Cannabinoids in Chronic Pain: Clinical Outcomes, Adverse Effects and Legal Challenges
Abstract
1. Introduction
2. Historical Perspectives on Medical Cannabis
3. Endocannabinoid System and Analgesic Mechanisms
3.1. Cannabinoid Receptors (CB1, CB2, TRPV1, and PPAR)
3.2. Endogenous Ligands and Enzymatic Regulation (AEA, 2-AG, FAAH, and MAGL)
3.3. Central vs. Peripheral Mechanisms of Pain Modulation
3.4. Synaptic Plasticity and Maladaptive Changes
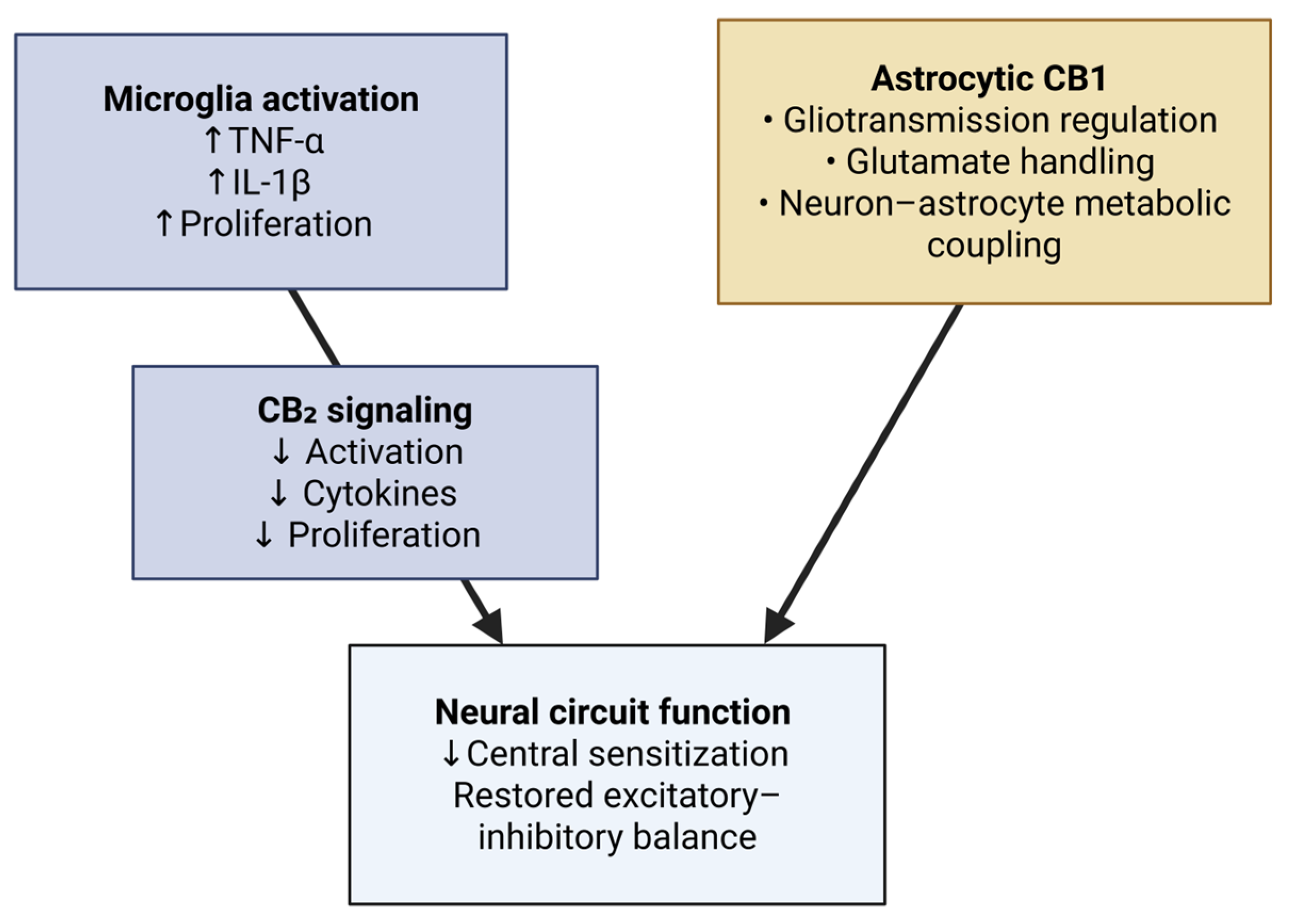
3.5. Glial Modulation and Neuroinflammation
3.6. Descending Pain Modulation
4. Cannabinoids in Pain Management
5. Ongoing Trials and Emerging Therapeutic Data
6. Regulatory Barriers and Patient Access
6.1. United States and North America
6.2. Europe and International Perspectives
6.3. Global Guidelines and Future Directions
7. Adverse Effects, Safety Concerns, and Misuse Potential
8. Discussion and Limitations
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rikard, S.M.; Strahan, A.E.; Schmit, K.M.; Guy, G.P., Jr. Chronic Pain Among Adults—United States, 2019–2021. Morb. Mortal. Wkly. Rep. 2023, 72, 379–385. [Google Scholar] [CrossRef]
- Gaskin, D.J.; Richard, P. The Economic Costs of Pain in the United States. J. Pain 2012, 13, 715–724. [Google Scholar] [CrossRef]
- Bruun-Plesner, K.; Bye-Møller, L.; Vægter, H.B. High-Impact Chronic Pain. IASP Fact Sheet, International Association for the Study of Pain. 2023. Available online: https://www.iasp-pain.org/resources/fact-sheets/high-impact-chronic-pain/ (accessed on 24 July 2025).
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic Pain: A Review of Its Epidemiology and Associated Factors in Population-Based Studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- The NNT Group. Non-Steroidal Anti-Inflammatory Drugs for Acute Low Back Pain. 2020. Available online: https://thennt.com/nnt/non-steroidal-anti-inflammatory-drugs-acute-low-back-pain (accessed on 24 August 2025).
