T.T.H.; Robert, R.; Hickey, M.J.; et al. Deficiency of Dietary Fiber Modulates Gut Microbiota Composition, Neutrophil Recruitment and Worsens Experimental Colitis. Front. Immunol. 2021, 12, 619366. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Belzer, C.; Goossens, M.; Kleerebezem, M.; De Vos, W.M.; Thas, O.; De Weirdt, R.; Kerckhof, F.M.; Van de Wiele, T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013, 7, 949–961. [Google Scholar] [CrossRef]
- Martinez, I.; Lattimer, J.M.; Hubach, K.L.; Case, J.A.; Yang, J.; Weber, C.G.; Louk, J.A.; Rose, D.J.; Kyureghian, G.; Peterson, D.A.; et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013, 7, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Azpiroz, F.; Dubray, C.; Bernalier-Donadille, A.; Cardot, J.M.; Accarino, A.; Serra, J.; Wagner, A.; Respondek, F.; Dapoigny, M. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: A randomized, double blind, placebo controlled study. Neurogastroenterol. Motil. 2017, 29, e12911. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; Kjølbæk, L.; Gómez Del Pulgar, E.M.; Brahe, L.K.; Astrup, A.; Matysik, S.; Schött, H.F.; Krautbauer, S.; Liebisch, G.; Boberska, J.; et al. A Multi-omics Approach to Unraveling the Microbiome-Mediated Effects of Arabinoxylan Oligosaccharides in Overweight Humans. mSystems 2019, 4, e00209-19. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, S.M.; Voordouw, G. Effect of sulfide on growth physiology and gene expression of Desulfovibrio vulgaris Hildenborough. Antonie Van Leeuwenhoek 2010, 97, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, X.; Luan, C.; Li, Z. Hydrogen Sulfide Attenuates Hydrogen Peroxide-Induced Injury in Human Lung Epithelial A549 Cells. Int. J. Mol. Sci. 2019, 20, 3975. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Xie, X.Q.; Zhou, L.; Guan, Q.; Ren, Y.; Mao, Y.; Shi, J.S.; Xu, Z.H.; Geng, Y. Desulfovibrio fairfieldensis-Derived Outer Membrane Vesicles Damage Epithelial Barrier and Induce Inflammation and Pyroptosis in Macrophages. Cells 2022, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Loubinoux, J.; Bronowicki, J.P.; Pereira, I.A.; Mougenel, J.L.; Faou, A.E. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol. Ecol. 2002, 40, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Rowan, F.; Docherty, N.G.; Murphy, M.; Murphy, B.; Calvin Coffey, J.; O’Connell, P.R. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis. Colon. Rectum 2010, 53, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Fite, A.; Macfarlane, S.; Furrie, E.; Bahrami, B.; Cummings, J.H.; Steinke, D.T.; Macfarlane, G.T. Longitudinal analyses of gut mucosal microbiotas in ulcerative colitis in relation to patient age and disease severity and duration. J. Clin. Microbiol. 2013, 51, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, G.; Yang, Q.; Ye, J.; Cai, X.; Tsering, P.; Cheng, X.; Hu, C.; Zhang, S.; Cao, P. Gut microbiota drives the attenuation of dextran sulphate sodium-induced colitis by Huangqin decoction. Oncotarget 2017, 8, 48863–48874. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Yue, Y.; Guan, Q.; Ren, Y.; Guo, L.; Fan, Y.; Lu, Z.M.; Shi, J.S.; Xu, Z.H. Cereal Vinegar Sediment Alleviates Spontaneous Ulcerative Colitis in Il-10 Deficient Mice. Mol. Nutr. Food Res. 2021, 65, e2001227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Yuan, X.; Cui, Y.Y.; Liu, J.; Shen, J.; Jin, B.Y.; Feng, B.C.; Zhai, Y.J.; Zheng, M.Q.; Kou, G.J.; et al. Melatonin Mitigates Oxazolone-Induced Colitis in Microbiota-Dependent Manner. Front. Immunol. 2021, 12, 783806. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xie, Q.; Yue, Y.; Chen, Q.; Zhao, L.; Evivie, S.E.; Li, B.; Huo, G. Gut microbiota modulation and anti-inflammatory properties of mixed lactobacilli in dextran sodium sulfate-induced colitis in mice. Food Funct. 2021, 12, 5130–5143. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Peng, K.; Xiao, S.; Long, Y.; Yu, Q. The role of Lactobacillus in inflammatory bowel disease: From actualities to prospects. Cell Death Discov. 2023, 9, 361. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).