- Mason, L.; Moore, R.A.; Edwards, J.E.; Derry, S.; McQuay, H.J. Topical NSAIDs for Chronic Musculoskeletal Pain: Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2004, 5, 28. [Google Scholar] [CrossRef]
- Lunn, M.P.; Hughes, R.A.C.; Wiffen, P.J. Duloxetine for Treating Painful Neuropathy or Chronic Pain. Cochrane Database Syst. Rev. 2009, 2009, CD007115. [Google Scholar] [CrossRef]
- Qaseem, A.; Wilt, T.J.; McLean, R.M.; Forciea, M.A. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef]
- IASP Presidential Task Force on Cannabis and Cannabinoid Analgesia. Cannabis and Cannabinoids for Pain Management: An IASP Position Statement. Pain 2021, 162, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Dahlhamer, J.M.; Connor, E.M.; Bose, J.; Lucas, J.W.; Zelaya, C.E. Prescription Opioid Use Among Adults with Chronic Pain: United States, 2019. Natl. Health Stat. Rep. 2021, 162, 1–8. [Google Scholar] [CrossRef]
- Dydyk, A.M.; Jain, N.K.; Gupta, M. Opioid Use Disorder: Evaluation and Management. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553166/ (accessed on 24 July 2025).
- Matos, C.; Pereira, A.T.; Dias, M.J.; Sousa, C.; Vinha, A.F.; Moutinho, C.; Carvalho, M. Cannabis for Chronic Pain: Mechanistic Insights and Therapeutic Challenges. Stresses 2025, 5, 7. [Google Scholar] [CrossRef]
- Leroux, T.; Ajrawat, P.; Sundararajan, K.; Syed, Y.A.; Ladak, S.; Zhang, C.; Clarke, H. Understanding the Epidemiology and Perceived Efficacy of Cannabis Use in Patients with Chronic Musculoskeletal Pain. J. Cannabis Res. 2024, 6, 28. [Google Scholar] [CrossRef]
- Crocq, M.A. History of Cannabis and the Endocannabinoid System. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.P.; Palastro, M.D.; Johnson, B.; Ditre, J.W. Cannabis and Pain: A Clinical Review. Cannabis Cannabinoid Res. 2017, 2, 96–104. [Google Scholar] [CrossRef]
- Baron, E.P. Comprehensive Review of Medicinal Marijuana, Cannabinoids, and Therapeutic Implications in Medicine and Headache: What a Long Strange Trip It’s Been…. Headache 2015, 55, 885–916. [Google Scholar] [CrossRef]
- Fine, P.G.; Rosenfeld, M.J. The Endocannabinoid System, Cannabinoids, and Pain. Rambam Maimonides Med. J. 2013, 4, e0022. [Google Scholar] [CrossRef]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef]
- Simankowicz, P.; Stępniewska, J. The Role of Endocannabinoids in Physiological Processes and Disease Pathology: A Comprehensive Review. J. Clin. Med. 2025, 14, 2851. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Landín, I.; García-Baos, A.; Castro-Zavala, A.; Valverde, O. Reviewing the Role of the Endocannabinoid System in the Pathophysiology of Depression. Front. Pharmacol. 2021, 12, 762738. [Google Scholar] [CrossRef] [PubMed]
- Khara, L.S.; Amin, M.R.; Ali, D.W. Inhibiting the endocannabinoid degrading enzymes FAAH and MAGL during zebrafish embryogenesis alters sensorimotor function. J. Exp. Biol. 2022, 225, jeb244146. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, S.; Yew, W.P.; Brookes, S.; Zagorodnyuk, V. A combination of peripherally restricted CB1 and CB2 cannabinoid receptor agonists reduces bladder afferent sensitisation in cystitis. Eur. J. Pharmacol. 2024, 985, 177078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, T.; Meng, F.; Jiang, M.; Wu, S.; Xu, H. The endocannabinoid system in the brain undergoes long-lasting changes following neuropathic pain. iScience 2024, 27, 111409. [Google Scholar] [CrossRef]
- Zorrilla, E.; Della Pietra, A.; Russo, A.F. Interplay between cannabinoids and the neuroimmune system in migraine. J. Headache Pain. 2024, 25, 178. [Google Scholar] [CrossRef]
- Martinez Ramirez, C.E.; Ruiz-Pérez, G.; Stollenwerk, T.M.; Behlke, C.; Doherty, A.; Hillard, C.J. Endocannabinoid signaling in the central nervous system. Glia 2023, 71, 5–35. [Google Scholar] [CrossRef] [PubMed]
- Albarran, E.; Sun, Y.; Liu, Y.; Raju, K.; Dong, A.; Li, Y.; Wang, S.; Sudhof, T.C.; Ding, J.B. Postsynaptic synucleins mediate endocannabinoid signaling. Nat. Neurosci. 2023, 26, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Barti, B.; Dudok, B.; Kenesei, K.; Zöldi, M.; Miczán, V.; Balla, G.Y.; Zala, D.; Tasso, M.; Sagheddu, C.; Kisfali, M.; et al. Presynaptic nanoscale components of retrograde synaptic signaling. Sci. Adv. 2024, 10, eado0077. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, S.-J. Neural Circuitry Polarization in the Spinal Dorsal Horn (SDH): A Novel Form of Dysregulated Circuitry Plasticity during Pain Pathogenesis. Cells 2024, 13, 398. [Google Scholar] [CrossRef]
- Spicarova, D.; Palecek, J. Anandamide-Mediated Modulation of Nociceptive Transmission at the Spinal Cord Level. Physiol. Res. 2024, 73 (Suppl. S1), S435–S448. [Google Scholar] [CrossRef]
- Sharma, P.; Daksh, R.; Khanna, S.; Mudgal, J.; Lewis, S.A.; Arora, D.; Nampoothiri, M. Microglial cannabinoid receptor 2 and epigenetic regulation: Implications for the treatment of depression. Eur. J. Pharmacol. 2025, 995, 177422. [Google Scholar] [CrossRef]
- Xu, K.; Wu, Y.; Tian, Z.; Xu, Y.; Wu, C.; Wang, Z. Microglial Cannabinoid CB2 Receptors in Pain Modulation. Int. J. Mol. Sci. 2023, 24, 2348. [Google Scholar] [CrossRef]
- Adermark, L.; Stomberg, R.; Söderpalm, B.; Ericson, M. Astrocytic Regulation of Endocannabinoid-Dependent Synaptic Plasticity in the Dorsolateral Striatum. Int. J. Mol. Sci. 2024, 25, 581. [Google Scholar] [CrossRef]
- Pagliusi, M., Jr.; Gomes, F.V. The Role of The Rostral Ventromedial Medulla in Stress Responses. Brain Sci. 2023, 13, 776. [Google Scholar] [CrossRef]
- Lubejko, S.T.; Livrizzi, G.; Buczynski, S.A.; Patel, J.; Yung, J.C.; Yaksh, T.L.; Banghart, M.R. Inputs to the locus coeruleus from the periaqueductal gray and rostroventral medulla shape opioid-mediated descending pain modulation. Sci. Adv. 2024, 10, eadj9581. [Google Scholar] [CrossRef]
- Sainsbury, B.; Bloxham, J.; Hassan Pour, M.; Padilla, M.; Enciso, R. Efficacy of Cannabis-Based Medications Compared to Placebo for the Treatment of Chronic Neuropathic Pain: A Systematic Review with Meta-Analysis. J. Dent. Anesth. Pain Med. 2021, 21, 479–506. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Chapter 4: Therapeutic Effects of Cannabis and Cannabinoids. In The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press: Washington, DC, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK425767/ (accessed on 22 July 2025).
- Hansen, J.S.; Hansen, R.M.; Petersen, T.; Gustavsen, S.; Oturai, A.B.; Sellebjerg, F.; Sædder, E.A.; Kasch, H.; Rasmussen, P.V.; Finnerup, N.B.; et al. The Effect of Cannabis-Based Medicine on Neuropathic Pain and Spasticity in Patients with Multiple Sclerosis and Spinal Cord Injury: Study Protocol of a National Multicenter Double-Blinded, Placebo-Controlled Trial. Brain Sci. 2021, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Yanes, J.A.; McKinnell, Z.E.; Reid, M.A.; Busler, J.N.; Michel, J.S.; Pangelinan, M.M.; Sutherland, M.T.; Younger, J.W.; Gonzalez, R.; Robinson, J.L. Effects of Cannabinoid Administration for Pain: A Meta-Analysis and Meta-Regression. Exp. Clin. Psychopharmacol. 2019, 27, 370–382. [Google Scholar] [CrossRef]
- Lo Castro, F.; Baraldi, C.; Pellesi, L.; Guerzoni, S. Clinical Evidence of Cannabinoids in Migraine: A Narrative Review. J. Clin. Med. 2022, 11, 1479. [Google Scholar] [CrossRef]
- Biringer, R.G. Treatment of Migraine with Phytocannabinoids, the Involvement of Endocannabinoids in Migraine, and Potential Mechanisms of Action. Pain Res. Manag. 2025, 2025, 7181066. [Google Scholar] [CrossRef] [PubMed]
- Schuster, N.M.; Wallace, M.S.; Marcotte, T.D.; Buse, D.C.; Lee, E.; Liu, L.; Sexton, M. Vaporized Cannabis versus Placebo for Acute Migraine: A Randomized Controlled Trial. medRxiv 2024. [Google Scholar] [CrossRef]
- van de Donk, T.; Niesters, M.; Kowal, M.A.; Olofsen, E.; Dahan, A.; van Velzen, M. An Experimental Randomized Study on the Analgesic Effects of Pharmaceutical-Grade Cannabis in Chronic Pain Patients with Fibromyalgia. Pain 2019, 160, 860–869. [Google Scholar] [CrossRef]
- Chaves, C.; Bittencourt, P.C.T.; Pelegrini, A. Ingestion of a THC-Rich Cannabis Oil in People with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pain Med. 2020, 21, 2212–2218. [Google Scholar] [CrossRef]
- Abrams, D.I.; Couey, P.; Dixit, N.; Sagi, V.; Hagar, W.; Vichinsky, E.; Kelly, M.E.; Connett, J.E.; Gupta, K. Effect of Inhaled Cannabis for Pain in Adults with Sickle Cell Disease: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010874. [Google Scholar] [CrossRef]
- Eibach, L.; Scheffel, S.; Cardebring, M.; Lettau, M.; Özgür Celik, M.; Morguet, A.; Roehle, R.; Stein, C. Cannabidivarin for HIV-Associated Neuropathic Pain: A Randomized, Blinded, Controlled Clinical Trial. Clin. Pharmacol. Ther. 2021, 109, 1055–1062. [Google Scholar] [CrossRef]
- Heineman, J.T.; Forster, G.L.; Stephens, K.L.; Cottler, P.S.; Timko, M.P.; DeGeorge, B.R., Jr. A Randomized Controlled Trial of Topical Cannabidiol for the Treatment of Thumb Basal Joint Arthritis. J. Hand Surg. Am. 2022, 47, 611–620. [Google Scholar] [CrossRef]
- Almog, S.; Aharon-Peretz, J.; Vulfsons, S.; Ogintz, M.; Abalia, H.; Lupo, T.; Hayon, Y.; Eisenberg, E. The Pharmacokinetics, Efficacy, and Safety of a Novel Selective-Dose Cannabis Inhaler in Patients with Chronic Pain: A Randomized, Double-Blinded, Placebo-Controlled Trial. Eur. J. Pain 2020, 24, 1505–1516. [Google Scholar] [CrossRef]
- Pramhas, S.; Thalhammer, T.; Terner, S.; Pickelsberger, D.; Gleiss, A.; Sator, S.; Kress, H.G. Oral Cannabidiol (CBD) as Add-On to Paracetamol for Painful Chronic Osteoarthritis of the Knee: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Lancet Reg. Health Eur. 2023, 35, 100777. [Google Scholar] [CrossRef] [PubMed]
- A Study of Medical Cannabis Aerosol Via the Fixed-Dose Syqe Inhaler as an Add-on Treatment of Diabetic Peripheral Neuropathic Pain (DPNP); ClinicalTrials.gov Identifier: NCT06490445; ClinicalTrials.gov. 2024. Available online: https://clinicaltrials.gov/study/NCT06490445 (accessed on 24 July 2025).
- Cannabidiol After Multi-Trauma for Pain and Opioid Therapy (CAM-POT); ClinicalTrials.gov Identifier: NCT06448923; ClinicalTrials.gov. 2025. Available online: https://clinicaltrials.gov/study/NCT06448923 (accessed on 24 July 2025).
- Brain Mechanisms Supporting Cannabis-induced Pain Relief; ClinicalTrials.gov Identifier: NCT04982965; ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/study/NCT04982965 (accessed on 24 July 2025).
- Outcomes Mandate National Integration with Cannabis as Medicine (OMNI-Can). ClinicalTrials.gov Identifier: NCT03944447. Available online: https://clinicaltrials.gov/study/NCT03944447 (accessed on 24 July 2025).
- Dronabinol As an Adjunct for Reducing Pain (DARP). ClinicalTrials.gov Identifier: NCT06834997. Available online: https://clinicaltrials.gov/study/NCT06834997 (accessed on 24 July 2025).
- MIVetsCan: Cannabidiol (CBD)-Care Trial. ClinicalTrials.gov Identifier: NCT06213233. Available online: https://clinicaltrials.gov/study/NCT06213233 (accessed on 24 July 2025).
- Proof of Concept Trial of Cannabis Derivatives in Neuropathic Pain; ClinicalTrials.gov Identifier: NCT05351801; ClinicalTrials.gov. 2023. Available online: https://clinicaltrials.gov/study/NCT05351801 (accessed on 24 July 2025).
- Improving Pain Disability with the Use of Oral Cannabinoids; ClinicalTrials.gov Identifier: NCT05351905; ClinicalTrials.gov. 2022. Available online: https://clinicaltrials.gov/study/NCT05351905 (accessed on 25 July 2025).
- Christo, P.J.; Vortsman, E.; Gharibo, C.; LeQuang, J.A.K.; Pergolizzi, J.V. Considering Long-Acting Synthetic Cannabidiol for Chronic Pain: A Narrative Review. Cureus 2025, 17, e81577. [Google Scholar] [CrossRef]
- Massachusetts Health Policy Forum. Medical Cannabis: Time to Act. Evidence-Based Strategies for Patient-Centered Care in Massachusetts; The Heller School for Social Policy and Management, Brandeis University: Waltham, MA, USA, 2025; Available online: https://heller.brandeis.edu/mass-health-policy-forum/categories/public-health/pdfs/medical-cannabis/medical-cannabis-issue-brief-2.26.25.pdf (accessed on 24 July 2025).
- Mehta, S.K.; Tusing, L.D.; Higazy, A.; Piper, B.J. Medical Cannabis Certifications for Severe Chronic and Intractable Pain: Discerning Geographic Patterns across Pennsylvania, USA. medRxiv 2025. [Google Scholar] [CrossRef]
- Health Canada. Regulations Amending Certain Regulations Concerning Cannabis (Streamlining of Requirements)—SOR/2025-43. Canada Gazette, Part II. 2025. Available online: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/summary-changes-following-streamlining-regulations.html (accessed on 24 August 2025).
- Cassels. Federally Proposed Amendments Now in Effect. 2025. Available online: https://cassels.com/insights/streamlining-the-cannabis-regulations-federally-proposed-amendments-now-in-effect/ (accessed on 24 August 2025).
- Etges, T.; Karolia, K.; Grint, T.; Taylor, A.; Lauder, H.; Daka, B.; Wright, S. An Observational Postmarketing Safety Registry of Patients in the UK, Germany, and Switzerland Who Have Been Prescribed Sativex® (THC:CBD, Nabiximols) Oromucosal Spray. Ther. Clin. Risk Manag. 2016, 12, 1667–1675. [Google Scholar] [CrossRef]
- Canadian Centre on Substance Use and Addiction (CCSA). Clearing the Smoke on Cannabis: Medical Use of Cannabis and Cannabinoids—2024 Update; CCSA: Ottawa, ON, Canada, 2024. Available online: https://www.ccsa.ca/sites/default/files/2024-04/Clearing-the-Smoke-on-Cannabis-Medical-Use-of-Cannabis-and-Cannabinoids-2024-Update-en.pdf (accessed on 24 July 2025).
- Manthey, J.; Rehm, J.; Verthein, U. Germany’s Cannabis Act: A Catalyst for European Drug Policy Reform? Lancet Reg. Health Eur. 2024, 42, 100929. [Google Scholar] [CrossRef]
- Baltes-Flückiger, L.; Steinauer, R.; Meyer, M.; Vogel, M.; Walter, M. Effects of Cannabis Regulation in Switzerland: Study Protocol of a Randomized Controlled Trial (“Weed Care”). Front. Psychiatry 2023, 14, 1139325. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). FDA and Cannabis: Research and Drug Approval Process. 2024. Available online: https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process (accessed on 25 July 2025).
- U.S. Department of Health and Human Services; Food and Drug Administration (FDA). Basis for the Recommendation to Reschedule Marijuana into Schedule III of the Controlled Substances Act; U.S. Public Health Service: Washington, DC, USA, 2024. Available online: https://www.dea.gov/sites/default/files/2024-05/2016-17954-HHS.pdf (accessed on 24 July 2025).
- Kansagara, D.; Kansagara, D.; Hill, K.P.; Yost, J.; Humphrey, L.L.; Shaw, B.; Obley, A.J.; Haeme, R.; Akl, E.A.; Qaseem, A. Cannabis or Cannabinoids for the Management of Chronic Noncancer Pain: Best Practice Advice from the American College of Physicians. Ann. Intern. Med. 2025, 178, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.D.; MacCallum, C.; Margolese, S.; Walsh, Z.; Wright, P.; Daeninck, P.J.; Mandarino, E.; Lacasse, G.; Kaur Deol, J.; de Freitas, L.; et al. Clinical Practice Guidelines for Cannabis and Cannabinoid-Based Medicines in the Management of Chronic Pain and Co-Occurring Conditions. Cannabis Cannabinoid Res. 2024, 9, 669–687. [Google Scholar] [CrossRef]
- Bhaskar, A.; Bell, A.; Boivin, M.; Blais Coste, J.; Sanchez, T.; MacCallum, C.; Lefebvre, D.; Blais Morin, C.; Moulin, D.E.; Perrot, S.; et al. Consensus Recommendations on Dosing and Administration of Medical Cannabis to Treat Chronic Pain: Results of a Modified Delphi Process. J. Cannabis Res. 2021, 3, 22. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Cannabis-Based Medicinal Products: Evidence-Based Recommendations for Chronic Pain. NICE Guideline [NG144]. 2019. Available online: https://www.nice.org.uk/guidance/ng144 (accessed on 24 August 2025).
- Therapeutic Goods Administration (TGA). Guidance for the Use of Medicinal Cannabis in Australia—Overview. 2025. Available online: https://www.tga.gov.au/products/unapproved-therapeutic-goods/medicinal-cannabis-hub/medicinal-cannabis-guidance-documents/guidance-use-medicinal-cannabis-australia-overview (accessed on 24 August 2025).
- Johnson, B.W.; Strand, N.H.; Raynak, J.C.; Jara, C.; Habtegiorgis, K.; Hand, B.A.; Hong, S.; Maloney, J.A. Cannabinoids in Chronic Pain Management: A Review of the History, Efficacy, Applications, and Risks. Biomedicines 2025, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Riccitelli, G.C.; Disanto, G.; Bogousslavsky, J.; Cavelti, A.; Czell, D.; Kamm, C.P.; Kliesch, U.; Ramseier, S.P.; Gobbi, C.; et al. Effectiveness, Safety and Patients’ Satisfaction of Nabiximols (Sativex®) on Multiple Sclerosis Spasticity and Related Symptoms in a Swiss Multicenter Study. J. Clin. Med. 2024, 13, 2907. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Kaur, V.; Bui, H.L.; Yang, T.; Erridge, S.; Holvey, C.; Coomber, R.; Rucker, J.J.; Weatherall, M.W.; Sodergren, M.H. Clinical Outcome Analysis of Patients with Multiple Sclerosis—Analysis from the UK Medical Cannabis Registry. Mult. Scler. Relat. Disord. 2024, 87, 105665. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Volkow, N.D.; Friemel, C.M.; Schneider, M. Cannabis, Cannabinoids and Health: A Review of Evidence on Risks and Medical Benefits. Eur. Arch. Psychiatry Clin. Neurosci. 2025, 275, 281–292. [Google Scholar] [CrossRef]
- Prieto González, J.M.; Vila Silván, C. Safety and Tolerability of Nabiximols Oromucosal Spray: A Review of Real-World Experience in Observational Studies, Registries, and Case Reports. Expert Rev. Neurother. 2021, 21, 547–558. [Google Scholar] [CrossRef]
- Cortez-Resendiz, A.; Leiter, T.J.; Riela, S.M.; Graziane, N.M.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacology of Cannabinoids in Chronic Pain. Med. Cannabis Cannabinoids 2025, 8, 31–46. [Google Scholar] [CrossRef]
- Division of Workers’ Compensation. Cannabis Guideline. In Evidence-Based Update to the Medical Treatment Utilization Schedule (MTUS), Section 9792.24.8; California Department of Industrial Relations: Oakland, CA, USA, 2025. Available online: https://www.dir.ca.gov/dwc/DWCPropRegs/2025/MTUS-Evidence-Based-Update/Cannabis-Guideline.pdf (accessed on 25 August 2025).
- Al-Husinat, L.; Obeidat, S.; Azzam, S.; Al-Gwairy, Y.; Obeidat, F.; Al Sharie, S.; Haddad, D.; Haddad, F.; Rekatsina, M.; Leoni, M.L.G.; et al. Role of Cannabis in the Management of Chronic Non-Cancer Pain: A Narrative Review. Clin. Pract. 2025, 15, 16. [Google Scholar] [CrossRef]
- Florian, J.; Salcedo, P.; Burkhart, K.; Shah, A.; Chekka, L.M.S.; Keshishi, D.; Patel, V.; Yang, S.; Fein, M.; DePalma, R.; et al. Cannabidiol and Liver Enzyme Level Elevations in Healthy Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2025, in press. [Google Scholar] [CrossRef]
- Balachandran, P.; Elsohly, M.; Hill, K.P. Cannabidiol Interactions with Medications, Illicit Substances, and Alcohol: A Comprehensive Review. J. Gen. Intern. Med. 2021, 36, 2074–2084. [Google Scholar] [CrossRef]
- National Institute on Drug Abuse (NIDA). Cannabis Potency Data. National Institute on Drug Abuse. Available online: https://nida.nih.gov/research/research-data-measures-resources/cannabis-potency-data (accessed on 28 August 2025).
- Axios. Chart: THC Levels in Cannabis Have Soared. Axios Local San Francisco. Available online: https://www.axios.com/local/san-francisco/2025/04/18/cannabis-thc-high-levels-potency-chart (accessed on 28 August 2025).
- Snooks, T.; Stewart, S.H.; Romero-Sanchiz, P.; DeGrace, S.; Barrett, S.P.; Bernusky, H.C.R.; Tibbo, P.G. The roles of cannabis potency and gender in cannabis dependence and anxiety in recent cannabis users with trauma exposure histories. Pharmacol. Res. 2025, 212, 107586. [Google Scholar] [CrossRef]
- Ahrens, J.; Ford, S.D.; Schaefer, B.; Reese, D.; Khan, A.R.; Tibbo, P.; Rabin, R.; Cassidy, C.M.; Palaniyappan, L. Convergence of Cannabis and Psychosis on the Dopamine System. JAMA Psychiatry 2025, 82, 609–617. [Google Scholar] [CrossRef]
- King’s College London. High-Potency Cannabis Linked to Higher Rates of Psychosis. King’s College London Archive News, 19 March 2019. Available online: https://www.kcl.ac.uk/archive/news/ioppn/records/2019/march/high-potency-cannabis-linked-to-higher-rates-of-psychosis (accessed on 27 August 2025).
- Schoeler, T.; Ferris, J.; Winstock, A.R. Rates and Correlates of Cannabis-Associated Psychotic Symptoms in over 230,000 People Who Use Cannabis. Transl. Psychiatry 2022, 12, 369. [Google Scholar] [CrossRef]
- Billion, Z.; Hein, M. Impact de la Légalisation du Cannabis à Usage Récréatif sur le Risque de Psychose: Une Revue Systématique de la Littérature [Impact of the Legalization of Recreational Cannabis on the Risk of Psychosis: A Systematic Review of the Literature]. Encephale 2025, 51, 186–201. [Google Scholar] [CrossRef]
- Myran, D.T.; Pugliese, M.; Harrison, L.D.; Solmi, M.; Anderson, K.K.; Fiedorowicz, J.G.; Finkelstein, Y.; Manuel, D.; Taljaard, M.; Webber, C.; et al. Changes in Incident Schizophrenia Diagnoses Associated with Cannabis Use Disorder After Cannabis Legalization. JAMA Netw. Open 2025, 8, e2457868. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Ganae-Motan, E.D.; Allende, S.; Yanagihara, R.; Shaiova, L.; Weinstein, S.; McQuade, R.; Wright, S.; Fallon, M.T. Nabiximols for Opioid-Treated Cancer Patients with Poorly-Controlled Chronic Pain: A Randomized, Placebo-Controlled, Graded-Dose Trial. J. Pain 2012, 13, 438–449. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, M.S.; Wagner, J.; Ahmed, A.Y.; Fu, R.; Griffin, J.C.; Morasco, B.J.; Kansagara, D. Living Systematic Review on Cannabis and Other Plant-Based Treatments for Chronic Pain: Surveillance Report 2: Literature Update Period: August 2021 through October 2021. In Living Systematic Review on Cannabis and Other Plant-Based Treatments for Chronic Pain: Interim Progress and Surveillance Reports; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK589234/ (accessed on 26 July 2025).
- Bicket, M.C.; Stone, E.M.; McGinty, E.E. Use of Cannabis and Other Pain Treatments among Adults with Chronic Pain in US States with Medical Cannabis Programs. JAMA Netw. Open 2023, 6, e2249797. [Google Scholar] [CrossRef]
- Ware, M.A.; Wang, T.; Shapiro, S.; Collet, J.P.; COMPASS Study Team. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J. Pain 2015, 16, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Giossi, R.; Carrara, F.; Padroni, M.; Bertora, P.; Avenali, M.; Sormani, M.P.; Ferini-Strambi, L.; Gialdini, G. Systematic Review and Meta-Analysis Seem to Indicate That Cannabinoids for Chronic Primary Pain Treatment Have Limited Benefit. Pain Ther. 2022, 11, 1341–1358. [Google Scholar] [CrossRef]
- Jylkkä, J.; Hupli, A.; Nikolaeva, A.; Karjalainen, M.; Malinen, M.; Hakkarainen, P. The Holistic Effects of Medical Cannabis Compared to Opioids on Pain Experience in Finnish Patients with Chronic Pain. J. Cannabis Res. 2023, 5, 38. [Google Scholar] [CrossRef]
- Piper, B.J.; Beals, M.L.; Abess, A.T.; Nichols, S.D.; Martin, M.W.; Cobb, C.M.; DeKeuster, R.M. Chronic Pain Patients’ Perspectives of Medical Cannabis. Pain 2017, 158, 1373–1379. [Google Scholar] [CrossRef]
- Soliman, N.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Liao, J.; Macleod, M.; Segelcke, D.; Sena, C.; Thomas, J.; Vollert, J.; et al. Systematic Review and Meta-Analysis of Cannabinoids, Cannabis-Based Medicines, and Endocannabinoid System Modulators Tested for Antinociceptive Effects in Animal Models of Injury-Related or Pathological Persistent Pain. Pain 2021, 162 (Suppl. S1), S26–S44. [Google Scholar] [CrossRef]
- Ayub, S.; Bachu, A.K.; Jain, L.; Parnia, S.; Bhivandkar, S.; Ahmed, R.; Kaur, J.; Karlapati, S.; Prasad, S.; Kochhar, H.; et al. Non-Opioid Psychiatric Medications for Chronic Pain: Systematic Review and Meta-Analysis. Front. Pain Res. 2024, 5, 1398442. [Google Scholar] [CrossRef]
- Dey, S.; Sanders, A.E.; Martinez, S.; Kopitnik, N.L.; Vrooman, B.M. Alternatives to Opioids for Managing Pain. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK574543/ (accessed on 25 August 2025).
- Moore, R.A.; Wiffen, P.J.; Derry, S.; Toelle, T.; Rice, A.S. Gabapentin for Chronic Neuropathic Pain and Fibromyalgia in Adults. Cochrane Database Syst. Rev. 2014, 4, CD007938. [Google Scholar] [CrossRef]
- Mayoral, V.; Gálvez, R.; Ferrándiz, M.; Miguéns Vázquez, X.; Cordero-García, C.; Alcántara Montero, A.; Pérez, C.; Pérez-Páramo, M. Pregabalin vs. Gabapentin in the Treatment of Neuropathic Pain: A Comprehensive Systematic Review and Meta-Analysis of Effectiveness and Safety. Front. Pain Res. 2024, 5, 1513597. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; Delanerolle, G.; Cavalini, H.; Deng, C.; Yang, X.; Boyd, A.; Fernandez, T.; Phiri, P.; Bhaskar, A.; Shi, J.Q. A Systematic Review and Network Meta-Analysis of Pharmaceutical Interventions Used to Manage Chronic Pain. Sci. Rep. 2024, 14, 1621. [Google Scholar] [CrossRef]
- Hameed, M.; Prasad, S.; Jain, E.; Dogrul, B.N.; Al-Oleimat, A.; Pokhrel, B.; Chowdhury, S.; Co, E.L.; Mitra, S.; Quinonez, J.; et al. Medical Cannabis for Chronic Nonmalignant Pain Management. Curr. Pain Headache Rep. 2023, 27, 57–63. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Beilby, J.; Frans, M.; Bonomo, Y.; McGregor, I.S.; Arkell, T.R. Medicinal Cannabis for Pain: Real-World Data on Three-Month Changes in Symptoms and Quality of Life. Drug Sci. Policy Law 2023, 9, 20503245231172535. [Google Scholar] [CrossRef]
- Chesney, E.; Oliver, D.; Green, A.; Sovi, S.; Wilson, J.; Englund, A.; Freeman, T.P.; McGuire, P. Adverse effects of cannabidiol: A systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology 2020, 45, 1799–1806. [Google Scholar] [CrossRef]
- Serpell, M.; Ratcliffe, S.; Hovorka, J.; Schofield, M.; Taylor, L.; Lauder, H.; Ehler, E. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD oromucosal spray in patients with central neuropathic pain due to multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1344–1349. [Google Scholar] [CrossRef]
- Almuntashiri, N.; El Sharazly, B.M.; Carter, W.G. Are Cannabis-Based Medicines a Useful Treatment for Neuropathic Pain? A Systematic Review. Biomolecules 2025, 15, 816. [Google Scholar] [CrossRef] [PubMed]
- George, B.J.; Aban, I.B. An Application of Meta-Analysis Based on DerSimonian and Laird Method. J. Nucl. Cardiol. 2016, 23, 690–692. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.E.; Veroniki, A.A. A Brief Note on the Random-Effects Meta-Analysis Model and Its Relationship to Other Models. J. Clin. Epidemiol. 2024, 174, 111492. [Google Scholar] [CrossRef]
- Woo, J.J.; van Reekum, E.A.; Rosic, T.; Samaan, Z. Children and Youth Who Use Cannabis for Pain Relief: Benefits, Risks, and Perceptions. Adolesc. Health Med. Ther. 2020, 11, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.C.; Vilela de Faria, A.O.; Bizzi, I.; Moreira, F.A.; Colasanti, A.; Ghezzi, P. Online Information on Medical Cannabis May Rise Unrealistic Expectations and Downplay Potential Side Effects. arXiv 2020. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Chapter 15: Challenges and Barriers in Conducting Cannabis Research. In The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press: Washington, DC, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK425757/ (accessed on 25 August 2025).
- The Guardian. US Bill Seeks to Ease Restrictions on Medical Cannabis Research. 10 May 2025. Available online: https://www.theguardian.com/us-news/2025/may/10/cannabis-medical-research-bill (accessed on 25 August 2025).
- Reuters. Update: DEA’s Efforts to Reschedule Cannabis—What You Need to Know. 11 September 2024. Available online: https://www.reuters.com/legal/litigation/update-deas-efforts-reschedule-cannabis-what-you-need-know-2024-09-11/ (accessed on 25 August 2025).
- Baratta, F.; Simiele, M.; Pautasso, A.; Mercadante, S.; De Luca, M.L.; Brusa, P.; De Luca, A. Analysis of Recent Clinical Trials on Medical Cannabis: Regulatory Legislation in Countries Allowing Medical Use. Front. Pharmacol. 2022, 13, 888903. [Google Scholar] [CrossRef]
- Ruheel, M.A.; Gomes, Z.; Usman, S.; Khan, A.; Hussain, S.; Monaghan, T. Facilitators and barriers to the regulation of medical cannabis: A scoping review of the peer-reviewed literature. Harm Reduct. J. 2021, 18, 106. [Google Scholar] [CrossRef]





| Study (Author, Year) | Population | Intervention | Control | Outcome | Conclusion |
|---|---|---|---|---|---|
| van de Donk et al., 2019 [42] | 20 patients with fibromyalgia (chronic muscle pain) | Single inhalation of pharmaceutical cannabis: 3 strains (THC-dominant, CBD-dominant, and THC + CBD combination) | Inhaled placebo (without THC/CBD) | No difference in spontaneous pain between cannabis and placebo. However, the THC + CBD combination showed a higher proportion of patients achieving ≥30% pain reduction (90% vs. 55%; p = 0.01). THC-containing strains increased pressure pain threshold (p < 0.01). No serious adverse effects. | Limited short-term analgesic effect of THC (with/without CBD) in fibromyalgia. |
| Chaves et al., 2020 [43] | 17 women with fibromyalgia (low socioeconomic status) | Oral THC-rich cannabis oil (24 mg/mL THC; 0.5 mg/mL CBD); titrated to ~30 mg THC/day for 8 weeks | Oral placebo oil | Significant reduction in Fibromyalgia Impact Questionnaire (FIQ) scores in THC group compared with placebo (p = 0.005). Improvements noted in well-being, pain intensity, work ability, and fatigue. No severe adverse effects. | THC oil improved fibromyalgia symptoms and was well tolerated. |
| Abrams et al., 2020 [44] | 23 adults with sickle cell disease (SCD) and chronic pain (crossover study) | Inhaled cannabis (vaporizer) with 4.4% THC and 4.9% CBD, 3×/day for 5 days (in-hospital) | Inhaled placebo (no active components) | No significant difference in daily average pain ratings. Only minor mood-related improvements (p = 0.02). No effect on sleep, physical activity, or opioid use. Well tolerated. | Cannabis not superior to placebo for pain relief in SCD. |
| Eibach et al., 2021 [45] | 32 patients with HIV-related neuropathic pain (crossover RCT) | Oral cannabidivarin (CBDV), 400 mg/day for 4 weeks | Oral placebo (identical format) | No reduction in neuropathic pain with CBDV vs. placebo (pain score slightly worse with CBDV; p = 0.16). No impact on analgesic use, pain features, or quality of life. Well tolerated. | CBDV was safe but ineffective for HIV-related neuropathic pain. |
| Heineman et al., 2022 [46] | 18 patients with base-of-thumb osteoarthritis (hand joint pain; crossover) | Topical CBD gel (6.2 mg/mL in shea butter), applied 2× daily for 2 weeks | Topical placebo gel (same base) | CBD gel significantly reduced pain compared with placebo (VAS 5.0→2.2 vs. 4.9→4.0). Improved hand function (DASH score). No adverse events or local irritation. | Topical CBD effective for localized joint pain without side effects. |
| Almog et al., 2020 [47] | 27 patients with chronic neuropathic pain or CRPS | Inhaled metered-dose THC (0.5 mg and 1.0 mg) via Syqe Inhaler | Inhaled placebo (identical device) | 1.0 mg of THC significantly reduced pain intensity (p = 0.014); 0.5 mg showed milder, non-significant effect. No cognitive decline or serious AEs. | Low-dose inhaled THC provided dose-dependent analgesia and was well tolerated. |
| Pramhas et al., 2023 [48] | 86 patients with chronic knee osteoarthritis pain (~63 years old; 8 weeks of treatment) | Oral CBD capsules (600 mg/day) + acetaminophen (3 g/day) | Placebo capsules + same acetaminophen | No additional pain relief with CBD vs. placebo after 8 weeks (WOMAC pain score reduced equally in both groups; p = 0.80). Adverse effects more common in CBD group, including elevated liver enzymes. | High-dose CBD not effective and associated with more side effects. |
| Study ID | Location(s) | Type of Cannabinoid | Target Population | Status | Expected Completion |
|---|---|---|---|---|---|
| NCT06490445 [49] | USA and other countries (multicenter aerosol study) | Aerosolized medical cannabis (~0.25–1.0 mg of Δ9-THC per inhalation via Syqe inhaler) | Adults with diabetic peripheral neuropathic pain | Recruiting (phase 2) | Nov 2025 |
| NCT06448923 [50] | Montreal, Canada (trauma center) | Oral CBD (synthetic, two-dose regimen) | Patients with polytrauma (long bone fracture) and acute pain (preventing chronic pain) | Not yet recruiting (phase 2) | Sep 2026 |
| NCT04982965 [51] | United States (UC San Diego and others) | Inhaled (vaporized) cannabis (5% THC by weight) vs. placebo | Adults (21–65; healthy volunteers and chronic pain model) | Recruiting (phase 1) | Mar 2027 |
| NCT03944447 [52] | Multiple U.S. states (multicenter) | Inhaled medical cannabis (doses/strains vary by condition; via RYAH inhaler) | ~200,000 patients across ≥33 chronic conditions (including chronic pain, neuropathic pain, cancer pain, PTSD, etc.) | Recruiting (open-label phase 2) | Dec 2025 |
| NCT06834997 [53] | Texas Children’s Hospital (Houston, USA) | Oral dronabinol capsules (synthetic Δ9-THC, up to 30 mg/day) | ~75 women with endometriosis-related chronic pelvic pain (refractory to standard treatment) | Not yet recruiting (phase 2 pilot RCT) | Dec 2027 |
| NCT06213233 [54] | Michigan and partner VA sites (USA) | Oral CBD solution vs. placebo | 468 military veterans with chronic pain (musculoskeletal, neuropathic, etc.) | Enrolling (phase 2 RCT) | Dec 2026 |
| NCT05351801 [55] | VA San Diego (CA), Seattle (WA), and San Antonio (TX), USA | Oral THC, CBD, THC + CBD (4 arms: THC [Syndros], CBD [Epidiolex], THC + CBD [nabiximols] vs. placebo) | Veterans with chronic neuropathic pain (high-impact CNP), ≥21 years | Active, recruiting (phase 2 RCT) | Jun 2027 |
| NCT05351905 [56] | Toronto General Hospital (Ontario, Canada) | Oral CBD (alone) or CBD + THC oil vs. placebo | ~51 adults with chronic non-palliative pain (Toronto General Transitional Pain Service) | Recruiting (pilot RCT, phase 2) | Apr 2026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sic, A.; George, C.; Gonzalez, D.F.; Tseriotis, V.-S.; Knezevic, N.N. Cannabinoids in Chronic Pain: Clinical Outcomes, Adverse Effects and Legal Challenges. Neurol. Int. 2025, 17, 141. https://doi.org/10.3390/neurolint17090141
Sic A, George C, Gonzalez DF, Tseriotis V-S, Knezevic NN. Cannabinoids in Chronic Pain: Clinical Outcomes, Adverse Effects and Legal Challenges. Neurology International. 2025; 17(9):141. https://doi.org/10.3390/neurolint17090141
Chicago/Turabian StyleSic, Aleksandar, Conor George, Daniela Ferrer Gonzalez, Vasilis-Spyridon Tseriotis, and Nebojsa Nick Knezevic. 2025. "Cannabinoids in Chronic Pain: Clinical Outcomes, Adverse Effects and Legal Challenges" Neurology International 17, no. 9: 141. https://doi.org/10.3390/neurolint17090141
APA StyleSic, A., George, C., Gonzalez, D. F., Tseriotis, V.-S., & Knezevic, N. N. (2025). Cannabinoids in Chronic Pain: Clinical Outcomes, Adverse Effects and Legal Challenges. Neurology International, 17(9), 141. https://doi.org/10.3390/neurolint17090141










